Abstract
Objective:
The study aims to determine whether apparent diffusion coefficient (ADC) can help differentiate benign and malignant bone tumors.
Materials and Methods:
From January 2012 to February 2013, we prospectively included 26 patients. Of these 15 patients were male and 11 were female; ranging in age from 8 to 76 years (mean age, 34.5 years). Diffusion-weighted magnetic resonance (MR) imaging was obtained with a single-shot echo-planar imaging sequence using a 1.5T MR scanner. We grouped malignant lesions as primary, secondary, and primary tumor with chondroid matrix. The minimum ADC was measured in the tumors and mean minimum ADC values were selected for statistical analysis. ADC values were compared between malignant and benign tumors using the Mann-Whitney U-test and receiver operating curve analysis were done to determine optimal cut-off values.
Results:
The mean ADC values from the area with lowest ADC values of benign and malignant tumors were 1.99 ± 0.57 × 10−3 mm2/s and 1.02 ± 1.0 × 10−3 mm2/s, respectively. The mean minimum ADC values of benign and malignant tumors were statistically different (P = 0.029). With cut-off value of 1.37 (10−3 mm2/s), sensitivity was 77.8% and specificity was 82.4%, for distinguishing benign and malignant lesion. Benign and secondary malignant tumors showed statistically significant difference (P = 0.002). There was some overlap in ADC values between benign and malignant tumors. The mean minimum ADC values of benign and malignant chondroid tumors were high. Giant cell tumor, non-ossifying fibroma and fibrous dysplasia showed lower ADC values.
Conclusion:
Although there is some overlap, ADC values of benign and malignant bone tumors seem to be different. Further studies with larger patient groups are needed to find an optimal cut-off ADC value.
Keywords: Bone, diffusion-weighted magnetic resonance imaging, neoplasm
INTRODUCTION

Diffusion-weighted (DW) magnetic resonance imaging (MRI) provides information about the mobility of water molecules.[1] The different tissue contrast obtained using DW imaging makes it a useful tool for identifying benign and malignant lesions in the body.[2]
Most bone tumors have classical radiographic appearance and they can be diagnosed and correlated with patient age and clinical data. MRI can detect non-mineralized tumor tissue, evaluate the local extent of a malignant process for the purpose of staging and assess bone tumor therapeutic responses.[3] However, lesions that have high T2 signal and low enhancement constitute diagnostic challenge in daily practice.[4] In addition, a few benign and malignant tumors show atypical features and need further investigation. Some benign lesions in patients with known primary malignancies also constitute a diagnostic problem. DW MRI has been applied to evaluate certain musculoskeletal tumors and has been reported to be a useful diagnostic aide.[4,5] In this study, we aim to determine whether apparent diffusion coefficient (ADC) values can help differentiate benign and malignant bone tumors.
MATERIALS AND METHODS
Patients
The study was approved by a regional ethics committee and all participants provided written informed consent.
From January 2012 to February 2013 we prospectively included 26 patients (15 male and 11 female; ranging in age from 8 to 76 years (mean age, 34.5 years) with bone neoplasm in this study. None of the patients had biopsy, surgery, or any treatment before imaging. The diagnosis of all masses was confirmed by biopsy and/or surgery after MRI. Of the 26 lesions, six were located in the distal femur, four were in the proximal femur, three were in the proximal tibia, three were in the distal tibia, two were in the humerus, one was in the proximal fibula, one was in the proximal ulna, one was in the calcaneus, one was in the phalanx, one was in the sacrum, and three were in the pelvic bones.
Magnetic resonance imaging
All studies were conducted using a 1.5-T system (Achieva, Philips, Best, The Netherlands). All images were obtained with a flexible circular surface coil, flex phased-array coil or body coil. MRI protocol includes the following sequences: Axial, coronal, and/or sagittal T1-weighted, fat-suppressed T2-weighted, diffusion weighted images, dynamic MRI, and contrast-enhanced fat-suppressed axial, coronal, and/or sagittal T1-weighted images.
All DW images were obtained before contrast administration. The pulse sequence used for obtaining the DW images was a single-shot echo-planar imaging technique with the following parameters: Repetition time: 4500 ms, echo time: 105 ms, directions of the motion-probing gradients: Three orthogonal axes, b value: 0 and 1000 s/mm2, field of view: 220 mm, matrix size: 128 × 80, section thickness: 5 mm with 0.2 mm intersection gaps and two signals acquired. Parallel imaging techniques, sensitivity encoding with a reduction factor of 1-1.5 were used. In all images a fat-saturated pulse was used to exclude chemical-shift artifacts. The DW images were obtained within an acquisition time of 1-2 min.
ADC maps were automatically generated on the operating console from concurrent images. The ADC values were calculated by using the following equation: ADC = −ln (S[b]/S[0])/b, where b indicates the b value and S (b) and S (0) are the signal intensities of images with b values equal to 1000 and 0, respectively.
Image analysis
Images were transferred to a workstation (Easy Vision; Philips Medical Systems). The measurements were performed by two radiologists, who did not have any knowledge of tumor type, patient clinical data, and of prior images.
We performed all measurements in the axial plane. The most solid and/or homogeneous portion of the lesion according to T2-weighted and contrast-enhanced images were selected for measurement. A region of interest (ROI) was placed around the margin of the suspicious area and the mean minimum ADC values were obtained. When heterogeneity in signal intensity was observed, multiple small, at least three, uniform round or oval ROIs (area, minimum 10 mm2, maximum 55 mm2) were placed on the ADC map including the areas of enhancing tumor with the lowest ADC determined by visual inspection. The ROIs position was always checked with reference to conventional MRI to avoid contamination from different adjacent tissues. The mean ADC values from the area with lowest ADC values were selected for statistical analyses.
ADC of normal fatty bone marrow was 0 × 10− 3 mm2/s because of the fat suppression technique used for DW imaging. However, it was above 0 in hematopoietic bone marrow. Normal bone cortex ADC values were 0 × 10− 3 mm2/s. We avoided selecting voxels with ADC values of almost 0, which corresponded to the fatty marrow, the cortex and tumor calcification-ossification.
Statistical analysis
Data analyses were performed using statistical software (SPSS 15.0 for windows; SPSS Inc., Chicago, IL, USA). For statistical analysis, we grouped lesions as benign and malignant tumors and subdivided malignant tumors as primary malignant tumors, malignant tumors with chondroid matrix and secondary malignant tumors according to the data obtained from previous studies.[4,5] The mean minimum ADC values were compared between malignant and benign tumors using the Mann-Whitney U-test. The mean minimum ADC values of malignant lesions (primary malignant tumors, malignant tumors with chondroid matrix and secondary malignant tumors) were compared using the Kruskal-Wallis analysis. A difference with P < 0.05 was considered to be significant. We did a receiver operating curve (ROC) analysis to determine optimal cut-off mean minimum ADC value for distinguishing benign from a malignant tumor. Inter-observer agreement for the ADC measurements was analyzed with the method of Bland and Altman and the intraclass correlation coefficient (ICC) regarding the measurements were calculated. ICC > 0.75 was considered as a good agreement.
RESULTS
The ICCs for inter-observer agreement about the measurements were 0.83 (95% confidence interval: 0.78, 0.84). The variability between ADC measurements was larger by using single ROI for measurement than using multiple small ROIs.
The histologic diagnosis, male-female distribution, patient age and tumor ADC values are presented in Table 1.
Table 1.
List of the bone tumors and mean minimum ADC values
The mean minimum ADC values of benign and malignant tumors were 1.99 ± 0.57 × 10− 3 mm2/s and 1.02 ± 1.0 × 10− 3 mm2/s (mean ± standard deviation), respectively. The mean minimum ADC values of benign and malignant tumors were statistically different (P = 0.029). The three bone cysts in our study group were complicated with fracture. According to ROC analyses, for distinguishing benign from malignant lesions, the cut-off mean minimum ADC value was 1.37 (×10− 3 mm2/s). Sensitivity and specificity for differentiation were 77.8% and 82.4%, respectively (area under the curve: 0.765) [Figure 1].
Figure 1.
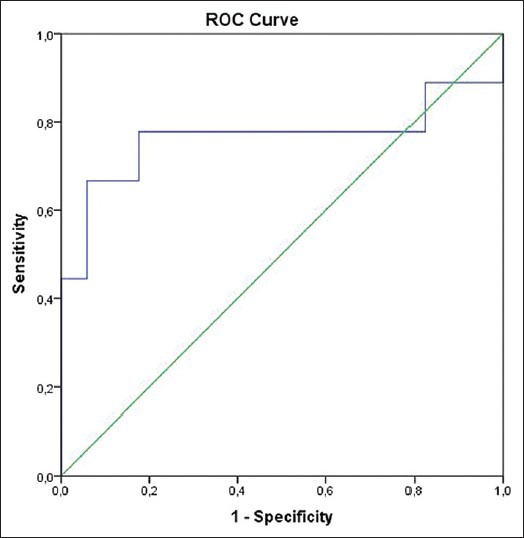
Receiver operating curve (ROC) curve of mean minimum apparent diffusion coefficient value for differentiation malignant and benign bone tumors. The area under the ROC curve is 0.765 (95% confidence interval: 0.518-1.000).
There was some overlap in ADC values between benign and malignant tumors. The mean ADC values of benign and malignant tumors with chondroid matrix were high. Giant cell tumor, non-ossifying fibroma and fibrous dysplasia had lower ADC values.
DISCUSSION
Our preliminary results indicate that although there is some overlap, ADC values of benign and malignant bone tumors seem to be different. However, further studies with larger patient groups are needed to demonstrate the role of DW MRI in differentiating benign and malignant tumors and to find an optimal cut-off ADC value. Contrast between different tissues seen on DW MRI makes this imaging modality a useful tool for identifying bone lesions. It is known that the whole body DW MRI detection capability is superior to positron emission tomography and scintigraphy.[6] DW imaging has also been used in monitoring therapeutic response and a higher ADC values after treatment were found be related to good response.[7]
Differentiating benign and malignant tumors in the musculoskeletal system may be possible with DW imaging. Increased ADC values represent an increase in extracellular water or loss of the cell membrane integrity, whereas decreased ADC values reflect the decrease in extracellular water content or increase in cell number or size.[2] Malignant tumors tend to have lower ADC values and benign tumors tend to have higher ADC values, but there are some exceptions. Threshold ADC values for brain tumors, breast tumors, etc., have been reported but to the best of our knowledge there are no defined cut-off ADC values for bone tumors.[8,9]
We used DW MRI and found that ADC values of benign and malignant tumors might be different. Bone cyst and aneurysmal bone cyst were the benign bone tumors that had the highest ADC values in our study like a previous study by Hayashida et al.[4] [Figure 2a–e]. All the three patients with bone cysts included in our study had old fractures that made diagnosis difficult with radiography and conventional MRI. However, they were hyperintense on DW imaging and ADC map and had high ADC values. Osteoblastoma and benign tumors that have chondroid matrix (chondroblastoma and enchondroma) had higher ADC values than fibrous dysplasia and non-ossifying fibroma. Lower ADC values in fibrous dysplasia and non-ossifying fibroma might be related to their content that restricted the diffusion.[5] Giant cell tumor in our study also had low ADC values. Nagata et al.,[10] reported low ADC values in giant cell tumors of soft-tissue. This is probably due to its histologic features, spindle-shaped stromal cells and multinucleated giant cells.[11] These low ADC values and high signal in DW imaging could be used to differentiate tumor residue or recurrence from post-operative findings.[12]
Figure 2.
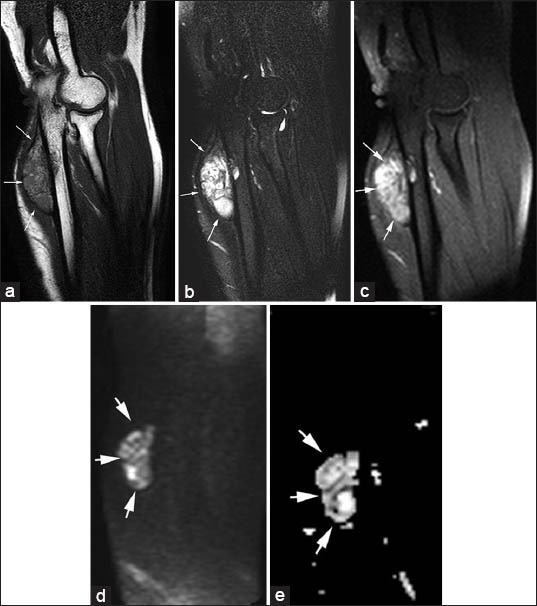
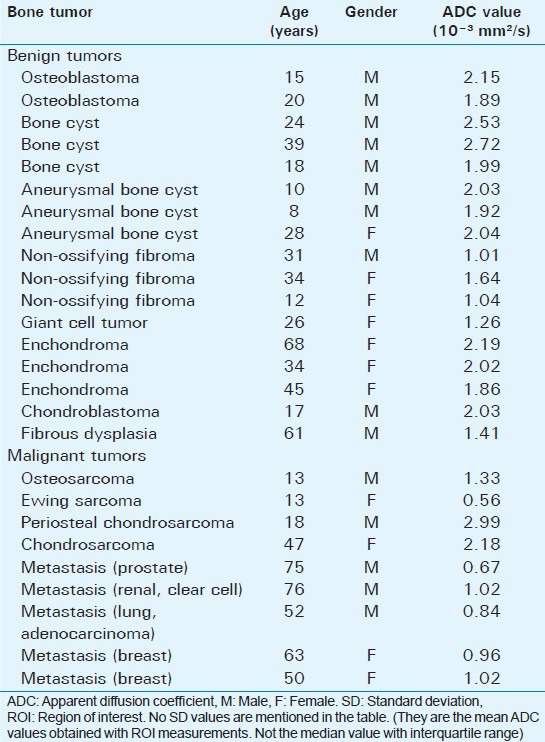
(a-e) A 28-year-old female with aneurysmal bone cysts. (arrows). (a) Sagittal T1-weighted image demonstrates well-marginated, expansive, eccentrically located lesion in the ulna that is hyperintense to muscle. (b) Fat-suppressed T2-weighted and (c) Post-contrast fat-suppressed T1-weighted images show septated hyperintense mass with heterogenous enhancement. (d) On diffusion-weighted image (b 1000) and (e) Apparent diffusion coefficient. (ADC) map show lesion is hyperintense. Mean minimum ADC value was 2.04 × 10-3 mm2/s.
Malignant chondroid tumors had the highest ADC values among malignant tumors. Hayashida et al.,[4] found significantly higher ADC values in chondroblastic osteosarcomas when compared with other types of osteosarcoma. However, they did not find any statistically significant difference between chondroblastic osteosarcoma and chondrosarcoma. The ADC values of malignant chondroid tumors were higher than benign tumors in our study. Nagata et al.,[13] also reported similar findings. Hence, tumors that have chondroid matrix should be a different group and ADC values between benign and malignant chondroid tumors should be compared in further studies with more patients.
Ewing sarcoma was the tumor that showed the lowest ADC value in our study [Figure 3a–e]. Ewing sarcoma is a member of small round blue cell tumors, undifferentiated aggressive embryonal tumors, which have similar histological features.[14] Our findings were similar with previous studies.[15,16] Small round blue cell tumors should be in the differential diagnosis of the tumors with very low ADC values.
Figure 3.
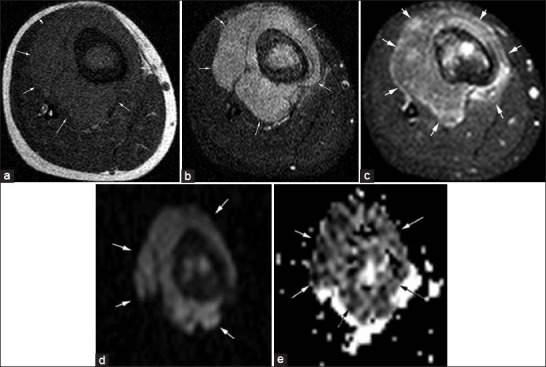
(a-e) 13-year-old female with Ewing sarcoma (arrows). (a) Axial T1-weighted image shows hypointense mass with prominent soft-tissue component in the proximal tibia. (b) Fat-suppressed T2-weighted and (c) Post-contrast fat-suppressed T1-weighted images demonstrate hyperintense mass with diffuse enhancement. (d) On diffusion-weighted image. (b1000) the mass was hyperintense and (e) On apparent diffusion coefficient. (ADC) map the mass was very hypointense. Mean minimum ADC value was 0.56 × 10-3 mm2/s.
The ADC values between benign and secondary malignant tumors were significantly different in our study. Differentiation benign tumors and bone metastases, especially solitary bone metastases, might be difficult in patients with a known primary tumor[17] [Figure 4a–e]. We think DW imaging could be used as a problem solving tool in these patients.
Figure 4.
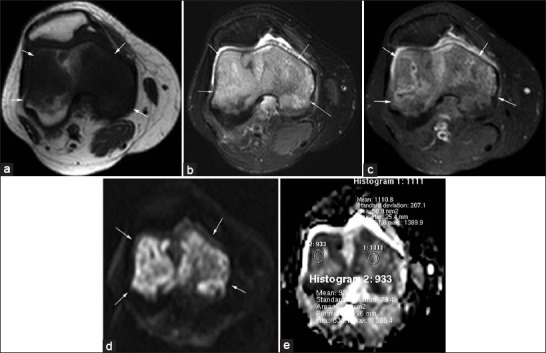
(a-e) 50-year-old female with breast cancer and bone metastasis (arrows). (a) Axial T1-weighted image shows poorly marginated hypointense mass in the distal femur. (b) Fat-suppressed T2-weighted and (c) Post-contrast fat-suppressed T1-weighted images demonstrate hyperintense mass with diffuse heterogeneous enhancement. (d) On diffusion-weighted image (b1000) the mass was hyperintense and (e) On apparent diffusion coefficient (ADC) map the lesion was hypointense. Five small region of interests were placed (area, 50-55 mm2). Mean minimum ADC value was 1.02 × 10-3 mm2/s.
Although, there was some overlap, mean ADC values of the benign tumors were always found to be above 1 in our study. A recent study demonstrated that the mean ADC value below 1.03 × 10− 3 mm2/s was a strong indicator of malignancy in pediatric patients.[18]
Limitations
There are several limitations to our study. The number of patients, especially cases of malignant tumors was limited in this study. In light of the small number of patients, further studies will have to be conducted to show the relevance of these conclusions. We did histogram analysis and used mean minimum ADC values. We did not calculate perfusion effects. The two most important components of signal attenuation on DW imaging are diffusion of water molecules in the extracellular space and perfusion.[15] Perfusion fractions of malignant tumors are higher than that of the benign tumor and perfusion contributes more to ADC values in malignant lesions. However, by increasing the diffusion gradient strength the contribution of the perfusion effect is considerably reduced. We used high b values (b 1000) to reduce perfusion effects.
CONCLUSION
In conclusion, our preliminary results indicate that although there is some overlap, ADC values of benign and malignant bone tumors seem to be different. Adding DW imaging to routine tumor MRI protocol would improve our diagnostic confidence without increasing study time.
Footnotes
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2013/3/1/63/124094
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: Application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161:401–7. doi: 10.1148/radiology.161.2.3763909. [DOI] [PubMed] [Google Scholar]
- 2.Bley TA, Wieben O, Uhl M. Diffusion-weighted MR imaging in musculoskeletal radiology: Applications in trauma, tumors, and inflammation. Magn Reson Imaging Clin N Am. 2009;17:263–75. doi: 10.1016/j.mric.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Baur A, Stäbler A, Brüning R, Bartl R, Krödel A, Reiser M, et al. Diffusion-weighted MR imaging of bone marrow: Differentiation of benign versus pathologic compression fractures. Radiology. 1998;207:349–56. doi: 10.1148/radiology.207.2.9577479. [DOI] [PubMed] [Google Scholar]
- 4.Hayashida Y, Hirai T, Yakushiji T, Katahira K, Shimomura O, Imuta M, et al. Evaluation of diffusion-weighted imaging for the differential diagnosis of poorly contrast-enhanced and T2-prolonged bone masses: Initial experience. J Magn Reson Imaging. 2006;23:377–82. doi: 10.1002/jmri.20512. [DOI] [PubMed] [Google Scholar]
- 5.Yakushiji T, Oka K, Sato H, Yorimitsu S, Fujimoto T, Yamashita Y, et al. Characterization of chondroblastic osteosarcoma: Gadolinium-enhanced versus diffusion-weighted MR imaging. J Magn Reson Imaging. 2009;29:895–900. doi: 10.1002/jmri.21703. [DOI] [PubMed] [Google Scholar]
- 6.Goudarzi B, Kishimoto R, Komatsu S, Ishikawa H, Yoshikawa K, Kandatsu S, et al. Detection of bone metastases using diffusion weighted magnetic resonance imaging: Comparison with (11) C-methionine PET and bone scintigraphy. Magn Reson Imaging. 2010;28:372–9. doi: 10.1016/j.mri.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Hayashida Y, Yakushiji T, Awai K, Katahira K, Nakayama Y, Shimomura O, et al. Monitoring therapeutic responses of primary bone tumors by diffusion-weighted image: Initial results. Eur Radiol. 2006;16:2637–43. doi: 10.1007/s00330-006-0342-y. [DOI] [PubMed] [Google Scholar]
- 8.Yamasaki F, Kurisu K, Satoh K, Arita K, Sugiyama K, Ohtaki M, et al. Apparent diffusion coefficient of human brain tumors at MR imaging. Radiology. 2005;235:985–91. doi: 10.1148/radiol.2353031338. [DOI] [PubMed] [Google Scholar]
- 9.Noguchi K, Watanabe N, Nagayoshi T, Kanazawa T, Toyoshima S, Shimizu M, et al. Role of diffusion-weighted echo-planar MRI in distinguishing between brain brain abscess and tumour: A preliminary report. Neuroradiology. 1999;41:171–4. doi: 10.1007/s002340050726. [DOI] [PubMed] [Google Scholar]
- 10.Nagata S, Nishimura H, Uchida M, Sakoda J, Tonan T, Hiraoka K, et al. Diffusion-weighted imaging of soft tissue tumors: Usefulness of the apparent diffusion coefficient for differential diagnosis. Radiat Med. 2008;26:287–95. doi: 10.1007/s11604-008-0229-8. [DOI] [PubMed] [Google Scholar]
- 11.Goldenberg RR, Campbell CJ, Bonfiglio M. Giant-cell tumor of bone. An analysis of two hundred and eighteen cases. J Bone Joint Surg Am. 1970;52:619–64. [PubMed] [Google Scholar]
- 12.Costa FM, Ferreira EC, Vianna EM. Diffusion-weighted magnetic resonance imaging for the evaluation of musculoskeletal tumors. Magn Reson Imaging Clin N Am. 2011;19:159–80. doi: 10.1016/j.mric.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Nagata S, Nishimura H, Uchida M, Hayabuchi N. Usefulness of diffusion-weighted MRI in differentiating benign from malignant musculoskeletal tumors. Nihon Igaku Hoshasen Gakkai Zasshi. 2005;65:30–6. [PubMed] [Google Scholar]
- 14.Reichardt W, Juettner E, Uhl M, Elverfeldt DV, Kontny U. Diffusion-weighted imaging as predictor of therapy response in an animal model of Ewing sarcoma. Invest Radiol. 2009;44:298–303. doi: 10.1097/RLI.0b013e31819dcc84. [DOI] [PubMed] [Google Scholar]
- 15.Baur A, Reiser MF. Diffusion-weighted imaging of the musculoskeletal system in humans. Skeletal Radiol. 2000;29:555–62. doi: 10.1007/s002560000243. [DOI] [PubMed] [Google Scholar]
- 16.Garrett KM, Kim HK, Stanek J, Emery KH. MR findings of primary bone lymphoma in a 15-year-old girl: Emphasis on diffusion-weighted imaging. Pediatr Radiol. 2011;41:658–62. doi: 10.1007/s00247-010-1893-2. [DOI] [PubMed] [Google Scholar]
- 17.Su MG, Tian R, Fan QP, Tian Y, Li FL, Li L, et al. Recognition of fibrous dysplasia of bone mimicking skeletal metastasis on 18F-FDG PET/CT imaging. Skeletal Radiol. 2011;40:295–302. doi: 10.1007/s00256-010-0999-9. [DOI] [PubMed] [Google Scholar]
- 18.Neubauer H, Evangelista L, Hassold N, Winkler B, Schlegel PG, Köstler H, et al. Diffusion-weighted MRI for detection and differentiation of musculoskeletal tumorous and tumor-like lesions in pediatric patients. World J Pediatr. 2012;8:342–9. doi: 10.1007/s12519-012-0379-8. [DOI] [PubMed] [Google Scholar]


