SUMMARY
Oligodendrocyte progenitor cells (OPCs) can repair demyelinated lesions by maturing into myelin-producing oligodendrocytes. However, the OPC potential to differentiate can be prevented by inhibitory signals present in the pathological lesion environment. Identification of these signals is essential to promote OPC differentiation and lesion repair. We identified an endogenous inhibitor of remyelination, Endothelin-1 (ET-1), which is highly expressed in reactive astrocytes of demyelinated lesions. Using both gain- and loss-of-function approaches, we demonstrate that ET-1 drastically reduces the rate of remyelination. We also discovered that ET-1 acts mechanistically by promoting Notch activation in OPCs during remyelination through induction of Jagged1 expression in reactive astrocytes. Pharmacological inhibition of ET-signaling prevented Notch activation in demyelinated lesions, and accelerated remyelination. These findings reveal that ET-1 is a negative regulator of OPC differentiation and remyelination, and is potentially a novel therapeutic target to promote lesion repair in demyelinated tissue.
INTRODUCTION
Current multiple sclerosis (MS) therapies can be effective in patients with relapsing and remitting MS, but have little impact in promoting remyelination in tissue, leading to permanently demyelinated lesions with substantial axonal loss (Buck and Hemmer, 2011; Compston and Coles, 2008). Repair of demyelinated MS plaques is carried out by endogenous oligodendrocyte progenitor cells (OPCs) in a process called remyelination (Ffrench-Constant and Raff, 1986). However, several studies have shown that OPCs often fail to differentiate in chronic MS lesions (Chang et al., 2002; Wolswijk, 1998). The molecular mechanisms that prevent OPC maturation and OL regeneration under pathological conditions are largely unknown.
OPCs migrate to demyelinated lesions, proliferate, and eventually differentiate into mature OLs to produce myelin (Franklin and Ffrench-Constant, 2008). This transition from a progenitor cell to a myelinating OL can be negatively regulated by signals which are present in the pathological lesion environment. This is created, in part, by a dense network of reactive astrocytes (RAs) (Compston and Coles, 2008; McKhann, 1982). It is still poorly understood how RAs impact OPC development, and whether signals released or expressed by astrocytes limit remyelination (Moore et al., 2010; Nair et al., 2008). Interestingly, recent studies have identified the Notch activator Jagged1 as a signal expressed by RAs in MS tissue that might limit OPC differentiation and remyelination (John et al., 2002; Stidworthy et al., 2004; Zhang et al., 2009). However, it is still unknown how Jagged1 expression or Notch activation is regulated in demyelinated lesions, and whether these pathways are beneficial or detrimental to the overall remyelination process.
In a previous study, we identified endothelin-1 (ET-1) as a signaling molecule synthesized in the corpus callosum (CC) following demyelinating injury (Gadea et al., 2008). ET-1 is a secreted signaling peptide, which has systemic roles as a vasomodulator in the cardiovascular system (Rubanyi and Botelho, 1991). Interestingly, RAs produce ET-1 following various brain injuries and we found that this peptide promotes reactive astrogliosis in demyelinated tissue (Gadea et al., 2008; Jiang et al., 1993). Despite the abundance of ET-1 following injury, and its essential role in inducing reactive astrogliosis, the role or mechanistic action of ET-1 during remyelination have not been defined.
Here we use the well-established lysolecithin model of focal demyelination to recapitulate some aspects of the focal lesions that are found in MS tissue. Specifically, this model allows us to investigate the time course and cell-specificity of ET-1 signaling, and how it regulates remyelination efficiency in vivo. Using both genetic and pharmacological approaches, we are the first to demonstrate the mechanistic action of ET-1 during remyelination. We show that astrocyte-derived ET-1 inhibits OPC differentiation and remyelination through activation of Notch signaling, and that this effect can be reversed by a clinically used ET-R pan-antagonist. Our results present a new therapeutic candidate to promote repair in demyelinated lesions where OPC differentiation is stalled or limited.
RESULTS
ET-1 is expressed by reactive astrocytes in MS and murine demyelinated lesions
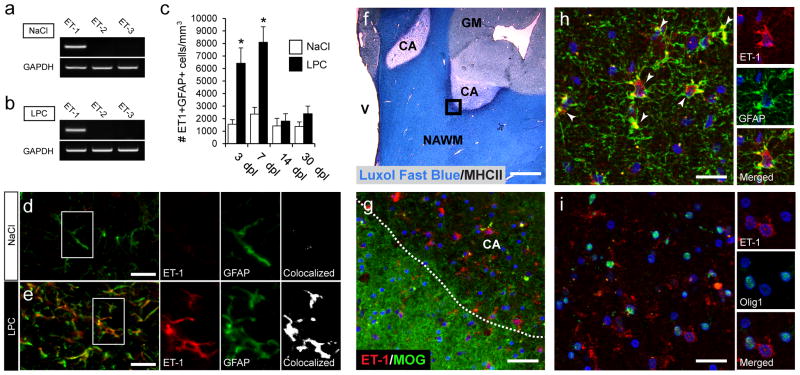
We have previously demonstrated that the neuropeptide ET-1 is upregulated in the CC following lysolecithin (LPC)-induced focal demyelination, and that overall ET-1 levels peak at 5 days post lesion (dpl) (Gadea et al., 2008). While we found ET-1 co-expression in GFAP+ cells in the SVZ during development (Gadea et al., 2009), expression of ET-1 in astrocytes in LPC lesions had not been analyzed. Of the three endothelin isoforms, only ET-1 mRNA was found in the micro-dissected tissue from the CC and cingulum, in either saline- or LPC-injected tissue (Fig 1a,b). Further ET-1 expression analysis revealed that ET-1 was specifically upregulated in GFAP+ astrocytes within LPC lesions (Fig 1d,e). The total number of ET-1+GFAP+ cells peaked between 3 and 7dpl, and gradually decreased until 30dpl, when only very few double-labeled cells were found (Fig 1c). Co-staining with CD31, an endothelial cell marker, showed a small increase in the number of CD31+ET-1+ cells at 3 and 7dpl, but there was no difference between vehicle- and LPC-injected hemispheres (Supp. Fig 1a,b,f). CD31+ET-1+ cells made up approximately 22% and 14% of the total number of ET-1+ cells at 3 and 7 dpl, respectively. (Supp. Fig 1e). We found a slight upregulation of ET-1 in MAC1+ microglia (Supp. Fig 1c,d,g), but these cells only comprised 13% and 5% of the total number of ET-1+ cells at 3 and 7 dpl, respectively. Staining was also comparatively weak as compared to ET-1 staining in astrocytes (Supp. Fig 1d).
Figure 1. ET-1 expression is upregulated in astrocytes in demyelinated LPC and MS lesions.
In dissected tissue at 3 days post lesion (dpl), RT-PCR analysis revealed that only ET-1 was expressed in control (a) or LPC lesions (b). (c) At 3 and 7 dpl there was a strong upregulation of ET-1 in astrocytes in LPC lesions (N=4, *p<0.05, unpaired t-test, mean +/− SEM). Confocal images from NaCl (d) and LPC (e) lesions at 7 dpl co-stained with anti-GFAP and anti-ET1 antibodies. Scale bar=25 μm (f) Luxol fast blue/MHCII staining of chronic active MS lesions (CA). V=ventricle, NAWM=normal white matter, GM=gray matter. Scale bar=2.5mm. (g) Confocal images of the active border co-stained with anti-ET-1 and anti-MOG antibodies. Increased numbers of ET-1+ cells were detected in the demyelinated tissue. Scale bar=50μm (h) Co-immunolabeling revealed strong ET-1 expression in GFAP+ astrocytes. White arrows indicate colocalized cells. (i) Elevated ET-1 expression coincided with large numbers of Olig1+ (nuclear) OPCs. No expression of ET-1 was found in OPCs. For h–i, scale bar=30μm. See also Supplemental Fig 1.
Examination of ET-1 expression in the active borders of chronic active (CA) MS lesions (Fig 1f), revealed that ET-1 was specifically upregulated in demyelination regions of the tissue (Fig 1f,g). In these areas, large numbers of ET-1 expressing astrocytes were found (Fig 1h). Furthermore, consistent with our previous observation that a high density of immature Olig1+ OPCs (Arnett et al., 2004) populate the active borders of CA MS lesions (Moll et al., 2013), we found large numbers of OPCs in close proximity to ET-1+ cells in the same active borders (Fig 1i). There was no evidence of ET-1 expression by immature OPCs (Fig 1i), but ET-1 expression was also found in MHCII+ T-cells (data not shown).
These results demonstrate that demyelination leads to an abrupt increase in ET-1 expression within the lesion. This increase in ET-1 was conserved between experimentally induced lesions in mice and in human MS tissue. The majority of ET-1 expression was found in astrocytes, demonstrating that they are the predominant source of ET-1 following injury.
ET-1 directly limits OPC differentiation after demyelination
While the effects of ET-1 on specific cell types (including astrocytes) have been studied in detail (Schinelli, 2006), the effect of ET-1 on the important endogenous repair process following demyelination, has not been examined. We have previously shown that ET-1 directly inhibits OPC differentiation in vitro and elicits pro-migratory effects during the development of the subcortical white matter (Gadea et al., 2009). Therefore, we sought to understand the potential role of ET-1 following LPC-induced demyelination in vivo using both gain- and loss-of-function approaches.
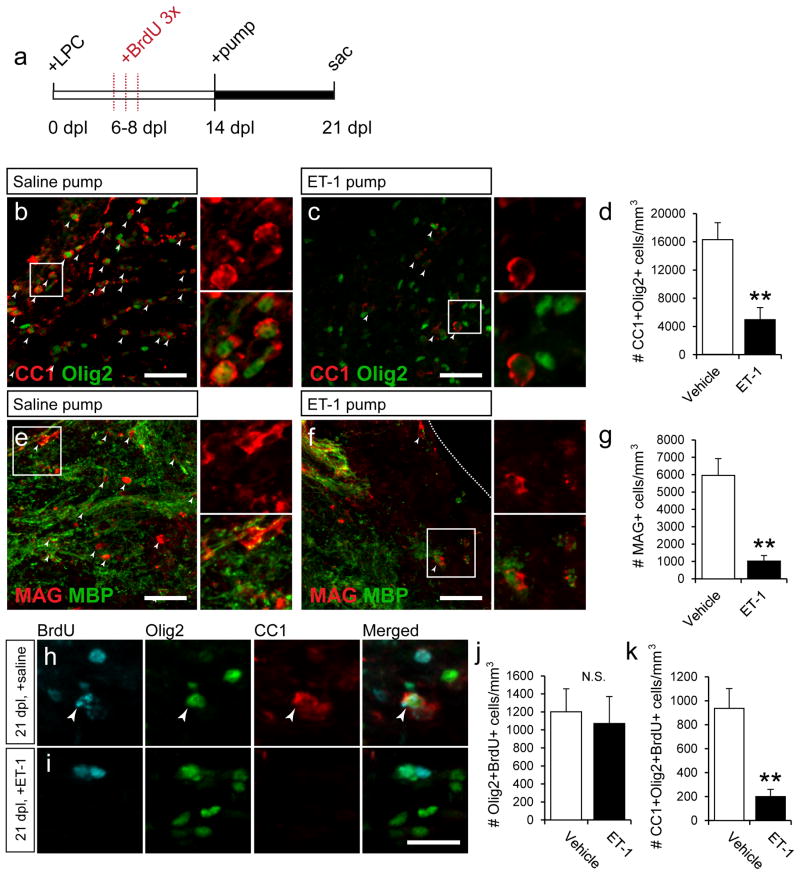
First, we infused exogenous ET-1 into remyelinating lesions and measured the extent of mature OL regeneration, using the mature OL markers CC1 and MAG. We and others have found that, following LPC-induced demyelination, OPC differentiation into mature OLs begins to occur at approximately 14dpl (Aguirre et al., 2007). Based on our immunohistochemical (Fig 1e) and previous Western blot analysis (Gadea et al., 2008), endogenous ET-1 levels peak during the first week of remyelination, and are very low at 14dpl. Therefore, we extended the natural window of ET-1 release following LPC demyelination by infusing exogenous ET-1 beginning at 14dpl by using mini-osmotic pumps.
Mini-osmotic pumps containing 100nM ET-1 were installed at 14 dpl, and left until 21dpl (Fig 2a). In the vehicle-infused LPC lesions at 21dpl, a large number of CC1+Olig2+ and MAG+ cells were found, indicating substantial levels of repair (Fig 2b,e,d,g). In contrast, in the ET-1-infused LPC lesions, a significant reduction in the number of mature OLs (CC1+Olig2+ and MAG+ cells) was found (Fig 2c,d,f,g). To further characterize and label newly generated OLs, BrdU was injected once per day at 6, 7, and 8dpl, when OPCs are proliferating within the lesion (Fig 2a). We found that, while there was little change in the number of Olig2+BrdU+ cells between the vehicle and ET-1-infused lesions (Fig 2j), there was a significant decrease in the number of CC1+Olig2+BrdU+ cells in the ET-1-infused lesions as compared to saline controls (Fig 2k). This showed that fewer Olig2+ OPCs had matured into CC1+ OLs, and that similar numbers of early OPCs were present following the infusion. Additionally, these results confirmed that the mature CC1+ cells that we observed in the vehicle-infused lesions were newly generated. Altogether, these results indicated that when the window of ET-1 release is extended into the OPC differentiation phase of remyelination (14–21 dpl), OL differentiation was delayed.
Figure 2. ET-1 limits OPC differentiation following demyelination.
(a) Following LPC demyelination, mini-osmotic pumps containing ET-1 were installed at 14 dpl, when endogenous ET-1 levels are low, and left until 21 dpl. BrdU was injected once a day, from 6–8 dpl to label proliferating OPCs. (b) Large numbers of CC1+Olig2+ mature OLs were found in saline-infused control mice at 21 dpl but not ET-1-infused mice (c). White arrows indicate colocalized cells. (d) A significant decrease in the number of CC1+Olig2+ OLs was found in ET-1-infused mice. (N=6, **p<0.01, unpaired t-test, mean +/− SEM). Reduced expression of myelin proteins MAG and MBP were also found in ET-1-infused lesions (f), as compared to controls (e). White arrows indicate MAG+ cells. (g) A significant decrease in the number of MAG+ cells was found in ET-1-infused samples. (N=6, **p<0.01, unpaired t-test, mean +/− SEM). Scale bars=50μm for b,c,e,f. More BrdU+Olig2+CC1+ cells were found in saline-infused (h), but not ET-1-infused (i) samples. Scale bars=25μm. White arrows indicate triple-labeled cells. No change was found in the total number of BrdU+Olig2+ cells (j), but a significant decrease in the number of BrdU+Olig2+CC1+ cells was found in ET-1-infused samples (k). (For j–k, N=5, **p<0.01, N.S=not significant, unpaired t-test, mean +/− SEM).
Loss of ET-1 expression in astrocytes is sufficient to accelerate OPC differentiation and remyelination
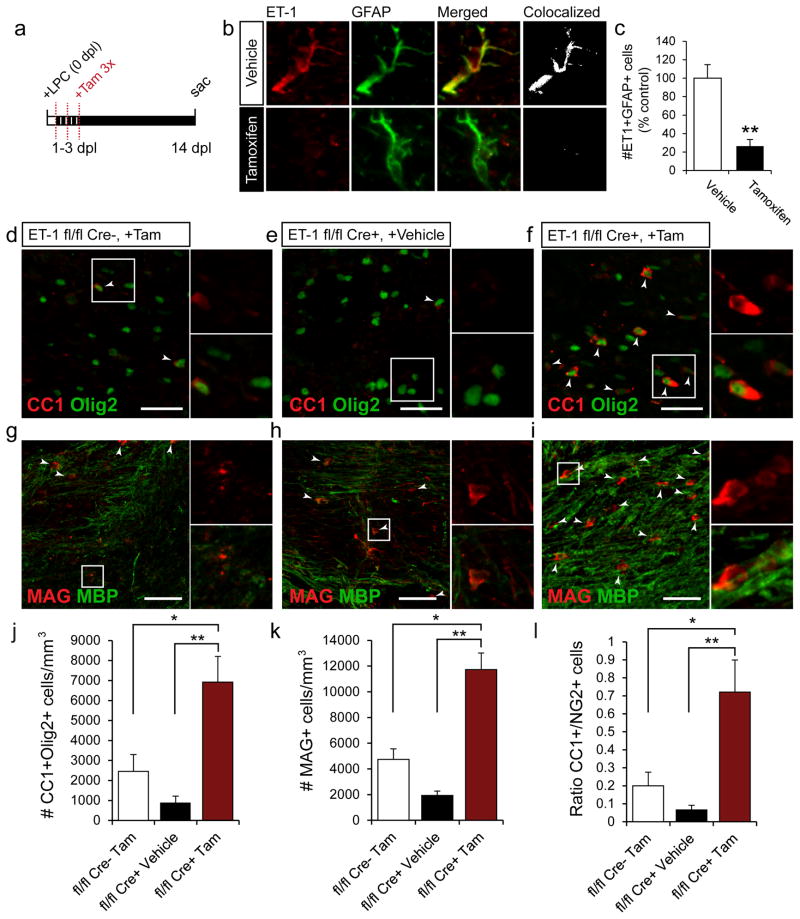
RAs specifically upregulate ET-1 expression during the first week after demyelination, when OPC expansion occurs at the expense of differentiation. The vast majority of ET-1+ cells following demyelination were also RAs (Supp. Fig 1e). Therefore, we eliminated ET-1 expression in astrocytes following demyelination to specifically assess the role of astrocyte-derived ET-1 on remyelination efficiency. An ET-1flox/flox mouse was bred with a hGFAP-Cre-ERT2mouse, to selectively eliminate ET-1 expression in astrocytes (hGFAP-Cre-ERT2;ET-1flox/flox mouse). First, we examined the expression of ET-1 in white matter RAs from demyelinated hGFAP-Cre-ERT2;ET-1flox/flox mice with Tamoxifen or vehicle-injections (Fig 3a–c). At the peak of ET-1 expression (5 dpl), a 76% reduction was found in the total number of ET-1+GFAP+ cells in the lesion (Fig 3b,c).
Figure 3. Selective elimination of ET-1 in astrocytes accelerates OPC differentiation following demyelination.
(a) hGFAP-Cre-ERT2;ET-1flox/flox mice were generated to selectively reduce ET-1 expression in astrocytes. Following LPC demyelination, tamoxifen was injected once a day, from 1–3 dpl to induce recombination. (b) Confocal images of anti-ET-1 and anti-GFAP stained astrocytes. Strong ET-1 expression was seen in astrocytes in the vehicle-injected mice, but not the tamoxifen-induced mice at 5 dpl. (c) A significant reduction (76%) in the number of ET-1+GFAP+ astrocytes was found in the tamoxifen-induced mice at 5 dpl. (N=4, **p<0.01, unpaired t-test, mean +/− SEM). Confocal images of ET-1 fl/fl Creneg +Tamoxifen (d,g), ET-1 fl/fl Cre+ +Vehicle (e,h), and ET-1 fl/fl Cre+ +Tamoxifen (f,i) mice at 14 dpl co-immunolabeled with anti-CC1 and anti-Olig2 (d–f); or anti-MAG and anti-MBP (g–i). Scale bar=40μm for d–f, 50μm for g–i. White arrows indicate CC1+Olig2+ or MAG+ cells. Analysis of the three groups reveals a significant increase in the number of CC1+Olig2+ OLs (j) and MAG+ OLs (k) in the ET-1 fl/fl Cre+ +Tamoxifen mice, as compared to ET-1 fl/fl Creneg +Tamoxifen and ET-1 fl/fl Cre+ +Vehicle mice. (l) A significant increase in the ratio of CC1+ to NG2+ cells was also found in the ET-1 fl/fl Cre+ +Tamoxifen mice, as compared to controls. (For j–l, N=4, *p<0.05, **p<0.01, ANOVA Bonferroni post hoc, mean +/− SEM).
Infusion of exogenous ET-1 during remyelination limited OPC differentiation, so we measured OPC maturation following genetic ablation of ET-1 in astrocytes to determine the effects on OPC development. Three experimental groups were established: 1.) ET-1 fl/fl Creneg +Tamoxifen; 2.) ET-1 fl/fl Cre+ +Vehicle; and 3.) ET-1 fl/fl Cre+ +Tamoxifen. Interestingly, we found a significant increase in the number of CC1+Olig2+ (Fig 3d–f,j) and MAG+ cells (Fig 3g–i,k) in the ET-1 fl/fl Cre+ +Tamoxifen mice, as compared to ET-1 fl/fl Creneg +Tamoxifen and ET-1 fl/fl Cre+ +vehicle littermates. We also observed an increase in the CC1+/NG2+ ratio in the ET-1 fl/fl Cre+ +Tamoxifen as compared to controls (Fig 3l).
Altogether, these results demonstrate that selective deletion of ET-1 in astrocytes significantly increased the number of mature OLs in LPC lesions after two weeks, and shifted the OL ratio from an immature to mature phenotype. Conversely, extended expression of ET-1 during remyelination led to a reduction in the number of mature OLs generated in LPC lesions. These findings indicate that astrocyte-derived ET-1 acts as an inhibitor of OPC differentiation and remyelination.
ET-1 induces Jagged1 expression in astrocytes
We wanted to identify the mechanisms by which ET-1 acts to limit OPC maturation. There are two potential mechanisms of ET-1 action: 1.) Direct signaling to OPCs through ET-Rs; 2.) Indirect signaling to OPCs through astrocytes. We have previously shown that ET-1 can act directly on OPCs to limit their differentiation by activation of ET-Rs on their cell surface, particularly during migration (Gadea et al., 2009). Therefore, we wanted to investigate the effect of ET-1 signaling through astrocytes, and the resulting effects of OPC differentiation. We previously identified ET-1 as a potent activator of astrocytes (Gadea et al., 2008), but expression of signals by those RAs that inhibit OPC differentiation following ET-1 exposure was not explored. It has been previously described that RAs in MS lesions express high levels of Jagged1, a ligand for the Notch1 receptor (John et al., 2002). In fact, elevated Jagged1 expression by astrocytes was found in the active borders of CA MS lesions (John et al., 2002), the same areas where found high ET-1 expression in astrocytes and a high density of immature OPCs (Figure 1g). In the same study, Notch1 expression was also found on OPCs. Independent studies have also shown that Notch1 inhibits OPC differentiation during both development and remyelination (Genoud et al., 2002; Zhang et al., 2009). We tested the functional relevance of Jagged1/Notch1 signaling as a possible mechanism underlying the effects of ET-1 on OPC differentiation during remyelination.
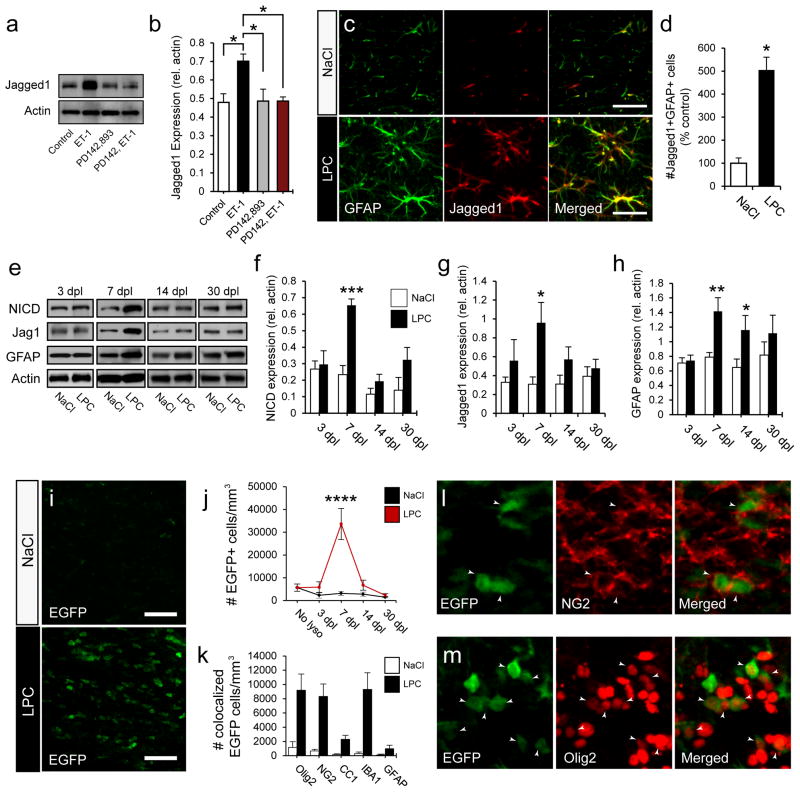
First we sought to determine whether ET-1 has a role in regulating the expression of Jagged1. In primary cultured astrocyte monolayers ET-1 induced a significant increase in Jagged1 protein expression after 48 h (Fig 4a). These increases in Jagged1 were blocked by pre-incubation with the ET-receptor (ET-R) pan-antagonist PD142,893 (Fig 4a,b). These results indicate that astrocytes express increased levels of Jagged1 following ET-1 exposure, and that this increase is mediated by ET-Rs.
Figure 4. ET-1 promotes Jagged1 expression in astrocytes and Notch signaling is activated in OPCs during the first week following demyelination.
(a) Cultured primary astrocytes were treated with ET-1 for 48 h, and expression of Jagged1 was measured by Western blot. (b) Significant increases in Jagged1 expression were found following ET-1 exposure, as compared to control and PD142,893 treated groups. (N=3 independent cultures, with multiple replicates per sample, *p<0.05, ANOVA Bonferroni post hoc, mean +/− SEM). (c) Confocal images of NaCl and LPC lesions at 7 dpl labeled with anti-GFAP and anti-Jagged1. (d) A large increase in Jagged1 expression was found in astrocytes in LPC lesions. (N=4, *p<0.05, unpaired t-test, mean +/− SEM, Scale bar=30μm). (e) Western blot analysis of micro-dissected tissue from NaCl and LPC lesions at 3, 7, 14, and 30 dpl. Significant increases in NICD (f), Jagged1 (g), and GFAP (h) expression were found at 7 dpl. (N=7–10, *p<0.05, **p<0.01, ***p<0.001, unpaired t-test, mean +/− SEM). The TNR mouse was used to measure Notch activation in LPC lesions. (i) Confocal image of EGFP expression in NaCl and LPC lesions at 7 dpl. Scale bar=50μm. (j) Quantification of the number of EGFP+ cells following demyelination. A significant increase was found at 7 dpl in LPC samples. (N=4, ****p<0.0001, ANOVA Bonferroni post hoc, mean +/− SEM) (k) Colocalization analysis of LPC lesions at 7 dpl. A large number of cells were EGFP+Olig2+ and EGFP+NG2+ in LPC lesions. Confocal images of TNR LPC lesions at 7 dpl co-immunolabeled with anti-NG2 (l) and anti-Olig2 (m). See also Supplemental Fig 2.
Notch signaling analysis in LPC lesions during remyelination
It has been previously shown that components of Notch signaling are present in both MS lesions and in experimentally induced LPC lesions in mice (John et al., 2002; Stidworthy et al., 2004). Specifically, it was shown that astrocytes express Jagged1, and OPCs express Notch1. However, to our knowledge, functional activation of Notch signaling in demyelinated lesions has not been directly demonstrated. We utilized the Transgenic Notch reporter (TNR) mouse, in which EGFP is expressed upon canonical Notch activation (Mizutani et al., 2007), to study the cell specific activation and time course of Notch signaling following demyelination.
As demonstrated in previous studies (John et al., 2002; Stidworthy et al., 2004), Jagged1 was specifically upregulated five-fold in astrocytes in LPC lesions at 7 dpl (Fig 4c,d). Further analysis of Notch component expression in micro-dissected WM tissue revealed increases in Notch intracellular cleaved domain (NICD), Jagged1 (Jag1), and GFAP protein levels (Fig 4e–h). Increases in Notch intracellular domain (NICD) levels (Fig 4e,f) coincided with elevated Jagged1 expression at 7 dpl (Fig 4e,g). In the same set of experiments, we also observed high levels of GFAP expression at 7dpl (Fig 4e,h), which indicated that the highest levels of astrogliosis coincide with maximal Notch activation. We have previously shown that total ET-1 expression peaks at 5dpl (Gadea et al., 2008), and the greatest number of ET-1 expressing astrocytes peaks between 3 and 7dpl (Fig 1e), which, as predicted, immediately precedes the peak of Notch activation at 7dpl.
Analysis of functional Notch activation in the TNR mouse, supported our earlier results. EGFP expression was specifically upregulated in LPC lesions (Fig 4i), with the greatest number of EGFP+ cells found at 7 dpl (Fig 4j). Co-staining of these EGFP+ cells with Hes1, a direct downstream target of the CSL/CBF1/RPGJ transcriptional regulators, confirmed the reliability of the TNR mouse as an indicator of canonical Notch activation (Supp. Fig 2a). Co-localization analysis demonstrated that approximately half of the EGFP+ cells were Olig2+ (Fig 4k,m) and that, as expected, the majority of these cells were NG2+ OPCs (Fig 4k,l). Interestingly, we also found a significant population of EGFP+IBA1+ microglia in the core of the lesion (Fig 4k). Little or no Notch activation was found in astrocytes (GFAP) (Fig 4k).
Altogether, these results demonstrate that Notch signaling is activated at high levels in demyelinated tissue, and that astrocytes are likely a major regulator of Notch activation in these lesions. ET-1 also acts to promote Jagged1 expression in astrocytes, indicating that it might regulate Notch activation in vivo. Components of both the ET-1 and Notch signaling pathways are activated during the first week following demyelination, with the peak of ET-1 expression immediately preceding increases in GFAP and Jagged1 levels, indicating a possible functional connection.
The ET-R antagonist PD142,893 blocks Notch activation in vivo
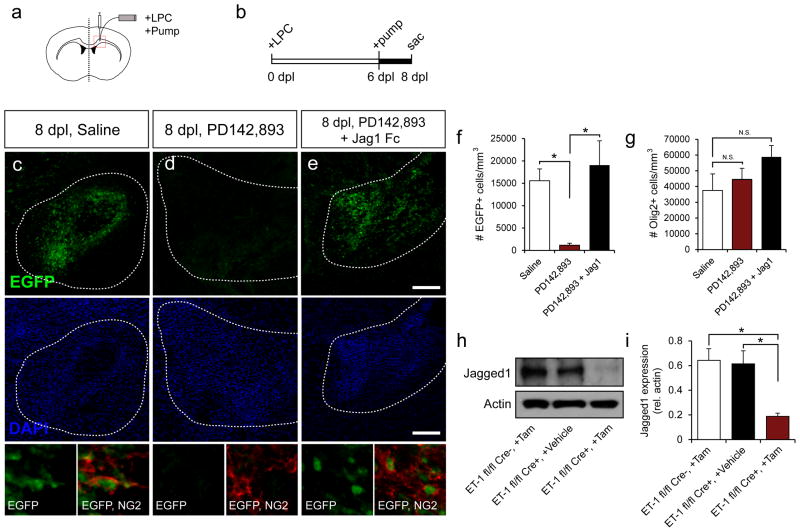
We showed in vitro that ET-1 strongly upregulated Jagged1 expression in astrocytes, and that these increases could be blocked using PD142,893 (Fig 4a,b). We also found that activation of Notch signaling in vivo coincided with increases in Jagged1 expression in astrocytes in demyelinated lesions (Fig 4c,d,e,g). Therefore, we hypothesized that, by blocking ET-R activation using PD142,893, we could reduce Notch activation in vivo. To test this hypothesis, PD142,893 (50 μM) was infused into the remyelinating lesions of TNR mice using mini-osmotic pumps (Fig 5a,b), when Notch activation remained high in OPCs (Fig 5c, bottom panels). As predicted, strong reductions were found in total Notch activation following PD142,893 infusion (Fig 5c–e,f). Notch activation was recovered when recombinant Jagged1 Fc (2 μg/mL) was added to a mixture containing PD142,893 (Fig 5f). No overall changes in the total number of OL lineage cells caused by infusion of the antagonist were observed between groups (Fig 5g). To further confirm that ET-1 induces Jagged1 expression, leading to Notch activation, Jagged1 protein expression was examined in the hGFAP-Cre-ERT2;ET-1flox/flox mouse. Jagged1 levels were significantly reduced at 7 dpl in microdissected tissue from the ET-1 fl/fl Cre+ +Tamoxifen, as compared to ET-1 fl/fl Creneg +Tamoxifen and ET-1 fl/fl Cre+ +vehicle littermates (Fig 5h,i).
Figure 5. Inhibition of ET-signaling blocks Notch activation in LPC lesions.
(a) Mini-osmotic pumps were used to infuse drugs into LPC lesions from 6–8 dpl (b), to coincide with the peak of Notch activation. Low magnification confocal images of EGFP expression in the core of LPC lesions at 8 dpl in saline- (c), PD142,893- (d), and PD142,893+Jagged1Fc-infused mice (e). Scale bar=200μm for c-e. NG2+EGFP+ cells (below DAPI) show EGFP activation in OPCs within the lesions. (f) Quantification of the number of EGFP+ cells for each condition. A significant reduction was found in the PD142,893-infused samples. Infusion of PD142,893 in combination with Jagged1Fc restored Notch activation in the lesion (N=4–5, *p<0.05, ANOVA Bonferroni post hoc, mean +/− SEM). (g) No significant (N.S.) change was found in the total number of Olig2+ cells per condition. (h) Western blot analysis of Jagged1 expression in micro-dissected tissue from the hGFAP-Cre-ERT2;ET-1flox/flox mice at 7 dpl. (i) A significant reduction in Jagged1 levels was found in the ET-1 fl/fl Cre+ +Tamoxifen mice, as compared to ET-1 fl/fl Creneg +Tamoxifen and ET-1 fl/fl Cre+ +Vehicle mice. (N=4–7, *p<0.05, ANOVA Bonferroni post hoc, mean +/− SEM).
These results demonstrate the functional connection of ET-1 and Notch signaling in vivo. PD142,893 strongly blocked Notch activation in LPC lesions, and exogenous Jagged1 rescues Notch activation, even when ET-1-signaling is blocked. We also confirm in our hGFAP-Cre-ERT2;ET-1flox/flox that a reduction in ET-1 produced by astrocytes is sufficient to reduce overall Jagged1 levels in the lesion. These findings also confirm that ET-1 signaling is upstream of Notch activation during remyelination.
ET-1-treated astrocytes inhibit OPC differentiation through Notch signaling
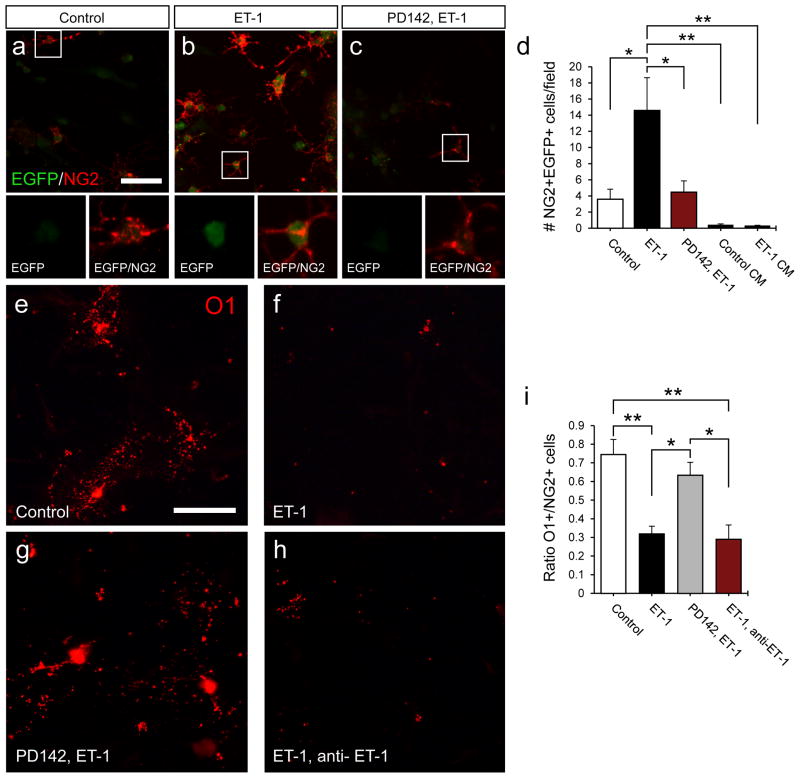
ET-1 signaling inhibits remyelination, and limits OPC differentiation. We propose that one mechanism regulating this effect is expression of Jagged1 by astrocytes and resulting Notch activation in OPCs. To specifically assess the functional interaction between astrocytes and OPCs, we used a co-culture system. We have shown that ET-1 promotes Jagged1 expression in astrocytes, and that ET-1 signaling promotes Notch activation in vivo. To assess Notch activation in co-cultures, OPCs from the TNR mouse were plated on ET-1 pre-treated astrocytes. There was a significant increase in the number of EGFP+NG2+ cells in ET-1 pre-treated co-cultures, as compared to control cultures, indicating enhanced Notch signaling in OPCs (Fig 6a–d). This signaling activation was blocked by pre-incubation with PD142,893 (Fig 6a–d). To ensure that Notch activation was mediated by astrocyte-OPC contact and not soluble factors, co-cultures were performed in which TNR mouse OPCs were plated on glass coverslips, rather than in direct contact with astrocytes (Supp. Fig 3a). This prevented the cell-to-cell contact required for Notch receptor/ligand interaction, but still allowed soluble factors to be released into the cell culture media. Under these conditions, very few EGFP+NG2+ OPCs were found, with no difference between the control (Control CM) or ET-1 (ET-1 CM) pretreated groups (Fig 6d).
Figure 6. ET-1 pretreated astrocytes limit OPC differentiation through Notch activation.
OPCs from the TNR mouse were used to assess Notch activation in astrocyte-OPC co-cultures. (a–c) Flourescent image of control (a), ET-1 (b), and PD142,ET-1 (c) pretreated co-cultures co-stained with anti-EGFP and anti-NG2 antibodies. (d) A significant increase in the number of NG2+EGFP+ OPCs was found in the ET-1 pretreated co-cultures after 48 h, as compared to control and PD142,893 pre-treated co-cultures. Little or no Notch activation was found in control (control CM) or ET-1 pre-treated (ET-1 CM) barrier co-cultures. (N=3–5 independent cultures with multiple replicates per sample, *p<0.05, **p<0.01, ANOVA Bonferroni post hoc, mean +/− SEM). In wild-type co-cultures, OPC differentiation was analyzed by measuring the ratio of O1+ to NG2+ cells. Flourescent images of control (e), ET-1 (f), PD142,ET-1 (g), and ET-1, anti-ET-1 co-cultures (h) immunolabeled with anti-O1 antibody. (i) Quantification of the O1+ to NG2+ ratio for each co-culture condition. Significant decreases in the ratio were found in ET-1 pretreated and ET-1, anti-ET-1 treated groups after 48 h. (N=4 independent cultures with multiple replicates per sample, *p<0.05, **p<0.01, ANOVA Bonferroni post hoc, mean +/− SEM). Scale bar=50μm for all images. See also Supplemental Fig 3.
Analysis of mature OL formation in these co-cultures revealed a drastic reduction in the number of mature O1+ OLs, when plated on astrocytes pre-treated with ET-1, as compared to untreated astrocytes (Fig 6e,f). Furthermore, a reduction in the O1+/NG2+ (mature/immature) cell ratio was seen in ET-1-pre-treated co-cultures, indicating a delay in OL lineage progression (Fig 6i). This effect was blocked by PD142,893 pre-incubation (Fig 6g,i). To ensure that OPC development was not directly influenced by ET-1 remaining in the culture media following astrocyte pre-treatment, an anti-ET-1 antibody was added to the culture media during OPC differentiation. We have previously shown that this anti-ET-1 antibody can functionally block ET-1 signaling in slice cultures (Gadea et al., 2009). As expected, there was no difference between the ET-1 pretreated and the ET-1 pretreated/anti-ET-1 groups, indicating that there was little or no ET-1 remaining in the culture media (Fig 6h,i). There was also no difference in the O1+/NG2+ cell ratio in OPCs plated on glass coverslips (Supp. Fig 3a–d). This is consistent with our TNR results, showing little or no Notch activation in these barrier co-cultures (Fig 6d).
Our findings show that Notch signaling is activated during OPC differentiation in co-cultures with astrocytes, and Notch activation can be increased by pre-exposing astrocytes to ET-1. These effects require cell-cell contact between astrocytes and OPCs, and are not mediated by soluble factors released by astrocytes following ET-1 exposure. Finally, when OPCs are plated on astrocytes that were pre-exposed to ET-1, they exhibit a more immature phenotype, and are less likely to differentiate into mature OLs. These results establish that astrocytes limit OPC differentiation, particularly when primed using ET-1.
The ET-R antagonist PD142,893 increases the rate of OPC differentiation and myelination in vivo
Using a genetic loss-of-function approach, we demonstrated that OPC differentiation is accelerated following reduction of ET-1 levels in the lesion (Fig 3). Our data indicate that this is due, in part, to reduced Notch activation. In fact, selective elimination of Notch1 receptors on OPCs accelerated remyelination in vivo (Zhang et al., 2009). We also found that PD142,893 was a potent inhibitor of Notch activation in vivo (Fig 5), therefore we tested whether PD142,893 was a therapeutic candidate to promote remyelination by enhancing OPC differentiation in demyelinated lesions.
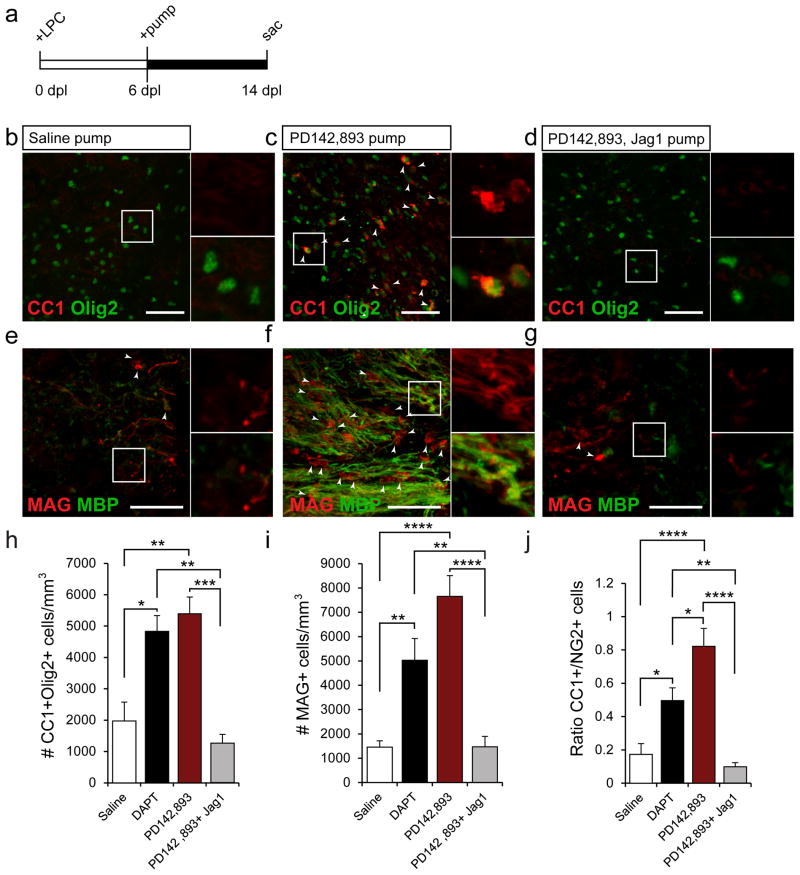
PD142,893 was directly infused into demyelinated lesions using mini-osmotic pumps beginning at 6 dpl (Fig 7a). Relatively few mature OLs were found in the saline-infused samples (Fig 7b,e,h,i), but a robust increase in CC1+ and MAG+ was seen following PD142,893 infusion (Fig 7c,f,h,i) at 14 dpl. This effect was blocked when Jagged1 Fc was infused with PD142,893 (Fig 7d,g,h,i). Increases in mature OL number were accompanied with a shift in the O1+/NG2+ cell ratio (Fig 7j). BrdU was used to further characterize proliferating OPCs in the lesion for each condition (Supp. Fig 4a). At 14dpl, we observed a significant increase in the number of CC1+Olig2+BrdU+ cells in the PD142,893-infused lesions, as compared to SIC and PD142,893 +Jag1Fc-infused mice (Supp. Fig 4b–e). This indicated that a greater number of OPCs had differentiated into mature OLs. It also showed that the CC1+ cells we observed at 14dpl in the PD142,893-infused lesions were newly generated following demyelination.
Figure 7. PD142,893 accelerates OPC differentiation.
(a) Mini-osmotic pumps were used to infuse drugs into LPC lesions from 6–14 dpl. Confocal images of anti-Olig2 and anti-CC1 or anti-MAG and anti-MBP stained samples from saline- (b,e), PD142,893- (c,f), and PD142,893+Jagged1Fc- infused mice (d,g) at 14 dpl. White arrows indicate CC1+Olig2+ or MAG+ cells. Scale bar=50μm for b–g. (h) Quantification of the number of CC1+Olig2+ cells in four groups. A significant increase was seen in PD142,893- and DAPT-infused samples. (i) A significant increase was also seen in the number of MAG+ cells in PD142,893- and DAPT-infused samples. (j) An increase in the CC1 to NG2 ratio was also found in PD142,893-infused samples. (For h–j, N=7, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, ANOVA Bonferroni post hoc, mean +/− SEM). See also Supplemental Fig 4.
Since ET-1 can act directly on OPCs to promote their migration and inhibit their differentiation (Gadea et al., 2009), it is unlikely that the effects we observed on OPC differentiation were entirely Notch-mediated. To isolate the effects of Notch signaling alone, the γ-secretase inhibitor DAPT (50 μM) was infused into demyelinated lesions. γ-secretase is responsible for NICD cleavage, which happens upon binding of the Notch receptor by Jagged1. While there was a significant increase in mature OLs and in the CC1+/NG2+ ratio following DAPT infusion, the average increase was lower than in PD142,893-infused samples (Fig 7h,i). This indicated that the positive effects of PD142,893 on oligodendrogenesis after demyelination are not entirely Notch-mediated, and are likely due to direct block of ET-1-signaling to OPCs, which would lead to enhanced differentiation (Gadea et al., 2009).
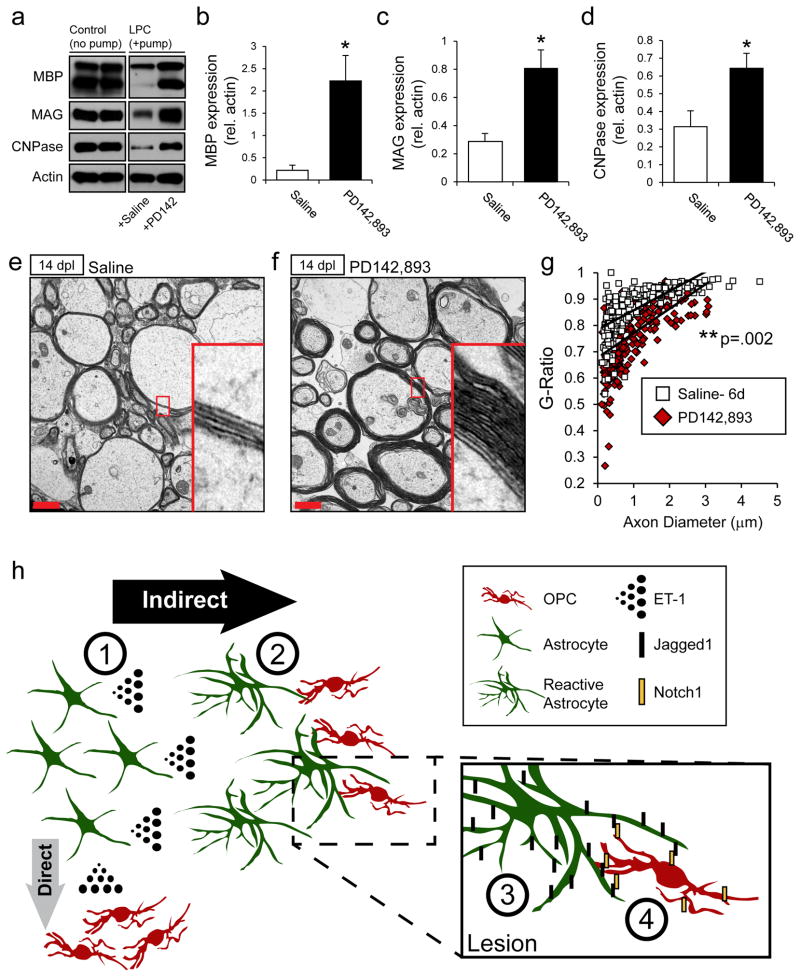
Markers of OL lineage progression indicate maturation, but do not necessarily imply increased production of myelin proteins and formation of compact myelin. Therefore, analysis of myelin production was examined in micro-dissected tissue and at the ultrastructural level. As expected, myelin protein analysis revealed a sharp decrease in MBP, MAG, and CNPase levels in LPC-injected tissue infused with saline (Fig 8a, +Saline), compared to non-demyelinated controls (Fig 8a, left panels). Conversely, there was a strong recovery in myelin levels in PD142,893-infused samples (Fig 8a–d). These increases were sustained until 21 dpl, at which point myelin levels in the SIC samples had also recovered (Supp. Fig 5b–e).
Figure 8. PD142,893 accelerates remyelination.
Mini-osmotic pumps were used to infuse drugs into LPC lesions from 6–14 dpl. (a) Western blot analysis of myelin protein expression in micro-dissected tissue from control non-demyelinated tissue (left 2 lanes), and from LPC-injected lesions at 14 dpl infused with saline- (third lane) or PD142,893 (fourth lane). Significant increases in MBP (b), MAG (c), and CNPase (d) were observed. (N=4–6, *p<0.05, unpaired t-test, mean +/− SEM). Electron micrograph of axonal cross-sections in saline- (e) and PD142,893-infused (f) samples at 14 dpl. Red scale bar=1μm. (g) G-ratios were calculated from electron micrographs of saline- and PD142,893-infused samples. A significant decrease in the g-ratio was found in the PD142,893-infused samples, indicating thicker myelin sheaths. (N=3, unpaired t-test, p=0.002). See also Supplemental Fig 5. (h) Model of the effects of ET-1 on astrocytes and remyelination. (1) ET-1 is released at high levels by astrocytes following demyelination. ET-1 then acts through an astrocyte-mediated indirect pathway, or to a lesser extent through direct activation of ET-Rs on OPCs (See Gadea et al., 2009). (2) High levels of ET-1 in the lesion promote a reactive phenotype in astrocytes, (3) including an upregulation of Jagged1. (4) Jagged1 on reactive astrocytes binds to Notch1 receptors on OPCs, which have migrated to the demyelinated lesion to initiate the remyelination process. Activation of Notch1 receptors on OPCs by reactive astrocytes inhibits OPC differentiation, leading to delayed remyelination and fewer mature, myelinating oligodendrocytes.
Examination of the myelin sheaths at the ultrastructural level also showed the benefits conferred by PD142,893 infusion. EM analysis revealed large increases in myelin thickness in the PD142,893-infused samples as compared to SIC (Fig 8e,f). Due to increases in myelin thickness, we observed a significant decrease in the average g-ratio of the PD142,893 samples as compared to SIC (Fig 8g). We also observed no compaction or wrapping abnormalities in the myelin sheaths produced in the PD142,893-infused mice (Fig 8e,f).
Altogether, these results indicate that infusion of PD142,893 promotes early differentiation of OPCs into mature, myelinating OLs. Early differentiation also led to thicker myelin sheaths in the PD142,893-infused samples. PD142,893 was a more potent promoter of OPC differentiation than DAPT-infusion alone, which indicates that multiple mechanisms contribute to the observed beneficial effect. PD142,893 offers a promising therapeutic avenue for lesion repair, as it strongly limits Notch activation and promotes remyelination in LPC lesions.
DISCUSSION
Here we demonstrate that ET-1 - which is a soluble factor released by astrocytes following demyelination - inhibits the rate of OPC differentiation and remyelination through induction of Jagged1 in astrocytes and activation of Notch signaling in OPCs (See model; Fig 8h). We found that ET-1 regulates the rate of OPC differentiation both in vitro and in vivo, and ET-R antagonists promote remyelination, at least in part, by preventing Notch activation. These findings are the first to demonstrate the importance of ET-1 signaling in myelin pathology, and as a potential therapeutic target to promote remyelination and prevent OPC differentiation failure in MS lesions.
Although astrocytes are not the only cells that produce and express ET-1, we found that the largest increases in ET-1 expression following demyelination were in astrocytes. Both non-selective infusion of ET-R antagonists and selective elimination of ET-1 expression in astrocytes resulted in the acceleration of OPC differentiation. This suggests that astrocytes are the primary source of elevated ET-1 in the sub-cortical WM following demyelination. In further support of this notion, our hGFAP-Cre-ERT2;ET-1flox/flox data showed that the contribution of ET-1 from microglia or endothelial cells is dispensible to the observed remyelination phenotype. Although other ET-isoforms exists, we found no evidence of ET-2 or ET-3 expression in the normal or demyelinated subcortical WM. Previous studies have shown that ET-1 is the predominant endothelin isoform in the brain (Schinelli, 2006), and here we demonstrate that genetic ablation of ET-1 produced major effects on Jagged1/Notch1 signaling and OPC differentiation. Interestingly, a recent study demonstrated a divergent role for ET-2 as a promoter of remyelination in the rat cerebellum (Yuen et al., 2013). This opposing role of ET-2 could be accounted for by differential ET-R distribution in these brain regions. Since periventricular lesions are seen in the subcortical WM of over 80% of MS cases (Adams et al., 1987; Ge, 2006), our data demonstrating a beneficial effect of blocking ET-1 in this region is particularly relevant. Interestingly, studies of ET-1 following other CNS injuries have been well documented. Increases in ET-1 levels have been detected in Alzheimer’s (Minami et al., 1995), and in astrocytes following hypoxia/ischemia in mice (Tsang et al., 2001). ET-1 is itself also an activator of astrocytes (Gadea et al., 2008; Rogers et al., 2003). ET-receptor antagonists have been used to block increases in the number of RAs following cortical stab wound and optic nerve crush experiments (Koyama et al., 1999; Rogers et al., 2003). This is consistent with our finding that infusion of the ET-R pan-antagonist Bosentan following demyelination reduces the number of RAs in the corpus callosum (Gadea et al., 2008). Therefore, our results could shed further light onto the role of astrocytes in mediating recovery and repair in other CNS disorders where ET-1 release is elevated.
There has been a long-standing debate about whether extensive astrogliosis following injury is detrimental or beneficial to remyelination (Brosnan and Raine, 2013; Nair et al., 2008; Williams et al., 2007). Early evidence showed that astrocytic scars formed in chronic MS tissue are impediments to OPC survival, differentiation, migration, and axonal connectivity (Fawcett and Asher, 1999; Nair et al., 2008; Silver and Miller, 2004; Su et al., 2011; Wang et al., 2011; Zhang et al., 2010). More recent evidence has demonstrated that astrocytes can create a permissive environment for OPC expansion and differentiation within the lesion (Moore et al., 2010; Nair et al., 2008; Schulz et al., 2012). In this study we show that reactive astrocytes express two major inhibitory signals, first ET-1 and then Jagged1, which ultimately lead to the inhibition of OPC differentiation. Recent studies have demonstrated that reactive astrogliosis is not an all-or-none cellular transformation, and that specific inflammatory cues or injuries can induce distinct transcriptional responses in astrocytes both in vivo and in vitro (Hamby et al., 2012; Zamanian et al., 2012). Interestingly, it has been shown that TGF-β1 also causes an increase in Jagged1 expression in astrocytes, suggesting that increases in Notch ligand expression might be triggered by a host of inflammatory cues (Zhang et al., 2010). However, we demonstrate that ablation of ET-1 in astrocytes during remyelination is sufficient to reduce the overall levels of Jagged1 in the lesion, despite the presence of other inflammatory signals. This strongly suggests that ET-1 is a major signal that regulates Jagged1 expression in RAs under pathological conditions. By modulating astrocytic ET-1, our results also demonstrate that RAs prevent OPC differentiation during remyelination, and may therefore promote a non-permissive environment for recovery.
Here we show that astrocytic expression of Jagged1 plays a key role in Notch regulation in demyelinated lesions. Notch signaling plays an essential role in neural development and its role in the adult brain has gained increased importance in recent years (Ables et al., 2011). Both Jagged1 and Notch1 have been found in human and in murine demyelinated lesions in the adult brain (John et al., 2002; Seifert et al., 2007; Stidworthy et al., 2004), but there are conflicting reports on whether Notch inhibits OPC differentiation during remyelination (Stidworthy et al., 2004; Zhang et al., 2009). Our analysis reveals that the Notch activator Jagged1 inhibits OPC differentiation, and the Notch inhibitor DAPT accelerates OPC differentiation in vivo. Therefore, our results support the notion that Notch acts as an inhibitor of remyelination, and newly demonstrate that Notch signaling is regulated by ET-1 through astrocytes. Due to its importance in a wide-variety of adult brain functions, understanding how Notch is regulated, and identifying endogenous regulators of Notch activation is crucial. Our evidence that ET-1 plays such an essential role as an activator of Notch following demyelination could improve our understanding of the mechanisms underlying cell differentiation and repair in other types of brain injury involving reactive astrogliosis and ET-1 release.
There is extensive evidence demonstrating that OPC maturation and remyelination in LPC lesions occur spontaneously around 2–3 weeks post lesion, depending on the age of the animal and location of the lesion (Aguirre et al., 2007; Arnett et al., 2004; Fancy et al., 2011). Our results suggest that ET-1 release during early remyelination induces Jagged1 expression in RAs, which likely sustains expansion of the OPC population in the lesion prior to differentiation. We hypothesize that extended or aberrant expression of ET-1 in human MS lesions could account for stalled OPC differentiation in this disease (Chang et al., 2002; Wolswijk, 1998). When we tested this hypothesis in LPC lesions, we found that extended ET-1 expression was sufficient to delay remyelination. Other studies have shown that there could be a critical time window for OPC differentiation (Franklin, 2002), and our present results demonstrate that a prolonged increase in ET-1 levels is a major contributor of the OPC differentiation failure under pathological conditions.
Despite strong evidence that ET-1 indirectly inhibits OPC differentiation through an astrocyte- dependent pathway, we cannot exclude direct signaling to OPCs, which also express ET receptors (Gadea et al., 2009; Yuen et al., 2013). In fact, our lab has shown that ET-1 stimulates OPC migration from the SVZ in situ and stalls OPCs in a premyelinating state without any effect on OPC proliferation (Gadea et al., 2009). This direct function of ET-1 inhibits OPC differentiation during migration and possibly within the lesion as well (See model: Fig 8h). These findings, together with the present report, indicate that ET-1 could act via both direct and indirect pathways to limit OPC differentiation. However, it is worth noting that direct exposure of OPCs to ET-1 has produced mixed results, and ET-1 or ET-R agonists have been found in other studies to promote OPC differentiation in vitro (Jung et al., 2011; Yuen et al., 2013). This conflicting evidence suggests that the direct effect of ET-1 on OPCs in vitro might not reflect their response in vivo. Our present studies further support the notion that direct ET-1 signaling to OPCs may not play a significant role in regulating differentiation within the lesion itself. The infusion of DAPT, to isolate Notch effects, strongly promoted OPC differentiation at a rate almost equivalent to PD142,896 infusion, suggesting that astrocyte-derived ET-1 and the Jagged1-mediated indirect pathway contributes much more significantly to limiting the rate of remyelination within the lesion.
To our knowledge, this is the first study which successfully identifies an ET-R antagonist as a pro-myelinating compound in vivo. PD142,893, a very potent inhibitor of ET-1 signaling, prevented Jagged1 induction and Notch activation. For ET-1 MS therapy, one of the major challenges will be finding a white matter-and astrocyte-specific delivery method to minimize systemic side-effects (Schnyder and Huwyler, 2005; Shi et al., 2001).The ET-R pan-antagonist Bosentan (Tracleer) (Rubin et al., 2002) is an approved drug currently used to treat pulmonary arterial hypertension (PAH), and displays a similar affinity to ET-Rs as PD142,893 (Maguire et al., 2012). Some MS patients with PAH respond to Bosentan (Ledinek et al., 2009). Therefore, PD142,893 and other ET-R pan-antagonists could offer a promising therapeutic avenue to promote lesion repair for patients with MS.
EXPERIMENTAL PROCEDURES
Animals
TNR (#005854) and C57bl/6n mice were purchased from the Jackson Laboratory and Charles River Labs, respectively. Floxed ET-1 mice were obtained from Dr. Ralph Shohet at the University of Hawaii and are described here: (Shohet et al., 2004). The hGFAP-Cre-ERT2 mice were obtained from Dr. Flora Vaccarino at Yale University, and were generated as previously described (Ganat et al., 2006). Mice used for all experiments were 8–12 weeks old unless otherwise specified. All mouse colonies were maintained in the animal facility of Children’s National Medical Center, and all animal procedures complied with the guidelines of the National Institute of Health, and with the Children’s Research Institute Institutional Animal Care and Use Committee (IACUC) guidelines.
Antibodies
For immunohistochemical, immunocytochemical, and Western blot procedures please reference the Supplemental Experimental Procedures. Antibodies used for immunohistochemistry were anti-GFP (Abcam, 1:500), anti-Brdu (Accurate,1:200, 30 min 2N HCl followed by 15 min 0.1M Boric Acid brain section pre-treatment), anti-NG2 (Millipore, 1:500), anti-GFAP (Sigma, 1:500), anti-ET-1 (Abbiotec, 1:200), anti-CD31 (BD Biosciences, 1:500), anti-Jagged-1 (Iowa Hybridoma Bank, 1:200), anti-IBA1 (Wako, 1:500), anti-MAG (Santa Cruz, 1:200), anti-MBP (Covance (SMI-99p), 1:1000), anti-Hes1 (Millipore, 1:1000), anti-CD11b/MAC1 (ABD Serotec, 1:400), anti-Olig2 (Millipore, 1:500), and anti-APC (Ab-7) (CC-1) (Calbiochem, 1:500). Antibodies used for immunocytochemistry were anti-GFP (Abcam, 1:500), anti-O1 (R&D systems, 1:500), anti-GFAP (Sigma, 1:500), and anti-NG2 (Millipore, 1:500). Antibodies used for Western Blot analysis include anti-MBP (Covance (SMI-99p), 1:5000), anti-MAG (Santa Cruz (sc-15324), 1:200), anti-CNPase (Covance, 1:500), anti-Jagged1 (Santa Cruz (sc-135955), 1:200), anti-β-actin(C4) (Millipore, 1:5000), anti-GFAP (Sigma, 1:5000), and anti-NICD (Iowa Hybridoma Bank C17.9C6, 1:1000).
Lysolecithin injection
Mice were deeply anaesthetized using 100 mg/kg Ketamine and 10mg/kg Xylazine. Lysolecithin (1% Lyso, 2μL, EMD chemicals) was injected unilaterally into the external capsule of 8–12wk old TNR or C57bl/6n mice using a Hamilton syringe. On the contralateral side, 2μL of 0.9% NaCl was injected for control purposes. Injections were made using a stereotaxic apparatus at the following coordinates: 1.0 mm anterior to Bregma, 1.5 mm lateral, 3.0 mm deep. The date of injection was denoted as 0 days post lesion (dpl). Mice were then left for a period of 3, 7, 14, or 30 dpl and then perfused for immunohistochemical analysis.
Mini-osmotic pump installation
Mice were deeply anaesthetized using 100 mg/kg Ketamine and 10mg/kg Xylazine and unilateral lysolecithin injections were performed in 8–12wk old TNR and C57bl/6n mice. Mini-osmotic pumps (Durect) were assembled using a brain infusion kit (#0008851, Durect) with a 3mm low profile 30 gauge stainless steel cannula and approximately 1 inch of polyethylene catheter tubing. Both the catheter tubing and mini-osmotic pumps were preloaded with 0.9% saline, ET-1 (EMD Chemicals), PD142,893 (Enzo life sciences), DAPT (Tocris), or a cocktail of PD142,893 and recombinant rat Jagged1 Fc (R&D systems) and left overnight at 37°C in 0.9% saline to initiate the pumping process and ensure steady state operation. For 6–8, 6–14, and 14–21dpl infusions, we pre-loaded pumps (pump #107D) with 100nM ET-1, 50 μM PD142,893, 50 μM DAPT, or 50 μM PD142,893 + 2 μg/mL Jagged1 Fc, and for 15 day infusions, we loaded pumps with 100 μM PD142,893 (pump #1002). Due to differences in flow rates between the 7 d (#107D) and 14 d pumps (#1002), we had to adjust the concentration of PD142,893. The approximate PD142,893 delivery rates for both pumps were 300 pmol/day. For ET-1, the delivery rate was 60 pmol/day. Mice were re-anaesthetized and the pumps were installed into a subcutaneous pocket at the base of the neck. The catheter tubing and cannula were lead to the initial injection site and the cannula was inserted into the same skull perforation used for lysolecithin injection, which was still visible. The cannula was attached to the skull using cyanoacrylate adhesive (#0008670, Durect). Brains were then used for immunohistochemical and Western blot analysis.
Cell cultures
Purified astrocyte cultures were prepared from 20-d-old Sprague Dawley rat embryos as previously described (Gallo and Armstrong, 1995; Schinelli et al., 2001). Astrocytes were left to grow until 70–80% confluency before addition of 100nM ET-1 or 100μM PD142,893. Cell culture media was changed in both control and ET-1/antagonist treated groups every 24 h, including new ET-1/antagonist for the treated groups. For cells treated with both ET-1 and the antagonist, PD142,893 was added 2 h prior to the addition of ET-1. For Western blot analysis, cells were rinsed twice in ice-cold PBS and collected in RIPA lysis buffer with protease inhibitor cocktail. Co-cultures: Glass coverslips (Fisher, 25mM HCl treated) were coated in poly-L-lysine in 6-well plates. Purified astrocytes were plated at 75,000 cells per coverslip and left to grow until 70–80% confluent in 10% FBS/DMEM. Three experimental groups of astrocytes were then established: 1.) untreated 2.) ET-1 treated 3.) PD142,893 treated, then ET-1 treated, as described above.
Purified cortical oligodendrocyte progenitor (OP) cultures were prepared from embryonic day 20 rats or P5 TNR mice as previously described (Gallo et al., 1996; Ghiani et al., 1999). OPC cultures were maintained in DMEM-N1 biotin-containing medium (penicillin, 100 U/ml; streptomycin, 100 μg/ml; human apo-transferrin, 50 μg/ml; biotin, 10 ng/ml; Na selenium, 25 nM; insulin, 2.5 μg/ml; putrescine, 100 μM; progesterone 20 nM) with added PDGF-AA and bFGF (10 ng/mL each, R&D systems, Minneapolis, MN) to inhibit differentiation. Following astrocyte treatment, OPCs were plated on the astrocytes at a density of 75,000 cells per coverslip in the DMEM-N1 biotin-containing medium minus growth factors to allow differentiation. For co-cultures treated with anti-ET-1 antibody (Calbiochem, 1:500), astrocyte monolayers were pretreated with ET-1, and during the OPC differentiation phase of the culture anti-ET-1 was added once per day. Co-cultures were then left for a period of 48 h to differentiate. Coverslips were then used for immunocytochemical analysis.
Statistical analysis
Specific numbers of animals or cultures are denoted in each figure legend. Significance was calculated using GraphPad Prism software (graphpad.com) using unpaired t-tests for comparisons between two groups. For multi-group comparisons, a one-way ANOVA with a Bonferroni post-hoc analysis was used.
Supplementary Material
Acknowledgments
This work was completed as part of a dissertation for the George Washington Institute for Biomedical Sciences Ph.D. training program. We thank GWU IBS committee members Drs. Josh Corbin, Anthony-Samuel LaMantia, and Anne Chiaramello. We also thank Drs. Li-Jin Chew, Joseph Scafidi, and Matthew Raymond for discussion and for critically reading this manuscript. This work was supported by the National Multiple Sclerosis Foundation (grant # RG4019; V.G.) and partially supported by R01NS045702 (V.G.), R01NS056427 (V.G.) and by NIH IDDRC P30HD40677 (V.G.).
Footnotes
CONTRIBUTIONS
T.R.H., A.G., and V.G. designed and conceptualized all the experiments. T.R.H. performed and analyzed all the experiments in this project with the exception of EM and MS tissue staining. J.D. performed EM and EM analysis. B.N. and C.K. performed MS tissue staining and analysis. A.A. assisted with LPC injections and osmotic pump installations, and helped with the overall analysis of results from these experiments. T.R.H. and V.G. wrote and edited the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ables JL, Breunig JJ, Eisch AJ, Rakic P. Not(ch) just development: Notch signalling in the adult brain. Nat Rev Neurosci. 2011;12:269–283. doi: 10.1038/nrn3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams CW, Abdulla YH, Torres EM, Poston RN. Periventricular lesions in multiple sclerosis: their perivenous origin and relationship to granular ependymitis. Neuropathol Appl Neurobiol. 1987;13:141–152. doi: 10.1111/j.1365-2990.1987.tb00177.x. [DOI] [PubMed] [Google Scholar]
- Aguirre A, Dupree JL, Mangin JM, Gallo V. A functional role for EGFR signaling in myelination and remyelination. Nat Neurosci. 2007;10:990–1002. doi: 10.1038/nn1938. [DOI] [PubMed] [Google Scholar]
- Arnett HA, Fancy SP, Alberta JA, Zhao C, Plant SR, Kaing S, Raine CS, Rowitch DH, Franklin RJ, Stiles CD. bHLH transcription factor Olig1 is required to repair demyelinated lesions in the CNS. Science. 2004;306:2111–2115. doi: 10.1126/science.1103709. [DOI] [PubMed] [Google Scholar]
- Brosnan CF, Raine CS. The astrocyte in multiple sclerosis revisited. Glia. 2013;61:453–465. doi: 10.1002/glia.22443. [DOI] [PubMed] [Google Scholar]
- Buck D, Hemmer B. Treatment of multiple sclerosis: current concepts and future perspectives. J Neurol. 2011;258:1747–1762. doi: 10.1007/s00415-011-6101-2. [DOI] [PubMed] [Google Scholar]
- Chang A, Tourtellotte WW, Rudick R, Trapp BD. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med. 2002;346:165–173. doi: 10.1056/NEJMoa010994. [DOI] [PubMed] [Google Scholar]
- Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- Fancy SP, Harrington EP, Yuen TJ, Silbereis JC, Zhao C, Baranzini SE, Bruce CC, Otero JJ, Huang EJ, Nusse R, et al. Axin2 as regulatory and therapeutic target in newborn brain injury and remyelination. Nat Neurosci. 2011;14:1009–1016. doi: 10.1038/nn.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull. 1999;49:377–391. doi: 10.1016/s0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant C, Raff MC. Proliferating bipotential glial progenitor cells in adult rat optic nerve. Nature. 1986;319:499–502. doi: 10.1038/319499a0. [DOI] [PubMed] [Google Scholar]
- Franklin RJ. Why does remyelination fail in multiple sclerosis? Nat Rev Neurosci. 2002;3:705–714. doi: 10.1038/nrn917. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9:839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- Gadea A, Aguirre A, Haydar TF, Gallo V. Endothelin-1 regulates oligodendrocyte development. J Neurosci. 2009;29:10047–10062. doi: 10.1523/JNEUROSCI.0822-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadea A, Schinelli S, Gallo V. Endothelin-1 regulates astrocyte proliferation and reactive gliosis via a JNK/c-Jun signaling pathway. J Neurosci. 2008;28:2394–2408. doi: 10.1523/JNEUROSCI.5652-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V, Armstrong RC. Developmental and growth factor-induced regulation of nestin in oligodendrocyte lineage cells. J Neurosci. 1995;15:394–406. doi: 10.1523/JNEUROSCI.15-01-00394.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V, Zhou JM, McBain CJ, Wright P, Knutson PL, Armstrong RC. Oligodendrocyte progenitor cell proliferation and lineage progression are regulated by glutamate receptor-mediated K+ channel block. J Neurosci. 1996;16:2659–2670. doi: 10.1523/JNEUROSCI.16-08-02659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganat YM, Silbereis J, Cave C, Ngu H, Anderson GM, Ohkubo Y, Ment LR, Vaccarino FM. Early postnatal astroglial cells produce multilineage precursors and neural stem cells in vivo. J Neurosci. 2006;26:8609–8621. doi: 10.1523/JNEUROSCI.2532-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y. Multiple sclerosis: the role of MR imaging. AJNR Am J Neuroradiol. 2006;27:1165–1176. [PMC free article] [PubMed] [Google Scholar]
- Genoud S, Lappe-Siefke C, Goebbels S, Radtke F, Aguet M, Scherer SS, Suter U, Nave KA, Mantei N. Notch1 control of oligodendrocyte differentiation in the spinal cord. J Cell Biol. 2002;158:709–718. doi: 10.1083/jcb.200202002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiani CA, Eisen AM, Yuan X, DePinho RA, McBain CJ, Gallo V. Neurotransmitter receptor activation triggers p27(Kip1)and p21(CIP1) accumulation and G1 cell cycle arrest in oligodendrocyte progenitors. Development. 1999;126:1077–1090. doi: 10.1242/dev.126.5.1077. [DOI] [PubMed] [Google Scholar]
- Hamby ME, Coppola G, Ao Y, Geschwind DH, Khakh BS, Sofroniew MV. Inflammatory mediators alter the astrocyte transcriptome and calcium signaling elicited by multiple G-protein-coupled receptors. J Neurosci. 2012;32:14489–14510. doi: 10.1523/JNEUROSCI.1256-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang MH, Hoog A, Ma KC, Nie XJ, Olsson Y, Zhang WW. Endothelin-1-like immunoreactivity is expressed in human reactive astrocytes. Neuroreport. 1993;4:935–937. doi: 10.1097/00001756-199307000-00024. [DOI] [PubMed] [Google Scholar]
- John GR, Shankar SL, Shafit-Zagardo B, Massimi A, Lee SC, Raine CS, Brosnan CF. Multiple sclerosis: re-expression of a developmental pathway that restricts oligodendrocyte maturation. Nat Med. 2002;8:1115–1121. doi: 10.1038/nm781. [DOI] [PubMed] [Google Scholar]
- Jung KJ, Kim DW, Lee HN, Lee YS, Lee SJ, Che JH, Lee YH, Kang BC. The role of endothelin receptor A during myelination of developing oligodendrocytes. J Korean Med Sci. 2011;26:92–99. doi: 10.3346/jkms.2011.26.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama Y, Takemura M, Fujiki K, Ishikawa N, Shigenaga Y, Baba A. BQ788, an endothelin ET(B) receptor antagonist, attenuates stab wound injury-induced reactive astrocytes in rat brain. Glia. 1999;26:268–271. doi: 10.1002/(sici)1098-1136(199905)26:3<268::aid-glia8>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Ledinek AH, Jazbec SS, Drinovec I, Rot U. Pulmonary arterial hypertension associated with interferon beta treatment for multiple sclerosis: a case report. Mult Scler. 2009;15:885–886. doi: 10.1177/1352458509104593. [DOI] [PubMed] [Google Scholar]
- Maguire JJ, Kuc RE, Davenport AP. Defining the affinity and receptor sub-type selectivity of four classes of endothelin antagonists in clinically relevant human cardiovascular tissues. Life Sci. 2012 doi: 10.1016/j.lfs.2012.05.008. [DOI] [PubMed] [Google Scholar]
- McKhann GM. Multiple sclerosis. Annu Rev Neurosci. 1982;5:219–239. doi: 10.1146/annurev.ne.05.030182.001251. [DOI] [PubMed] [Google Scholar]
- Minami M, Kimura M, Iwamoto N, Arai H. Endothelin-1-like immunoreactivity in cerebral cortex of Alzheimer-type dementia. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19:509–513. doi: 10.1016/0278-5846(95)00031-p. [DOI] [PubMed] [Google Scholar]
- Mizutani K, Yoon K, Dang L, Tokunaga A, Gaiano N. Differential Notch signalling distinguishes neural stem cells from intermediate progenitors. Nature. 2007;449:351–355. doi: 10.1038/nature06090. [DOI] [PubMed] [Google Scholar]
- Moll NM, Hong E, Fauveau M, Naruse M, Kerninon C, Tepavcevic V, Klopstein A, Seilhean D, Chew LJ, Gallo V, Oumesmar BN. SOX17 is expressed in regenerating oligodendrocytes in experimental models of demyelination and in multiple sclerosis. Glia. 2013;61:1659–1672. doi: 10.1002/glia.22547. [DOI] [PubMed] [Google Scholar]
- Moore CS, Abdullah SL, Brown A, Arulpragasam A, Crocker SJ. How factors secreted from astrocytes impact myelin repair. J Neurosci Res. 2010;89:13–21. doi: 10.1002/jnr.22482. [DOI] [PubMed] [Google Scholar]
- Nair A, Frederick TJ, Miller SD. Astrocytes in multiple sclerosis: a product of their environment. Cell Mol Life Sci. 2008;65:2702–2720. doi: 10.1007/s00018-008-8059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SD, Peters CM, Pomonis JD, Hagiwara H, Ghilardi JR, Mantyh PW. Endothelin B receptors are expressed by astrocytes and regulate astrocyte hypertrophy in the normal and injured CNS. Glia. 2003;41:180–190. doi: 10.1002/glia.10173. [DOI] [PubMed] [Google Scholar]
- Rubanyi GM, Botelho LH. Endothelins. FASEB J. 1991;5:2713–2720. doi: 10.1096/fasebj.5.12.1916094. [DOI] [PubMed] [Google Scholar]
- Rubin LJ, Badesch DB, Barst RJ, Galie N, Black CM, Keogh A, Pulido T, Frost A, Roux S, Leconte I, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346:896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- Schinelli S. Pharmacology and physiopathology of the brain endothelin system: an overview. Curr Med Chem. 2006;13:627–638. doi: 10.2174/092986706776055652. [DOI] [PubMed] [Google Scholar]
- Schinelli S, Zanassi P, Paolillo M, Wang H, Feliciello A, Gallo V. Stimulation of endothelin B receptors in astrocytes induces cAMP response element-binding protein phosphorylation and c-fos expression via multiple mitogen-activated protein kinase signaling pathways. J Neurosci. 2001;21:8842–8853. doi: 10.1523/JNEUROSCI.21-22-08842.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnyder A, Huwyler J. Drug transport to brain with targeted liposomes. NeuroRx. 2005;2:99–107. doi: 10.1602/neurorx.2.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz K, Kroner A, David S. Iron efflux from astrocytes plays a role in remyelination. J Neurosci. 2012;32:4841–4847. doi: 10.1523/JNEUROSCI.5328-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert T, Bauer J, Weissert R, Fazekas F, Storch MK. Notch1 and its ligand Jagged1 are present in remyelination in a T-cell- and antibody-mediated model of inflammatory demyelination. Acta Neuropathol. 2007;113:195–203. doi: 10.1007/s00401-006-0170-9. [DOI] [PubMed] [Google Scholar]
- Shi N, Zhang Y, Zhu C, Boado RJ, Pardridge WM. Brain-specific expression of an exogenous gene after i.v. administration. Proc Natl Acad Sci U S A. 2001;98:12754–12759. doi: 10.1073/pnas.221450098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohet RV, Kisanuki YY, Zhao XS, Siddiquee Z, Franco F, Yanagisawa M. Mice with cardiomyocyte-specific disruption of the endothelin-1 gene are resistant to hyperthyroid cardiac hypertrophy. Proc Natl Acad Sci U S A. 2004;101:2088–2093. doi: 10.1073/pnas.0307159101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Stidworthy MF, Genoud S, Li WW, Leone DP, Mantei N, Suter U, Franklin RJ. Notch1 and Jagged1 are expressed after CNS demyelination, but are not a major rate-determining factor during remyelination. Brain. 2004;127:1928–1941. doi: 10.1093/brain/awh217. [DOI] [PubMed] [Google Scholar]
- Su Z, Yuan Y, Chen J, Zhu Y, Qiu Y, Zhu F, Huang A, He C. Reactive astrocytes inhibit the survival and differentiation of oligodendrocyte precursor cells by secreted TNF-alpha. J Neurotrauma. 2011;28:1089–1100. doi: 10.1089/neu.2010.1597. [DOI] [PubMed] [Google Scholar]
- Tsang MC, Lo AC, Cheung PT, Chung SS, Chung SK. Perinatal hypoxia-/ischemia-induced endothelin-1 mRNA in astrocyte-like and endothelial cells. Neuroreport. 2001;12:2265–2270. doi: 10.1097/00001756-200107200-00044. [DOI] [PubMed] [Google Scholar]
- Wang Y, Cheng X, He Q, Zheng Y, Kim DH, Whittemore SR, Cao QL. Astrocytes from the contused spinal cord inhibit oligodendrocyte differentiation of adult oligodendrocyte precursor cells by increasing the expression of bone morphogenetic proteins. J Neurosci. 2011;31:6053–6058. doi: 10.1523/JNEUROSCI.5524-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A, Piaton G, Lubetzki C. Astrocytes--friends or foes in multiple sclerosis? Glia. 2007;55:1300–1312. doi: 10.1002/glia.20546. [DOI] [PubMed] [Google Scholar]
- Wolswijk G. Chronic stage multiple sclerosis lesions contain a relatively quiescent population of oligodendrocyte precursor cells. J Neurosci. 1998;18:601–609. doi: 10.1523/JNEUROSCI.18-02-00601.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen TJ, Johnson KR, Miron VE, Zhao C, Quandt J, Harrisingh MC, Swire M, Williams A, McFarland HF, Franklin RJ, Ffrench-Constant C. Identification of endothelin 2 as an inflammatory factor that promotes central nervous system remyelination. Brain. 2013;136:1035–1047. doi: 10.1093/brain/awt024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA. Genomic analysis of reactive astrogliosis. J Neurosci. 2012;32:6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Argaw AT, Gurfein BT, Zameer A, Snyder BJ, Ge C, Lu QR, Rowitch DH, Raine CS, Brosnan CF, John GR. Notch1 signaling plays a role in regulating precursor differentiation during CNS remyelination. Proc Natl Acad Sci U S A. 2009;106:19162–19167. doi: 10.1073/pnas.0902834106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang J, Navrazhina K, Argaw AT, Zameer A, Gurfein BT, Brosnan CF, John GR. TGFbeta1 induces Jagged1 expression in astrocytes via ALK5 and Smad3 and regulates the balance between oligodendrocyte progenitor proliferation and differentiation. Glia. 2010;58:964–974. doi: 10.1002/glia.20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.