Abstract
Estragole is a volatile terpenoid, which occurs naturally as a constituent of the essential oils of many plants. It has several pharmacological and biological activities. The objective of the present study was to investigate the mechanism of action of estragole on neuronal excitability. Intact and dissociated dorsal root ganglion neurons of rats were used to record action potential and Na+ currents with intracellular and patch-clamp techniques, respectively. Estragole blocked the generation of action potentials in cells with or without inflexions on their descendant (repolarization) phase (Ninf and N0 neurons, respectively) in a concentration-dependent manner. The resting potentials and input resistances of Ninf and N0 cells were not altered by estragole (2, 4, and 6 mM). Estragole also inhibited total Na+ current and tetrodotoxin-resistant Na+ current in a concentration-dependent manner (IC50 of 3.2 and 3.6 mM, respectively). Kinetic analysis of Na+ current in the presence of 4 mM estragole showed a statistically significant reduction of fast and slow inactivation time constants, indicating an acceleration of the inactivation process. These data demonstrate that estragole blocks neuronal excitability by direct inhibition of Na+ channel conductance activation. This action of estragole is likely to be relevant to the understanding of the mechanisms of several pharmacological effects of this substance.
Keywords: Estragole, Local anesthetic, Essential oil, Dorsal root ganglia, Phenylpropanoid, Na+ current
Introduction
Estragole is a constituent of essential oils of many plants such as Ravensara anisata, Ocimum basilicum, and Croton zehntneri. These plants are widely used in folk medicine and popular cooking (1,2). Their essential oils are extensively employed in aromatherapy (3). Estragole is also used as a flavoring agent in pharmaceutical, cosmetic, and food industries (4,5) and as an antimicrobial for food preservation (6).
Estragole has many biological effects, including antioxidant and antimicrobial activities (7,8). Its pharmacological activities are reported to be anxiolytic (9), to induce contraction of skeletal muscle (10), and to relax ileal and other vascular smooth muscles (11,12). In addition to these activities, estragole has been shown to have an anti-inflammatory activity (13), which is pharmacologically potent (3-30 mg/kg per os) and is effective at oral doses smaller than those considered toxic (LD50 values of 1200 and 1800 mg/kg for rats and 1250 mg/kg for mice) (14). In this connection it is interesting that its analogues, anethole and eugenol, block the effects of tumor necrosis factor-α, which is a well-known proinflammatory cytokine (15).
Using an extracellular recording technique, we demonstrated that estragole blocks the excitability of rat sciatic nerve (16). However, the mechanism underlying this effect is unknown, and understanding how estragole affects excitable membranes may explain the mechanism of action of other pharmacological activities induced by this essential oil constituent.
Estragole is a phenylpropanoid, and some substances of this class are local anesthetics: they block excitability predominantly through direct inhibitory effects on Na+ channel conductance activation (17-20). For this reason, we hypothesized that estragole blocks excitability through a local anesthetic-like activity. To test this hypothesis we examined action potential (AP) generation and inward Na+ currents in intact and isolated neurons derived from dorsal root ganglia (DRG). Our results revealed that estragole blocks the generation of AP in DRG neurons by a direct inhibition of voltage-gated activation of Na+ channel conductance. Thus, estragole appears to possess potent local anesthetic activity.
Material and Methods
Animals, tissue dissection, and cell preparation
Wistar rats (250-350 g) of both sexes were used throughout the study. They were maintained at constant temperature (22±2°C) with a 12:12-h light-dark cycle and free access to food and water. All animals were cared for in compliance with the Guide for the Care and Use of Laboratory Animals, published by the United States National Institutes of Health (NIH Publication 27-89, revised 1996). All procedures were approved by the animal Ethics Committee of Universidade Estadual do Ceará (CEUA-UECE process #06379067-0).
After rats were killed by CO2 inhalation, DRG were dissected and immediately placed in an ice-cold modified Locke's solution. For intracellular recordings, intact tissues were used on the same day of dissection. For patch-clamp recording, dissociated DRG neurons were prepared as described previously (21). Briefly, DRG were placed in Ca2+- and Mg2+-free Hanks' balanced salt solutions, then placed in a dissociation solution, which consisted of 1.0 mg/mL collagenase type I, for 75 min and in 2.5 mg/mL trypsin for 15 min, both in Hanks' balanced salt solutions at 37°C. After they were incubated in the dissociation solutions, the cells were freed from the tissue by gently titrating the DRG using a fire-polished Pasteur pipette and transferred to Dulbecco's modified Eagle's medium containing 10% fetal bovine serum, 100 U/mL streptomycin, and 0.1 mg/mL penicillin, and then plated onto coverslips coated with 0.01% poly-D-lysine. The neurons were incubated in a 5% CO2 atmosphere at 37°C and used within 6-48 h. All electrophysiological experiments were performed at room temperature (∼22°C).
Solutions and drugs
The composition of modified Locke's solution was: 140 mM NaCl, 5.6 mM KCl, 1.2 mM MgCl2, 2.2 mM CaCl2, 10 mM glucose, and 10 mM Tris-(hydroxymethyl-aminomethane). Hanks' balanced salt solution used in the dissociation protocol had the following composition: 137.93 mM NaCl, 5.33 mM KCl, 0.44 mM KH2PO4, 4.0 mM NaHCO3, 0.3 mM Na2HPO4, and 5.6 mM glucose. For patch-clamp recording, the composition of the bath solution was: 140 mM NaCl, 5.0 mM KCl, 1.8 mM CaCl2, 0.5 mM MgCl2, 5.0 mM HEPES, and 5.0 mM glucose. To study the Na+ current in dissociated DRG neurons we used an external solution with the following composition: 40 mM NaCl, 70 mM choline chloride, 3.0 mM KCl, 1.0 mM CaCl2, 1.0 mM MgCl2, 20 mM tetraethylammonium chloride, 0.1 mM CdCl2, 10 mM HEPES, and 10 mM glucose. The pH of all solutions was adjusted to 7.4 with HCl, except for the modified external solution, which was adjusted with tetraethylammonium hydroxide. The internal solution contained 10 mM NaCl, 100 mM CsCl, 10 mM HEPES, 11 mM ethylene glycol tetraacetic acid, 10 mM tetraethylammonium chloride, and 5.0 mM MgCl2, and the pH was adjusted to 7.2 with CsOH. In some patch-clamp experiments, we added 300 nM tetrodotoxin (TTX) to the modified external solution in order to block the TTX-sensitive (TTX-S) component of the Na+ current. The Na+ current remaining in the presence of TTX was designated TTX-resistant (TTX-R). We used neurons of different sizes to investigate the effects of estragole on total and TTX-R Na+ currents. To improve the quality of voltage clamp and data acquired, we chose to lower the extracellular Na+ concentration in the external solution to reduce the electrochemical gradient and effectively decrease Na+ current amplitudes. Thus, we used choline as a nonpermeate monovalent cation in partial substitution of external Na+. Cs+ and tetraethylammonium were used to block K+ channels, and Cd2+ was used to block Ca2+ channels.
Estragole was dissolved in dimethylsulfoxide (DMSO), and stock solutions were prepared daily. Stock solutions were added to the modified Locke's solution for intracellular recording and to modified external solutions for patch-clamp recording so as to provide a desired concentration of estragole with a final concentration of DMSO always lower than 0.2% (v/v). At this concentration DMSO did not alter any measurable electrophysiological parameters.
Estragole concentrations used for intracellular and patch-clamp recordings were 1-6 and 0.6-14 mM, respectively. All salts and drugs were purchased from Sigma Chemical (USA) or Reagen (Brazil) and were of analytical grade.
Electrophysiological measurements and analysis
Intracellular recordings
DRG were fixed to the floor of an acrylic chamber specially designed to permit superfusion with modified Locke's solution. The superfusion was maintained by a gravity flux and adjusted to 1.0-1.5 mL/min. The chamber was placed on a magnifying glass (model College Stereo, MLW Intermed, Germany), and the electrode movement and impalement were performed by a hydraulic micromanipulator (model MWO-3, Narishige International Inc., USA). Reservoirs containing control and drug solution were connected to the chamber by three-way valves that could rapidly switch between the main reservoir and test solutions. After impalement, cells were followed for 3-5 min to allow the stabilization of neuronal membrane properties. Neurons were exposed for up to 5 min to a given estragole concentration or until it blocked AP. Subsequently, a washout period began by switching to drug-free solutions.
Intracellular recordings were made with thin-walled borosilicate glass microelectrodes (1.0 mm OD, 0.5 mm ID, WPI Corp., USA) filled with a 3.0-M KCl solution. These microelectrodes were pulled with a micropipette puller (P-97 micropipette puller model, Sutter Instruments, USA) and had resistance ranging from 40 to 100 MΩ. Pipettes were connected via an Ag-AgCl wire to an Axoclamp-2B amplifier (Axon Instruments, USA).
Neurons were considered to be acceptable for study when they stabilized with resting potential (Em) more negative than -50 mV and with input resistance (Rin) larger than 10 MΩ for 3-5 min after impalement. Voltage response and APs were elicited in response to depolarizing current pulses, which were 20-25% above the AP threshold. The recording was performed in bridge mode (bandwidth filter at 30 kHz) and in discontinuous (switched; ∼1.5 kHz) current injection mode. Headstage (unsampled) voltage was continuously monitored to ensure that it settled completely prior to sampling. Current and voltage outputs were sampled at 20 kHz, and data acquisition and storage were performed using computer acquisition hardware (Digidata 1200 model, Molecular Devices, USA).
The electrophysiological measurements were Em, Rin, and AP parameters: amplitude, duration, maximum rate of rise [ascendant, dV/dt(asc)], and maximum rate of fall of AP [descendant inclinations, dV/dt(desc), the absolute value of the minimum value of negative dV/dt]. Rin was measured by means of Ohm's law (dividing the maximum voltage response due to hyperpolarizing current pulse) and AP amplitude by measuring the difference between maximum voltage amplitude in an AP and Em. AP duration was measured at 50% AP amplitude.
Patch-clamp recordings
Coverslips with dissociated DRG neurons were placed in a recording chamber on the stage of an inverted phase-contrast microscope (model Axiovert 200, Carl Zeiss Inc., USA) and perfused with bath solution. The perfusion system was composed of electronic valves (LFAA series, The Lee Co., USA) and a glass pipette located in the vicinity of the cell patch-clamped for recording the Na+ current.
The recording electrodes were pulled from thick-walled flint glass tubing with a micropipette puller (P-97 micropipette puller model, Sutter Instruments) and had a resistance range of 1.5-3.0 MΩ. Seal resistances were larger than 1 GΩ and voltage-clamp recordings were made in whole-cell patch-clamp configuration using an Axopatch 200B amplifier (Axon Instruments) driven by a Clampex program (version 10.2).
The neurons were voltage-clamped at -80 mV for all experimental manipulations. A 100-ms voltage step to 0 mV, 0.2-Hz interval, was employed to elicit Na+ currents. The experimental time points were: control, with external solution perfusion only (∼30 s); drug exposure, with estragole-containing solution (120-150 s); and washout/recovery, back to test perfusion solution. Capacitance and leakage subtraction were performed using a P/6 subtraction protocol. Series resistance compensation (70-90%) was routinely employed to reduce voltage error. The liquid junction potential was not corrected in this set of experiments. The current was sampled at 40 kHz and low-pass filtered at 2 kHz, and data acquisition and storage were performed using computer acquisition hardware (Digidata 1440A model, Axon Instruments).
To assess current kinetics, we measured time-to-peak for activation and inactivation decay time constants. Time-to-peak was determined by the time elapsed from initiation of voltage pulse test to the peak of Na+ current. Inactivation decay time constants were estimated by fitting the decay phase of current trace to a double-exponential function, denoted here as slow and fast inactivation time constants.
Statistical analysis
Data are reported as means±SE with n indicating the number of experiments. The Student t-test was used for parametric data and the Mann-Whitney test for nonparametric data. The difference in percentage of neurons blocked by estragole in intracellular recordings was determined by a chi-square test. For all concentration-response curves, IC50 values (concentration that inhibited the effect to 50% of its maximal magnitude) were measured by Hill equation adjustment of data points. Data were considered to be significant when P<0.05.
Results
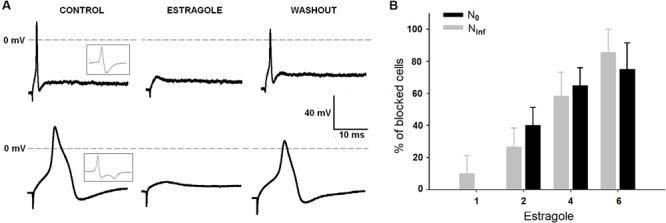
The effects of estragole on DRG neurons were studied on a total of 100 cells using intracellular recordings. Two different shapes of AP were observed (Figure 1A). Some neurons exhibited an inflexion or shoulder on the falling of repolarization phase of AP and were classified as Ninf; others did not (Figure 1A, left traces) and were designated N0 (22,23). Previous studies demonstrated that the neurons here classified as Ninf generally correspond to nociceptors, while N0 were classified as non-nociceptors (22,24,25). The classification of a neuron as Ninf or N0 was based on the first derivative of the AP (inset panel in Figure 1A). The dV/dt of the neurons, after crossing a zero value corresponding to the peak of the AP, decreased to a minimum and then, for the N0 but not for the Ninf, increased monotonically toward zero.
Figure 1. Effects of estragole on action potential recorded in N0 and Ninf cells. Tracings in panel A show the effect of 4 mM estragole on action potentials; insets show first derivative of voltage responses. Panel B shows the percentage of neurons blocked by estragole (1.0-6.0 mM). The total number of cells was 8, 20, 20, and 8 for N0, and 10, 15, 12, and 7 cells for Ninf, for each estragole concentration, respectively. Data are reported as means±SE. *P<0.05, chi-square test.
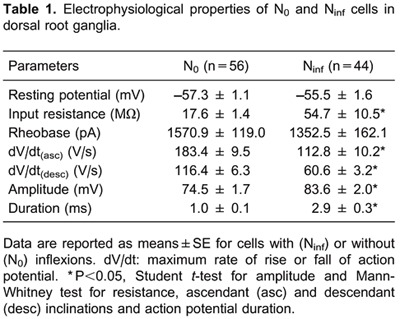
The number of N0 and Ninf cells was 56 and 44, respectively. All passive and AP parameters differed significantly between these two types of neurons (Table 1), except for rheobase (P>0.05, Mann-Whitney test) and for Em (P>0.05, Student t-test).
Estragole possesses an inhibitory effect on AP generation, as seen in Figure 1. It blocked the AP for both N0 and Ninf groups of DRG neurons within minutes (up to 5 min at lower concentrations) and its effects were reversible after wash. Figure 1B reports the percentage of N0 and Ninf neurons whose AP was blocked by estragole exposure, and the percentage of neurons was significantly blocked in a concentration-dependent manner (P<0.05, chi-square test).
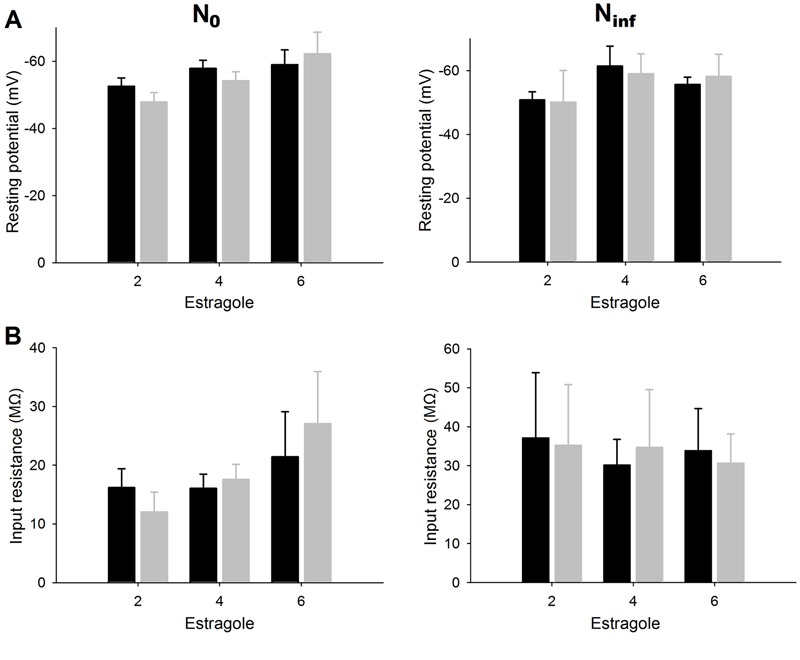
The Em and Rin values in N0 and Ninf neurons that underwent complete blockade of AP generation were measured in different concentrations of estragole (2-6 mM; Figure 2). Over a variable concentration range, estragole (2-6 mM) did not alter the Em (Figure 2A) and Rin (Figure 2B) of N0 and Ninf cells (P>0.05, Student t-test).
Figure 2. Effects of estragole on passive electrical properties of dorsal root ganglia neurons. Panels A and B report resting potential and input resistance, respectively, recorded in N0 and Ninf cells. At the estragole concentrations employed (2-6 mM), there were no significant differences between control and estragole treatment for either type of cells (P>0.05, Student t-test). Data are reported as means±SE. The number of experiments was n=6-12 and n=4-7 for N0 and Ninf, respectively.
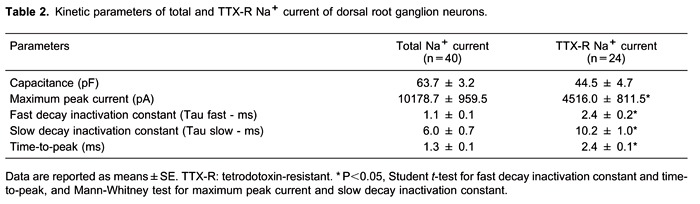
AP inhibition in the absence of measurable alterations of Em and Rin suggests that estragole may have a direct effect on Na+ channel conductance activation. In order to further elucidate an effect on Na+ channel function, DRG neurons were dissociated and Na+ currents were recorded with patch-clamp techniques. To ensure that we were dealing with currents produced selectively by Na+, we performed pilot experiments where all Na+ from the external solution was replaced by choline. Under these conditions no current was observed (data not shown).
Total Na+ current (a mixture of TTX-S and TTX-R Na+ currents) and TTX-R Na+ currents were recorded in isolated DRG neurons. The neuronal capacitances in cells with total Na+ current and TTX-R current were 63.7±3.2 (n=40) and 44.5±4.7 pF (n=24), respectively. The current kinetics was also different for the two types of Na+ currents (Table 2).
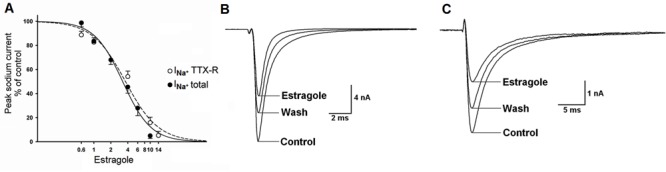
Estragole inhibited total and TTX-R Na+ currents in a voltage- and concentration-dependent manner (Figure 3A). These effects were partially reversible upon wash (Figure 3B and C). The time courses of Na+ current inhibition developed within 150 s and were the same for both currents. The pharmacological potency was similar for total and TTX-R Na+ currents: IC50 values were 3.2±0.6 and 3.6±1.1 mM, respectively.
Figure 3. Estragole-induced inhibitory effect on total (INa+ total) and on tetrodotoxin-resistant (INa+ TTX-R) Na+ current recorded in N0 and Ninf neurons. Panel A shows concentration-response curves (0.6-14 mM) for the effect of estragole on total Na+ current and on TTX-R Na+ current. Panels B and C show representative traces of the effects of 4 mM estragole on total (panel B) and TTX-R (panel C) Na+ currents. There was partial recovery with washout. For INa+ total, the number of experiments was n=4-12 and for INa+ TTX-R, it was n=4-5 for each estragole concentration used.
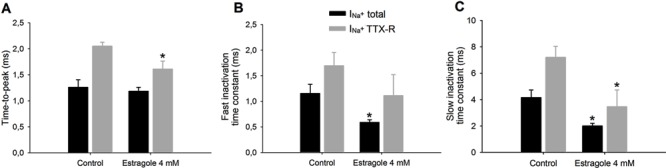
Because calculated IC50 values were similar for both currents (3.2 and 3.6 mM for total and TTX-R Na+ currents), we next analyzed the actions of 4 mM estragole on kinetics parameters, time-to-peak, and inactivation time constants (Figure 4). There was a statistically significant reduction for the time-to-peak value of TTX-R Na+ current (P<0.05, Student t-test), but no reduction was observed in total Na+ current (Figure 4). Fast and slow inactivation time constants (Figure 4B and C) were reduced for both currents, indicating an acceleration of current inactivation in the presence of 4 mM estragole. For the slow-time constant, there were significant differences in total and TTX-R Na+ currents following 4 mM estragole when compared with control (P<0.05, Student t-test). For the fast-time constant, statistical significance was found only for total Na+ current (P<0.05, Student t-test).
Figure 4. Effects of 4 mM estragole on kinetic parameters of total (INa+ total) and tetrodotoxin-resistant (INa+ TTX-R) Na+ current. Panel A shows time-to-peak parameter and panels B and C show fast and slow inactivation time constants. Data are reported as means±SE for n=5-6. *P<0.05, statistical difference of 4 mM estragole effects on INa+ total or INa+ TTX-R compared to control (Student t-test).
Discussion
The main finding of the present research is that estragole blocks AP detonation and causes inhibition of Na+ channel conductance activation in DRG neurons. Both these effects were observed at similar pharmacological concentrations, suggesting that the blockade of voltage-dependent Na+ current underlies the inhibition of AP generation. Our current results confirm our hypothesis for the mechanism of action of estragole-induced blockade of neuronal excitability - namely, that this substance inhibits neuronal excitability by primary blockade of voltage-dependent activation of Na+ channel conductance. This mechanism is a novel and relevant finding. These results may be important for an understanding of the pharmacological effects of estragole and of the widespread use of this substance in industry, and as a constituent of several essential oils in aromatherapy.
Besides the blockade of APs and Na+ current, other aspects of estragole action on DRG neurons deserve consideration. At the concentrations that block AP and Na+ currents, estragole produced no significant changes in Em and Rin (passive electrophysiological properties). Modifications in membrane Rin are frequently associated with changes in Em. This is because many K+ conductances are active at or near Em (26). On the other hand, changes in Na+ conductance had little or no visible changes in Em or Rin; these passive membrane properties are usually unaffected by alterations in Na+ conductances (27). Thus, our data, while supporting the hypothesis that estragole inhibits excitability by blocking voltage-dependent Na+ current, gave no indication that estragole acts on K+ channels. The direct action of estragole on Na+ channels indicates that this essential oil constituent possesses potent local anesthetic activity (28).
The dependence of excitability inhibition on Na+ current blockade and independence on changes in passive electrophysiological parameters shown by estragole were found for other terpenes and terpenoids like thymol (29), linalool (21), eugenol (19,20), carvacrol (17), and menthol (30). This is not always the case, however, as 1,8-cineole, a terpenoid, was demonstrated to cause depolarization of the resting membrane and to block excitability, mainly indirectly, via the effect of depolarization voltage-dependent Na+ current activation (31).
Data from intracellular recordings suggested that the mechanism of action of estragole on excitability is that it has a direct effect on Na+ channels. Two distinct Na+ currents can be characterized in different DRG neurons: TTX-S and TTX-R. These currents are observed in different populations of DRG neurons based on their soma diameters (32,33). APs can be triggered solely by TTX-S, by TTX-R currents, or by a mixture of both (33-35). According to the classification used in this study (N0 and Ninf cells), we expected that N0 and Ninf cells would possess a mixture of TTX-S and TTX-R Na+ currents, but that Ninf cells would have a predominance of TTX-R.
We observed that Ninf cells had much longer AP durations. This increased AP duration may be produced by an inward Ca2+ current that is activated during the AP depolarization. It is also a possible contributor to the inflection observed on the repolarization limb of the AP (35). However, it is worth mentioning that Ca2+ currents cannot promote an AP with the characteristics of N0 or Ninf here described. Our patch-clamp data showed that estragole blocked the amplitude of both currents (total and TTX-R) with similar IC50 values (3.2 and 3.6 mM for TTX-S and TTX-R, respectively; Figure 3), indicating similar pharmacological potency. This suggests that estragole has no preference for Na+ current subtypes.
Besides estragole's effect on the amplitude of Na+ current, it also reduced kinetic parameters of Na+ current (time-to-peak and inactivation time constants; Figure 4). At 4 mM (approximately the IC50 for excitability and Na+ current blockade), estragole might have influenced neuron excitability via changes in Na+ current kinetics. The kinetic parameters shown in Table 2 are similar to those in the literature for the Na+ currents in DRG neurons (33). Also, the reduction in inactivation time constants promoted by estragole is in agreement with the effects of other terpenes and terpenoids like eugenol (20) and menthol (30).
Estragole possesses several biological and pharmacological activities. The present study adds an additional effect - namely, a local anesthetic effect. This new local anesthetic effect adds to the list of other pharmacological actions of estragole, such as antimicrobial (7), antioxidant (8), anti-edematogenic, and anti-inflammatory (13) effects, and places this substance as a candidate for therapeutic usefulness to treat ulcerations (infected or not), for example, and similar illnesses. In addition to these activities, one can consider that anethole, with a molecular structure very similar to estragole, has been documented to have procicatrizing activity (36). This prospect also increases when one takes into consideration recent studies showing that long-lasting per os administration of 37.5 mg/kg estragole showed no toxic effects (37), suggesting that extracts having estragole as the principal constituent, like fennel seed extract, have anticancer (for certain types of cancer) and antioxidant activities, and radical scavenging potential (38).
It is noteworthy that the local anesthetic activities of estragole may help shed light on the actions of other essential oils that contain estragole. For example, the essential oil of Croton zehntneri contains estragole and is documented to have anti-nociceptive activity (39). It is known that part of the inflammatory mechanism is mediated through the nervous system, and a local anesthetic activity may contribute to the anti-inflammatory effect (40).
Previous studies of our group (16) investigated the effects of estragole on myelinated fibers of the sciatic nerve with conduction velocity between 40 and 100 m/s; myelinated fibers with lower conduction velocities and unmyelinated fibers were not recorded. The present investigation, using intracellular recordings, investigated the effect of estragole on N0 and Ninf neurons and demonstrated that estragole had similar pharmacological potency and efficacy in both types of cells. The similar actions of estragole on these two types of neurons indicate that this terpenoid affects a wide range of axon diameters. Ninf represents predominantly Aδ afferents and C-neurons (slowly conducting fibers that are specified as nociceptors), while N0 represents Aα/β afferent neurons (intermediate conducting fibers associated with mechanoreceptors) (22,24,25). Thus, estragole has no preference for any particular type of primary sensory afferent axon.
In conclusion, our data demonstrate that estragole-induced blockade of the excitability in peripheral nerves is very probably due to a local anesthetic mechanism. Thus, estragole may be a potentially useful drug for therapeutic use.
Acknowledgments
The authors thank Mr. Pedro Militão de Albuquerque Neto for technical assistance and Dr. Daniel Weinreich for improving and correcting the manuscript. Research supported by CNPq, CAPES, Fundação Cearense de Apoio ao Desenvolvimento Cientifico e Tecnológico (FUNCAP), and Instituto Superior de Ciências Biomédicas, Universidade Estadual do Ceará (ISCB-UECE).
Footnotes
First published online December 2, 2013.
References
- 1.Tisserand R, Balacs T. Essential oil safety: a guide for health care professionals. New York: Churchill Livingstone; 1995. [Google Scholar]
- 2.Leal-Cardoso JH, Fonteles MC. Pharmacological effects of essential oils of plants of the northeast of Brazil. An Acad Bras Cienc. 1999;71:207–213. [PubMed] [Google Scholar]
- 3.Craveiro AA, Fernandes AG, Andrade CHS, Matos FJA, Alencar JW, Machado MIL. =leos essenciais de plantas do Nordeste. Fortaleza: Edições UFC; 1981. [Google Scholar]
- 4.De Vincenzi M, Silano M, Maialetti F, Scazzocchio B. Constituents of aromatic plants: II. Estragole. Fitoterapia. 2000;71:725–729. doi: 10.1016/S0367-326X(00)00153-2. [DOI] [PubMed] [Google Scholar]
- 5.Masten S. Terpinolene. Review of toxicological literature. North Carolina: Research Triangle Park; 1999. [Google Scholar]
- 6.Lachowicz KJ, Jones GP, Briggs DR, Bienvenu FE, Wan J, Wilcock A, et al. The synergistic preservative effects of the essential oils of sweet basil (Ocimum basilicum L.) against acid-tolerant food microflora. Lett Appl Microbiol. 1998;26:209–214. doi: 10.1046/j.1472-765X.1998.00321.x. [DOI] [PubMed] [Google Scholar]
- 7.Friedman M, Henika PR, Mandrell RE. Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica . J Food Prot. 2002;65:1545–1560. doi: 10.4315/0362-028x-65.10.1545. [DOI] [PubMed] [Google Scholar]
- 8.Morais SM, Júnior FEAC, Silva ARA, Neto JSM, Rondina D, Leal-Cardoso JH. Atividade antioxidante de óleos essenciais de espécies de Croton do Nordeste do Brasil. Quím Nova. 2006;29:907–910. doi: 10.1590/S0100-40422006000500004. [DOI] [Google Scholar]
- 9.Cosentino RM, Norte MC, Lazarini CA. Estragole-induced behavioural changes in rats. Phytother Res. 2004;18:921–924. doi: 10.1002/ptr.1583. [DOI] [PubMed] [Google Scholar]
- 10.Albuquerque AA, Sorenson AL, Leal-Cardoso JH. Effects of essential oil of Croton zehntneri, and of anethole and estragole on skeletal muscles. J Ethnopharmacol. 1995;49:41–49. doi: 10.1016/0378-8741(95)01301-6. [DOI] [PubMed] [Google Scholar]
- 11.Coelho-de-Souza AN, Barata EL, Magalhães PJC, Lima CC, Leal-Cardoso JH. Effects of the essential oil of Croton zehntneri, and its constituent estragole on intestinal smooth muscle. Phytother Res. 1997;11:299–304. doi: 10.1002/(SICI)1099-1573(199706)11:4<299::AID-PTR99>3.0.CO;2-A. [DOI] [Google Scholar]
- 12.Soares PM, Lima RF, de Freitas PA, Souza EP, Assreuy AM, Criddle DN. Effects of anethole and structural analogues on the contractility of rat isolated aorta: Involvement of voltage-dependent Ca2+-channels. Life Sci. 2007;81:1085–1093. doi: 10.1016/j.lfs.2007.08.027. [DOI] [PubMed] [Google Scholar]
- 13.Ponte EL, Sousa PL, Rocha MV, Soares PM, Coelho-de-Souza AN, Leal-Cardoso JH, et al. Comparative study of the anti-edematogenic effects of anethole and estragole. Pharmacol Rep. 2012;64:984–990. doi: 10.1016/s1734-1140(12)70895-2. [DOI] [PubMed] [Google Scholar]
- 14.Opdyke DL. Monographs on fragrance raw materials. Food Cosmet Toxicol. 1976;14:601–633. doi: 10.1016/S0015-6264(76)80015-6. [DOI] [PubMed] [Google Scholar]
- 15.Chainy GB, Manna SK, Chaturvedi MM, Aggarwal BB. Anethole blocks both early and late cellular responses transduced by tumor necrosis factor: effect on NF-kappaB, AP-1, JNK, MAPKK and apoptosis. Oncogene. 2000;19:2943–2950. doi: 10.1038/sj.onc.1203614. [DOI] [PubMed] [Google Scholar]
- 16.Leal-Cardoso JH, Matos-Brito BG, Lopes-Junior JE, Viana-Cardoso KV, Sampaio-Freitas AB, Brasil RO, et al. Effects of estragole on the compound action potential of the rat sciatic nerve. Braz J Med Biol Res. 2004;37:1193–1198. doi: 10.1590/S0100-879X2004000800009. [DOI] [PubMed] [Google Scholar]
- 17.Joca HC, Cruz-Mendes Y, Oliveira-Abreu K, Maia-Joca RP, Barbosa R, Lemos TL, et al. Carvacrol decreases neuronal excitability by inhibition of voltage-gated sodium channels. J Nat Prod. 2012;75:1511–1517. doi: 10.1021/np300050g. [DOI] [PubMed] [Google Scholar]
- 18.Ravens U, Wettwer E, Hala O. Pharmacological modulation of ion channels and transporters. Cell Calcium. 2004;35:575–582. doi: 10.1016/j.ceca.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 19.Moreira-Lobo DC, Linhares-Siqueira ED, Cruz GM, Cruz JS, Carvalho-de-Souza JL, Lahlou S, et al. Eugenol modifies the excitability of rat sciatic nerve and superior cervical ganglion neurons. Neurosci Lett. 2010;472:220–224. doi: 10.1016/j.neulet.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Cho JS, Kim TH, Lim JM, Song JH. Effects of eugenol on Na+ currents in rat dorsal root ganglion neurons. Brain Res. 2008;1243:53–62. doi: 10.1016/j.brainres.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 21.Leal-Cardoso JH, da Silva-Alves KS, Ferreira-da-Silva FW, dos Santos-Nascimento T, Joca HC, de Macedo FH, et al. Linalool blocks excitability in peripheral nerves and voltage-dependent Na+ current in dissociated dorsal root ganglia neurons. Eur J Pharmacol. 2010;645:86–93. doi: 10.1016/j.ejphar.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Harper AA, Lawson SN. Electrical properties of rat dorsal root ganglion neurones with different peripheral nerve conduction velocities. J Physiol. 1985;359:47–63. doi: 10.1113/jphysiol.1985.sp015574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amir R, Devor M. Chemically mediated cross-excitation in rat dorsal root ganglia. J Neurosci. 1996;16:4733–4741. doi: 10.1523/JNEUROSCI.16-15-04733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harper AA, Lawson SN. Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurons. J Physiol. 1985;359:31–46. doi: 10.1113/jphysiol.1985.sp015573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Villiere V, McLachlan EM. Electrophysiological properties of neurons in intact rat dorsal root ganglia classified by conduction velocity and action potential duration. J Neurophysiol. 1996;76:1924–1941. doi: 10.1152/jn.1996.76.3.1924. [DOI] [PubMed] [Google Scholar]
- 26.Gutman GA, Chandy KG, Grissmer S, Lazdunski M, McKinnon D, Pardo LA, et al. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol Rev. 2005;57:473–508. doi: 10.1124/pr.57.4.10. [DOI] [PubMed] [Google Scholar]
- 27.Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev. 2005;57:397–409. doi: 10.1124/pr.57.4.4. [DOI] [PubMed] [Google Scholar]
- 28.Altman RS, Smith-Coggins R, Ampel LL. Local anesthetics. Ann Emerg Med. 1985;14:1209–1217. doi: 10.1016/S0196-0644(85)81031-3. [DOI] [PubMed] [Google Scholar]
- 29.Haeseler G, Maue D, Grosskreutz J, Bufler J, Nentwig B, Piepenbrock S, et al. Voltage-dependent block of neuronal and skeletal muscle sodium channels by thymol and menthol. Eur J Anaesthesiol. 2002;19:571–579. doi: 10.1017/s0265021502000923. [DOI] [PubMed] [Google Scholar]
- 30.Gaudioso C, Hao J, Martin-Eauclaire MF, Gabriac M, Delmas P. Menthol pain relief through cumulative inactivation of voltage-gated sodium channels. Pain. 2012;153:473–484. doi: 10.1016/j.pain.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 31.Ferreira-da-Silva FW, Barbosa R, Moreira-Junior L, dos Santos-Nascimento T, de Oliveira-Martins MD, Coelho-de-Souza AN, et al. Effects of 1,8-cineole on electrophysiological parameters of neurons of the rat superior cervical ganglion. Clin Exp Pharmacol Physiol. 2009;36:1068–1073. doi: 10.1111/j.1440-1681.2009.05188.x. [DOI] [PubMed] [Google Scholar]
- 32.Ogata N, Tatebayashi H. Ontogenic development of the TTX-sensitive and TTX-insensitive Na+ channels in neurons of the rat dorsal root ganglia. Brain Res Dev Brain Res. 1992;65:93–100. doi: 10.1016/0165-3806(92)90012-L. [DOI] [PubMed] [Google Scholar]
- 33.Elliott AA, Elliott JR. Characterization of TTX-sensitive and TTX-resistant sodium currents in small cells from adult rat dorsal root ganglia. J Physiol. 1993;463:39–56. doi: 10.1113/jphysiol.1993.sp019583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scholz A, Vogel W. Tetrodotoxin-resistant action potentials in dorsal root ganglion neurons are blocked by local anesthetics. Pain. 2000;89:47–52. doi: 10.1016/S0304-3959(00)00345-6. [DOI] [PubMed] [Google Scholar]
- 35.Renganathan M, Cummins TR, Waxman SG. Contribution of Na(v)1.8 sodium channels to action potential electrogenesis in DRG neurons. J Neurophysiol. 2001;86:629–640. doi: 10.1152/jn.2001.86.2.629. [DOI] [PubMed] [Google Scholar]
- 36.Cavalcanti JM, Leal-Cardoso JH, Diniz LR, Portella VG, Costa CO, Linard CF, et al. The essential oil of Croton zehntneri and trans-anethole improves cutaneous wound healing. J Ethnopharmacol. 2012;144:240–247. doi: 10.1016/j.jep.2012.08.030. [DOI] [PubMed] [Google Scholar]
- 37.Bristol DW. NTP 3-month toxicity studies of estragole (CAS No. 140-67-0) administered by gavage to F344/N rats and B6C3F1 mice. Toxic Rep Ser. 2011;82:1–111. [PubMed] [Google Scholar]
- 38.Mohamad RH, El-Bastawesy AM, Abdel-Monem MG, Noor AM, Al-Mehdar HA, Sharawy SM, et al. Antioxidant and anticarcinogenic effects of methanolic extract and volatile oil of fennel seeds (Foeniculum vulgare) J Med Food. 2011;14:986–1001. doi: 10.1089/jmf.2008.0255. [DOI] [PubMed] [Google Scholar]
- 39.Oliveira AC, Leal-Cardoso JH, Santos CF, Morais SM, Coelho-de-Souza AN. Antinociceptive effects of the essential oil of Croton zehntneri in mice. Braz J Med Biol Res. 2001;34:1471–1474. doi: 10.1590/s0100-879x2001001100016. [DOI] [PubMed] [Google Scholar]
- 40.Murakami M, Hirano T. The molecular mechanisms of chronic inflammation development. Front Immunol. 2012;3:323. doi: 10.3389/fimmu.2012.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]


