Abstract
BACKGROUND
Genetic and environmental factors interact in determining the risk of venous thromboembolism (VTE). The risk associated with the polymorphic variants G1691A of factor V (Factor V Leiden,FVL), G20210A of prothrombin (PT20210A) and C677T of methylentetrahydrofolate reductase (C677T MTHFR) genes has been investigated in many studies.
METHODS
We performed a pooled analysis of case-control and cohort studies investigating in adults the association between each variant and VTE, published on Pubmed, Embase or Google through January 2010. Authors of eligible papers, were invited to provide all available individual data for the pooling. The Odds Ratio (OR) for first VTE associated with each variant, individually and combined with the others, were calculated with a random effect model, in heterozygotes and homozygotes (dominant model for FVL and PT20210A; recessive for C677T MTHFR).
RESULTS
We analysed 31 databases, including 11,239 cases and 21,521 controls. No significant association with VTE was found for homozygous C677T MTHFR (OR: 1.38; 95% confidence intervals [CI]: 0.98–1.93), whereas the risk was increased in carriers of either heterozygous FVL or PT20210 (OR=4.22; 95% CI: 3.35–5.32; and OR=2.79;95% CI: 2.25–3.46, respectively), in double hterozygotes (OR=3.42; 95%CI 1.64-7.13), and in homozygous FVL or PT20210A (OR=11.45; 95%CI: 6.79-19.29; and OR: 2.79; 95%CI: 2.25 – 3.46, respectively). The stratified analyses showed a stronger effect of FVL on individuals ≤45 years (p-value for interaction = 0.036) and of PT20210A in women using oral contraceptives (p-value for interaction = 0.045).
CONCLUSIONS
In this large pooled analysis, inclusive of large studies like MEGA, no effect was found for C677T MTHFR on VTE; FVL and PT20210A were confirmed to be moderate risk factors. Notably, double carriers of the two genetic variants produced an impact on VTE risk significantly increased but weaker than previously thought.
Keywords: Venous thromboembolism, genetic susceptibility, Factor V Leiden, Prothrombin G202010A, Methylentetrahydrofolate reductase C677T
INTRODUCTION
Venous thromboembolism (VTE) is a complex disease with a yearly incidence rate of 100 per 100,000 adults in the United States. The risk of VTE rises exponentially from <5 cases per 100,000 persons <15 years old, to 500 cases per 100,000 persons at age 80 years [1]. VTE includes the common manifestation of deep venous thrombosis (DVT) of the lower extremities, and the less frequent life-threatening pulmonary embolism (PE) [2]. Epidemiological studies clearly indicate that genetic and environmental factors interact in determining the risk of VTE, and strong differences in the incidence have been shown between different ethnicities [3]. Factor V Leiden G1691A (FVL), prothrombin G2021A (PT20210A), and methylenetethraydrofolate reductase C677T (C677T MTHFR) are the most common polymorphic variants investigated in the past decades as putative genetic risk factors for VTE. While it is apparent that FVL and PT20210A represent by themselves significant risk factors for VTE, more controversial is the role of C677T MTHFR. According to a recently published meta-analysis [4], heterozygosity for FVL significantly increases the risk of VTE by 9.45-fold, and PT20210A by 3.17-fold compared with wild-types. As for C677T MTHFR, a significantly 57% increased risk of VTE was shown in a Chinese population for homozygotes compared with heterozygotes and homozygous wild-types, whereas in Caucasians no significant association was found.
Between 3 and 8 percent of the Caucasian U.S. and European populations carry one copy of the FVL variant. The variant is less common in Hispanics and it is extremely rare in people of African or Asian descent [5]. Heterozygous carriers of the PT20210A variant are found in about 2% of the US white population. The mutation is uncommon in African Americans (approximately 0.5%) and is rare in Asians, Africans, and Native Americans [6]. The homozygous variant of C677T MTHFR ranges from less than 1% among African Americans to 20% and more among some Caucasian populations and Hispanics. Asian populations have a prevalence of around 11% [7].
Due to the relatively low prevalence of FVL and PT20210A variants, large studies of VTE are needed to achieve enough power to provide reliable risk estimates, and even more to explore gene-gene and gene-environment interaction. The effect of these three genes, in fact, seems to be influenced by modifiable risk factors such as oral contraceptives, pregnancy, surgery and trauma [8, 9]. Additionally, the combined effect of more than one genetic variant can double or triple the risk from a single variant. To examine to what extent FVL, PT2120A, and C677T MTHFR alone, and in combination with each other and with several environmental risk factors, affect the risk of VTE, we conducted an individual patient (IPD) data analysis by pooling data from 36 published studies. We also evaluated the association of each of the polymorphic variants with the occurrence of thromboembolic events stratified by type and site, and among individuals at higher risk such as women using oral contraceptives.
METHODS
Literature search
A detailed literature search on the association of VTE occurrence and presence of the FVL, PT20210A and C677T MTHFR polymorphic variants was conducted on Medline and Embase databases, and on Google. The Medline query was structured using a string made of two parts, with the first was repeated for the three polymorphic variants considered: “(“Venous Thrombosis”[Mesh] OR Venous Thrombosis OR “deep vein thrombosis” OR “thrombosis” OR thrombosis OR Venous Thromboembolism OR Thromboembolism OR DVT OR pulmonary embolism OR VTE)”, followed by the second part, specific for each of the three polymorphisms as detailed below:
- FVL: “(“factor V Leiden”[Substance Name] OR “factor V Leiden” OR “factor V G1691A”)”.
- PT20210A: “(“Prothrombin”[Mesh] OR “factor II”) AND (“G20210A”[Mesh] OR G20210A OR “20210”[Mesh] OR 20210 OR 20210GA OR 20210G/A OR 20210A)”.
- C677T MTHFR: “(“Methylenetetrahydrofolate Reductase (NADPH2)”[Mesh] OR “Methylenetetrahydrofolate reductase” OR “MTHFR” OR Methylenetetrahydrofolate Reductase OR MTHFR OR “MTHFR-677” OR MTHFR677”)”.
The two parts were searched using the Boolean operator “AND”.
The literature search was repeated on EMBASE using a generic part “(“venous thrombosis” or “deep vein thrombosis” or “venous thromboembolism” or thromboembolism or DVT or “pulmonary embolism” or PE or VTE).tea. [mp=title, abstract, and full text, caption text]” and the following keywords for each polymorphism:
- FVL: “(FVL or “leiden”).mp”
- PT20210A: “(prothrombin or “factor II”).ti, ab. and (20210$ or g20210$).mp”
- C677T MTHFR: “(MTHFR or “tetrahydrofolate reductase”).mp”
Finally, the search was completed by consulting Google as a non-specific search engine and by reading of the references of the eligible articles retrieved.
Language restrictions were not given, and the search was updated on January 30th 2010.
Inclusion criteria
The studies identified by the search were considered eligible when they met all inclusion criteria:
- Case-controls or cohort studies reporting the occurrence of at least one of the three polymorphic variants considered, both in the population of cases and in that of the healthy controls;
- Clear definition of cases (individuals affected by VTE) and of controls (individual not affected by VTE). To avoid overlaps, studies retrieved with each of the three strings were cross-referenced and duplicates were excluded.
Exclusion criteria
Studies were not considered eligible when at least one of the following situations was present:
- Studies focusing solely on the association between the polymorphic variants and VTE occurrence in individuals affected by other conditions that are known risk factors for thromboembolic events (tumors, inflammatory bowel diseases, autoimmune conditions, Behçet disease, transplant);
- Studies focusing solely on individuals younger than 16 years old;
- Case-only and case-series studies, with no control population;
- Studies solely on families or on recurrences;
- Studies where the allelic frequencies of the polymorphic variants considered in the control population did not follow Hardy-Weinberg equilibrium (p ≤ 0.10 using the χ2 test).
Responders
Authors of the eligible studies were invited to send their datasets via email. Formal invitations were sent to either first or last authors of the papers. In case of no response, or when the email addresses were no longer in use, other co-authors were contacted. Also, authors were asked to state whether they had used their data on more than one publication, and, if that was the case, to merge the data and provide only the larger dataset. Authors were asked to hand in the most updated information available, including unpublished data.
Non responders
Four reminders were sent to non-responders. Authors who did not want to collaborate, or could not, were asked to explicitly decline the invitation and, if possible, state the reasons for non-participation. We kept a record of missed contributions to the study and collected available data (polymorphic variants considered, number of exposed cases/controls, number of non exposed cases/controls, general notes) from the relative publications.
Case definition
Most studies included validated their endpoints via physicians review of medical records. In some instances the International Classification of Diseases, 9th Edition or 10th Edition (ICD-9, ICD-10) discharge codes were used without further validation.
For the purpose of this pooled analysis, venous thromboembolism cases were grouped as venous thrombosis with no evidence of pulmonary embolism; venous thrombosis with pulmonary embolism; cerebral venous sinus thrombosis; splanchnic venous thrombosis; other types of events, i.e. multiple, undetermined, unspecified site.
Individual data collection
For the pooled analysis, participating authors were asked to send their dataset, coding the variables in a standard format. For each case enrolled we asked the status of the polymorphic variants of FVL, PT20210A and C677T MTHFR, (wild type homozygous, heterozygous carrier, homozygous carrier) and several potential confounders\effect modifiers:
- Age at the first thromboembolic event
- Gender
- Body mass index (BMI)
- Ethnic group
- Type of VTE
- Known VTE family history (defined as having one or more first-degree relatives with a history of venous thrombosis) [11]
- VTE recurrence
- Plasma or serum concentration of folates [12]
- Plasma or serum vitamin B12 levels [12]
- Homocysteinemia or homocysteine levels [13]
- Diagnosis of Diabetes Mellitus (DM)
- Smoking habits (current smoker, former smoker, never smoker; pack/years)
- Pregnancy status
- Occurrence of adverse pregnancy events (preeclampsia, defined as hypertension and proteinuria detected for the first time after 20 weeks' gestation with systolic blood pressure ≥140 mm; spontaneous recurrent abortion, defined as ≥3 unexplained foetal loss in the first trimester; intrauterine growth restriction, defined as birth weight <10th centile for gestational age; placental abruption, defined as presence of tender, hypertonic uterus and disseminated intravascular coagulation and/or retroplacental haematoma with/without signs of infarction) [14]
- Use of oral contraceptives (OCs) in women at a reproductive age and of hormonal replacement therapy in post-menopausal women
- Other VTE predisposing factors, such as major trauma, long journeys, prolonged bed rest and leg cast
- Oncologic diagnosis
- Presence of autoimmune diseases
- Organ transplant
- Diagnosis of Behçet disease.
The same data were requested for controls, except for: age at thromboembolic event (age at enrollment in the study was requested instead), type of VTE and VTE recurrence.
After all the individual data were collected, some subjects were excluded on an individual basis:
- Occurrence of known conditions associated with highly increased risk of thromboembolism (tumors, inflammatory bowel diseases, autoimmune conditions, Behçet disease, transplant)
- Age under 16 years old
Where the studies included were multi-centric, we considered the database from each centre separately.
The databases received were extracted and merged in one global database.
Statistical analysis
Data from cohort studies were extracted and used as if they were nested case-control samples. All individuals followed in the cohort who developed a DVT, at the time when the database was sent, were considered cases. Individuals who had not developed a DVT were considered controls.
For each study included, we reported the p-values of the Hardy-Weinberg equilibrium (HWE) for the whole population and for the controls; and the value of the minor allelic frequency (MAF).
The association between the three polymorphic variants and VTE was estimated from each study included by unconditional logistic regression models to estimate odds ratios (ORs) and 95% confidence intervals (CIs). Adjusted models controlled for age and gender. Summary ORs and 95% CI were estimated by pooling study-specific ORs using a random effects model for taking into account the heterogeneity between studies. Multivariate-adjusted models among all subjects were further stratified by age groups (<45 and ≥45 years old: this treshold was chosen based on the studies included in the pooled analysis), gender, use of oral contraceptives, recurrence of thromboembolic events, family history of VTE, and type of VTE event (venous thrombosis with no evidence of pulmonary embolism; pulmonary embolism; cerebral venous sinus thrombosis; splanchnic thrombosis; and other types of events). A test of interaction was then performed to assess for statistically significant differences among strata estimates (data not reported in the tables, only in the text if p-value for interaction <0.05). Lastly, a gene-gene interaction analysis was performed.
Based on the relative frequencies of heterozygous and homozygous variants of each polymorphism in populations, from previous examples in literature, and on a biological plausibility criterion, we applied a dominant model to determine the effect of the polymorphic variants of FVL and PT20210A on the occurrence of VTE (carriers [both heterozygous and homozygous] versus non-carriers). Conversely, a recessive model was used with regards to C677T MTHFR (homozygous carriers versus heterozygous and non-carriers) [15]. These assumptions were also used when performing gene-gene interaction analyses and stratified analyses.
Publication bias was evaluated both graphically and statistically, using Beggs and Egger tests and with funnel plots. Presence of inter-study heterogeneity in the overall and stratified analyses was assessed with the I2 method. When high heterogeneity (I2 ≥ 50%) was found, a sensitivity analysis was performed to single out the accountable studies and reasons for heterogeneity described.
Additional analyses of the pooled data
All the statistical analyses described above were performed after exclusion of individuals with known triggering factors or at increased risk, that is: long journeys, immobilisation, trauma, major surgery, pregnancy, use of OCs or hormonal replacement therapy.
Studies not included
In order to measure bias due to non responders, we performed a tabular meta-analysis of the studies not included.
We compared the overall measures of association yielded by the analysis of studies not included with those yielded by the pooled analysis, using a heterogeneity test.
All calculations were performed using the Stata 11.0 ® software package.
RESULTS
Study selection
The literature search yielded over 4000 results. Of these, after application of the inclusion and exclusion criteria and deletion of duplicates, 115 were deemed eligible. Once the authors of these papers were contacted, 31 papers were included [9, 16-45]. Of the remaining 84 [46-129], 20 papers were excluded on the grounds that authors could not provide the information required. Most common reasons for non-participation were: data no longer available, scarcity of time available for the retrieval and codification of the data, and laboratory no longer existed. 59 papers could not be included because the authors never replied (Figure 1).
Figure 1.
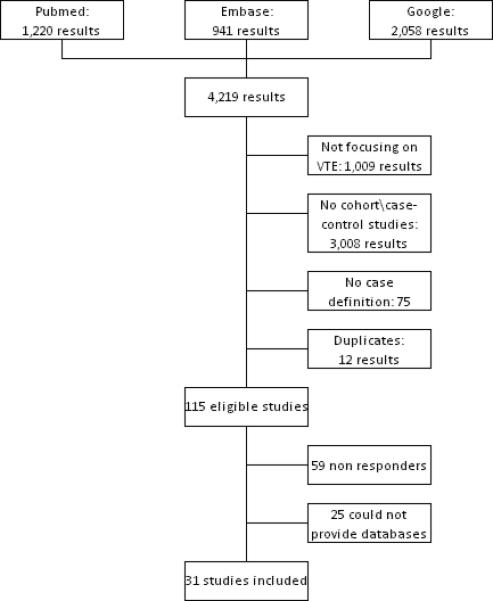
Flow chart of the selection process for including articles
Because in several cases the same database was used to produce more than one article on the topic, authors were asked to send in their largest database available, including unpublished data, and to communicate whether their data were used for more than one publication. Two studies were multi-centric [9, 27-28], so each database from these studies was considered independently from the others. For these two reasons, the number of databases included does not coincide with the number of papers considered: 37 databases were included in the pooled analysis (Table 1).
Table 1.
Characteristics of the databases included in the analysis and crude and adjusted Odds Ratio (OR) for Factor V Leiden (FVL), prothrombin (PT) 20210A and methylenetetraydrofolate reductase (MTHFR) C677T polymorphisms.
| First Author, year of publication | No. of cases | No. of controls | Country | Source of controls | FVL (Dominant model) |
PT 20210A (Dominant
model) |
MTHFR C667A (Recessive
model) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crude OR | CIa | Adj ORb | CI | Crude OR | CI | Adj OR | CI | Crude OR | CI | Adj OR | CI | |||||
| Akar, 2000 [16] | 328 | 108 | Turkey | Blood donors | 2.46 | (1.33-4.54) | 2.26 | (1.19-4.29) | 0.87 | (0.39-1.93) | 0.91 | (0.39-2.14) | 0.81 | (0.35-1.90) | 0.90 | (0.35-2.31) |
| Almawi, 2005 [17] | 324 | 733 | Lebanon | Blood donors | 6.53 | (4.74-8.99) | 6.56 | (4.76-9.03) | 5.75 | (3.33-9.91) | 5.83 | (3.38-10.07) | 3.44 | (2.37-4.98) | 3.42 | (2.36-4.95) |
| Angchaisuksiri, 2000 [18] | 40 | 500 | Thailand | Blood donors | . | . | . | . | . | . | . | . | . | . | . | . |
| Aznar, 2000 [19] | 120 | 447 | Spain | Blood donors | 4.70 | (2.48-8.90) | 3.86 | (2.01-7.43) | 1.24 | (0.62-2.5) | 1.20 | (0.59-2.44) | . | . | . | . |
| Bedencic, 2007c [20] | 30 | 56 | Slovenia | Hospital staff, students and their acquaintances | 6.30 | (1.53-26.01) | 6.59 | (1.56-27.79) | 4.64 | (1.26-17.01) | 4.70 | (1.27-17.40) | 1.02 | (0.31-3.38) | 1.02 | (0.31-3.38) |
| Cantu, 2004d [21] | 44 | 90 | Mexico | Friends and relatives of patients | . | . | . | . | . | . | . | . | 2.35 | (0.90-6.17) | 2.33 | (0.88-6.16) |
| Cushman, 2004e [22] | 147 | 513 | USA | Blood donors | 3.18 | (1.68-6.03) | 3.19 | (1.68-6.04) | 0.87 | (0.32-2.36) | 0.87 | (0.32-2.37) | 0.68 | (0.36-1.27) | 0.67 | (0.36-1.27) |
| De Stefano, 2003 [23] | 1,678 | 1,137 | Italy | Blood donors, hospital staff, outpatients | 7.94 | (5.56-11.35) | 8.35 | (5.83-11.96) | 2.78 | (1.99-3.88) | 2.87 | (2.05-4.02) | . | . | . | . |
| Diaz, 2005 [24] | 100 | 101 | Spain | Patients admitted with no VTE-related illness | . | . | . | . | . | . | . | . | . | . | . | . |
| Ducros, 2009 [25] | 151 | 155 | France | Patients with suspect VTE negative at ultrasonography | 15.91 | (3.70-68.34) | 18.99 | (4.34-83.14) | 2.14 | (0.78-5.87) | 2.04 | (0.73-5.65) | 1.10 | (0.55-2.18) | 1.03 | (0.51-2.07) |
| Emmerich (1), 2001 [9] | 427 | 557 | Italy | Blood donors and hospital staff | 8.05 | (4.63-14.00) | 8.77 | (4.91-15.67) | 5.14 | (2.80-9.44) | 4.70 | (2.50-8.83) | . | . | . | . |
| Emmerich (2), 2001 [9] | 345 | 850 | Italy | Blood donors and hospital staff | 4.27 | (2.84-6.44) | 4.37 | (2.90-6.60) | 3.69 | (2.39-5.71) | 3.71 | (2.40-5.74) | . | . | . | . |
| Emmerich (3), 2001 [9] | 472 | 474 | Netherlands | Blood donors and hospital staff | 8.02 | (4.50-14.30) | 7.37 | (4.09-13.27) | 2.77 | (1.37-5.62) | 2.36 | (1.15-4.87) | . | . | . | . |
| Emmerich (4), 2001 [9] | 174 | 130 | Brazil | Blood donors and hospital staff | 1.33 | (0.54-3.28) | 0.99 | (0.39-2.48) | 3.49 | (0.74-16.44) | 2.96 | (0.61-14.25) | . | . | . | . |
| Emmerich (5), 2001 [9] | 194 | 161 | UK | Blood donors and hospital staff | 3.84 | (1.63-9.03) | 4.58 | (1.89-11.08) | 4.65 | (1.02-21.32) | 4.55 | (0.95-21.77) | . | . | . | . |
| Emmerich (6), 2001 [9] | 264 | 398 | France | Blood donors and hospital staff | 6.56 | (3.61-11.91) | 6.96 | (3.79-12.80) | 4.89 | (2.42-9.90) | 4.52 | (2.20-9.28) | . | . | . | . |
| Emmerich (7), 2001 [9] | 99 | 281 | Sweden | Blood donors and hospital staff | 3.07 | (1.73-5.43) | 2.71 | (1.50-4.88) | 4.20 | (1.30-13.55) | 3.79 | (1.14-12.61) | . | . | . | . |
| Fernandez-Miranda, 2005f [26] | 100 | 177 | Spain | Hospital staff and their acquaintances | . | . | . | . | . | . | . | . | 1.94 | (1.05-3.58) | 1.99 | (1.07-3.72) |
| Folsom (1), 2002, 2007g [27, 28] | 210 | 421 | USA | Nested - randomly selected from cohort | 2.11 | (1.01-4.42) | 2.14 | (1.02-4.48) | 3.46 | (1.41-8.49) | 3.47 | (1.41-8.52) | . | . | . | . |
| Folsom (2), 2002, 2007g [27, 28] | 338 | 676 | USA | Nested - randomly selected from cohort | 4.59 | (2.57-8.21) | 4.57 | (2.55-8.17) | 1.19 | (0.46-3.04) | 1.18 | (0.46-3.02) | . | . | . | . |
| Frederiksen, 2004 [29] | 463 | 8,790 | Denmark | Selected from the Copenhagen City Heart Study | 2.48 | (1.86-3.31) | 2.49 | (1.86-3.32) | 1.12 | (0.61-2.08) | 1.20 | (0.64-2.22) | 0.91 | (0.65-1.27) | 0.91 | (0.65-1.28) |
| Gemmati (1), 1999 [30] | 235 | 220 | Italy | Blood donors | . | . | . | . | . | . | . | . | 1.81 | (1.16-2.84) | 1.87 | (1.18-2.96) |
| Gemmati (2), 1999 [31] | 180 | 200 | Italy | Blood donors | . | . | . | . | . | . | . | . | 1.67 | (0.99-2.81) | 1.77 | (1.04-3.01) |
| Ivanov, 2008h [32] | 51 | 98 | Bulgaria | Outpatients with unrelated diagnosis | 4.00 | (1.46-10.92) | 5.59 | (1.70-18.31) | 3.00 | (0.48-18.56) | 1.42 | (0.16-12.80) | 0.45 | (0.12-1.67) | 0.36 | (0.07-1.76) |
| Keijzer, 2002 [33] | 185 | 500 | Netherlands | General practice, health survey | 5.35 | (3.26-8.79) | 5.18 | (3.06-8.78) | 1.89 | (0.83-4.29) | 1.79 | (0.74-4.29) | 0.81 | (0.43-1.51) | 0.97 | (0.50-1.87) |
| Le Cam-Duchez, 2005d [34] | 51 | 100 | France | Healthy volunteers | 2.75 | (0.59-12.80) | 4.58 | (0.84-24.95) | 11.95 | (2.51-56.95) | 9.93 | (2.03-48.64) | 0.63 | (0.16-2.44) | 0.54 | (0.13-2.18) |
| Lichy, 2006d> [35] | 89 | 202 | Germany | Random selection from population registries | 2.16 | (0.98-4.76) | 2.41 | (1.02-5.68) | 4.49 | (1.46-13.81) | 3.80 | (1.18-12.22) | . | . | . | . |
| Martinelli, 2003 [36] | 3,042 | 1,803 | Italy | Friends and partners of patients | 5.34 | (3.98-7.18) | 5.19 | (3.86-6.98) | 3.42 | (2.60-4.51) | 3.33 | (2.52-4.39) | . | . | . | . |
| Nizankowska-Mogilnicka, 2003 [37] | 111 | 100 | Poland | Healthy hospital staff | 9.40 | (2.74-32.23) | 9.85 | (2.84-34.11) | . | . | . | . | 1.22 | (0.41-3.64) | 1.29 | (0.42-3.89) |
| Ogunyemi, 2003i [38] | 30 | 30 | USA | Blood donors | 11.05 | (1.28-95.16) | 11.85 | (1.35-104.19) | . | . | . | . | 0.14 | (0.03-0.73) | 0.08 | (0.01-0.51) |
| Oguzulgen, 2009h [39] | 143 | 181 | Turkey | Outpatients with unrelated diagnosis | 3.17 | (1.61-6.24) | 3.26 | (1.65-6.47) | 2.07 | (0.78-5.49) | 2.04 | (0.77-5.42) | . | . | . | . |
| Okumus, 2008 [40] | 273 | 215 | Turkey | Hospital workers and their relatives | 2.69 | (1.56-4.63) | 3.09 | (1.77-5.40) | 2.46 | (0.96-6.31) | 2.81 | (1.08-7.27) | . | . | . | . |
| Penco, 2005 [41] | 321 | 315 | Italy | Blood donors and hospital staff | 3.27 | (1.57-6.79) | 3.96 | (1.39-11.25) | 2.46 | (1.31-4.59) | 2.68 | (1.18-6.10) | 1.16 | (0.79-1.70) | 1.34 | (0.79-2.24) |
| Shmeleva, 2003 [42] | 62 | 30 | Russia | Members of hospital staff | . | . | . | . | . | . | . | . | 2.63 | (0.69-9.96) | 2.75 | (0.71-10.71) |
| Straczek, 2005e [43] | 236 | 547 | France | Patients from hospital and selection from electoral roll | 3.81 | (2.27-6.4) | 3.71 | (2.21-6.24) | 5.75 | (2.93-11.29) | 5.61 | (2.85-11.06) | . | . | . | . |
| Torres, 2006 [44] | 104 | 60 | Colombia | Blood donors and hospital staff | . | . | . | . | . | . | . | . | 1.43 | (0.64-3.16) | 1.74 | (0.63-4.79) |
| Vaya, 2003j [45] | 78 | 165 | Spain | Healthy volunteers recruited in routine screenings | 1.27 | (0.30-5.46) | 1.47 | (0.33-6.47) | 3.43 | (1.18-10.02) | 3.36 | (1.14-9.85) | . | . | . | . |
| Total | 11,239 | 21,521 | ||||||||||||||
95% Confidence intervals
Odds Ratio (OR) adjusted by age and gender
Women only
Cerebral venous sinus thrombosis only
Post-menopausal only
<50 years old
45-64 years old
Pulmonary embolism only
Pregnant women
upper extremities only.
Odds ratios (ORs) and 95% confidence intervals (CIs) for each database included are reported in Table 1. It is worth mentioning that crude ORs are not the same as those of the referenced articles, as new data were provided, and some subjects were excluded on an individual basis as detailed in the methods section.
The IPD analysis was performed on an overall sample of 11,239 cases and 21,521 controls. 28 databases out of 37 (76%) came from European countries [9, 16, 19, 20, 23-26, 29-37, 39, 40-43, 45], 3 from Latin America [9, 21, 44], 4 from North America [22, 27, 28, 38] and 2 from Asia [17, 18].
The p-values of the HWE for each study overall and for the control population, as well as the MAF, are reported in table 2.
Table 2.
Minor allelic frequency and p-value for the Hardy-Weinberg Equilibrium for the control population and for the overall sample of the databases included in the analysis for Factor V Leiden (FVL), prothrombin (PT) 20210A and methylenetetraydrofolate reductase (MTHFR) C677T polymorphisms.
| First Author, year of publication | FVL |
PT20210A |
MTHFR C667A |
||||||
|---|---|---|---|---|---|---|---|---|---|
| MAF | HWE controls | HWE overall | MAF | HWE controls | HWE overall | MAF | HWE controls | HWE overall | |
| Akar, 2000 [16] | 0.13 | 0.476 | 0.024 | 0.04 | 0.096 | 0.667 | 0.28 | 0.345 | 0.100 |
| Almawi, 2005 [17] | 0.12 | 0.101 | 0.010 | 0.03 | 0.026 | 0.975 | 0.33 | 0.501 | 0.002 |
| Angchaisuksiri, 2000 [18] | 0.00 | 0.982 | 0.983 | 0.00 | 0.982 | 0.983 | . | . | . |
| Aznar, 2000 [19] | 0.36 | 0.843 | 0.794 | 0.45 | 0.811 | 0.619 | . | . | . |
| Bedencic, 2007 [20] | 0.06 | 0.835 | 0.524 | 0.07 | 0.780 | 0.484 | 0.33 | 0.098 | 0.019 |
| Cantu, 2004 [21] | 0.05 | . | . | 0.09 | . | . | 0.43 | 0.235 | 0.133 |
| Cushman, 2004 [22] | 0.04 | 0.189 | 0.012 | 0.02 | 0.642 | 0.608 | 0.34 | 0.476 | 0.386 |
| De Stefano, 2003 [23] | 0.07 | 0.174 | 0.000 | 0.04 | 0.486 | 0.837 | . | . | . |
| Diaz, 2005 [24] | 0.05 | . | . | 0.05 | . | . | . | . | . |
| Ducros, 2009 [25] | 0.05 | 0.936 | 0.692 | 0.03 | 0.806 | 0.180 | 0.38 | 0.513 | 0.109 |
| Emmerich (1), 2001 [9] | 0.04 | 0.728 | 0.096 | 0.04 | 0.764 | 0.292 | . | . | . |
| Emmerich (2), 2001 [9] | 0.06 | 0.449 | 0.105 | 0.02 | 0.494 | 0.171 | . | . | . |
| Emmerich (3), 2001 [9] | 0.04 | 0.744 | 0.067 | 0.02 | 0.798 | 0.506 | . | . | . |
| Emmerich (4), 2001 [9] | 0.05 | 0.717 | 0.513 | 0.02 | 0.930 | 0.748 | . | . | . |
| Emmerich (5), 2001 [9] | 0.05 | 0.777 | 0.313 | 0.03 | 0.936 | 0.723 | . | . | . |
| Emmerich (6), 2001 [9] | 0.08 | 0.701 | 0.156 | 0.02 | 0.780 | 0.387 | . | . | . |
| Emmerich (7), 2001 [9] | 0.04 | 0.311 | 0.095 | 0.06 | 0.880 | 0.754 | . | . | . |
| Fernandez-Miranda, 2005 [26] | 0.04 | . | . | . | . | . | 0.42 | 0.306 | 0.622 |
| Folsom (1), 2002, 2007 [27, 28] | 0.03 | 0.698 | 0.523 | 0.02 | 0.838 | 0.657 | . | . | . |
| Folsom (2), 2002, 2007 [27, 28] | 0.03 | 0.717 | 0.001 | 0.01 | 0.810 | 0.756 | . | . | . |
| Frederiksen, 2004 [29] | 0.04 | 0.761 | 0.739 | 0.01 | 0.998 | 0.952 | 0.31 | 0.369 | 0.377 |
| Gemmati (1), 1999 [30] | . | . | . | . | . | . | 0.48 | 0.303 | 0.796 |
| Gemmati (2), 1999 [31] | . | . | . | . | . | . | 0.43 | 0.410 | 0.893 |
| Ivanov, 2008 [32] | 0.07 | 0.714 | 0.115 | 0.02 | 0.919 | 0.835 | 0.35 | 0.385 | 0.310 |
| Keijzer, 2002 [33] | 0.06 | 0.459 | 0.771 | 0.02 | 0.726 | 0.616 | 0.30 | 0.362 | 0.718 |
| Le Cam-Duchez, 2005 [34] | 0.02 | 0.879 | 0.771 | 0.04 | 0.920 | 0.155 | 0.34 | 0.116 | 0.033 |
| Lichy, 2006 [35] | 0.05 | 0.584 | 0.734 | 0.02 | 0.859 | 0.674 | . | . | . |
| Martinelli, 2003 [36] | 0.05 | 0.346 | 0.000 | 0.04 | 0.588 | 0.000 | . | . | . |
| Nizankowska-Mogilnicka, 2003 [37] | 0.07 | 0.879 | 0.997 | . | 0.960 | 0.763 | 0.23 | 0.999 | 0.228 |
| Ogunyemi, 2003 [38] | 0.08 | 0.926 | 0.526 | . | . | . | 0.41 | 0.240 | 0.042 |
| Oguzulgen, 2009 [39] | 0.08 | 0.588 | 0.002 | 0.03 | 0.791 | 0.607 | . | . | . |
| Okumus, 2008 [40] | 0.09 | 0.474 | 0.070 | 0.02 | 0.836 | 0.578 | . | . | . |
| Penco, 2005 [41] | 0.03 | 0.775 | 0.008 | 0.04 | 0.665 | 0.455 | 0.44 | 0.704 | 0.087 |
| Shmeleva, 2003 [42] | . | . | . | . | . | . | 0.36 | 0.994 | 0.019 |
| Straczek, 2005 [43] | 0.04 | 0.554 | 0.705 | 0.03 | 0.779 | 0.584 | . | . | . |
| Torres, 2006 [44] | . | . | . | . | . | . | 0.48 | 0.284 | 0.678 |
| Vaya, 2003 [45] | 0.02 | 0.843 | 0.794 | 0.01 | 0.811 | 0.619 | . | . | . |
MAF: Minor allele frequency; HWE: p-value for Hardy-Weinberg Equilibrium
Synthesis of results
FVL
Based on 9,081 cases and 17,513 controls, FVL was associated with an elevated risk of developing VTE, with an overall OR: 4.38 (CI: 3.48-5.51; I-squared = 70.3%, 95% CI: 54.6% to 80.5%; table 3, figure 2). The risk was significantly higher in younger individuals (<45 years old, OR: 5.43; ≥45 years old, OR: 3.71; p-value for interaction = 0.036). Men had a higher, but not statistically significant, risk of developing VTE with FVL compared to women (male population, OR: 5.06; female population, OR 3.82), with no significant difference between strata estimates.
Table 3.
Overall and stratum-specific adjusted odds ratios (OR) by polymorphic variants: Factor V Leiden (FVL), prothrombin (PT) 20210A and methylenetetraydrofolate reductase (MTHFR) C677T
| Cases/Controls Exposed | Cases/Controls Not exposed | ORa | CIa | I-sq (CI), % | p-valueb | p-valuefor interaction | |
|---|---|---|---|---|---|---|---|
| FVL Carriers (Dominant model) | |||||||
| Overall | 1,758/1,201 | 7,323/16,312 | 4.38 | (3.48-5.51) | 70.3 (50.6-80.5) | 0.000 | |
| Age category | |||||||
| <45 y.o. | 1,003/436 | 3,911/6,228 | 5.43 | (4.20-7.03) | 43.7 (5.8-66.4) | 0.017 | 0.036 |
| >= 45 y.o. | 716/764 | 3,258/9,921 | 3.71 | (2.90-4.75) | 45.9 (9.7-67.6) | 0.012 | |
| Gender (and associated covariates) | |||||||
| Men | 809/545 | 2,997/7,324 | 5.06 | (3.89-6.59) | 48.4 (15.3-68.6) | 0.006 | 0.184 |
| Women | 939/656 | 4,255/8,905 | 3.82 | (2.78-5.26) | 70.9 (55.3-81.1) | 0.000 | |
| - Not using OCc | 137/435 | 591/5,655 | 3.91 | (2.18-7.02) | 70.9 (39.9-85.9) | 0.001 | 0.418 |
| - Using OC | 121/66 | 284/1,133 | 6.10 | (2.47-15.04) | 73.5 (39.3-88.4) | 0.002 | |
| Recurrence | |||||||
| Yes | 305/321 | 1,343/4,248 | 5.81 | (4.03-8.38) | 40.0 (0.0-72.4) | 0.101 | 0.120 |
| No | 492/321 | 2,496/4,248 | 3.95 | (2.87-5.43) | 52.8 (3.4-77.0) | 0.025 | |
| Family history | |||||||
| Yes | 90/95 | 217/1,091 | 9.70 | (3.96-23.76) | 43.9 (0.0-79.4) | 0.129 | 0.982 |
| No | 121/95 | 260/1,091 | 9.59 | (6.71-13.71) | 0.0 (0.0-54.9) | 0.797 | |
| Trigger factorsd | |||||||
| Yes | 486/482 | 2,045/6,706 | 4.87 | (3.66-6.49) | 54.7 (20.4-74.1) | 0.004 | 0.971 |
| No | 954/482 | 3,888/6,706 | 4.91 | (3.75-6.41) | 58.9 (29.9-75.9) | 0.001 | |
| PT 20210A Carriers (Dominant model) | |||||||
| Overall | 1,007/807 | 8,127/16,799 | 2.80 | (2.25-3.48) | 46.1 (11.2-67.3) | 0.010 | |
| Age category | |||||||
| < 45 y.o. | 609/362 | 4,329/4,331 | 3.19 | (2.44-4.17) | 32.3 (0.0-60.6) | 0.082 | 0.233 |
| >= 45 y.o. | 383/380 | 3,558/10,241 | 2.57 | (2.04-3.24) | 8.2 (0.0-44.2) | 0.358 | |
| Gender (and associated covariates) | |||||||
| Men | 441/366 | 3,343/7,487 | 2.91 | (2.36-3.58) | 0.0 (0.0-23.8) | 0.840 | 0.952 |
| Women | 561/441 | 4,695/9,196 | 2.88 | (2.18-3.80) | 40.0 (0.0-64.4) | 0.031 | |
| - Not using OC | 52/147 | 817/6,226 | 2.73 | (1.58-4.72) | 37.4 (0.0-72.3) | 0.131 | 0.045 |
| - Using OC | 69/38 | 449/1,480 | 5.58 | (3.60-8.63) | 0.0 (0.0-68.6) | 0.473 | |
| Recurrence | |||||||
| Yes | 239/479 | 1,433/4,266 | 4.38 | (3.44-5.58) | 0.0 (0.0-0.0) | 0.989 | 0.081 |
| No | 389/479 | 2,646/4,266 | 3.08 | (2.24-4.22) | 36.4 (0.0-69.7) | 0.117 | |
| Family history | |||||||
| Yes | 39/42 | 294/1,423 | 4.49 | (2.39-8.43) | 23.5 (0.0-68.7) | 0.265 | 0.711 |
| No | 60/42 | 544/1,423 | 3.86 | (2.38-6.27) | 19.0 (0.0-63.7) | 0.290 | |
| Trigger factors | |||||||
| Yes | 291/566 | 2,198/6,698 | 3.09 | (2.47-3.86) | 11.3 (0.0-48.6) | 0.324 | 0.670 |
| No | 608/566 | 4,235/6,698 | 3.32 | (2.60-4.23) | 31.3 (0.0-62.3) | 0.113 | |
| MTHFR C677T Homozygous Carriers (Recessive model) | |||||||
| Overall | 384/1,111 | 2,117/10,298 | 1.38 | (0.99-1.93) | 69.6 (46.2-82.8) | 0.000 | |
| Age category | |||||||
| <45 y.o. | 163/340 | 663/3,047 | 1.52 | (0.99-2.32) | 49.5 (2.0-74.0) | 0.026 | 0.698 |
| >=45 y.o. | 212/756 | 1,395/7,129 | 1.36 | (0.98-1.90) | 36.8 (0.0-69.9) | 0.114 | |
| Gender (and associated covariates) | |||||||
| Men | 183/487 | 879/4,583 | 1.84 | (1.24-2.74) | 52.6 (6.0-76.1) | 0.020 | 0.191 |
| Women | 201/615 | 1,202/5,644 | 1.30 | (0.93-1.82) | 42.9 (0.0-70.2) | 0.050 | |
| - Not using OC | 40/39 | 162/352 | 1.94 | (0.94-4.01) | 43.1 (0.0-80.9) | 0.153 | 0.911 |
| - Using OC | 28/25 | 137/224 | 2.05 | (1.11-3.79) | 0.0 (0.0-0.0) | 0.643 | |
| Recurrence | |||||||
| Yes | 1/19 | 13/171 | 3.41 | (0.29-40.70) | nce | nc | 0.527 |
| No | 12/19 | 69/171 | 1.38 | (0.38-5.03) | 54.6 (0.0-88.9) | 0.138 | |
| Family history | |||||||
| Yes | 121/186 | 424/1,540 | 1.93 | (1.17-3.17) | 57.3 (6.4-80.6) | 0.022 | 0.994 |
| No | 118/186 | 546/1,540 | 1.93 | (1.42-2.63) | 15.4 (0.0-58.3) | 0.309 | |
| Trigger factors | |||||||
| Yes | 103/183 | 432/1,513 | 1.77 | (1.21-2.58) | 34.6 (0.0-72.4) | 0.164 | 0.788 |
| No | 122/183 | 490/1,513 | 1.92 | (1.24-2.98) | 49.9 (0.0-78.8) | 0.062 | |
Odds ratio (OR) adjusted for age and gender; CI: 95% Confidence Interval
from Q-square test, random effects model
OC: oral contraceptives
long journeys, immobilisation, trauma, major surgery, pregnancy, use of oral contraceptives or hormonal replacement therapy
nc: not computable
Figure 2.
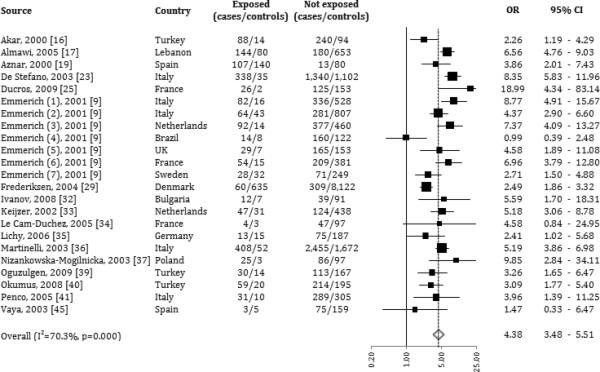
Forrest plot: association between Factor V Leiden and risk of venous thromboembolism (odds ratios are represented on log scale).
As expected, the VTE OR for FVL was higher in women using OCs (OR: 6.10) than women not using OCs (OR: 3.91), but this difference was not significant. No significant differences in ORs for FVL emerged when stratifying by presence of triggering conditions. Complete data on triggering conditions, however, were only available for 20% of the databases included. Family history did not seem to interact with FVL in risk of developing VTE. On the other hand, FVL was associated with recurrences (recurrent VTE event, OR: 5.81; no recurrence reported, OR: 3.95).
The stratification by type of outcome showed that the polymorphic variant was more strongly associated with the risk of developing venous thrombosis without evidence of pulmonary embolism (OR: 4.49) or a cerebral venous sinus thrombosis (OR: 4.14); the risk of developing a splanchnic thrombosis was not statistically significant (Table 4).
Table 4.
Risk of venous thromboembolic accidents stratified by the type of event in subjects carriers of Factor V Leiden (FVL), prothrombin (PT) 20210A and methylenetetraydrofolate reductase (MTHFR) C677T polymorphism
| Cases/Controls Exposed | Cases/Controls Not exposed | ORa | CIa | I-sq (CI), % | p-valueb | |
|---|---|---|---|---|---|---|
| All FVL Carriers (Dominant model) | ||||||
| Venous thrombosis (w\o pulmonary embolism) | 855/1,063 | 3,396/14,498 | 4.49 | (3.23-6.24) | 75.1 (59.5-84.7) | 0.000 |
| Venous thromboembolism | 409/1,048 | 1,959/14,756 | 3.46 | (2.71-4.42) | 39.6 (0.0-65.6) | 0.043 |
| Cerebral venous sinus thrombosis | 53/101 | 291/2,394 | 4.14 | (2.46-6.97) | 34.3 (0.0-77.0) | 0.206 |
| Splanchnic venous thrombosis | 8/62 | 200/1,977 | 1.30 | (0.61-2.79) | 0.0 (0.0-0.0) | 0.819 |
| Otherc | 189/90 | 797/2,871 | 7.89 | (5.99-10.38) | 1.3 (0.0-89.7) | 0.363 |
| All PT 20210A Carriers Dominant model) | ||||||
| Venous thrombosis (w\o pulmonary embolism) | 510/754 | 3,798/14,900 | 2.60 | (1.94-3.47) | 45.5 (0.3-70.2) | 0.029 |
| Venous thromboembolism | 250/758 | 2,085/14,894 | 3.00 | (2.30-3.90) | 30.2 (0.0-61.8) | 0.122 |
| Cerebral venous sinus thrombosis | 51/100 | 303/2,723 | 4.40 | (2.18-8.91) | 39.9 (0.0-77.8) | 0.155 |
| Splanchnic venous thrombosis | 17/78 | 192/1,969 | 2.10 | (1.17-3.77) | 0.0 (0.0-0.0) | 0.941 |
| Otherc | 92/118 | 919/2,859 | 2.62 | (1.79-3.84) | 26.6 (0.0-92.4) | 0.256 |
| MTHFR C677T Homozygous Carriers (Recessive model) | ||||||
| Venous thrombosis (w\o pulmonary embolism) | 163/1,021 | 789/9,367 | 1.33 | (1.03-1.72) | 9.1 (0.0-68.0) | 0.360 |
| Venous thromboembolism | 116/1,033 | 891/9,453 | 1.15 | (0.82-1.62) | 30.9 (0.0-67.0) | 0.162 |
| Cerebral venous sinus thrombosis | 22/130 | 164/888 | 1.34 | (0.64-2.82) | 35.4 (0.0-75.8) | 0.185 |
| Splanchnic venous thrombosis | 5/63 | 13/252 | 1.92 | (0.50-7.45) | ncd | nc |
| Otherc | 1/6 | 3/94 | 3.02 | (0.17-54.24) | nc | nc |
Odds ratio (OR) adjusted for age and gender; CI: Confidence Interval
from Q-square test, random effects model
multiple, undetermined, unspecified site
nc, not computable
Lastly, the OR for FVL homozygotes was 11.45 (CI 95% 6.79-19.29), including 116 subjects among cases and 24 controls (data shown in appendix 1).
PT20210A
Based on 9,134 cases and 17,606 controls, PT20210A was associated with an increased risk of developing VTE (overall OR 2.80, CI 2.25-3.48; I-squared= 46.1%, 95% CI: 11.2% to 67.3%), albeit not as strongly as FVL (Table 3, figure 3). Again, younger carriers of the polymorphic variant were at higher risk from PT20210A than their older counterparts (< 45 years old, OR: 3.19; ≥ 45 years old, OR: 2.57), although the difference between the two groups was not as remarkable as for FVL and not significantly different. Gender did not seem to affect the risk of developing VTE in the presence of the polymorphic variant: the risks for men (OR: 2.91) and women (OR: 2.88) were similar. As expected, the use of OCs affected risk of PT20210A for developing VTE (women not using OCs, OR: 2.73; women using OCs, OR: 5.58; p-value for interaction = 0.045) was confirmed in the present study. As with FVL, the presence of triggering conditions did not affect risk of developing VTE in the presence of PT20210A. Complete data on triggering conditions, again, were only available for 20% of the databases included. Individuals who reported a family history of VTE and individuals with recurrences had a higher risk of VTE in the presence of PT20210A (positive family history, OR: 4.49; negative family history: 3.86; recurrence, OR: 4.38; no recurrence: OR, 3.08), although in neither instance was the difference statistically significant.
Figure 3.
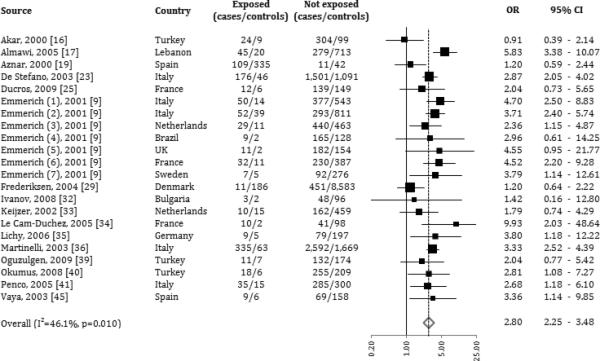
Forrest plot: association between Prothrombin 20210A and risk of venous thromboembolism (odds ratios are represented on log scale).
The stratification by outcome showed a very strong association of the polymorphic PT20210A variant with regard to the risk of developing a thrombosis of the cerebral venous sinus (OR: 4.52). The polymorphic PT20210A variant was significantly associated also with the other types of thrombotic events considered, with ORs ranging from 2.10 to 3.53, as shown in Table 4. Lastly, the OR for PT20210A homozygotes was 6.74 (CI 95% 2.19-20.72), including 28 subjects among cases and 4 controls (data shown in appendix 1).
C677T MTHFR
The overall analysis (OR: 1.38, CI: 0.98 – 1.93; I-squared= 69.6%, 95% CI: 46.2% to 82.8%. Table 4, figure 4) and the stratification analyses indicated that the C677T MTHFR variant was associated with no consistent risk of developing a VTE (see Table 3). From the stratified analysis, only men with the polymorphic variant had a significantly increased risk of developing VTE (OR: 1.84, CI: 1.24 – 2.74).
Figure 4.
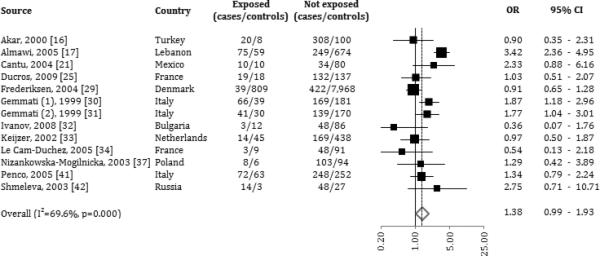
Forrest plot: association between Methylenetetrahydrofolate reductase C677T and venous thromboembolism (odds ratios are represented on log scale).
Women using OCs had elevated risk associated with the C677T MTHFR variant (OR: 2.05). The C677T MTHFR variant carried a borderline significantly increased risk to develop a venous thrombosis without evidence of pulmonary embolism (OR: 1.33, CI: 1.03 – 1.72). No other thromboembolic event type was significantly associated with the presence of the polymorphic variant (Table 4).
Gene-Gene interactions
Notably, the presence of both FVL and PT20210A did not seem to confer additional risk for first VTE beyond the sole presence of FVL. However, given the scarcity of carriers of both variants, the confidence intervals were broad (OR: 3.423, CI: 1.65 – 7.13) (Table 5).
Table 5.
Overall and stratum-specific adjusted odds ratios (OR) in the presence of gene-gene interaction: Factor V Leiden (FVL), prothrombin (PT) 20210A and methylenetetraydrofolate reductase (MTHFR) C677T
| Cases/Controls Exposed | Cases/Controls Not exposed | ORa | CIa | I-sq (CI), % | p-valueb | p-value for interaction | |
|---|---|---|---|---|---|---|---|
| FVL & PT 20210A Carriers | |||||||
| Overall | 187/121 | 4,156/3,604 | 3.43 | (1.64-7.13) | 59.1 (10.7-81.3) | 0.017 | |
| <45 y.o. | 64/61 | 363/811 | 3.54 | (0.93-13.40) | 67.6 (5.8-88.9) | 0.026 | 0.666 |
| >= 45 y.o. | 82/59 | 1,756/1,593 | 2.54 | (1.26-5.12) | 28.4 (0.0-69.2) | 0.211 | |
| Men | 92/59 | 1,587/1,234 | 3.45 | (1.47-8.10) | 39.5 (0.0-77.6) | 0.158 | 0.847 |
| Women | 89/61 | 2,252/2,309 | 3.04 | (1.16-7.95) | 55.0 (0.0-80.7) | 0.038 | |
| - Not using OCc | --- | --- | ncd | . | nc | nc | |
| - Using OC | 14/2 | 92/225 | 17.03 | (3.79-76.47) | nc | nc | |
| MTHFR C677T Homozygous & FVL Carriers | |||||||
| Overall | 44/71 | 1,466/10,298 | 3.79 | (1.71-8.40) | 37.2 (0.0-78.4) | 0.189 | |
| <45 y.o. | 21/10 | 352/777 | 4.86 | (2.23-10.58) | 0.0 (0.0-59.9) | 0.772 | 0.527 |
| >=45 y.o. | 13/45 | 549/7,186 | 3.19 | (1.11-9.12) | 48.7 (0.0-85.1) | 0.142 | |
| Men | 11/27 | 356/4,370 | 3.13 | (1.30-7.52) | 0.0 (0.0-79.4) | 0.603 | 0.759 |
| Women | 22/43 | 656/5,578 | 3.91 | (1.27-12.07) | 42.3 (0.0-80.6) | 0.158 | |
| - Not using OC | 4/1 | 88/209 | 9.31 | (1.02-84.78) | nc | nc | 0.834 |
| - Using OC | 12/4 | 94/223 | 7.13 | (2.24-22.70) | nc | nc | |
| MTHFR C677T Homozygous & PT 20210A Carriers | |||||||
| Overall | 12/25 | 1,096/9,154 | 1.94 | (0.71-5.33) | 0.0 (0.0-19.5) | 0.879 | |
| <45 y.o. | 3/3 | 161/181 | 1.76 | (0.32-9.53) | 0.0 (0.0-0.0) | 0.787 | 0.791 |
| >= 45 y.o. | 9/16 | 678/6.940 | 2.34 | (0.64-8.54) | 0.0 (0.0-0.0) | 0.893 | |
| Men | 7/13 | 329/4.111 | 3.17 | (1.00-10.05) | 0.0 (0.0-0.0) | 0.329 | 0.506 |
| Women | 1/1 | 182/71 | 1.10 | (0.06-19.98) | nc | nc | |
| - Not using OC | --- | --- | nc | . | nc | nc | |
| - Using OC | --- | --- | nc | . | nc | nc | |
Odds ratio (OR) adjusted for age and gender; CI: Confidence Interval
from Q-square test, random effects model
OC: oral contraceptives
nc: not computable
There was no interaction of the C677T MTHFR variant with FVL and with PT20210A in risk of developing VTE, confirming the notion that C677T MTHFR is not a risk factor for VTE. (Table 5) Because of the rarity of the variants in the general population (especially when combined), and in spite of the ample sample size considered, the analysis yielded very wide confidence intervals.
The stratification by outcome showed particularly increased risks for individuals with both FVL and PT20210A variants to develop a cerebral venous sinus thrombosis, although not statistically significant (OR: 7.38, CI: 0.99 – 55.06). Only 5 cases and no control carried a triple combination of the gene variants investigated (double heterozygosity for FVL and PT20210A with homozygous C677T MTHFR), producing a high OR but with quite unstable confidence intervals due to the rarity of combination (Table 5).
Risk of bias within studies
Statistical tests for publication bias were not statistically significant for FVL (p-values for Beggs test: 0.492, Egger: 0.706), PT20210A (p-values for Beggs test: 0.822, Egger: 0.470) and C677T MTHFR (p-values for Beggs test: 0.360, Egger 0.373). This was also confirmed graphically by the Funnel plots for the three polymorphic variants (figures 5, 6 and 7).
Figure 5.
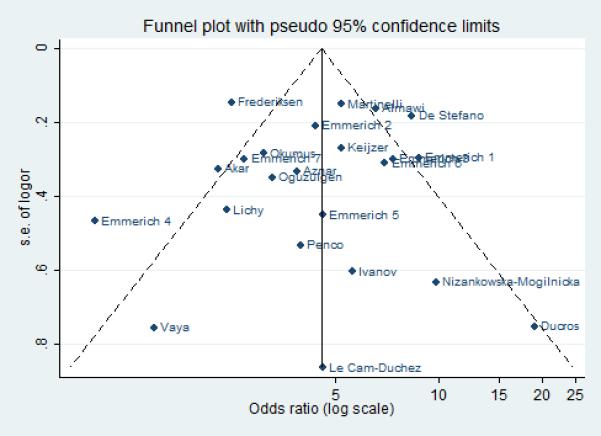
Funnel plot: publication bias for the studies on Factor V Leiden.
Figure 6.
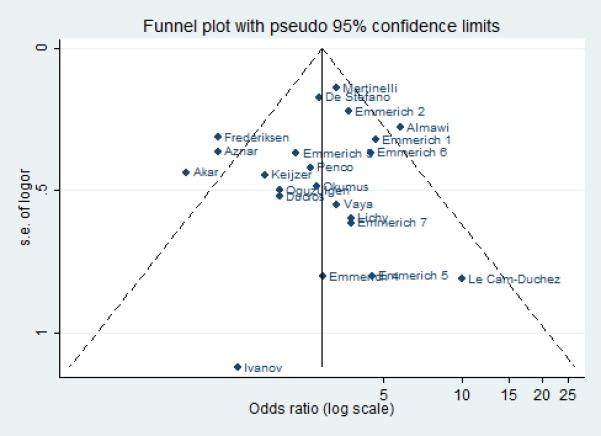
Funnel plot: publication bias for the studies on Prothrombin 202010A.
Figure 7.
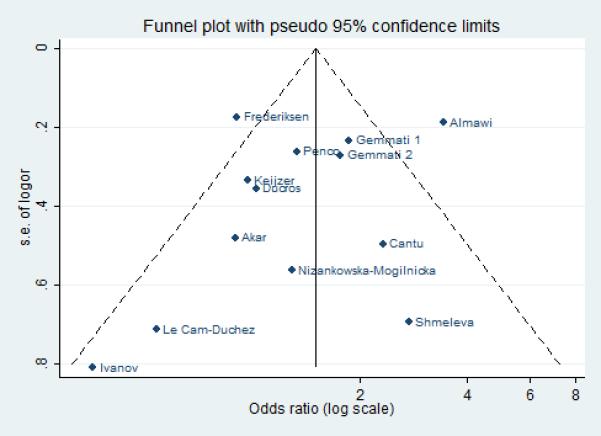
Funnel plot: publication bias for the studies on Methylenetetrahydrofolate reductase C677T.
Inter-study heterogeneity was high for the FVL and C677T MTHFR. Sensitivity analysis singled out the study by Almawi et al. [17] as the main source of heterogeneity for C677T MTHFR (excluding Almawi, OR: 1.25; CI: 0.98 – 1.59; I2= 31%, p = 0.144). As for FVL, the heterogeneity remained still high even after exclusion of the two greater contributors of heterogeneity (Emmerich et al., database 4 [9], Frederiksen et al. [29]: OR: 4.88; 95% CI: 4.00 - 5.96; I2= 52%, p = 0.003).
Additional analyses of the pooled data
Results did not change significantly after the exclusion of homozygous carriers of the FVL and PT20210A variants from the analyses (FVL, OR: 4.22; CI: 3.35 – 5.32; I2 = 70%, p < 0.001. PT20210A, OR: 2.79; CI: 2.25 – 3.46; I2 = 44%, p = 0.016). If it is true that both FVL and PT20210A homozygous carriers are at a higher risk of developing VTE than the heterozygous counterparts, the frequencies of homozygosity in the general population are so low that the results do not change whether they are included or excluded.
Analysis of the studies not included
Results from the tabular meta-analyses of the studies not included [46-129] did not differ significantly from those yielded by the pooled analysis (Forrest plots reported in appendixes 2-4).
Carriers of FVL had a 5-fold increase in the odds of developing VTE (OR: 4.23; CI: 4.23-5.59). This result is in line with that from the pooled analysis (p-value for heterogeneity: 0.448).
Similarly, PT20210A yielded an OR of 3.07 (CI: 2.72-3.48; p-value for heterogeneity: 0.472) and MTHFR an OR of 1.10 (CI: 0.84-1.45; p-value for heterogeneity: 0.308).
DISCUSSION
Inherited deficiency of antithrombin (AT), protein C (PC) and its co-factor, protein S (PS), were the first identified causes of increased risk for DVT. More recently, two common gene polymorphisms were recognized as additional causes of hypercoagulability: FVL, resistant to the anticoagulant action of activated protein C, and PT20210A, associated with increased levels of circulating prothrombin. Mild hyperhomocysteinemia is also an established risk factor for thrombophilia [130].
Overall, the rare deficiencies of natural coagulation inhibitors (AT, PC, and PS) are detectable in less than 1% of the general population and in less than 10% of unselected patients with VTE [130]. FVL is present almost exclusively among Caucasians, with a prevalence of 5% in the general population with European ancestry and 18% among patients with VTE. In some European areas (Sweden, Alsace, Cyprus) the prevalence of FVL in the general population has been reported to be 10 to 15% [131]. Finally, the PT20210A allele is present in 2% of healthy individuals and in 7% of patients with VTE [132]. Acquired factors (low intake of pyridoxine, cobalamin, folate) can produce mild hyperhocysteinemia interacting with gene factors, such as the C677T polymorphism in the MTHFR gene. Homozygous carriers can develop hyperhomocysteinemia especially in the presence of low folate levels. Among Caucasians the prevalence of the TT genotype is 13.7%, quite similar to that found among patients with VTE, suggesting that search for this genotype is not useful per se [133]. Such figures have been recently confirmed in a large case-control Dutch study (Multiple Environmental and Genetic Assessment, MEGA, of risk factors for VTE study), recruiting 4,375 patients and 4,856 control subjects. Among the controls, the frequency of FVL, PT20210A, heterozygous MTHFR C677T, and homozygous MTHFR C677T was 5%, 2%, 43%, and 11%, respectively. In the patients the distribution of FVL, PT20210A, heterozygous MTHFR C677T, and homozygous MTHFR C677T was 16%, 5%, 43%, and 10%. Accordingly, FVL and PT20210A were confirmed to be risk factors for VTE, whereas the carriership of the MTHFR C677T polymorphism had no effect on the risk for VTE [134].
VTE is a common complex (multifactorial) disease, being the resultant of gene-gene and gene-environment interactions. Unfortunately, a simple model expressing the presence or the absence of two dichotomous factors (high-risk allele and exposure to an environmental risk factor) is not sufficient. This is due to incomplete clinical penetrance of genotypes, since not all carriers develop VTE during life, and to variable expressivity of severity and age of onset of the disease; moreover, the onset of disease is modulated also by gene-gene interactions, in the large majority of cases still obscure, and by multiple effects of environmental risk factors: these act on the genotype in a additive or multiplicative way.
The influence of genetics is supported by the fact that family history of VTE has been consistenly reported to be a risk factor for VTE independent of the presence of known thrombophilic abnormalities [11, 135, 136]. Moreover, the carriers of FVL with a family history of VTE have been reported to be more prone to VTE than those without [11].
Acquired risk factors are far more common than inherited thrombophilias. Advanced age, cancer, immobility, and recent trauma, surgery, or hospitalization are well-recognized risk factors [137-139]. Important gender risk factors for VTE are pregnancy and hormone treatment. Compared to age-matched nonpregnant women, the risk of VTE is increased 5- to 10-fold during the antepartum period and 15- to 35-fold after delivery [140]; current oral contraceptive use or hormonal replacement therapy are associated with a 2 to 6-fold increased risk of VTE [141].
Our analysis confirmed a significant risk of VTE associated with the common polymorphisms FVL and PT20210A, with an overall odds ratio of 4.4 and 2.8, respectively. These figures are consistent with the large meta-analysis of Gohil et al [4], who reported an overall odds ratio of 4.9 for FVL and 3.2 for PT20210A. Homozygous carriers of FVL were confirmed to be at higher risk of VTE (11.4-fold ), consistently with the results obtained by Gohil et al [4] (odds ratio for VTE 9.4 for FVL homozygotes. Moreover, we were able to estimate the risk for VTE associated with homozygous PT20210A, (6.7-fold), which was unexplored by Gohil et al [4]. In agreement with current knowledge, the association of the MTHFR C677T homozygous variant with VTE was negligible, substantially confirming the results of Gohil et al [4]. Our meta-analysis included fewer cases and controls than that of Gohil et al [4]: 9,081/17,513 cases/controls versus 22,225/37,566 for FVL, 9,134/17,606 cases/controls versus 21,605/27,947 for PT20210A, 2,501/11,409 versus 13,570/20,935 for MTHFR C677T homozygous variant. This could be due to some differences in the selection criteria adopted in our meta-analysis in respect to those of Gohil et al [4]: in fact, we excluded the studies focusing on individuals younger than 16 years, on recurrent thrombotic events, and family studies. More importantly, we invited the authors of the eligible studies to furnish the last version of their database. Although this policy reduced by two thirds the number of studies included in our analysis, we are highly confident that the cases and the controls recruited in the final pooled database did non include duplicated groups of cases or controls. Contrary to Gohil et al [4], we included cases with post-surgical or pregnancy-related VTE, to allow an evaluation of the impact of common thrombophilia on the risk of VTE more representative of real life. Finally, we also included cases with cerebral or splanchnic vein thrombosis not related to overt cancer or myeloproliferative neoplasms.
The strength of our analysis in respect to previous studies consists in the application of a multivariate model having as covariates age, gender, and presence of FVL, PT20210A and MTHFR 677.
We confirmed that the use of OCs further increases the risk of VTE associated with FVL or PT20210A, with a greater effect with the former, as previously reported [142, 143]. No extensive data are available on the interaction between the MTHFR 677 TT genotype and the use of OCs; in our analysis the use of OC produced a small increase in risk which seems not different from that expected in non-thrombophilic women. The occurrence of other triggering situations did not significantly affect the overall risk of VTE associated with FVL or PT20210A.
Our study is the largest meta-analysis so far carried out on individuals with combined abnormalities. In a previous multicenter study (included in the present meta-analysis) recruiting 51 cases of VTE with double heterozygosity for FVL and PT20210A the risk of VTE was 20-fold increased compared to the controls, none of them was a double heterozygote [144]. In the present analysis we collected the data of 187 cases and 121 controls carriers of both FVL and PT20210A (among 4,343 cases and 3,725 controls investigated for both genes) allowing a more reliable statistical analysis. The risk of first VTE resulted 3.4-fold increased, quite similar to the risk associated with either heterozygous abnormality (OR=4.2 for FVL and 2.8 for PT20210A). Risk of first VTE associated with double heterozygosity not greatly exceeding that associated with heterozygous FVL alone could be interpreted by a weak or null additional risk due to the presence of PT20210A, as recently suggested in the clinical settings of pregnancy-related VTE [144]. Consistently with this finding, a pooled analysis of appropriate family studies showed that double heterozygotes for FVL and PT20210A, who are relatives of VTE index patients, had an increased risk for an initial episode of VTE not much higher than that estimated for those relatives who were only FVL heterozygous (pooled OR 6.7 vs. 3.5, respectively) [145].
Consistently with the null effect of the MTHFR 677 TT genotype on the risk of VTE, the combination of MTHFR 677 TT with either FVL or PT20210A, did not produce any increase in the thrombotic risk. This is in agreement with the results obtained in the aforementioned MEGA Study [134].
Despite the large sample size of this study, there are several limitations that need to be taken into account in the interpretation of results. Firstly, the studies included in the analyses come from very different populations. Sources of controls and methods for data collection were different. All these factors account, at least in part, for the observed heterogeneity among studies.
Data relative to potential confounders / effect modifiers were also collected using different methods (self-administered questionnaires, clinical assessments, interviews), and possibly different wordings. Furthermore, some potential confounders / effect modifiers could not be considered in the analyses for lack or incompleteness of the data retrieved (pregnancy status and pregnancy-related risk factors, homocysteinaemia, body mass index, diagnosis of diabetes mellitus).
Missing articles and non-responding authors are a potential source of bias. However, our results are in line with the more inclusive meta-analysis produced by Gohil et al [4], and with the meta-analyses of Marjot et al [146], focusing on CVTs only, and Gouveia et al [147], relative to MTHFR only. The comparability of results is suggestive that the bias due to high proportion of non-responders is unlikely to have affected our estimates systematically. Table 6 compares the meta-analyses available in literature on this topic, in terms of endpoints considered, population included, number of studies and sample size, inter-study heterogeneity (I-squared test, %). The statistical assessment also does not show a significant publication bias for our study.
Table 6.
Risk of cardiovascular thromboembolic accidents stratified by the type of event in the presence of gene-gene interaction: Factor V Leiden (FVL), prothrombin (PT) 20210A and methylenetetraydrofolate reductase (MTHFR) C677T
| Cases/Controls Exposed | Cases/Controls Not exposed | ORa | CIa | I-sq (CI), % | p-valueb | |
|---|---|---|---|---|---|---|
| FVL & PT 20210A Carriers | ||||||
| Venous thrombosis (w\o embolism) | 101/111 | 1,708/2,231 | 3.56 | (1.50-8.46) | 36.0 (0.0-76.1) | 0.181 |
| Thromboembolism | 50/115 | 1,147/2,877 | 2.39 | (1.13-5.04) | 28.3 (0.0-69.1) | 0.213 |
| Cerebral venous sinus thrombosis | 5/6 | 199/2,169 | 7.38 | (0.99-55.06) | 50.0 (0.0-87.2) | 0.157 |
| Splanchnic | --- | --- | ncd | . | nc | nc |
| Otherc | 4/3 | 573/1,703 | 4.37 | (0.97-19.72) | nc | nc |
| MTHFR C677T Homozygous & FVL Carriers | ||||||
| Venous thrombosis (w\o embolism) | 2/58 | 225/8,794 | 1.65 | (0.24-11.65) | 26.0 (0.0-0.0) | 0.245 |
| Thromboembolism | 10/60 | 522/9,261 | 4.15 | (0.97-17.70) | 50.4 (0.0-85.7) | 0.133 |
| Cerebral venous sinus thrombosis | 2/2 | 62/467 | 11.40 | (1.37-94.66) | nc | nc |
| Splanchnic | --- | --- | nc | . | nc | nc |
| Otherc | --- | --- | nc | . | nc | nc |
| MTHFR C677T Homozygous & PT 20210A Carriers | ||||||
| Venous thrombosis (w\o embolism) | 6/7 | 221/416 | 1.97 | (0.40-9.80) | 0.0 (0.0) | 0.371 |
| Thromboembolism | 6/24 | 288/9,047 | 5.48 | (1.67-17.97) | 0.0 (0.0) | 0.783 |
| Cerebral venous sinus thrombosis | --- | --- | nc | . | nc | nc |
| Splanchnic | --- | --- | nc | . | nc | nc |
| Otherc | --- | --- | nc | . | nc | nc |
Odds ratio (OR) adjusted for age and gender; CI: Confidence Interval
from Q-square test, random effects model
multiple, undetermined, unspecified site
nc, not computable
Furthermore, as confirmed by the tabular analysis of the studies not included, the results yielded by our pooled analysis are, in all likelihood, reliable in spite of the high proportion of studies that could not be included. This might be also due to the fact that we were able to include all the biggest studies on the topic, and that most of the non responders were authors of relatively smaller studies. Our pooled analysis yielded more conservative results for FVL and PT20210A then Gohil [4] and then those from our meta-analysis of studies not included – albeit this difference is not statistically significant. Again, this could be due to the fact that bigger studies tend to be, generally, more conservative than smaller ones, and to the fact that, for each polymorphic variant, in the pooled analysis we adjusted by the presence of the other two polymorphic variants.
One limitation of this study is that ethnicity could not be analysed at an individual patient level, given the paucity of responses on this. Most of the studies in the analysis are from Western European countries, and in some of the studies it is specified that all – or most – subjects enrolled are white Caucasians. However, especially for what concerns studies from the Americas, or from European countries with high prevalence of non-Caucasian groups (such as France, the United Kingdom or the Netherlands), we felt that using the country of residence to assume ethnicity would have been too big of an assumption.
In conclusion, FVL and PT20210A are confirmed in a large meta-analysis to be moderate risk factors for VTE in the adult population, with an associated risk further doubled in the homozygotes in comparison with the heterozygotes, suggesting a gene-gene additive effect; in contrast, the MTHFR 677 TT homozygous genotype is not a risk factor. Finally, the double carriership of FVL and PT20210A seems to produce an impact on the risk of VTE weaker than previously thought.
Supplementary Material
Table 7.
Comparative table of tabular meta-analyses published on association between polymorphic variants of VFL, PT202010A, MTHFR and risk of first VTE
| Cases/Controls | Studies included | Endpoint | OR (95% CI)a | I-squared | Notes | |
|---|---|---|---|---|---|---|
| FVL | ||||||
| Marjot, 2011 [146] | 767/4020 | 19 | CVTb | 2.40 (1.75-3.30) | 0.0% | Adults, European descent |
| Gohil, 2009 [4] | 22225/37566 | 84 | VTEc | 4.93 (4.41-5.52) | 58.0% | Studies from European/American authors |
| PT20201A | ||||||
| Marjot, 2011 [146] | 646/3690 | 15 | CVT | 5.48 (3.88-7.74) | 0.0% | Adults, European descent |
| Gohil, 2009 [4] | 21605/27947 | 79 | VTE | 3.17 (2.19-3.46) | 0.0% | Studies from European/American authors |
| Gohil, 2009 [4] | 546/1734 | 4 | VTE | ncd | nc | Studies from Asian authors PT20201A not found |
| MTHFR C677T | ||||||
| Marjot, 2011 [146] | 233/1323 | 7 | 1.97 | 1.83 (0.88-3.80) | 68.0% | Adults, European descent |
| Gouveia, 2010 [147] | 382/1217 | 9 | 5.48 | 1.12 (0.80-1.58) | 60.5% | - |
| Gohil, 2009 [4] | 13570/20935 | 50 | nc | 1.57 (1.23-2.0) | 60.0% | Studies from European/American authors |
| Gohil, 2009 [4] | 827/2758 | 12 | nc | 1.09 (0.97-1.24) | 0.0% | Studies from Asian authors |
Odds ratio, 95% Confidence intervals
Cerebral venous thrombosis
Venous thromboembolism
Not computable
Acknowledgments
The authors gratefully acknowledge funding from the Italian Ministry for Education and University (PRIN 2007) for the project: Health Technology Assessment per gli screening genetici: lo studio dell'appropriatezza dei test genetici di suscettibilità alla malattia tromboembolica venosa come modello di studio (health thechnology assessment for genetic screening tests: appropriateness of susceptibility genetic tests to venous thromboembolism as a model of study).
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). The authors thank the staff and participants of the ARIC study for their important contributions.
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221. The authors thank the WHI investigators and staff for their dedication, and the study participants for making the program possible. A full listing of WHI investigators can be found at: http://www.whiscience.org/publications/WHI_investigators_shortlist.pdf
The Cardiovascular Health Study was supported by contracts HHSN268201200036C, HHSN268200800007C, N01 HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grant HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org.
Footnotes
Conflict of interests
The authors declare that they have no conflict of interest.
REFERENCES
- 1.White RH. The Epidemiology of Venous Thromboembolism MD. Circulation. 2003;107:I-4–I-8. doi: 10.1161/01.CIR.0000078468.11849.66. [DOI] [PubMed] [Google Scholar]
- 2.Snow V, Qaseem A, Barry P, Hornbake ER, Rodnick JE, Tobolic T, Ireland B, Segal JB, Bass EB, Weiss KB, Green L, Owens DK. American College of Physicians, American Academy of Family Physicians Panel on Deep Venous. Management of venous thromboembolism: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2007 Feb 6;146(3):204–10. doi: 10.7326/0003-4819-146-3-200702060-00149. [DOI] [PubMed] [Google Scholar]
- 3.White RH, Keenan CR. Effects of race and ethnicity on the incidence of venous thromboembolism. Thrombosis Research. 2009;123(Suppl. 4):S11–S17. doi: 10.1016/S0049-3848(09)70136-7. [DOI] [PubMed] [Google Scholar]
- 4.Gohil R, Peck G, Sharma P. The genetics of venous thromboembolism. A meta-analysis involving approximately 120,000 cases and 180,000 controls. Thromb Haemostas. 2009;102:360–70. doi: 10.1160/TH09-01-0013. [DOI] [PubMed] [Google Scholar]
- 5.Learning about Factor V Leiden thrombophilia. National Human Genome Research Institute; 2011. [September 20th 2012]. http://www.genome.gov/15015167#Q5. [Google Scholar]
- 6.Varga EA, Moll S. Prothrombin 20210 Mutation (Factor II Mutation). Circulation. 2004;110:e15–e18. doi: 10.1161/01.CIR.0000135582.53444.87. [DOI] [PubMed] [Google Scholar]
- 7.LD Botto Yang Q. 5,10-Methylenetetrahydrofolate reductase gene variants and congenital anomalies: a HuGE review. American Journal of Epidemiology. 2001;151(9):862–867. doi: 10.1093/oxfordjournals.aje.a010290. [DOI] [PubMed] [Google Scholar]
- 8.Brouwer JL, Veeger NJ, Kluin-Nelemans HC, van der Meer J. The pathogenesis of venous thromboembolism: evidence for multiple interrelated causes. Ann Intern Med. 2006;145:807–15. doi: 10.7326/0003-4819-145-11-200612050-00005. [DOI] [PubMed] [Google Scholar]
- 9.Emmerich J, Rosendaal FR, Cattaneo M, Margaglione M, De Stefano V, Cumming T, Arruda V, Hillarp A, Reny JL. Combined effect of factor V Leiden and prothrombin 20210A on the risk of venous thromboembolism--pooled analysis of 8 case-control studies including 2310 cases and 3204 controls. Study Group for Pooled-Analysis in Venous Thromboembolism. Thromb Haemost. 2001;86:809–16. [PubMed] [Google Scholar]
- 10. [September 20th 2012];International Statistical Classification of Diseases and Related Health Problems 10th Revision. 2010 http://apps.who.int/classifications/icd10/browse/2010/en.
- 11.Bezemer ID, van der Meer FJ, Eikenboom JC, Rosendaal FR, Doggen CJ. The value of family history as a risk indicator for venous thrombosis. Arch Intern Med. 2009 Mar 23;169(6):610–5. doi: 10.1001/archinternmed.2008.589. [DOI] [PubMed] [Google Scholar]
- 12.Snow CF. Laboratory diagnosis of vitamin B12 and folate deficiency: a guide for the primary care physician. Arch Intern Med. 1999;159(12):1289–98. doi: 10.1001/archinte.159.12.1289. 28. [DOI] [PubMed] [Google Scholar]
- 13.Graham IM, Daly LE, Refsum HM, Robinson K, Brattström LE, Ueland PM, Palma-Reis RJ, Boers GH, Sheahan RG, Israelsson B, Uiterwaal CS, Meleady R, McMaster D, Verhoef P, Witteman J, Rubba P, Bellet H, Wautrecht JC, de Valk HW, Sales Lúis AC, Parrot-Rouland FM, Tan KS, Higgins I, Garcon D, Andria G, et al. Plasma homocysteine as a risk factor for vascular disease. The European Concerted Action Project. JAMA. 1997;277(22):1775–81. doi: 10.1001/jama.1997.03540460039030. [DOI] [PubMed] [Google Scholar]
- 14.Practice Committee of the American Society for Reproductive Medicine Definitions of infertility and recurrent pregnancy loss. Fertil Steril. 2008;89:1603. doi: 10.1016/j.fertnstert.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Schwahn B, Rozen R. Polymorphisms in the methylenetetrahydrofolate reductase gene: clinical consequences. Am J Pharmacogenomics. 2001;1(3):189–201. doi: 10.2165/00129785-200101030-00004. [DOI] [PubMed] [Google Scholar]
- 16.Akar N, Akar E, Akcay R, Avcu F, Yalcin A, Cin S. Effect of methylenetetrahydrofolate reductase 677 C-T, 1298 A-C, and 1317 T-C on factor V 1691 mutation in Turkish deep vein thrombosis patients. Thromb Res. 2000;97:163–7. doi: 10.1016/s0049-3848(99)00157-7. [DOI] [PubMed] [Google Scholar]
- 17.Almawi WY, Tamim H, Kreidy R, Timson G, Rahal E, Nabulsi M, Finan RR, Irani-Hakime N. A case control study on the contribution of factor V-Leiden, prothrombin G20210A, and MTHFR C677T mutations to the genetic susceptibility of deep venous thrombosis. Thromb Thrombolysis. 2005 Jun;19(3):189–96. doi: 10.1007/s11239-005-1313-x. [DOI] [PubMed] [Google Scholar]
- 18.Angchaisuksiri P, Pingsuthiwong S, Sura T, Aryuchai K, Busabaratana M, Atichartakarn V. Prevalence of the C677T methylenetetra- hydrofolate reductase mutation in Thai patients with deep vein thrombosis. Acta Haematol. 2000;103(4):191–6. doi: 10.1159/000041048. [DOI] [PubMed] [Google Scholar]
- 19.Aznar J, Vayá A, Estellés A, Mira Y, Seguí R, Villa P, Ferrando F, Falcó C, Corella D, España F. Risk of venous thrombosis in carriers of the prothrombin G20210A variant and factor V Leiden and their interaction with oral contraceptives. Haematologica. 2000;85(12):1271–6. [PubMed] [Google Scholar]
- 20.Bedencic M, Bozic M, Peternel P, Stegnar M. Major and potential prothrombotic genotypes in patients with venous thrombosis and in healthy subjects from Slovenia. Pathophysiol Haemost Thromb. 2007;36(2):58–63. doi: 10.1159/000173722. [DOI] [PubMed] [Google Scholar]
- 21.Cantu C, Alonso E, Jara A, Martínez L, Ríos C, Fernández Mde L, Garcia I, Barinagarrementeria F. Hyperhomocysteinemia, low folate and vitamin B12 concentrations, and methylene tetrahydrofolate reductase mutation in cerebral venous thrombosis. Stroke. 2004;35(8):1790–4. doi: 10.1161/01.STR.0000132570.24618.78. [DOI] [PubMed] [Google Scholar]
- 22.Cushman M, Kuller LH, Prentice R, Rodabough RJ, Psaty BM, Stafford RS, Sidney S, Rosendaal FR. Estrogen plus progestin and risk of venous thrombosis. JAMA. 2004;292(13):1573–80. doi: 10.1001/jama.292.13.1573. [DOI] [PubMed] [Google Scholar]
- 23.De Stefano V, Rossi E, Paciaroni K, D'Orazio A, Cina G, Marchitelli E, Pepe R, Leone G. Different circumstances of the first venous thromboembolism among younger or older heterozygous carriers of the G20210A polymorphism in the prothrombin gene. Haematologica. 2003;88(1):61–6. [PubMed] [Google Scholar]
- 24.Díaz DE, Tuesta AM, Ribó MD, Belinchón O, Marchena PJ, Bruscas MJ, Val E, Cortés A, Nieto JA. Low levels of vitamin B12 and venous thromboembolic disease in elderly men. J Intern Med. 2005;258(3):244–9. doi: 10.1111/j.1365-2796.2005.01527.x. [DOI] [PubMed] [Google Scholar]
- 25.Ducros V, Barro C, Yver J, Pernod G, Polack B, Carpentier P, Desruets MD, Bosson JL. Should Plasma Homocysteine Be Used as a Biomarker of Venous Thromboembolism? A Case--Control Study Clin Appl Thromb Hemost. 2009;200915(5):517–22. doi: 10.1177/1076029608322548. [DOI] [PubMed] [Google Scholar]
- 26.Fernández-Miranda C, Coto A, Martínez J, Gómez P, Gómez de la Cámara A. [Effect of hyperhomocysteinemia and methylenetetrahydrofolate reductase 677C --> T mutation in venous thromboembolism risk of young adults]. Med Clin (Barc) 2005;124(14):532–4. doi: 10.1157/13073939. [DOI] [PubMed] [Google Scholar]
- 27.Folsom AR, Cushman M, Tsai MY, Aleksic N, Heckbert SR, Boland LL, Tsai AW, Yanez ND, Rosamond WD. A prospective study of venous thromboembolism in relation to factor V Leiden and related factors. Blood. 2002 Apr 15;99(8):2720–5. doi: 10.1182/blood.v99.8.2720. [DOI] [PubMed] [Google Scholar]
- 28.Folsom AR. Update on factor V Leiden association with venous thromboembolism in the LITE Study. Blood. 2007 Feb 1;109(3):1336–7. doi: 10.1182/blood-2006-10-052100. [DOI] [PubMed] [Google Scholar]
- 29.Frederiksen J, Juul K, Grande P, Jensen GB, Schroeder TV, Tybjaerg-Hansen A, Nordestgaard BG. Methylenetetrahydrofolate reductase polymorphism (C677T), hyperhomocysteinemia, and risk of ischemic cardiovascular disease and venous thromboembolism: prospective and case-control studies from the Copenhagen City Heart Study. Blood. 2004;104(10):3046–51. doi: 10.1182/blood-2004-03-0897. [DOI] [PubMed] [Google Scholar]
- 30.Gemmati D, Previati M, Serino ML, Moratelli S, Guerra S, Capitani S, Forini E, Ballerini G, Scapoli GL. Low folate levels and thermolabile methylenetetrahydrofolate reductase as primary determinant of mild hyperhomocystinemia in normal and thromboembolic subjects. Arterioscler Thrombvasc Biol. 1999;19:1761–7. doi: 10.1161/01.atv.19.7.1761. [DOI] [PubMed] [Google Scholar]
- 31.Gemmati D, Serino ML, Trivellato C, Fiorini S, Scapoli GL. C677T substitution in the methylenetetrahydrofolate reductase gene as a risk factor for venous thrombosis and arterial disease in selected patients. Haematologica. 1999;84:824–8. [PubMed] [Google Scholar]
- 32.Ivanov P, Komsa-Penkova R, Kovacheva K, Ivanov Y, Stoyanova A, Ivanov I, Pavlov P, Glogovska P, Nojarov V. Impact of thrombophilic genetic factors on pulmonary embolism: early onset and recurrent incidences. Lung. 2008;186(1):27–36. doi: 10.1007/s00408-007-9061-7. [DOI] [PubMed] [Google Scholar]
- 33.Keijzer MB, den Heijer M, Blom HJ, Bos GM, Willems HP, Gerrits WB, Rosendaal FR. Interaction between hyperhomocysteinemia, mutated methylenetetrahydrofolatereductase (MTHFR) and inherited thrombophilic factors in recurrent venous thrombosis. Thromb Haemost. 2002;88(5):723–8. [PubMed] [Google Scholar]
- 34.Le Cam-Duchez V, Bagan-Triquenot A, Ménard JF, Mihout B, Borg JY. Association of the protein C promoter CG haplotype and the factor II G20210A mutation is a risk factor for cerebral venous thrombosis. Blood Coagul Fibrinolysis. 2005 Oct;16(7):495–500. doi: 10.1097/01.mbc.0000184738.27723.b2. [DOI] [PubMed] [Google Scholar]
- 35.Lichy C, Dong-Si T, Reuner K, Genius J, Rickmann H, Hampe T, Dolan T, Stoll F, Grau A. Risk of cerebral venous thrombosis and novel gene polymorphisms of the coagulation and fibrinolytic systems. J Neurol. 2006;253(3):316–20. doi: 10.1007/s00415-005-0988-4. [DOI] [PubMed] [Google Scholar]
- 36.Martinelli I, Battaglioli T, Pedotti P, Cattaneo M, Mannucci PM. Hyperhomocysteinemia in cerebral vein thrombosis. Blood. 2003;102(4):1363–6. doi: 10.1182/blood-2003-02-0443. [DOI] [PubMed] [Google Scholar]
- 37.Nizankowska-Mogilnicka E, Adamek L, Grzanka P, Domagala TB, Sanak M, Krzanowski M, Szczeklik A. Genetic polymorphisms associated with acute pulmonary embolism and deep venous thrombosis. Eur Respir J. 2003;21(1):25–30. doi: 10.1183/09031936.03.00034302. [DOI] [PubMed] [Google Scholar]
- 38.Ogunyemi D, Cuellar F, Ku W, Arkel Y. Association between inherited thrombophilias, antiphospholipid antibodies, and lipoprotein A levels and venous thromboembolism in pregnancy. Am J Perinatol. 2003;20(1):17–24. doi: 10.1055/s-2003-37947. [DOI] [PubMed] [Google Scholar]
- 39.Oguzulgen IK, Yilmaz E, Demirtas S, Erkekol FO, Ekim N, Demir N, Numanoglu N, Ozel D, Ulu A, Akar The role of plasminogen activator inhibitor-1 polymorphism, factor-V-Leiden, and prothrombin-20210 mutations in pulmonary thromboembolism. Clin Appl Thromb Hemost. 2009 Feb;15(1):73–7. doi: 10.1177/1076029607305110. Epub 2007 Dec 26. [DOI] [PubMed] [Google Scholar]
- 40.Okumus G, Kiyan E, Arseven O, Tabak L, Diz-Kucukkaya R, Unlucerci Y, Abaci N, Unaltuna NE, Issever H. ereditary thrombophilic risk factors and venous thromboembolism in Istanbul, Turkey: the role in different clinical manifestations of venous thromboembolism. Clin Appl Thromb Hemost. 2008 Apr;14(2):168–73. doi: 10.1177/1076029607305620. Epub 2007 Sep 25. [DOI] [PubMed] [Google Scholar]
- 41.Penco S, Grossi E, Cheng S, Intraligi M, Maurelli G, Patrosso MC, Marocchi A, Buscema M. Assessment of the role of genetic polymorphism in venous thrombosis through artificial neural networks. Ann Hum Genet. 2005;69(Pt 6):693–706. doi: 10.1111/j.1529-8817.2005.00206.x. [DOI] [PubMed] [Google Scholar]
- 42.Shmeleva VM, Kapustin SI, Papayan LP, Sobczyńska-Malefora A, Harrington DJ, Savidge GF. Prevalence of hyperhomocysteinemia andù the MTHFR C677T polymorphism in patients with arterial and venous thrombosis from North Western Russia. Thromb Res. 2003;111(6):351–6. doi: 10.1016/j.thromres.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 43.Straczek C, Oger E, Yon de Jonage-Canonico MB, Plu-Bureau G, Conard J, Meyer G, Alhenc-Gelas M, Lévesque H, Trillot N, Barrellier MT, Wahl D, Emmerich J, Scarabin PY. Estrogen and Thromboembolism Risk (ESTHER) Study Group. Prothrombotic mutations, hormone therapy, and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration. Circulation. 2005;112(22):3495–500. doi: 10.1161/CIRCULATIONAHA.105.565556. [DOI] [PubMed] [Google Scholar]
- 44.Torres JD, Cardona H, Alvarez L, Cardona-Maya W, Castañeda SA, Quintero-Rivera F, Cadavid A, Bedoya G, Tobón L. Inherited thrombophilia is associated with deep vein thrombosis in a Colombian population. Am J Hematol. 2006;81(12):933–7. doi: 10.1002/ajh.20733. [DOI] [PubMed] [Google Scholar]
- 45.Vayá A, Mira Y, Mateo J, Falco C, Villa P, Estelles A, Corella D, Fontcuberta J, Aznar J. Prothrombin G20210A mutation and oral contraceptive use increase upper-extremity deep vein thrombotic risk. Thromb Haemost. 2003;89(3):452–7. [PubMed] [Google Scholar]
- 46.Alhenc-Gelas M, Arnaud E, Nicaud V, Aubry ML, Fiessinger JN, Aiach M, Emmerich J. Venous thromboembolic disease and the prothrombin, methylene tetrahydrofolate reductase and factor V genes. Thromb Haemost. 1999 Apr;81(4):506–10. [PubMed] [Google Scholar]
- 47.Alhenc-Gelas M, Nicaud V, Gandrille S, van Dreden P, Amiral J, Aubry ML, Fiessinger JN, Emmerich J, Aiach M. The factor V gene A4070G mutation and the risk of venous thrombosis. Thromb Haemost. 1999;81:193–197. [PubMed] [Google Scholar]
- 48.Arruda VR, von Zuben PM, Chiaparini LC, Annichino-Bizzacchi JM, Costa FF. The mutation Ala677-->Val in the methylene tetrahydrofolate reductase gene: a risk factor for arterial disease and venous thrombosis. Thromb Haemost. 1997;77:818–821. [PubMed] [Google Scholar]
- 49.Avdonin PV, Kirienko AI, Kozhevnikova LM, Shostak NA, Babadaeva NM, Leont'ev SG, Petukhov EB, Kubatiev AA, Savel'ev VS. C677T mutation in methylentetrahydrofolatereductase gene in patients with venous thromboses from the central region of Russia correlates with a high risk of pulmonary artery thromboembolism. Ter Arkh. 2006;78:70–76. [PubMed] [Google Scholar]
- 50.Balta G, Gurgey A. Methylenetetrahydrofolate reductase (MTHFR) C677T mutation in Turkish patients with thrombosis. Turk J Pediatr. 1999;41:197–199. [PubMed] [Google Scholar]
- 51.Barcellona D, Fenu L, Cauli C, Pisu G, Marongiu F. Allele 4G of gene PAI-1 associated with prothrombin mutation G20210A increases the risk for venous thrombosis. Thromb Haemost. 2003;90:1061–1064. doi: 10.1160/TH02-12-0305. [DOI] [PubMed] [Google Scholar]
- 52.Benson JM, Ellingsen D, El-Jamil M, Jenkins M, Miller CH, Dilley A, Evatt BL, Hooper WC. Factor V Leiden and factor V R2 allele: high-throughput analysis and association with venous thromboembolism. Thromb Haemost. 2001;86:1188–1192. [PubMed] [Google Scholar]
- 53.Bouaziz-Borgi L, Almawi WY, Mtiraoui N, Nsiri B, Keleshian SH, Kreidy R, Louzir B, Hezard N, Mahjoub T. Distinct association of factor V-Leiden and prothrombin G20210A mutations with deep venous thrombosis in Tunisia and Lebanon. Am J Hematol. 2006;81:641–643. doi: 10.1002/ajh.20582. [DOI] [PubMed] [Google Scholar]
- 54.Boyanovsky B, Russeva M, Ganev V, Penev M, Baleva M. Prevalence of factor V Leiden and prothrombin 20210 A variant in Bulgarian patients with pulmonary thromboembolism and deep venous thrombosis. Blood Coagul Fibrinolysis. 2001;12:639–642. doi: 10.1097/00001721-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 55.Brown K, Luddington R, Baglin T. A common polymorphism in the tumour necrosis factor-alpha gene associated with high TNF levels is not a risk factor for venous thromboembolism. Br J Haematol. 1998 Jun;101(3):480–2. doi: 10.1046/j.1365-2141.1998.00729.x. [DOI] [PubMed] [Google Scholar]
- 56.Brown K, Luddington R, Taylor SA, Lillicrap DP, Baglin TP. Risk of venous thromboembolism associated with the common hereditary haemochromatosis Hfe gene (C282Y) mutation. Br J Haematol. 1999;105:95–97. [PubMed] [Google Scholar]
- 57.Brown K, Luddington R, Williamson D, Baker P, Baglin T. Risk of venous thromboembolism associated with a G to A transition at position 20210 in the 3′-untranslated region of the prothrombin gene. Br J Haematol. 1997 Sep;98(4):907–9. doi: 10.1046/j.1365-2141.1997.3093130.x. [DOI] [PubMed] [Google Scholar]
- 58.Cappucci G, Margaglione M, Ames PR. Comparative prevalence of antiphospholipid antibodies and thrombophilic genotypes in consecutive patients with venous thrombosis. Blood Coagul Fibrinolysis. 2001;12:659–665. doi: 10.1097/00001721-200112000-00007. [DOI] [PubMed] [Google Scholar]
- 59.Cattaneo M, Chantarangkul V, Taioli E, Santos JH, Tagliabue L. The G20210A mutation of the prothrombin gene in patients with previous first episodes of deep-vein thrombosis: prevalence and association with factor V G1691A, methylenetetrahydrofolate reductase C677T and plasma prothrombin levels. Thromb Res. 1999;93:1–8. doi: 10.1016/s0049-3848(98)00136-4. [DOI] [PubMed] [Google Scholar]
- 60.Cattaneo M, Tsai MY, Bucciarelli P, Taioli E, Zighetti ML, Bignell M, Mannucci PM. A common mutation in the methylenetetrahydrofolate reductase gene (C677T) increases the risk for deep-vein thrombosis in patients with mutant factor V (factor V:Q506). Arterioscler Thromb Vasc Biol. 1997;17:1662–1666. doi: 10.1161/01.atv.17.9.1662. [DOI] [PubMed] [Google Scholar]
- 61.Chan DK, Hu G, Tao H, Owens D, Vun CM, Woo J, Chong BH. A comparison of polymorphism in the 3'- untranslated region of the prothrombin gene between Chinese and Caucasians in Australia. Br J Haematol. 2000;111:1253–1255. doi: 10.1046/j.1365-2141.2000.02477.x. [DOI] [PubMed] [Google Scholar]
- 62.Coen D, Zadro R, Honović L, Banfić L, Stavljenić Rukavina A. Prevalence and association of the factor V Leiden and prothrombin G20210A in healthy subjects and patients with venous thromboembolism. Croat Med J. 2001;42:488–492. [PubMed] [Google Scholar]
- 63.Colaizzo D, Amitrano L, Iannaccone L, Vergura P, Cappucci F, Grandone E, Guardascione MA, Margaglione M. Gain-of-function gene mutations and venous thromboembolism: distinct roles in different clinical settings. J Med Genet. 2007;44:412–416. doi: 10.1136/jmg.2006.048371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Corral J, González-Conejero R, Soria JM, González-Porras JR, Pérez-Ceballos E, Lecumberri R, Roldán V, Souto JC, Miñano A, Hernández-Espinosa D, Alberca I, Fontcuberta J, Vicente V. A nonsense polymorphism in the protein Z- dependent protease inhibitor increases the risk for venous thrombosis. Blood. 2006;108:177–183. doi: 10.1182/blood-2005-08-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Couturaud F, Oger E, Abalain JH, Chenu E, Guias B, Floch HH, Mercier B, Mottier D, Leroyer C. Methylenetetrahydrofolate reductase C677T genotype and venous thromboembolic disease. Respiration. 2000;67:657–661. doi: 10.1159/000056296. [DOI] [PubMed] [Google Scholar]
- 66.de Franchis R, Fermo I, Mazzola G, Sebastio G, Di Minno G, Coppola A, Andria G, D'Angelo A. Contribution of the cystathionine beta-synthase gene (844ins68) polymorphism to the risk of early-onset venous and arterial occlusive disease and of fasting hyperhomocysteinemia. Thromb Haemost. 2000;84:576–582. [PubMed] [Google Scholar]
- 67.de Moerloose P, Reber G, Perrier A, Perneger T, Bounameaux H. Prevalence of factor V Leiden and prothrombin G20210A mutations in unselected patients with venous thromboembolism. Br J Haematol. 2000;110:125–129. doi: 10.1046/j.1365-2141.2000.02039.x. [DOI] [PubMed] [Google Scholar]
- 68.de Paula Sabino A, Guimarães DA, Ribeiro DD, Paiva SG, Sant'Ana Dusse LM, das Graças Carvalho M, Fernandes AP. Increased Factor V Leiden frequency is associated with venous thrombotic events among young Brazilian patients. J Thromb Thrombolysis. 2007;24:261–266. doi: 10.1007/s11239-007-0024-x. [DOI] [PubMed] [Google Scholar]
- 69.Dilley A, Austin H, Hooper WC, Lally C, Ribeiro MJ, Wenger NK, Silva V, Rawlins P, Evatt B. Relation of three genetic traits to venous thrombosis in an African-American population. Am J Epidemiol. 1998;147:30–35. doi: 10.1093/oxfordjournals.aje.a009363. [DOI] [PubMed] [Google Scholar]
- 70.Dölek B, Eraslan S, Eroğlu S, Kesim BE, Ulutin T, Yalçiner A, Laleli YR, Gözükirmizi N. Molecular analysis of factor V Leiden, factor V Hong Kong, factor II G20210A, methylenetetrahydrofolate reductase C677T, and A1298C mutations related to Turkish thrombosis patients. Clin Appl Thromb Hemost. 2007;13:435–438. doi: 10.1177/1076029607303341. [DOI] [PubMed] [Google Scholar]
- 71.Dowling NF, Austin H, Dilley A, Whitsett C, Evatt BL, Hooper WC. The epidemiology of venous thromboembolism in Caucasians and African-Americans: the GATE Study. J Thromb Haemost. 2003;1:80–87. doi: 10.1046/j.1538-7836.2003.00031.x. [DOI] [PubMed] [Google Scholar]
- 72.Faioni EM, Castaman G, Asti D, Lussana F, Rodeghiero F. Association of factor V deficiency with factor V HR2. Haematologica. 2004;89:195–200. [PubMed] [Google Scholar]
- 73.Ferraresi P, Marchetti G, Legnani C, Cavallari E, Castoldi E, Mascoli F, Ardissino D, Palareti G, Bernardi F. The heterozygous 20210 G/A prothrombin genotype is associated with early venous thrombosis in inherited thrombophilias and is not increased in frequency in artery disease. Arterioscler Thromb Vasc Biol. 1997;17:2418–2422. doi: 10.1161/01.atv.17.11.2418. [DOI] [PubMed] [Google Scholar]
- 74.Francès F, Portolès O, Gabriel F, Corella D, Sorlí JV, Sabater A, Alfonso JL, Guillén M. Factor V Leiden (G1691A) and prothrombin-G20210A alleles among patients with deep venous thrombosis and in the general population from Spain. Rev Med Chil. 2006;134:13–20. doi: 10.4067/s0034-98872006000100002. [DOI] [PubMed] [Google Scholar]
- 75.Franco RF, Fagundes MG, Meijers JC, Reitsma PH, Lourenço D, Morelli V, Maffei FH, Ferrari IC, Piccinato CE, Silva WA, Jr, Zago MA. Identification of polymorphisms in the 5'-untranslated region of the TAFI gene: relationship with plasma TAFI levels and risk of venous thrombosis. Haematologica. 2001;86:510–517. [PubMed] [Google Scholar]
- 76.Franco RF, Morelli V, Lourenço D, Maffei FH, Tavella MH, Piccinato CE, Thomazini IA, Zago MA. A second mutation in the methylenetetrahydrofolate reductase gene and the risk of venous thrombotic disease. Br J Haematol. 1999;105:556–559. [PubMed] [Google Scholar]
- 77.García-Gala JM, Alvarez V, Pinto CR, Soto I, Urgellés MF, Menéndez MJ, Carracedo C, López-Larrea C, Coto E. Factor V Leiden (R506Q) and risk of venous thromboembolism: a case-control study based on the Spanish population. Clin Genet. 1997;52:206–210. doi: 10.1111/j.1399-0004.1997.tb02548.x. [DOI] [PubMed] [Google Scholar]
- 78.Gaustadnes M, Rüdiger N, Møller J, Rasmussen K, Bjerregaard Larsen T, Ingerslev J. Thrombophilic predisposition in stroke and venous thromboembolism in Danish patients. Blood Coagul Fibrinolysis. 1999;10:251–259. doi: 10.1097/00001721-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 79.González Ordóñez AJ, Fernández Alvarez CR, Rodríguez JM, García EC, Alvarez MV. Genetic polymorphism of methylenetetrahydrofolate reductase and venous thromboembolism: a case-control study. Haematologica. 1999;84:190–191. [PubMed] [Google Scholar]
- 80.González-Conejero R, Lozano ML, Corral J, Martínez C, Vicente V. The TFPI 536C-->T mutation is not associated with increased risk for venous or arterial thrombosis. Thromb Haemost. 2000;83:787–788. [PubMed] [Google Scholar]
- 81.Grossmann R, Schwender S, Geisen U, Schambeck C, Merati G, Walter U. CBS 844ins68, MTHFR TT677 and EPCR 4031ins23 genotypes in patients with deep-vein thrombosis. Thromb Res. 2002;107:13–15. doi: 10.1016/s0049-3848(02)00187-1. [DOI] [PubMed] [Google Scholar]
- 82.Hainaut P, Jaumotte C, Verhelst D, Wallemacq P, Gala JL, Lavenne E, Heusterspreute M, Zech F, Moriau M. Hyperhomocysteinemia and venous thromboembolism: a risk factor more prevalent in the elderly and in idiopathic cases. Thromb Res. 2002;106:121–125. doi: 10.1016/s0049-3848(02)00096-8. [DOI] [PubMed] [Google Scholar]
- 83.Hanson NQ, Aras O, Yang F, Tsai MY. C677T and A1298C polymorphisms of the methylenetetrahydrofolate reductase gene: incidence and effect of combined genotypes on plasma fasting and post-methionine load homocysteine in vascular disease. Clin Chem. 2001;47:661–666. [PubMed] [Google Scholar]
- 84.Hessner MJ, Luhm RA, Pearson SL, Endean DJ, Friedman KD, Montgomery RR. Prevalence of prothrombin G20210A, factor V G1691A (Leiden), and methylenetetrahydrofolate reductase (MTHFR) C677T in seven different populations determined by multiplex allele-specific PCR. Thromb Haemost. 1999 May;81(5):733–8. [PubMed] [Google Scholar]
- 85.Hoppe B, Tolou F, Radtke H, Kiesewetter H, Dörner T, Salama A. Marburg I polymorphism of factor VII-activating protease is associated with idiopathic venous thromboembolism. Blood. 2005;105:1549–1551. doi: 10.1182/blood-2004-08-3328. [DOI] [PubMed] [Google Scholar]
- 86.Ioannou HV, Mitsis M, Eleftheriou A, Matsagas M, Nousias V, Rigopoulos C, Vartholomatos G, Kappas AM. The prevalence of factor V Leiden as a risk factor for venous thromboembolism in the population of North-Western Greece. Int Angiol. 2000;19:314–318. [PubMed] [Google Scholar]
- 87.Isotalo PA, Donnelly JG. Prevalence of methylenetetrahydrofolate reductase mutations in patients with venous thrombosis. Mol Diagn. 2000;5:59–66. doi: 10.1007/BF03262024. [DOI] [PubMed] [Google Scholar]
- 88.Jackson A, Brown K, Langdown J, Luddington R, Baglin T. Effect of the angiotensin-converting enzyme gene deletion polymorphism on the risk of venous thromboembolism. Br J Haematol. 2000;111:562–564. doi: 10.1046/j.1365-2141.2000.02408.x. [DOI] [PubMed] [Google Scholar]
- 89.Junker R, Koch HG, Auberger K, Münchow N, Ehrenforth S, Nowak-Göttl U. Prothrombin G20210A gene mutation and further prothrombotic risk factors in childhood thrombophilia. Arterioscler Thromb Vasc Biol. 1999;19:2568–2572. doi: 10.1161/01.atv.19.10.2568. [DOI] [PubMed] [Google Scholar]
- 90.Kapur RK, Mills LA, Spitzer SG, Hultin MB. A prothrombin gene mutation is significantly associated with venous thrombosis. Arterioscler Thromb Vasc Biol. 1997 Nov;17(11):2875–9. doi: 10.1161/01.atv.17.11.2875. [DOI] [PubMed] [Google Scholar]
- 91.Kluijtmans LA, den Heijer M, Reitsma PH, Heil SG, Blom HJ, Rosendaal FR. Thermolabile methylenetetrahydrofolate reductase and factor V Leiden in the risk of deep-vein thrombosis. Thromb Haemost. 1998;79:254–258. [PubMed] [Google Scholar]
- 92.Koch HG, Nabel P, Junker R, Auberger K, Schobess R, Homberger A, Linnebank M, Nowak-Göttl U. The 677T genotype of the common MTHFR thermolabile variant and fasting homocysteine in childhood venous thrombosis. Eur J Pediatr. 1999;158(Suppl 3):S113–116. doi: 10.1007/pl00014332. [DOI] [PubMed] [Google Scholar]
- 93.Köppel H, Renner W, Gugl A, Cichocki L, Gasser R, Wascher TC, Pilger E. The angiotensin-converting-enzyme insertion/deletion polymorphism is not related to venous thrombosis. Thromb Haemost. 2004 Jan;91(1):76–9. doi: 10.1160/TH03-05-0266. [DOI] [PubMed] [Google Scholar]
- 94.Kostka H, Kuhlisch E, Schellong S, Siegert G. Polymorphisms in the TAFI gene and the risk of venous thrombosis. Clin Lab. 2003;49:645–647. [PubMed] [Google Scholar]
- 95.Kostka H, Schwarz T, Schellong S, Mix C, Kuhlisch E, Temelkova-Kurktschiev T, Henkel E, Köhler C, Gehrisch S, Siegert G. Coagulation factor V G allele and HR2 haplotype: factor V activity, activated protein C resistance and risk of venous thrombosis. Blood Coagul Fibrinolysis. 2003;14:49–56. doi: 10.1097/01.mbc.0000046197.72384.42. [DOI] [PubMed] [Google Scholar]
- 96.Legnani C, Cosmi B, Valdrè L, Boggian O, Bernardi F, Coccheri S, Palareti G. Venous thromboembolism, oral contraceptives and high prothrombin levels. J Thromb Haemost. 2003;1:112–117. doi: 10.1046/j.1538-7836.2003.00008.x. [DOI] [PubMed] [Google Scholar]
- 97.Leroyer C, Mercier B, Escoffre M, Férec C, Mottier D. Factor V Leiden prevalence in venous thromboembolism patients. Chest. 1997;111:1603–1606. doi: 10.1378/chest.111.6.1603. [DOI] [PubMed] [Google Scholar]
- 98.Lindmarker P, Schulman S, Sten-Linder M, Wiman B, Egberg N, Johnsson H. The risk of recurrent venous thromboembolism in carriers and non-carriers of the G1691A allele in the coagulation factor V gene and the G20210A allele in the prothrombin gene. DURAC Trial Study Group. Duration of Anticoagulation. Thromb Haemost. 1999;81:684–689. [PubMed] [Google Scholar]
- 99.Luddington R, Jackson A, Pannerselvam S, Brown K, Baglin T. The factor V R2 allele: risk of venous thromboembolism, factor V levels and resistance to activated protein C. Thromb Haemost. 2000;83:204–208. [PubMed] [Google Scholar]
- 100.Mansilha A, Araújo F, Sampaio S, Cunha Ribeiro LM, Braga A. The PORtromb Project: prothrombin G20210A mutation and venous thromboembolism in young people. Cardiovasc Surg. 2002;10:45–48. doi: 10.1016/s0967-2109(00)00150-2. [DOI] [PubMed] [Google Scholar]
- 101.Margaglione M, Bossone A, Brancaccio V, Ciampa A, Di Minno G. Factor XIII Val34Leu polymorphism and risk of deep vein thrombosis. Thromb Haemost. 2000;84:1118–1119. [PubMed] [Google Scholar]
- 102.Margaglione M, Brancaccio V, De Lucia D, Martinelli I, Ciampa A, Grandone E, Di Minno G. Inherited thrombophilic risk factors and venous thromboembolism: distinct role in peripheral deep venous thrombosis and pulmonary embolism. Chest. 2000;118:1405–1411. doi: 10.1378/chest.118.5.1405. [DOI] [PubMed] [Google Scholar]
- 103.Margaglione M, D'Andrea G, d'Addedda M, Giuliani N, Cappucci G, Iannaccone L, Vecchione G, Grandone E, Brancaccio V, Di Minno G. The methylenetetrahydrofolate reductase TT677 genotype is associated with venous thrombosis independently of the coexistence of the FV Leiden and the prothrombin A20210 mutation. Thromb Haemost. 1998 May;79(5):907–11. [PubMed] [Google Scholar]
- 104.Meyer M, Kutscher G, Vogel G. The prothrombin nt20210 A allele as a risk factor for venous thromboembolism: detection of heterozygous and homozygous carriers by alternative methods. Thromb Res. 2000;97:359–363. doi: 10.1016/s0049-3848(99)00177-2. [DOI] [PubMed] [Google Scholar]
- 105.Morelli VM, Lourenço DM, D'Almeida V, Franco RF, Miranda F, Zago MA, Noguti MA, Cruz E, Kerbauy J. Hyperhomocysteinemia increases the risk of venous thrombosis independent of the C677T mutation of the methylenetetrahydrofolate reductase gene in selected Brazilian patients. Blood Coagul Fibrinolysis. 2002;13:271–275. doi: 10.1097/00001721-200204000-00014. [DOI] [PubMed] [Google Scholar]
- 106.Ocal IT, Sadeghi A, Press RD. Risk of venous thrombosis in carriers of a common mutation in the homocysteine regulatory enzyme methylenetetrahydrofolate reductase. Mol Diagn. 1997;2:61–68. doi: 10.1054/MODI00200061. [DOI] [PubMed] [Google Scholar]
- 107.Oger E, Lacut K, Le Gal G, Couturaud F, Abalain JH, Mercier B, Mottier D, EDITH (Etude des Déterminants/Interaction de la THrombose veineuse) Collaborative Study Group Interrelation of hyperhomocysteinemia and inherited risk factors for venous thromboembolism.Results from the E.D.I.TH. study: a hospital based case-control study. Thromb Res. 2007;120:207–214. doi: 10.1016/j.thromres.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 108.Poort SR, Rosendaal FR, Reitsma PH, Bertina RM. A common genetic variation in the 3′-untranslated region of the prothrombin gene is associated with elevated plasma prothrombin levels and an increase in venous thrombosis. Blood. 1996;88:3698–3703. [PubMed] [Google Scholar]
- 109.Quéré I, Chassé JF, Dupuy E, Bellet E, Molho-Sabatier P, Tobelem G, Janbon C. Homocysteine, 5,10- methylenetetrahydrofolate reductase and deep venous thrombosis. Survey of 120 patients in internal medicine. Rev Med Interne. 1998;19:29–33. doi: 10.1016/s0248-8663(97)83696-x. [DOI] [PubMed] [Google Scholar]
- 110.Quéré I, Perneger TV, Zittoun J, Bellet H, Gris JC, Daurès JP, Schved JF, Mercier E, Laroche JP, Dauzat M, Bounameaux H, Janbon C, de Moerloose P. Red blood cell methylfolate and plasma homocysteine as risk factors for venous thromboembolism: a matched case-control study. Lancet. 2002;359:747–752. doi: 10.1016/S0140-6736(02)07876-5. [DOI] [PubMed] [Google Scholar]
- 111.Ray JG, Langman LJ, Vermeulen MJ, Evrovski J, Yeo EL, Cole DE. Genetics University of Toronto Thrombophilia Study in Women (GUTTSI): genetic and other risk factors for venous thromboembolism in women. Curr Control Trials Cardiovasc Med. 2001;2:141–149. doi: 10.1186/cvm-2-3-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Renner W, Köppel H, Hoffmann C, Schallmoser K, Stanger O, Toplak H, Wascher TC, Pilger E. Prothrombin G20210A, factor V Leiden, and factor XIII Val34Leu: common mutations of blood coagulation factors and deep vein thrombosis in Austria. Thromb Res. 2000;99:35–39. doi: 10.1016/s0049-3848(00)00219-x. [DOI] [PubMed] [Google Scholar]
- 113.Renner W, Winkler M, Hoffmann C, Köppel H, Seinost G, Brodmann M, Pilger E. The PlA1/A2 polymorphism of platelet glycoprotein IIIa is not associated with deep venous thrombosis. Int Angiol. 2001 Jun;20(2):148–51. [PubMed] [Google Scholar]
- 114.Reuner KH, Ruf A, Litfin F, Patscheke H. The mutation G20210-->A in the prothrombin gene is a strong risk factor for pulmonary embolism. Clin Chem. 1998;44:1365–1366. [PubMed] [Google Scholar]
- 115.Ruiz-Argüelles GJ, Garcés-Eisele J, Reyes-Núñez V, Ramírez-Cisneros FJ, et al. Primary thrombophilia in Mexico. II. Factor V G1691A (Leiden), prothrombin G20210A, and methylenetetrahydrofolate reductase C677T polymorphism in thrombophilic Mexican mestizos. Am J Hematol. 2001;66:28–31. doi: 10.1002/1096-8652(200101)66:1<28::AID-AJH1003>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 116.Salazar-Sánchez L, Leon MP, Cartin M, Schuster G, Wulff K, Schröder W, Jiménez-Arce G, Chacon R, Herrmann FH. The FXIIIVal34Leu, common and risk factors of venous thrombosis in early middle-age Costa Rican patients. Cell Biochem Funct. 2007;25:739–745. doi: 10.1002/cbf.1389. [DOI] [PubMed] [Google Scholar]
- 117.Salden A, Keeney S, Hay CR, Cumming AM. The C677T MTHFR variant and the risk of venous thrombosis. Br J Haematol. 1997;99:472. [PubMed] [Google Scholar]
- 118.Salomon O, Rosenberg N, Zivelin A, Steinberg DM, Kornbrot N, Dardik R, Inbal A, Seligsohn U. Methionine synthase A2756G and methylenetetrahydrofolate reductase A1298C polymorphisms are not risk factors for idiopathic venous thromboembolism. Hematol J. 2001;2:38–41. doi: 10.1038/sj.thj.6200078. [DOI] [PubMed] [Google Scholar]
- 119.Salomon O, Steinberg DM, Zivelin A, Gitel S, Dardik R, Rosenberg N, Berliner S, Inbal A, Many A, Lubetsky A, Varon D, Martinowitz U, Seligsohn U. Single and combined prothrombotic factors in patients with idiopathic venous thromboembolism: prevalence and risk assessment. Arterioscler Thromb Vasc Biol. 1999;19:511–518. doi: 10.1161/01.atv.19.3.511. [DOI] [PubMed] [Google Scholar]
- 120.Schobess R, Junker R, Auberger K, Münchow N, Burdach S, Nowak-Göttl U. Factor V G1691A and prothrombin G20210A in childhood spontaneous venous thrombosis- evidence of an age-dependent thrombotic onset in carriers of factor V G1691A and prothrombin G20210A mutation. Eur J Pediatr. 1999;158(Suppl 3):S105–108. doi: 10.1007/pl00014335. [DOI] [PubMed] [Google Scholar]
- 121.Seinost G, Renner W, Brodmann M, Winkler M, Köppel H, Pilger E. C677T mutation in the methylene tetrahydrofolate reductase gene as a risk factor for venous thrombotic disease in Austrian patients. Thromb Res. 2000;100:405–407. doi: 10.1016/s0049-3848(00)00341-8. [DOI] [PubMed] [Google Scholar]
- 122.Souto JC, Coll I, Llobet D, del Río E, Oliver A, Mateo J, Borrell M, Fontcuberta J. The prothrombin 20210A allele is the most prevalent genetic risk factor for venous thromboembolism in the Spanish population. Thromb Haemost. 1998;80:366–369. [PubMed] [Google Scholar]
- 123.Tosetto A, Missiaglia E, Frezzato M, Rodeghiero F. The VITA project: C677T mutation in the methylene tetrahydrofolate reductase gene and risk of venous thromboembolism. Br J Haematol. 1997;97:804–806. doi: 10.1046/j.1365-2141.1997.1422957.x. [DOI] [PubMed] [Google Scholar]
- 124.Varela ML, Adamczuk YP, Forastiero RR, Martinuzzo ME, Cerrato GS, Pombo G, Carreras LO. Major and potential prothrombotic genotypes in a cohort of patients with venous thromboembolism. Thromb Res. 2001;104:317–324. doi: 10.1016/s0049-3848(01)00384-x. [DOI] [PubMed] [Google Scholar]
- 125.Vargas M, Soto I, Pinto CR, Urgelles MF, Batalla A, Rodriguez-Reguero J, Cortina A, Alvarez V, Coto E. The prothrombin 20210A allele and the factor V Leiden are associated with venous thrombosis but not with early coronary artery disease. Blood Coagul Fibrinolysis. 1999 Jan;10(1):39–41. doi: 10.1097/00001721-199901000-00006. [DOI] [PubMed] [Google Scholar]
- 126.von Depka M, Czwalinna A, Wermes C, Eisert R, Scharrer I, Ganser A, Ehrenforth S. The deletion polymorphism in the angiotensin-converting enzyme gene is a moderate risk factor for venous thromboembolism. [PubMed] [Google Scholar]
- 127.von Depka M, Nowak-Göttl U, Eisert R, Dieterich C, Barthels M, Scharrer I, Ganser A, Ehrenforth S. Increased lipoprotein (a) levels as an independent risk factor for venous thromboembolism. Blood. 2000;96:3364–3368. [PubMed] [Google Scholar]
- 128.Wells PS, Rodger MA, Forgie MA, Langlois NJ, Armstrong L, Carson NL, Jaffey J. The ACE D/D genotype is protective against the development of idiopathic deep vein thrombosis and pulmonary embolism. Thromb Haemost. 2003;90:829–834. doi: 10.1160/TH03-03-0170. [DOI] [PubMed] [Google Scholar]
- 129.Zalavras ChG, Giotopoulou S, Dokou E, Mitsis M, Ioannou HV, Tzolou A, Kolaitis N, Vartholomatos G. Lack of association between the C677T mutation in the 5,10-methylenetetrahydrofolate reductase gene and venous thromboembolism in Northwestern Greece. Int Angiol. 2002;21:268–271. [PubMed] [Google Scholar]
- 130.De Stefano V, Rossi E, Paciaroni K, Leone G. Screening for inherited thrombophilia: indications and therapeutic implications. Haematologica. 2002;87:1095–108. [PubMed] [Google Scholar]
- 131.De Stefano V, Chiusolo P, Paciaroni K, Leone G. Epidemiology of factor V Leiden: clinical implications. Sem Thromb Haemost. 1998;24:367–79. doi: 10.1055/s-2007-996025. [DOI] [PubMed] [Google Scholar]
- 132.Rosendaal FR, Doggen CJM, Zivelin A, Arruda VR, Aiach M, Siscovick DS, Hillarp A, Watzke HH, Bernardi F, Cumming AM, Preston FE, Reitsma PH. Geographic distribution of the 20210 G to A prothrombin variant. Thromb Haemost. 1998;79:706–8. [PubMed] [Google Scholar]
- 133.De Stefano V, Casorelli I, Rossi E, Zappacosta B, Leone G. Interaction between hyperhomocysteinemia and inherited thrombophilic factors in venous thromboembolism. Sem Thromb Haemost. 2000;26:305–11. doi: 10.1055/s-2000-8473. [DOI] [PubMed] [Google Scholar]
- 134.Bezemer ID, Doggen CJ, Vos HL, Rosendaal FR. No association between the common MTHFR 677C >T polymorphism and venous thrombosis: results from the MEGA study. Arch Intern Med. 2007;167:497–501. doi: 10.1001/archinte.167.5.497. [DOI] [PubMed] [Google Scholar]
- 135.Zöller B, Li X, Sundquist J, et al. Parental history and venous thromboembolism: a nationwide study of age- specific and sex-specific familial risks in Sweden. J Thromb Haemost. 2011;9:64–70. doi: 10.1111/j.1538-7836.2010.04107.x. [DOI] [PubMed] [Google Scholar]
- 136.Sørensen HT, Riis AH, Diaz LJ, et al. Familial risk of venous thromboembolism: a nationwide cohort study. J Thromb Haemost. 2011;9:320–4. doi: 10.1111/j.1538-7836.2010.04129.x. [DOI] [PubMed] [Google Scholar]
- 137.Heit JA, Silverstein MD, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ. Risk factors for deep vein thrombosis and pulmonary embolism. A population-based case-control study. Arch Intern Med. 2000;160:809–15. doi: 10.1001/archinte.160.6.809. [DOI] [PubMed] [Google Scholar]
- 138.Heit JA O'Fallon WM, Petterson TM, Lohse CM, Silverstein MD, Mohr DN, Melton LJ. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism. A population-based case-control study. Arch Intern Med. 2002;162:1245–8. doi: 10.1001/archinte.162.11.1245. [DOI] [PubMed] [Google Scholar]
- 139.Cushman M, Tsai AW, White RH, Heckbert SR, Rosamond WD, Enright P, Folsom AR. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117:19–25. doi: 10.1016/j.amjmed.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 140.Bates SM. Pregnancy-associated venous thromboembolism: prevention and treatment. Semin Hematol. 2011;48:271–284. doi: 10.1053/j.seminhematol.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 141.van Hylckama Vlieg A, Middeldorp S. Hormone therapies and venous thromboembolism: where are we now? J Thromb Haemostasis. 2011;9:257–66. doi: 10.1111/j.1538-7836.2010.04148.x. [DOI] [PubMed] [Google Scholar]
- 142.Wu O, Robertson L, Langhorne P, Twaddle S, Lowe GD, Clark P, Greaves M, Walker ID, Brenkel I, Regan L, Greer IA. Oral contraceptives, hormone replacement therapy, thrombophilias and risk of venous thromboembolism: a systematic review. The Thrombosis: Risk and Economic Assessment of Thrombophilia Screening (TREATS) Study. Thromb Haemost. 2005;94:17–25. doi: 10.1160/TH04-11-0759. [DOI] [PubMed] [Google Scholar]
- 143.Mohllajee AP, Curtis KM, Martins SL, Peterson HB. Does use of hormonal contraceptives among women with thrombogenic mutations increase their risk of venous thromboembolism? A systematic review. Contraception. 2006;73:166–78. doi: 10.1016/j.contraception.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 144.Martinelli I, Battaglioli T, De Stefano V, Tormene D, Valdrè L, Grandone E, Tosetto A, Mannucci PM. GIT (Gruppo Italiano Trombofilia). The risk of first venous thromboembolism during pregnancy and puerperium in double heterozygotes for factor V Leiden and prothrombin G20210A. J Thromb Haemost. 2008;6:494–8. doi: 10.1111/j.1538-7836.2007.02880.x. [DOI] [PubMed] [Google Scholar]
- 145.Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group Recommendations from the EGAPP Working Group: routine testing for Factor V Leiden (R506Q) and prothrombin (20210G>A) mutations in adults with a history of idiopathic venous thromboembolism and their adult family members. Genet Med. 2011;13:67–76. doi: 10.1097/GIM.0b013e3181fbe46f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


