Abstract
This study addresses a much-debated effect on a much-debated region: the increase of left inferior frontal gyrus (LIFG) activation associated with object-extracted relative clauses. This haemodynamic result is one of the most central and most cited findings in the cognitive neuroscience of syntax and it has robustly contributed to the popular association of Broca's region with syntax. Our study had two goals: (1) to characterise the timing of this classic effect with magnetoencephalography (MEG) and (2) to connect it to psycholinguistic research on the effects of similarity-based interference during sentence processing. Specifically, behavioural studies have shown that object relatives are primarily only costly when the two preverbal noun phrases are parallel in their surface syntax, for example, both consisting of a definite determiner and a noun (e.g. the reporter who the senator attacked), as opposed to employing, for example, a definite noun phrase and a proper name (the reporter who Bill attacked). This finding suggests that the difficulty of object extraction lies not within its syntax but rather in similarity-based interference affecting working memory processes. Although working memory is a prominent hypothesis for the LIFG engagement in object extraction, the haemodynamic literature has routinely employed stimuli involving parallel as opposed to non-parallel syntax. Using written sentences presented word-by-word, we tested whether an LIFG effect of object extraction is obtained with MEG, allowing us to characterise its timing, and whether it reduces or disappears if the two preverbal noun phrases are non-parallel in their surface syntax. Our results show an LIFG increase for object relatives at around 600 ms after verb onset, but only when the preverbal arguments are parallel. These findings are consistent with memory and competition-based explanations of the LIFG effect of object extraction and challenge accounts attributing it to displacement.
Keywords: dependency formation, similarity-based interference, MEG, LIFG
One of the most contentious questions in the cognitive neuroscience of language has been the contribution of the left inferior frontal gyrus (LIFG) to syntactic processing. Within this literature, perhaps the most replicated finding has been the increase of haemodynamic activity from object-extracted relative clauses (the fireman who the deputy called saved the sailor) as compared to subject-extracted ones (the fireman who called the deputy saved the sailor) (Ben-Shachar, Hendler, Kahn, Ben-Bashat, & Grodzinsky, 2003; Ben-Shachar, Palti, & Grodzinsky, 2004; Caplan, Alpert, Waters, & Olivieri, 2000; Caplan, Stanczak, & Waters, 2008; Constable et al., 2004; Just, Carpenter, Keller, Eddy, & Thulborn, 1996; Keller, Carpenter, & Just, 2001; Rogalsky, Matchin, & Hickok, 2008; Stromswold, Caplan, Alpert, & Rauch, 1996), mirroring the behavioural finding that object relatives are more costly to process than subject relatives (e.g. Ford, 1983; Hakes, Evans, & Brannon, 1976; Holmes, 1973; Holmes & O'Regan, 1981; King & Just, 1991; Wanner & Maratsos, 1978; Waters, Caplan, & Hildebrandt, 1987). Consequently, this haemodynamic finding and related results on clefts (Caplan, Alpert, & Waters, 1999), scrambled expressions (Friederici, Fiebach, Schlesewsky, Bornkessel, & von Cramon, 2006), topicalisation (Ben-Shachar et al., 2004) and wh-movement (Fiebach, Schlesewsky, Lohmann, von Cramon, & Friederici, 2005; Santi & Grodzinsky, 2007) have played a prominent role in bolstering the hypothesis that left inferior frontal cortex, or ‘Broca's area’, is linked to syntactic processing, a proposal originally arising from the aphasia literature (Berndt & Caramazza, 1980; Damasio & Damasio, 1989; Grodzinsky, 2000; Zurif, 1995). Current theories of LIFG contributions to syntax include the processing of displacement (Ben-Shachar et al., 2003; Grodzinsky, 2000; Grodzinsky & Santi, 2008), ‘linearization’ (Bornkessel, Zysset, Friederici, von Cramon, & Schlesewsky, 2005; Grewe et al., 2005), and ‘unification’ (Hagoort, 2003, 2005), all of which contrast with more domain-general theories linking LIFG instead to working memory (Caplan et al., 2000, 2008; Fiebach et al., 2005; Fiebach, Schlesewsky, & Friederici, 2001; Kaan & Swaab, 2002; Rogalsky et al., 2008) or cognitive control (Botvinick, Braver, Barch, Carter, & Cohen, 2001; Miller & Cohen, 2001; Novick, Trueswell, & Thompson-Schill, 2005), both of which are resources relevant for much of the sentence processing.
The aims of the current study were twofold: first, to characterise the time course of activation in Broca's area during object extraction, and second, to better connect this literature to psycholinguistic findings on object relatives. As regards the former, although the LIFG literature on object relatives and similar structures is vast, no studies have yet attempted to characterise the timing of LIFG activation during object extraction. Only one Event-Related Potential (ERP) study has investigated object relatives, revealing an increased left anterior negativity for object relatives as compared to subject relatives at 300–500 ms (King & Kutas, 1995), but given the limited temporal resolution as Electroencephalography (EEG) but the conclusion that this effect originated in the LIFG is not warranted. In the present work, we addressed this question with magnetoencephalography (MEG), which offers the same temporal resolution as EEG but substantially superior spatial resolution.
As regards our second objective, although the choice of object vs. subject relatives as an experimental manipulation for brain research was originally guided by psycholinguistic results (Just et al., 1996; Stromswold et al., 1996), the subsequent literature has not always tightly connected with behavioural research on these types of structures. Specifically, although object relatives take longer to process than subject relatives, this effect is robustly modulated by whether or not the two noun phrase arguments in the structure are syntactically parallel, i.e. consistent in their surface syntax (Gordon, Hendrick, & Johnson, 2001; Gordon, Hendrick, Johnson, & Lee, 2006; Warren & Gibson, 2005;). In other words, in a four-way comparison such as in (1) below, only (1c) shows increased reading times, by hypothesis because the barber is identical in its surface syntax to the lawyer. On the other hand, no reliable differences are observed between subject and object extractions when the two noun phrases differ in their surface syntax (1b vs. 1d) (Gordon et al., 2001).
 |
Results such as these have been taken to show that the difficulty with object extractions lies not in their syntax but rather in similarity-based interference in working memory processes (Gordon et al., 2001, 2006; Warren & Gibson, 2005). Interestingly, although working memory and conflict resolution have both been proposed as explanations of the LIFG effect elicited by object-extracted clauses (Cooke et al., 2002; Fiebach et al. 2001, 2005; King & Kutas, 1995; Miller & Cohen, 2001; Novick et al., 2005; Rogalsky et al., 2008), no neurolinguistic investigation has yet directly addressed how this effect might be modulated by the syntactic parallelism of the two preverbal noun phrases. If computations in the LIFG reflect dependency formation (Ben-Shachar et al., 2003, 2004; Grodzinsky, 1986, 2000; Grodzinsky & Friederici, 2006; Santi & Grodzinsky, 2007; Grodzinsky & Santi 2008), parallel and non-parallel object relatives should engage the LIFG similarly. In contrast, if the role of the LIFG is instead related to working memory or conflict resolution among similar representations, only object relatives with syntactically parallel noun phrases should engage it, due to similarity-based interference. Prior imaging studies have overwhelmingly only used parallel syntactic structures (Ben-Shachar et al., 2004; Caplan et al., 1999, 2000, 2008; Fiebach et al., 2005; Friederici et al., 2006; Just et al., 1996; Rogalsky, et al., 2008; Santi & Grodzinsky, 2007; Stromswold et al., 1996), with just a handful of exceptions. Cooke et al. (2002) employed non-parallel proper names and definite noun phrases in short and long subject and object extractions, finding a left inferior frontal effect (BA 47) only for long object extractions, consistent with a working memory-based explanation. In contrast, Ben-Shachar et al. (2003), did observe an LIFG effect (BA 45) for object extractions with definite noun phrases and proper names, but this study used Hebrew where the definite determiner is a bound morpheme and thus the surface syntax of proper names and definite noun phrases is more similar than in English.
In this study, we examined the role of similarity-based interference in object extractions within an MEG paradigm that was designed to vary both the presence of dependency formation and the parallelism between the two preverbal noun phrases in an object-relative structure. To this end, we did not use subject relatives as a control condition, since subject relatives contain a dependency, but rather employed the embedded clause of our object relatives as the baseline condition, i.e. the ‘DP2 VP’ sequence of an object relative involving a ‘DP1 DP2 VP’ sequence. Although such a contrast would not be appropriate for haemodynamic techniques, the time resolution of MEG allowed us to focus our analysis on the final verb only, i.e. on the retrieval site. To achieve this contrast, the embedded clauses of our object relatives needed to employ verbs that only optionally take a direct object. Since transitivity alternations are typically accompanied by a morphological change (albeit often a zero derivation in English), it was critical to ensure that our contrast between simple sentences and object relatives was not confounded by a morphological complexity contrast in the verb. In other words, a possible effect of dependency formation should not be interpretable as an effect of increased morphological complexity on the verb, a possibility that would arise if the transitive verbs in the object relatives were all derived via affixation from the intransitive verbs in the simple sentence condition. To avoid such a confound, half of the verbs were reflexive alternating (e.g. bathe), and thus morphologically more basic in their transitive form (Mchombo, 1993); and half were causative alternating (e.g. walk), and thus morphologically more basic in their intransitive form (Pylkkänen, 2008). Thus, collapsing across these two types yielded a transitive and intransitive condition equated for morphological complexity (at least in light of the relevant theoretical literature).
Our stimuli employed the smallest possible phrases to achieve our desired contrasts, following our group's previous work on minimal composition (Bemis & Pylkkänen, 011, 2012, 2013). Thus instead of full sentences, our relative clause stimuli contained only the relevant complex noun phrase, as shown in (2).
 |
The verb was in all cases the target of the MEG data analysis. Although the critical stimuli varied in length, trial length was kept constant by inserting unpronounceable consonant strings at the beginning of the shorter expressions (cf., Bemis & Pylkkänen, 2011). Following the paradigm of Bemis and Pylkkänen (2011, 2012), each critical stimulus was followed by a picture that either matched or mismatched the verbal stimulus. This allowed us to monitor participants' attention continuously without asking them to perform explicit judgments on the critical stimuli themselves.
In sum, our aim was to contribute a timing dimension to neurolinguistic findings on object extraction and to test whether LIFG increases in response to them are dependent on syntactic parallelism between the two noun phrases of the expression. If such a dependency is observed, this would indicate that the LIFG effect does not reflect syntactic aspects of the computations involved in building object relatives, but rather interference caused by similarity-based retrieval.
Methods
Participants
Fifteen right-handed native English speakers participated in the study (9 females; age: mean: 27 years, range: 23–36 years, standard deviation: 6.5593 years). All had normal or corrected-to-normal vision and gave informed consent. Two subjects were excluded from the final analyses due to noisy data, resulting in a final N of 13.
Stimuli and task
Three types of visual stimuli, 88 trials per condition, were presented word-by-word to participants: (1) minimal sentences consisting of a subject and verb (SubjVerb: Sally bathed), (2) minimal relative clauses embedding the SubjVerb sequences of the baseline condition (ObjRel: the dog Sally bathed) and (3) relative clauses where the proper name of the ObjRel condition was replaced by a definite noun phrase, creating a sequence of two syntactically parallel noun phrases, intended to induce similarity-based interference (ObjRelSim: the dog the woman bathed). To match the verbs in the SubjVerb and object relative conditions for morphological complexity (see introduction), half of the trials in each 88-trial condition (i.e. 44 trials) employed 1 of 11 reflexive alternating verbs (bathed, dressed, undressed, disrobed, showered, shaved, washed, exercised, stretched, hid and rocked) and half of which employed 1 of 11 causative alternating verbs (walked, moved, ran, broke, bounced, floated, rolled, swung, rotated, turned and dropped). Four proper names were employed (Sally, Sam, Jean and Ted) and substituted by four definite noun phrases in the ObjRelSim condition (e.g. the woman for Sally; the man for Sam, the girl for Jean and the boy for Ted). The verb was always the target of the MEG analysis. To control for trial length and visual baseline, consonant strings were employed at the beginning of the trials, as shown in Figure 1.
Figure 1.
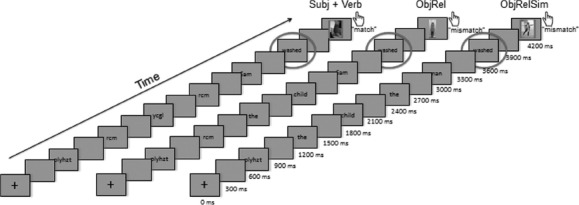
Trial structure.
Various fillers were employed to increase the variability of the materials and to lessen the predictability of the critical stimuli. In addition to the three frames of the critical conditions, each of the 88 stimulus sets also contained a stimulus where instead of initiating a relative clause, a definite noun phrase directly combined with a verb (the dog bathed) or occurred in isolation (the dog). Each set also contained a stimulus with an isolated proper name (Sally) and isolated verb (bathed). In total, each subject saw 616 text-picture trials in randomised order with four breaks (between every 154 trials).
Following the paradigm introduced in Bemis and Pylkkänen (2011), after each linguistic stimulus, participants were presented with a photograph that either matched or mismatched the verbal stimulus. For the purposes of this task, the participants were familiarised with the characters matching the proper names in the verbal stimuli prior to the MEG recording. Half of the photos matched and half mismatched the text. For a match, both the character and the action mentioned in the text needed to appear in the image. Mismatches were either full (both the character and the action mismatched) or partial (either the character or the action mismatched).
Procedure
Before the MEG recordings, participants were instructed about the experimental task and a Polhemus Fastrak® 3D digitiser was used to digitise their head shapes, which were then used to constrain source localisation during data analysis. During the experiment, participants lay in a dimly lit, magnetically shielded room. Using PsychToolbox, the experiment was presented on a 7.3 × 5.5 inch screen with a resolution of 1024 × 768 pixels placed approximately 16.5 inches above the subject's eye. Stimuli were presented word-by-word, 300 ms for each word, with a 300 ms blank screen between each word, followed by a picture shown for 300 ms (Figure 1). Using a button press, subjects expressed whether or not the picture depicted the previous linguistic expression. Subjects were given four rest periods and trial order was random.
MEG data were collected using a whole-head 157-channel axial gradiometer system (Kanazawa Institute of Technology, Nonoichi, Japan) sampling at 1000 Hz with a low-pass filter at 200 Hz using a DC recording and a notch filter at 60 Hz. Electro-oculography (EOG) is used to record eye-blinks. The entire recording took about 1 hour.
Data analysis
Behavioural data
Behavioural data were analysed with a repeated measures 2 × 3 ANOVA with Frame and Verb Type as factors. Incorrectly answered trials, along with those where the subjects' button presses were detected earlier than 100 ms or later than 7000 ms following the presentation of the image were removed.
Pre-processing of MEG data
Raw data were noise-reduced (CALM; Adachi, Shimogawara, Higuchi, Haruta, & Ochiai, 2001) and cleaned of artefacts (at a threshold of 4000 fT) including those trials that were removed in behavioural artefact rejection (i.e. incorrectly answered trials, or those where the subjects' button presses were not detected between 100 and 7000 ms following the presentation of the image). Artefacts also included eye-blinks which were removed manually by the examination of EOG recordings. On average, for each of the six sub-conditions (broken down by verb type), about 33 trials (76%) were kept after artefact rejection. Data were averaged by condition using a 200 ms pre-stimulus interval and an 800 ms post-stimulus interval and baseline corrected using the 200 ms pre-stimulus interval. Averages were created both for the three main conditions (collapsing over verb type) as well as for the six uncollapsed conditions broken down by verb type, allowing us to examine possible effects of verb type. Data were low-pass filtered at 40 Hz and high-pass filtered at 1 Hz before averaging, using the programme BESA® 5.1 (MEGIS Software GmbH). Additionally, two subjects were excluded as outliers because their data showed an amplitude that was twice as high as other subjects (either during the baseline or overall).
ROI and full brain analysis of minimum norm estimates
L2 minimum norm estimates of the averaged data were constructed in BESA 5.1 (MEGIS Software GmbH). These estimates contain 1426 distributed regional sources; 713 in a shell at 10% below a smoothed brain surface and 713 in a shell at 30% below. The activity of each of these sources is calculated by taking the root mean square (RMS) of the activity from a pair of dipoles that are perpendicular to one another at each source. The larger value from each source pair provided the modelled activation from 713 sources for each condition and each participant. Minimum norm images were depth weighted as well as spatiotemporally weighted, using a signal subspace correlation measure (Mosher & Leahy, 1998).
After the creation of the BESA minimum norms, statistical analysis on LIFG ROI activity was performed in MATLAB. The LIFG ROI comprised of all sources within the smooth BESA cortex whose Talairach coordinates were assigned to left Brodmann areas 44 and 45 by Talairach Daemon (Lancaster et al., 2000). The activity across these sources was averaged together within each subject and condition and the time course of this activity was analysed by non-parametric cluster-based permutation tests (Maris & Oostenveld, 2007) aimed at identifying temporal clusters of activity that were significantly affected by our stimulus manipulation, corrected for multiple comparisons. For initial cluster selection, the thresholds of Bemis and Pylkkänen (2011) were employed, i.e. clusters were required to extend 10 adjacent time points with an uncorrected p-value of 0.3. In what follows, the corrected p-values reflect the distribution of the tests statistics after 10,000 permutations of the original data (see Bemis & Pylkkänen, 2011; Maris & Oostenveld, 2007; for further details on the logic of the permutation test).
Our aim was to collapse activity across the two verb types to achieve the highest possible signal-to-noise ratio in our source estimates, but to assess the validity of this, we first tested whether verb type significantly affected LIFG ROI activity. To this end, data for each of the six sub-conditions were subjected to a cluster-based permutation test using a 2 × 3 repeated measures ANOVA with Verb type (Reflexive vs. Causative) and Frame (SubjVerb, ObjRel vs. ObjRelSim) as factors. The permutation test first calculated the cluster with the highest statistic in the data-set by performing F-tests for an ANOVA at each time point, then finding the time points which meet the cluster criteria described above. The F values were then summed within each cluster. For each subject, the matrix for each condition was then randomly assigned to any one of the other conditions within the ANOVA and the largest cluster level statistic was calculated on this repartitioning. This largest cluster statistic was compared to the original cluster statistic calculated prior to permutation. The number of cluster statistics in the permutation distribution greater than in the observed statistics divided by the total number of tests performed yields the corrected p-value.
To increase the possibility of observing verb effects (which we hoped to not observe), we performed the permutation test in two separate time windows, an early ‘N400’ time window (200–500 ms) and a late ‘P600’ time window (500–800 ms). The ANOVA revealed no main effects or interactions in either time window and thus we concluded it was safe to collapse the data across verb types for the primary analysis.
Source localisation was then performed on the collapsed averages and subjected to a non-parametric, cluster-based permutation analysis of LIFG ROI activity, consisting of pairwise t-tests on the resulting three conditions (SubjVerb, ObjRel, ObjRelSim). The cluster selection criteria, number of permutations and analysis intervals (200–500 ms and 500–800 ms) were as in the prior ANOVA. The two time windows fit well with prior electrophysiological findings on object extractions, which have object elicited increased negativities at the earlier time window both in relative clauses (King & Kutas, 1995) and in wh-clauses (Kaan, Harris, Gibson, & Holcomb, 2000) and increased late positivities in wh-clauses (Kaan et al., 2000; Penolazzi, Vincenzi, Angrilli, & Job, 2005).
Since our research questions were entirely LIFG focused – i.e. to characterise time course of LIFG activation during object extraction and to assess its sensitivity to similarity-based interference – we did not include in our analyses any ROIs other than the LIFG. A broader investigation would have been licenced if, for example, our goal had been to characterise where in the brain dependency or similarity-based interference effects occur, but our aim was narrower than this. A liberal uncorrected whole brain analysis was, however, conducted to assess whether the obtained ROI effects in fact correspond to effects within the LIFG, as opposed to potential spillover from nearby regions, a possibility given the somewhat blurry spatial resolution of MEG. The minimum norm estimates of the activity elicited by the experimental conditions were compared sample-by-sample in three pairwise analyses: ObjRelSim vs. ObjRel, ObjRel vs. SubjVerb and ObjRelSim vs. SubjVerb. A difference was considered significant if it remained reliable (p < 0.05, uncorrected for multiple comparisons) for at least five temporal samples and was observed in at least five spatially contiguous cortical sources.
Results
Behavioural data
Our behavioural task was simply intended to ensure attention and did not directly tap in to the processing of the critical stimuli. Accuracy in task was overall high and no reliable effects were observed except a main effect of Frame in accuracy (F(2,12) = 14.522, p < 0.0001) (average accuracy ± SD for reflexives: SubjVerb, 96.00 ± 19.20%; ObjRel, 92.08 ± 26.10%; ObjRelSim, 92.28 ± 25.80%; and reaction time: SubjVerb, 1105.7 ± 90.78 ms; ObjRel, 1198.9 ± 142.97 ms; ObjRelSim, 1232.9 ± 405.94 ms; and for causatives: SubjVerb, 96.00 ± 19.20%; ObjRel, 90.63 ± 28.00%; ObjRelSim, 94.07 ± 23.00%; and reaction time: SubjVerb, 1213.00 ± 2264.2 ms; ObjRel, 1055.4 ± 587.41 ms; ObjRelSim, 1165.00 ± 398.08 ms). Collapsing over Verb type, there was still a significant main effect of Frame in accuracy (F(2,12) = 2.6243, p < 0.0017) and no other effects (average accuracy ± SD collapsing across verb type: SubjVerb, 96 ± 19.25%; ObjRel, 92.05 ± 27.07%; ObjRelSim, 93.62 ± 24.45%; and reaction time: SubjVerb 1159.3 ± 756.08 ms, ObjRel 1127.1 ± 645.09 ms, ObjRelSim, 1199.0 ± 716.05 ms).
ROI results (LIFG)
Pairwise permutation t-tests on our 13 participants' LIFG activity revealed no reliable effects for the ObjRel vs. SubjVerb contrast, as shown in the top panel of Figure 2. However, the ObjRelSim condition showed an LIFG increase both in comparison to the ObjRel and to the SubjVerb condition. The ObjRelSim vs. ObjRel comparison showed a reliable increase in a cluster at 266407 ms (p = .0032) and the ObjRelSim vs. SubjVerb comparison at 208–280 ms (p = .0307) and at 574 ms to 666 ms (p = .0162). Thus the ROI results showed evidence of an LIFG effect of object extraction only in the presence of syntactic parallelism. This pattern was further confirmed in the whole brain analysis (below).
Figure 2.
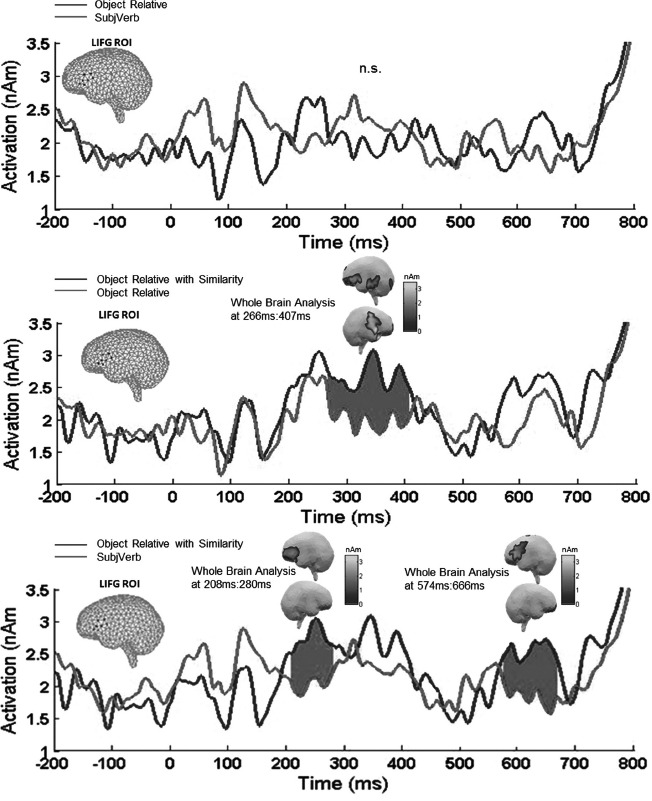
LIFG ROI results. The clusters of time points that were reliable in a clusterbased permutation t-test are shaded grey. Full-brain pair-wise subtractions are plotted for the significant intervals, replicating the LIFG increase for the ObjRelSim condition as compared to each of the other two.
Whole brain results
The lower two graphs in Figure 2 plot the same pairwise comparisons as reported above on loosely corrected whole brain minimum norms at the time windows of the significant effects in the ROI analysis. The aim of this analysis was to ascertain that the ROI results in fact correspond to activity localised in the LIFG. As the middle panel in Figure 2 reveals the ObjRelSim condition showed more activity in left inferior frontal cortex both in comparison to the non-parallel object relatives (ObjRel) and to the SubjVerb sequences. The full brain contrasts also showed that at the time of the reliable effects in the LIFG ROI analysis, effects extended beyond the LIFG for the ‘ObjRelSim – ObjRel’ comparison, showing an additional more posterior increase for ObjRelSim.
Discussion
This study used MEG to characterise the time course of well-documented LIFG effects of object extraction and to examine whether they are dependent on syntactic parallelism between the preverbal noun phrase arguments. Importantly, we replicate the LIFG increase associated with object extraction with a technique that has not yet been used to investigate it. Given the many differences between electrophysiological and haemodynamic measures (e.g. Huettel, Song, McCarthy, 2004; Maruyama, Pallier, Jobert, Sigman, & Dehaene, 2012; Vartiainen, Liljiestrom, Koskinen, Renvall, & Salmelin, 2011), this outcome is an important result in itself, as it opens up the possibility to employ a more time-sensitive method for the investigation of this well-established but controversial effect.
Regarding the time course of LIFG activation, the temporal resolution of MEG allowed us to address the basic but still open question of whether LIFG effects of object extraction occur primarily at the verb, i.e. the integration site of the dislocated element, or have already occurred earlier in the sentence. Within movement-based theories (Ben-Shachar et al., 2003, 2004; Grodzinsky 1986, 2000; Grodzinsky & Friederici, 2006; Grodzinsky & Santi 2008; Santi & Grodzinsky, 2007), a preverbal effect would be compatible with interpretations related to gap-anticipation as opposed to gap-filling. Within memory-based explanations (Cooke et al., 2002; Fiebach, et al. 2001, 2005; King & Kutas, 1995; Miller & Cohen, 2001; Novick et al., 2005; Rogalsky, et al., 2008), it would suggest similarity-based encoding interference – albeit its controversial status (e.g. Van Dyke & McElree, 2006) – as opposed to retrieval interference. Although our results do not rule out a preverbal effect, our study demonstrates that an LIFG increase is observed at the target verb, both in an early time window peaking around 250–350 ms and in a relatively late time-window peaking around 620 ms. However, this LIFG effect was only reliable for structures that involved noun phrases with a parallel syntactic structure, suggesting that the increase is primarily linked to similarity-based retrieval interference and not to gap-filling.
In sum, our findings suggest that the LIFG may not be sensitive to the presence of long-distance dependencies in the absence of interference-inducing parallel noun phrases. This result aligns the LIFG effect with the behavioural processing profile of object relatives, which are only robustly costly when gap-filling requires retrieval among noun phrases that are similar in their surface syntax (Gordon et al., 2001, 2006; Warren & Gibson, 2005). Of the various functional hypotheses regarding the LIFG, our results are most straightforwardly accounted for by theories that link the LIFG to memory operations sensitive to similarity-based interference or to conflict resolution more generally (Novick, et al., 2005; Thothathiri, Kim, Trueswell, & Thompson-Schill, 2012). Within memory-related hypotheses, the timing of our effect conforms best to a retrieval account (Öztekin, Curtis, McElree, 2009; Öztekin, McElree, Staresina, Davachi, 2008;), as our LIFG increase occurs at the gap-site. An alternative working memory-based theory has proposed that the LIFG effect of object extraction is due to the articulatory rehearsal during difficult to process sentences (Rogalsky et al., 2008). This type of account would most naturally predict a longer lasting effect covering much of the preverbal region in the current stimuli, whereas the effects we report are time-locked to the onset of the verb. As our study was designed for MEG analyses on the verb only, we cannot rule out the former type effect, and simply conclude that our findings conform well to retrieval but do not rule out rehearsal. Notably, it appears that a consensus is emerging in the field regarding the absence of LIFG effects driven by the sheer presence of a dependency (Santi & Grodzinsky, 2012), suggesting a possible closure to at least one corner of the debate regarding the role of Broca's region in language processing.
Acknowledgements
We thank Brian McElree and Julie Van Dyke for their comments on a previous version of this manuscript and Doug Bemis, Amanda Rysling, Rebecca Egbert and Paul Del Prato for their assistance at various stages of this project.
Funding
This study was supported by the National Science Foundation Grant [BCS-0545186] (LP), grant [G1001] from the NYUAD Institute, New York University Abu Dhabi (LP), and the Whitehead Fellowship for Junior Faculty Biomedical and Biological Sciences (LP).
References
- Adachi Y., Shimogawara M., Higuchi M., Haruta Y., Ochiai M. Reduction of non-periodic environmental magnetic noise in MEG measurement by continuously adjusted least squares method. IEEE Transactions on Applied Superconductivity. 2001;11(1):669–672. doi: 10.1109/77.919433. [DOI] [Google Scholar]
- Bemis D. K., Pylkkänen L. Simple composition: A magnetoencephalography investigation into the comprehension of minimal linguistic phrases. The Journal of Neuroscience. 2011;31:2801–2814. doi: 10.1523/JNEUROSCI.5003-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemis D. K., Pylkkänen L. Basic Linguistic Composition Recruits the Left Anterior Temporal Lobe and Left Angular Gyrus During Both Listening and Reading. Cerebral Cortex. 2012. bhs170. doi:10.1093/cercor/bhs170. [DOI] [PubMed]
- Bemis D. K., Pylkkänen L. Combination across domains: an MEG investigation into the relationship between mathematical, pictorial, and linguistic processing. Frontiers in Language Sciences. 2013;3:583. doi: 10.3389/fpsyg.2012.00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shachar M., Hendler T., Kahn I., Ben-Bashat D., Grodzinsky Y. The neural reality of syntactic transformations: Evidence from functional magnetic resonance imaging. Psychological Science. 2003;14:433–440. doi: 10.2307/40064164. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar M., Palti D., Grodzinsky Y. Neural correlates of syntactic movement: Converging evidence from two fMRI experiments. NeuroImage. 2004;21:1320–1336. doi: 10.1016/j.neuroimage.2003.11.027. [DOI] [PubMed] [Google Scholar]
- Berndt R. S., Caramazza A. A redefinition of the syndrome of Broca's Aphasia: Implications for a neurological model of language. Applied Psycholinguistics. 1980;1:225–278. doi: 10.1017/S0142716400000552. [DOI] [Google Scholar]
- Bornkessel I., Zysset S., Friederici A. D., von Cramon D. Y., Schlesewsky M. Who did what to whom? The neural basis of argument hierarchies during language comprehension. NeuroImage. 2005;26:221–233. doi: 10.1016/j.neuroimage.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Botvinick M. M., Braver T. S., Barch D. M., Carter C. S., Cohen J. D. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–652. doi: 10.1037/0033-295X.108.3.624. [DOI] [PubMed] [Google Scholar]
- Caplan D., Alpert N., Waters G. PET studies of syntactic processing with auditory sentence presentation. NeuroImage. 1999;9:343–351. doi: 10.1006/nimg.1998.0412. [DOI] [PubMed] [Google Scholar]
- Caplan D., Alpert N., Waters G., Olivieri A. Activation of Broca's area by syntactic processing under conditions of concurrent articulation. Human Brain Mapping. 2000;9(2):65. doi: 10.1002/(SICI)1097-0193(200002)9:2<65::AID-HBM1>3.3.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan D., Stanczak L., Waters G. Syntactic and thematic constraint effects on blood oxygenation level dependent signal correlates of comprehension of relative clauses. Journal of Cognitive Neuroscience. 2008;20:643–656. doi: 10.1162/jocn.2008.20044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constable R. T., Pugh K. R., Berroya E., Mencl W. E., Westerveld M., Ni W., Shankweiler D. Sentence complexity and input modality effects in sentence comprehension: An fMRI study. NeuroImage. 2004;22(1):11–21. doi: 10.1016/j.neuroimage.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Cooke A., Zurif E. B., DeVita C., Alsop D., Koenig P., Detre J., Grossman M. Neural basis for sentence comprehension: Grammatical and short-term memory components. Human Brain Mapping. 2002;15(2):80–94. doi: 10.1002/hbm.10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio H., Damasio A. R. Lesion analysis in neuropsychology. Oxford University Press; 1989. [Google Scholar]
- Fiebach C. J., Schlesewsky M., Friederici A. D. Syntactic working memory and the establishment of filler-gap dependencies: Insights from ERPs and fMRI. Journal of Psycholinguistic Research. 2001;30:321–338. doi: 10.1023/A:1010447102554. [DOI] [PubMed] [Google Scholar]
- Fiebach C. J., Schlesewsky M., Lohmann G., von Cramon D. Y., Friederici A. D. Revisiting the role of Broca's area in sentence processing: Syntactic integration versus syntactic working memory. Human Brain Mapping. 2005;24(2):79–91. doi: 10.1002/hbm.20070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford M. A method for obtaining measures of local parsing complexity throughout sentences. Journal of Verbal Learning and Verbal Behavior. 1983;22:203–218. doi: 10.1016/S0022-5371(83)90156-1. [DOI] [Google Scholar]
- Friederici A. D., Fiebach C. J., Schlesewsky M., Bornkessel I. D., von Cramon D. Y. Processing linguistic complexity and grammaticality in the left frontal cortex. Cerebral Cortex. 2006;16:1709–1717. doi: 10.1093/cercor/bhj106. [DOI] [PubMed] [Google Scholar]
- Gordon P. C., Hendrick R., Johnson M. Memory interference during language processing. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2001;27:1411–1423. doi: 10.1037/0278-7393.27.6.1411. [DOI] [PubMed] [Google Scholar]
- Gordon P. C., Hendrick R., Johnson M., Lee Y. Similarity-based interference during language comprehension: Evidence from eye tracking during reading. Journal of Experimental Psychology. Learning, Memory, and Cognition. 2006;32:1304–1321. doi: 10.1037/0278-7393.32.6.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewe T., Bornkessel I., Zysset S., Wiese R., Von Cramon D. Y., Schlesewsky M. The emergence of the unmarked: A new perspective on the language-specific function of Broca's area. Human Brain Mapping. 2005;26:178–190. doi: 10.1002/hbm.20154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodzinsky Y. Language deficits and the theory of syntax. Brain and Language. 1986;27(1):135–159. doi: 10.1016/0093-934X(86)90009-X. [DOI] [PubMed] [Google Scholar]
- Grodzinsky Y. The neurology of syntax: Language use without Broca's area. Target article with 36 commentaries. Behavioral and Brain Sciences. 2000;23:47–117. doi: 10.1017/S0140525X00502398. [DOI] [PubMed] [Google Scholar]
- Grodzinsky Y., Friederici A. D. Neuroimaging of syntax and syntactic processing. Current Opinion in Neurobiology. 2006;16:240–246. doi: 10.1016/j.conb.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Grodzinsky Y., Santi A. The battle for Broca's region. Trends in Cognitive Sciences. 2008;12:474–480. doi: 10.1016/j.tics.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Hagoort P. How the brain solves the binding problem for language: A neurocomputational model of syntactic processing. NeuroImage. 2003;20(Suppl. 1):S18–S29. doi: 10.1016/j.neuroimage.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Hagoort P. On Broca, brain, and binding: A new framework. Trends in Cognitive Sciences. 2005;9:416–423. doi: 10.1016/j.tics.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Hakes D. T., Evans J. S., Brannon L. L. Understanding sentences with relative clauses. Memory & Cognition. 1976;4:283–290. doi: 10.3758/BF03213177. [DOI] [PubMed] [Google Scholar]
- Holmes V. M. Order of main and subordinate clauses in sentence perception. Journal of Verbal Learning and Verbal Behavior. 1973;12:285–293. doi: 10.1016/S0022-5371(73)80072-6. [DOI] [Google Scholar]
- Holmes V. M., O'Regan J. K. Eye fixation patterns during the reading of relative-clause sentences. Journal of Verbal Learning and Verbal Behavior. 1981;20:417–430. doi: 10.1016/S0022-5371(81)90533-8. [DOI] [Google Scholar]
- Huettel S., Song A., McCarthy G. Functional magnetic resonance imaging [Sinauer Associates] 2004. Retrieved from http://www.amazon.ca/exec/obidos/redirect?tag=citeulike09-20&path=ASIN/0878932887.
- Just M. A., Carpenter P. A., Keller T. A., Eddy W. F., Thulborn K. R. Brain activation modulated by sentence comprehension. Science. 1996;274:114–116. doi: 10.1126/science.274.5284.114. [DOI] [PubMed] [Google Scholar]
- Kaan E., Harris A., Gibson E., Holcomb P. The P600 as an index of syntactic integration difficulty. Language and Cognitive Processes. 2000;15:159–201. doi: 10.1080/016909600386084. [DOI] [Google Scholar]
- Kaan E., Swaab T. Y. The brain circuitry of syntactic comprehension. Trends in Cognitive Sciences. 2002;6:350–356. doi: 10.1016/S1364-6613(02)01947-2. [DOI] [PubMed] [Google Scholar]
- Keller T. A., Carpenter P. A., Just M. A. The neural bases of sentence comprehension: A fMRI examination of syntactic and lexical processing. Cerebral Cortex. 2001;11:223–237. doi: 10.1093/cercor/11.3.223. [DOI] [PubMed] [Google Scholar]
- King J., Just M. A. Individual differences in syntactic processing: The role of working memory. Journal of Memory and Language. 1991;30:580–602. doi: 10.1016/0749-596X(91)90027-H. [DOI] [Google Scholar]
- King J. W., Kutas M. Who did what and when? Using word- and clause-level ERPs to monitor working memory usage in reading. Journal of Cognitive Neuroscience. 1995;7:376–395. doi: 10.1162/jocn.1995.7.3.376. [DOI] [PubMed] [Google Scholar]
- Lancaster J. L., Woldorff M. G., Parsons L. M., Liotti M., Freitas C. S., Rainey L., Fox P. T. Automated Talairach Atlas labels for functional brain mapping. Human Brain Mapping. 2000;10(3):120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E., Oostenveld R. Nonparametric statistical testing of EEG-and MEG-data. Journal of Neuroscience Methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Maruyama M., Pallier C., Jobert A., Sigman M., Dehaene S. The cortical representation of simple mathematical expressions. NeuroImage. 2012;16:1444–1460. doi: 10.1016/j.neuroimage.2012.04.020. [DOI] [PubMed] [Google Scholar]
- McHombo S. A., editor. Theoretical aspects of Bantu grammar. Stanford, CA: CSLI Publications; 1993. [Google Scholar]
- Miller E. K., Cohen J. D. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mosher J. C., Leahy R. M. Recursive MUSIC: A framework for EEG and MEG source localization. IEEE Transactions on Biomedical Engineering. 1998;45:1342–1354. doi: 10.1109/10.725331. [DOI] [PubMed] [Google Scholar]
- Novick J. M., Trueswell J. C., Thompson-Schill S. L. Cognitive control and parsing: Reexamining the role of Broca's area in sentence comprehension. Cognitive, Affective & Behavioral Neuroscience. 2005;5:263–281. doi: 10.3758/CABN.5.3.263. [DOI] [PubMed] [Google Scholar]
- Öztekin I., Curtis C. E., McElree B. The medial temporal lobe and the left inferior prefrontal cortex jointly support interference resolution in verbal working memory. Journal of Cognitive Neuroscience. 2009;21:1967–1979. doi: 10.1162/jocn.2008.21146. [DOI] [PubMed] [Google Scholar]
- Öztekin I., McElree B., Staresina B. P., Davachi L. Working memory retrieval: Contributions of the left prefrontal cortex, the left posterior parietal cortex, and the hippocampus. Journal of Cognitive Neuroscience. 2008;21:581–593. doi: 10.1162/jocn.2008.21016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penolazzi B., Vincenzi M. D., Angrilli A., Job R. Processing of temporary syntactic ambiguity in Italian “who”-questions: A study with event-related potentials. Neuroscience Letters. 2005;377:91–96. doi: 10.1016/j.neulet.2004.11.074. [DOI] [PubMed] [Google Scholar]
- Pylkkänen L. Introducing arguments. Cambridge, MA: MIT Press; 2008. [Google Scholar]
- Rogalsky C., Matchin W., Hickok G. Broca's area, sentence comprehension, and working memory: An fMRI study. Frontiers in Human Neuroscience. 2008;2:14. doi: 10.3389/neuro.09.014.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi A., Grodzinsky Y. Working memory and syntax interact in Broca's area. NeuroImage. 2007;37(1):8–17. doi: 10.1016/j.neuroimage.2007.04.047. [DOI] [PubMed] [Google Scholar]
- Santi A., Grodzinsky Y. Broca's area and sentence comprehension: A relationship parasitic on dependency, displacement or predictability? Neuropsychologia. 2012;50:821–832. doi: 10.1016/j.neuropsychologia.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromswold K., Caplan D., Alpert N., Rauch S. Localization of syntactic comprehension by positron emission tomography. Brain and Language. 1996;52:452–473. doi: 10.1006/brln.1996.0024. [DOI] [PubMed] [Google Scholar]
- Thothathiri M., Kim A., Trueswell J. C., Thompson-Schill S. L. Parametric effects of syntactic-semantic conflict in Broca's area during sentence processing. Brain and Language. 2012;120:259–264. doi: 10.1016/j.bandl.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke J. A., McElree B. Retrieval interference in sentence comprehension. Journal of Memory and Language. 2006;55:157–166. doi: 10.1016/j.jml.2006.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartiainen J., Liljeström M., Koskinen M., Renvall H., Salmelin R. Functional magnetic resonance imaging blood oxygenation level-dependent signal and magnetoencephalography evoked responses yield different neural functionality in reading. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2011;31:1048–1058. doi: 10.1523/JNEUROSCI.3113-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner E., Maratsos M. Halle M., editor; Bresnan J., Miller G., editors. An ATN approach to comprehension. Linguistic Theoryand Psychological Reality. 1978. pp. 119–161.
- Warren T., Gibson E. Effects of NP type in reading cleft sentences in English. Language and Cognitive Processes. 2005;20:751–767. doi: 10.1080/01690960500051055. [DOI] [Google Scholar]
- Waters G., Caplan D., Hildebrandt N. Working memory and written sentence comprehension. In: Coltheart M., editor. Attention and performance 12: The psychology of reading. Hillsdale, NJ: Lawrence Erlbaum Associates; 1987. pp. 531–555. [Google Scholar]
- Zurif E. B. Brain regions of relevance to syntactic processing. In: Gleitman L., Liberman M., editors. Invitation to Cognitive Sciences. 2nd edition. I. Cambridge, MA: MIT Press; 1995. [Google Scholar]


