SUMMARY
Background
Asimadoline, a kappa-opioid agonist, reduces visceral sensitivity in experimental animal models and may decrease satiation and postprandial fullness in healthy individuals. However, its effect on satiation in functional dyspepsia is unclear, and any symptom benefit has not been explored.
Aim
To evaluate the effects of asimadoline on satiation volume and postchallenge symptoms in functional dyspepsia.
Methods
A randomized, double-blind trial evaluated gastric satiation and symptoms before and after 8 weeks of asimadoline 0.5 mg (n = 13) or 1.0 mg (n = 13) or placebo (n = 14) b.d. in patients with functional dyspepsia (Rome II). Gastrointestinal Symptom Rating Scale and Nepean Dyspepsia Index were used to assess symptoms during the 8-week treatment.
Results
Over 8 weeks of treatment, asimadoline had no significant effect on maximum-tolerated volume or aggregate symptom score with nutrient drink challenge, and on the mean of the total daily symptom severity score compared to placebo. In a post hoc analysis, asimadoline 0.5 mg significantly increased the maximum-tolerated volume (1217 mL ± 90.2) compared to placebo (807 mL ± 111.8) in patients with higher postprandial fullness scores (P = 0.01).
Conclusion
Asimadoline overall did not significantly alter maximum-tolerated volume, symptoms postnutrient challenge or symptoms over 8 weeks in functional dyspepsia.
INTRODUCTION
Functional dyspepsia is a highly prevalent but heterogenous disorder in which multiple pathogenic mechanisms may be involved, although the underlying causal pathways remain obscure.1–7 One of the most important pathophysiologic mechanisms in functional dyspepsia is considered to be visceral hypersensitivity in response to mechanical or other stimuli.8–12 One pathway modulating pain sensation in the gastrointestinal tract is opioid driven.13 Peripherally acting kappa-opioid agonists have been developed which appear to have antinociceptive effects in the gastrointestinal tract without the side effects typical of other opioids including addiction, respiratory depression, sedation, dysphoria and hallucinations.13–16 Studies with the kappa receptor agonist fedotozine in patients with functional gastrointestinal symptoms appeared promising;17–19 however, the clinical development of fedotozine was suspended because of an inconsistent clinical response.20
Asimadoline is a selective agonist of the kappa-opioid receptor with a restricted ability to cross the blood–brain barrier.21–23 Asimadoline has been shown to attenuate responses to noxious gastric and colorectal distention especially after inflammation in animal models.24–26 In humans, asimadoline decreased perception of pain in response to colonic distention.27 Delvaux et al.27 reported that asimadoline decreased the overall perception of pain over a wide range of pressure distensions of the colon in patients with the irritable bowel syndrome, without altering colonic compliance. Delgado-Aros et al.28 also reported that single oral administration of asimadoline decreased satiation and postprandial fullness in healthy volunteers independent of its effects on gastric volumes. In addition, they showed that asimadoline has a good safety profile without adverse effects on gastrointestinal motility. However, the effects of asimadoline on satiation in functional dyspepsia remain unknown, and any symptom benefit has not been explored.
We, therefore, aimed to compare the effects of two doses of asimadoline (0.5 and 1.0 mg p.o. b.d.) and placebo on satiation volume, postprandial symptoms and symptoms of dyspepsia in patients with documented functional dyspepsia. We postulated that asimadoline would increase the maximum-tolerated volume (MTV) ingested during a nutrient drink test (NDT) challenge and reduce symptoms induced by the meal.
METHODS
Design
A randomized, parallel group, double-blind study was undertaken to evaluate the effects of 0.5 or 1.0 mg of asimadoline or matching identical placebo twice daily in patients with functional dyspepsia.
Study subjects
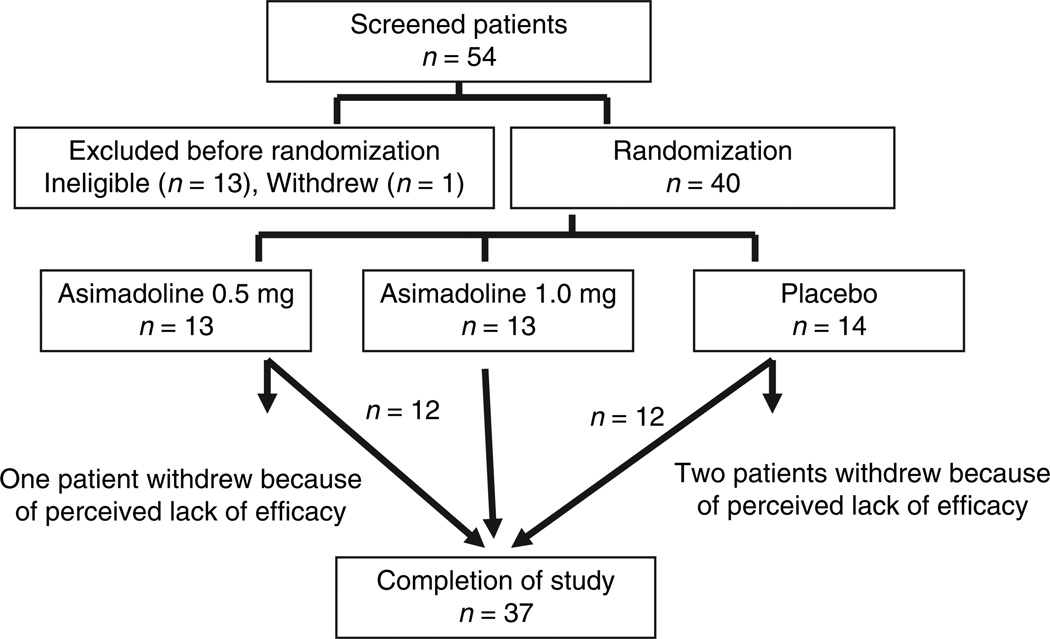
A total of 54 patients with functional dyspepsia were initially selected from the Mayo clinic practice through public advertisement. After screening visits, 13 patients were not eligible for our study by inclusion and exclusion criteria and one patient withdrew. Thus, 40 patients with functional dyspepsia were enrolled (Figure 1). These were either males (n = 13) or nonpregnant, nonbreastfeeding female volunteers (n = 27) with functional dyspepsia. The main inclusion criterion was based on Rome II criteria for functional dyspepsia; patients were required to have recurrent episodes of epigastric pain or discomfort including early satiety, fullness, bloating or nausea for at least 3 months in the previous year.
Figure 1.
Flow chart of the patients from initial screening to completion of the trial. The intention-to-treat (ITT) analysis included all patients randomized.
To exclude underlying organic disease, upper gastrointestinal endoscopy should have been performed in the previous 3 years, with no evidence of erosions, peptic ulcer (defined as a breach in mucosal continuity of >2 mm diameter and >1 mm depth), pyloric obstruction, oesophagitis or malignancy. They had to have no alarm indicators on clinical assessment (weight loss of more than 7 kg, gastrointestinal bleeding, recent recurrent vomiting, progressive dysphagia), and no history suggestive of small bowel obstruction. Other major structural or metabolic diseases that affect the gastrointestinal system were also excluded. Patients with irritable bowel syndrome or heartburn by the Talley Bowel Disease Questionnaire were excluded as well.29 Patients with a history of abdominal surgery, other than appendectomy, cholecystectomy, caesarean section or tubal ligation, were not eligible.
Subjects were not permitted to be on any other medications except for hormone replacement therapy, low-dose aspirin for cardioprotection, stable thyroid replacement, and birth control pills or depot injections. Medications that may alter gastrointestinal motility including laxatives, magnesium- or aluminium-containing antacids, prokinetics, erythromycin, narcotics, anticholinergics, tricyclic antidepressants, selective serotonin reuptake inhibitors and newer antidepressants, analgesic drugs including opiates, nonsteroidal anti-inflammatory drugs (including COX 2 inhibitors), systemic antifungal drugs, drugs inducing CYP 3A4 and 2D6 including carbamazepine, glucocorticoids, phenobarbital, phenytoin, rifampin, grapefruit juice, and benzodiazepines were all prohibited. Also excluded were subjects with a previous history of exposure to asimadoline, or a known history of hypersensitivity to asimadoline or other opioids.
The Hospital Anxiety and Depression Scale (HADS)30 was used to determine anxiety and depression scores at the start of the study. A score of 11 or higher on either the anxiety or depression scale suggests clinically relevant anxiety or depression. The study was approved by the Mayo Foundation’s Institutional Review Board. Eligible subjects freely gave their written consent before starting the study.
Randomization and concealed allocation
Each subject was assigned a unique patient number to ensure concealed allocation. Randomization was performed by the study statistician and communicated to the pharmacy in the Mayo General Clinical Research Center (GCRC). Treatment allocation was 1:1:1. Subjects entering the study were assigned the medication specified on the prestudy generated randomization schedule. All other investigators were blinded to treatment assignment.
Study medications
Following screening, subjects were randomized to receive 0.5 or 1.0 mg twice daily of asimadoline or identical placebo. Asimadoline was rapidly absorbed after oral administration with peak plasma concentrations being reached about 0.5–2 h after dosing.31 These two doses were chosen based on data from Delgado-Aros et al.28, 32 in healthy volunteers.
Symptom and quality of life measures
Daily severity of individual dyspeptic symptoms
Patients rated their individual dyspeptic symptoms intensity (abdominal pain, nausea, bloating and fullness) as recorded on a diary card according to the following intensity scale: 0, none; 1, mild; 2, moderate; 3, severe; 4, very severe.
Gastrointestinal Symptom Rating Scale
The Gastrointestinal Symptom Rating Scale (GSRS) was used to assess gastrointestinal symptoms in the preceding week at baseline and after 2, 4, 6 and 8 weeks of treatment and 4 weeks posttreatment.33 The questionnaire includes 15 symptoms and uses a seven-point Likert scale defined by verbal denominators. The highest score 6 denotes the most pronounced symptoms, and 0 denotes no symptoms.
Disease-specific quality of life
To assess the effects of treatment on disease-specific quality of life, we used the validated long version of the Nepean Dyspepsia Index (NDI)34 and calculated four subscales (interference, knowledge/control, eating/drinking and sleep disturbance) and the overall average quality-of-life score. The NDI quality-of-life scores range from 0 to 100, with higher scores indicating better quality of life.
Compliance and safety
At the 8-week visit, patients returned their medication containers, and pills were counted. At baseline and after 2 and 8 weeks, a physical examination and laboratory tests were performed, including liver- and renal-function tests, haematological tests and measurement of blood glucose. Twelve-lead resting electrocardiograms (ECGs) were obtained at the screening visit and after 2 and 8 weeks of treatment.
Nutrient drink test (NDT)
Subjects ingested 30 mL of a nutrient (Ensure®, Abbott Laboratories, USA; ∼1 kcal/mL; 11% fat, 73% carbohydrate and 16% protein) per minute.35 Thus, the participants were asked to drink 120 mL of Ensure every 4 min until maximal fullness was reached. They scored satiety at 5-min intervals using a rating scale that combined verbal descriptors on a scale of 0–5 (0 = no symptoms, 5 = maximum or unbearable fullness). Participants stopped the meal when a score of 5 was reached. At 30 min after reaching maximum satiation, the study subjects scored their symptoms of bloating, fullness, nausea and pain using a 100-mm visual analogue scale (VAS) anchored with the words unnoticeable and unbearable at the left and right ends. The aggregate symptom score was defined as the sum of VAS scores for each symptom (i.e. maximum of 400). The method has been thoroughly characterized and used extensively in our laboratory.36 We originally planned to enrol only patients with abnormal baseline maximum tolerated volume (MTV) (MTV <1000 mL), but to enrol successfully, we removed this restriction.
Study procedures
The study was performed over 12 weeks. A screening interview, assessment of a physical examination, baseline ECG, standard clinical laboratory tests (chemistry, haematology, urine analysis and urine drug screen), stool antigen test for Helicobacter pylori, HADS, Talley Bowel Disease Questionnaire, GSRS, NDI and a daily symptom diary were given. Consent was obtained within 1 week prior to the studies at the testing facility (GCRC, Mayo Clinic).
Day 1
The participant reported in a fasting condition to the study centre early in the morning. All participants underwent an NDT to assess postprandial symptoms. The participant took the study medication as prescribed two times a day for the next 8 weeks.
Day 7
All participants completed the GSRS, NDI and an NDT 30 min after receiving the morning dose of the study medication under supervision.
Day 14
A physical examination, ECG, standard clinical laboratory tests (chemistry, haematology, urine analysis and urine drug screen), GSRS, NDI and diaries were collected.
Day 28 and day 42
GSRS, NDI and diaries were collected.
Day 56
All participants underwent a physical examination, ECG, standard clinical laboratory tests (chemistry, haematology, urine analysis and urine drug screen), GSRS, NDI and diaries were collected. All participants underwent an NDT 30 min after the morning dose of the study medication. The study medication was stopped.
Day 84
The participant returned to the study centre early in the morning to undergo a physical examination, and a posttreatment GSRS and NDI. Diaries were collected.
Statistical analysis
The primary endpoints for analysis were MTV of the nutrient drink (posttreatment satiety testing) and aggregate symptom score 30 min after ingestion of the nutrient drink [the sum of the individual symptom (bloating, fullness, nausea and pain) scores]. The secondary endpoints for analysis were individual postprandial symptom scores (posttreatment satiation test), subscale scores for dyspepsia symptoms (GSRS) averaged over all follow-up, mean of diary dyspepsia symptom severity scores and quality of life (NDI) scores averaged over all follow-up. The effects of the three treatments on the primary and secondary response measures were assessed using an analysis of covariance (ancova). The covariates included in the analyses were age, gender, body mass index (BMI) and the baseline measurement of the corresponding endpoint (when measured). The assessment of treatment effects adjusted for baseline measures as these parameters often account for a significant proportion of the between-subjects variation. Additional post hoc ancova incorporating the baseline NDI epigastric pain score, postprandial discomfort score and nausea/vomiting score as covariates, along with the corresponding treatment group by NDI score interaction terms, were examined to assess the potential differential treatment effects depending on the degree or type of dyspepsia symptoms.
Following the intent-to-treat (ITT) paradigm, the analysis used all randomized patients. Subjects with missing posttreatment response values had their missing data imputed using the overall corresponding mean response value from subjects with nonmissing data. The overall mean value was computed from the 37 patients with complete data, rather than the mean for the treatment group in which the participant was assigned. Moreover, a concomitant adjustment in the error degrees of freedom (subtracting one for each missing value imputed) of the respective ancova model was made to reflect an appropriate residual error variance for the comparisons of treatment effects. Although this is an unbiased approach under the null hypothesis, it can be overly conservative if the number of subjects with missing data is substantial (e.g. >15% of the ITT population).
Sample size assessment
The primary endpoints were the posttreatment maximum volume in the satiety test and the aggregate symptom score, which had coefficients of variation (CV, %) of 62% and 13%, respectively, based on previous studies in healthy volunteers.28, 32 The estimated effect sizes detectable with 80% power based on a two sample t-test (at a two-sided a-level of 0.05) were 71% and 15%, respectively.28, 32 The effect size is the difference in group means as a percentage of the overall mean for each response, and assumes 13 subjects per group. The ancova would have provided 80% power to detect somewhat smaller (pairwise) differences using a pooled estimate of variation across all three groups and by adjusting for important covariates. The observed baseline CVs (%) for MTV and the aggregate symptom score were 43% and 27%, respectively. Thus, the power for detecting treatment effects for MTV was somewhat better than anticipated.
RESULTS
Participant characteristics
A total of 40 subjects were enrolled into the study. Two subjects on placebo and one on asimadoline 0.5 mg dropped out because of lack of efficacy from the study drug within 1 week (Figure 1). Thus, the ITT analyses imputed values for these three subjects and adjusted the error degrees of freedom downward by three in the ancovas.
The demographics of the subjects are shown in Table 1. There were no clinically important baseline differences in terms of age, gender, BMI, HADS score and the scores for predominant symptoms of dyspepsia (postprandial distress or epigastric pain computed from the NDI) amongst the three groups. Also, the baseline postprandial symptom scores 30 min postsatiety for nausea, fullness, bloating and the corresponding aggregate score were not clinically different among the three groups.
Table 1.
Patient characteristics and baseline satiety test results (symptom scores at 30- min postmaximum-tolerated volume)
| Asimadoline 0.5 mg (n = 13) |
Asimadoline 1.0 mg (n = 13) |
Placebo (n = 14) |
|
|---|---|---|---|
| Age | 50 (±4.8) | 44 (±4.4) | 41 (±3.5) |
| Female gender, n (%) | 9 (69) | 9 (69) | 9(64) |
| BMI (kg/m2) | 26.4 (±1.3) | 27.7 (± 1.8) | 27.1 (±1.4) |
| HADS score | 7.1 (±1.8) | 8.3 (±1.0) | 8.0 (±0.9) |
| HADS depression | 2.7 (±0.9) | 2.6 (±0.5) | 2.8 (±0.5) |
| HADS anxiety | 4.4 (±0.9) | 5.7 (±0.7) | 5.1 (±0.6) |
| NDI epigastric pain | 6.8 (±0.9) | 4.9 (±1.1) | 7.2 (±0.9) |
| NDI meal-related symptoms | 5.2 (±0.7) | 4.5 (±1.0) | 4.5 (±0.9) |
| Maximum-tolerated volume (minimum, maximum) |
777 (±73.9) (480, 1200) |
1116 (±140.8) (360, 2160) |
926 (±94.8) (530, 1710) |
| n (%) <1000 mL | 11 (85) | 7 (54) | 9 (64) |
| Nausea | 28 (±7) | 38 (±9) | 30 (±7) |
| Fullness | 82 (±3) | 75 (±4) | 78 (±4) |
| Bloating | 71 (±5) | 64 (±6) | 63 (±6) |
| Abdominal pain | 50 (±6) | 42 (±7) | 36 (±8) |
| Aggregate score | 229 (±14) | 220 (±17) | 208 (±18) |
Data are presented as mean (±S.E.M.) unless otherwise indicated. HADS score is used for evaluating the patients’ initial psychiatric features.
BMI, body mass index; HADS, Hospital Anxiety and Depression Scale; NDI, Nepean Dyspepsia Index.
MTV and postprandial symptoms with the NDT
A strong linear correlation (r = 0.83) between the MTV in NDT on 1 week of treatment and baseline over all subjects was observed (P < 0.001). After 8 weeks of therapy, the mean (±S.E.) MTV from the nutrient drink challenge (adjusted for age, gender, BMI and baseline MTV) on placebo was 1033 mL (±69) vs. 1073 mL (±76) on asimadoline 0.5 mg, and 999 mL (±74) on asimadoline 1.0 mg (overall P = 0.80) (Table 2).
Table 2.
Posttreatment satiety test results (symptom scores at 30-min postmaximum) at week 8 on asimadoline 0.5 or 1.0 mg or placebo
| Asimadoline 0.5 mg (n = 13) |
Asimadoline 1.0 mg (n = 13) |
Placebo (n = 14) |
P-value | |
|---|---|---|---|---|
| Maximum-tolerated volume (minimum, maximum) |
1073 (±75.6) (600, 2040) |
999 (±74.1) (480, 1926) |
1033 (±69.5) (490, 1680) |
0.80 |
| Nausea | 33 (±8) | 23 (±8) | 36 (±7) | 0.44 |
| Fullness | 80 (±4) | 74 (±4) | 79 (±4) | 0.48 |
| Bloating | 58 (±7) | 61 (±7) | 58 (±7) | 0.92 |
| Abdominal pain | 37 (±6) | 24 (±6) | 34 (±6) | 0.32 |
| Aggregate score | 209 (±19) | 183 (±19) | 207 (±18) | 0.55 |
Data are presented as mean (±S.E.M.), symptom scores on 100-mm visual analogue scale, adjusted for age, gender, body mass index and baseline satiation test.
Over 8 weeks of treatment, asimadoline had no significant effect on the mean postchallenge symptom score with NDT compared to placebo after adjusting for the individual subjects baseline postprandial symptom score with NDT (Table 2).
Effect on MTV by dyspepsia symptom subgroup
Post hoc analysis of treatment effects incorporating baseline dyspeptic symptom scores from the NDI as continuous covariates and corresponding interaction terms with treatment groups suggested influences on the treatment response. The overall treatment by epigastric pain symptom interaction was significant (P = 0.007). Specifically, in patients with lower epigastric pain scores at baseline, the treatment group effect indicated increased MTV values on asimadoline, while in subjects with higher epigastric pain scores at baseline, reduced MTV values were observed with asimadoline treatment. An interaction with postprandial discomfort scores was also detected (P = 0.005), with the opposite pattern observed among treatment groups depending on the level of discomfort score.
In patients with higher epigastric pain scores, asimadoline 0.5 and 1.0 mg decreased the MTV on postnutrient challenge compared to placebo, but this was not significant (P = 0.11 and P = 0.13, respectively). However, in patients with higher postprandial fullness scores, asimadoline 0.5 mg significantly increased the MTV on postnutrient challenge compared to placebo (P = 0.01), and asimadoline 1.0 mg also somewhat increased the MTV, but this was not significant (P = 0.15). Qualitatively similar results were obtained when analysing the subset of patients with abnormal baseline MTV (<1000 mL, n = 27, 68% of sample). However, the interactions between treatment and NDI scores were not significant (data not shown).
Mean of daily dyspeptic symptom severity scores during treatment and GSRS score
The mean (over 8 weeks) daily postprandial fullness symptom severity score was lower in the asimadoline 1.0 mg group, vs. asimadoline 0.5 mg and placebo, and this overall treatment effect was borderline significant (P = 0.09, Table 3). However, the pairwise treatment group differences were not significant (P = 0.15 for 0.5 mg vs. placebo, and P = 0.42 for 1.0 mg vs. placebo). The mean daily nausea symptom severity score was lower in the asimadoline 1.0 mg group vs. asimadoline 0.5 mg and placebo, but this was not significant (P = 0.52, Table 3).
Table 3.
Mean (±S.E.M.) of daily diary symptom severity scores* (range, 0–4) on asimadoline 0.5 or 1.0 mg or placebo during 8- week follow-up
| Asimadoline 0.5 mg (n = 13) |
Asimadoline 1.0 mg (n = 13) |
Placebo (n = 14) |
P-value | |
|---|---|---|---|---|
| Abdominal pain | 1.14 (±0.21) | 0.94 (±0.21) | 0.93 (±0.20) | 0.74 |
| Nausea | 0.57 (±0.17) | 0.54 (±0.16) | 0.78 (±0.16) | 0.52 |
| Postprandial fullness | 1.40 (±0.16) | 0.89 (±0.15) | 1.07 (±0.15) | 0.09 |
| Bloating | 1.40 (±0.20) | 1.22 (±0.19) | 1.14 (±0.19) | 0.65 |
| Aggregate symptom score | 1.12 (±0.14) | 0.90 (±0.14) | 0.98 (±0.14) | 0.53 |
Adjusted for age, gender and body mass index.
During the 8-week treatment period, asimadoline had no significant effect on the mean daily symptom severity score (range 0–4) compared to placebo (Table 4). Also, for the complete 12-week period, asimadoline had no significant effect on the mean of daily symptom severity score compared to placebo (data not shown).
Table 4.
Mean (±SEM) gastrointestinal symptom scores as evaluated with the Gastrointestinal Symptom Rating Scale during 8 weeks of treatment
| Mean scores* | Asimadoline 0.5 mg (n = 13) |
Asimadoline 1.0 mg (n = 13) |
Placebo (n = 14) |
P-value |
|---|---|---|---|---|
| Abdominal pain | 2.67 (±0.20) | 2.54 (±0.19) | 2.57 (±0.19) | 0.88 |
| Reflux | 2.25 (±0.24) | 2.41 (±0.23) | 2.34 (±0.23) | 0.89 |
| Diarrhoea | 1.88 (±0.19) | 1.80 (±0.18) | 2.01 (±0.18) | 0.72 |
| Indigestion | 2.64 (±0.22) | 2.84 (±0.21) | 2.71 (±0.21) | 0.79 |
| Constipation | 1.85 (±0.19) | 2.00 (±0.18) | 2.01 (±0.18) | 0.81 |
Adjusted for age, gender, body mass index and corresponding baseline score.
During the 8-week treatment period, asimadoline had no significant effect on the mean GSRS scores (range 0–6) compared to placebo (Table 4). The mean GSRS abdominal pain or indigestion symptom score also was not significantly changed during the treatment compared to placebo. Similarly, other gastrointestinal symptom dimensions, namely reflux, diarrhoea and constipation, were not significantly changed during the treatment compared to placebo.
Quality of life and asimadoline
During the 8-week treatment period, the mean NDI overall symptom score was not significantly different among the treatment groups and placebo. The overall group comparison was borderline significant (P = 0.10) for the mean tension/sleep QOL score. Pairwise differences showed a lower tension/sleep QOL on the asimadoline 0.5 mg treatment vs. placebo (P = 0.03). The other posttreatment response NDI scores were not significantly different among the treatment groups and placebo.
Safety and tolerability
No serious adverse events were reported. There were no reports of central effects, such as dizziness, headache or polyuria in those taking asimadoline or placebo. In the placebo group, four patients experienced nausea/vomiting, gas, heartburn or epigastric pain during the 8 weeks, but this did not lead to discontinuation. One patient experienced diarrhoea, which developed after an upper respiratory tract infection. In the 0.5-mg asimadoline group, five patients experienced nausea/vomiting, heartburn, bloating or epigastric pain during 8 weeks treatment; this did not lead to discontinuation. In the 1.0-mg asimadoline group, four patients experienced nausea/vomiting, heartburn or abdominal pain; this did not lead to discontinuation. The overall gastrointestinal-related adverse event rates were similar in the placebo and active treatment groups (P = 0.92, Fisher’s exact test).
DISCUSSION
We have shown that asimadoline is well tolerated, but after 8 weeks of treatment, this trial failed to demonstrate that asimadoline could significantly improve maximal-tolerated volume or postprandial symptoms induced by a nutrient challenge to the point of full satiation. We also did not observe an improvement of dyspepsia symptom scores over the 8-week treatment. However, in a post hoc (hypothesis generating) analysis where we attempted to divide subjects into the subgroups proposed by the new Rome III criteria,37 asimadoline appeared to increase MTV postnutrient challenge in the subgroup of patients with higher postprandial fullness.
Asimadoline is a kappa-opioid agonist and has antinociceptive effects in the gastrointestinal tract, at least after acute administration. Delgado-Aros et al.28 reported that asimadoline (0.15, 0.5, 1.5 mg) taken orally twice daily for 9 days increased MTV after a nutrient drink without altering postprandial symptoms; the drug also decreased fasting colonic tone in the gastrointestinal tract of healthy individuals. These effects were not accompanied by changes in gastric emptying, intestinal or colonic transit or postprandial contractions. Another healthy volunteer study by Delgado-Aros et al.32 also demonstrated that compared to placebo, a single dose of asimadoline 1.5 mg increased the maximum volume capable of being ingested during the NDT, and tended to decrease postprandial fullness. In addition, asimadoline 0.5 mg significantly increased fasting and postprandial gastric volumes in healthy females. A key difference between our study and studies of Delgado-Aros et al.28, 32 was treatment duration. It is well established that asimadoline or other peripheral kappa-opioid drugs have effects on antinociception of the gastrointestinal tract. However, it is not well established whether or not continued kappa-opioid drug use induces tachyphylaxis in humans. It is possible that chronic administration of a selective kappa-opioid agonist could lead to tolerance to subsequent drug administration.38, 39 Bhargava et al.38 reported that repeated administration of U50,488H (a selective kappa-opioid agonist) in rats caused profound tolerance to its analgesic effects. However, a study by Chen et al.40 examined the effect of asimadoline administration on the kappa-opioid receptor and found that receptors were neither down-regulated nor upregulated in the presence of asimadoline, suggesting that tachyphylaxis would be unlikely. If tachyphylaxis is an issue with chronic administration, other therapeutic approaches such as on-demand treatment may be worth testing.
The choice of the correct dose needs consideration. We chose 0.5 and 1 mg of asimadoline based on the data from a previous healthy volunteer study in our laboratory,28 but it is conceivable that higher doses may be more efficacious. In the studies by Delgado-Aros,32 asimadoline either increased MTV or decreased the sensation of fullness after the NDT at doses ranging from 0.5 to 1.5 mg twice a day. It was also observed that asimadoline 0.5 mg twice a day decreased visceral perception in human in a colonic distension model, while higher doses of asimadoline (1.5 mg twice a day) paradoxically increased visceral perception in this model.32
We did not show a major benefit of asimadoline on individual dyspeptic symptoms over 8 weeks of therapy, although the study was not powered for this endpoint. This observation is generally consistent with the 100 patients study from our group that used on-demand asimadoline for the on-demand treatment of abdominal pain in patients with irritable bowel syndrome.41
Conversely, Fraitag et al.18 reported that 6 weeks of fedotozine, another peripheral kappa-opioid receptor agonist, was significantly superior to placebo in relieving postprandial fullness, bloating, abdominal pain and nausea. Also Read et al.19 reported that fedotozine improved overall intensity of dyspeptic symptoms, epigastric pain and nausea in a multicentre trial. However, they also showed that overall physician assessment was not significant for fedotozine, and the symptom gains were very small. The development of fedotozine was ceased because efficacy was perceived to be too small.
Several studies27, 28, 32 have shown that asimadoline decreases visceral hypersensitivity in patients with functional gastrointestinal diseases. Our study also showed that asimadoline increased maximum-tolerated gastric volume but only in a post hoc analysis of patients with meal-related symptoms. The post hoc analyses must all be seen as hypothesis generating. However, low-dose asimadoline (0.5 mg) significantly increased the maximal-tolerated volume postnutrient challenge in the subjects with lower epigastric pain scores or higher postprandial fullness scores. Functional dyspepsia, however, is likely to be a heterogenous disorder with multiple aetiologies. In a study of 720 patients with functional dyspepsia where multiple putative pathophysiological disturbances were carefully assessed, predominant symptom patterns were not reliably linked to objective abnormalities, although satiety was most closely linked to impaired gastric accommodation.42 Targeting one pathophysiological disturbance alone may not be sufficient to improve dyspeptic symptoms, although a subset of patients with functional dyspepsia who have visceral hypersensitivity might respond to a visceral analgesic like asimadoline. Hence, theoretically the drug may provide maximal efficacy in those with postprandial distress syndrome as defined by Rome III.37 This hypothesis needs testing in a larger study designed to assess this specific patient population.
Notably, our data did not show any central or renal side effects, such as headache, dizziness and polyuria at the doses of asimadoline tested. Data from 24 healthy volunteers treated with high-dose asimadoline have shown that adverse events possibly of central origin occur at 5-mg doses, but no adverse events were seen at 1 mg.43
This study had some limitations. Notably, the study was not powered to detect an improvement of quality of life, and quality of life may even have deteriorated on drug. Also, our study could not detect if there was any gender difference in antinociception produced by kappa-opioid receptor agonists because of the sample size. There are other data which suggest that peripherally or centrally acting kappa receptor agonists produced greater antinociception in females compared to males, and an effect or interaction with female sex hormones has been postulated,44, 45 but others have not observed any significant gender effects with either peripheral or central kappa-opioid agonists.46
In conclusion, this pharmacodynamic randomized controlled trial failed to show that asimadoline at a dose of 0.5 or 1.0 mg two times daily for 8 weeks significantly improved satiation postnutrient challenge in patients with functional dyspepsia.
ACKNOWLEDGEMENTS
The authors wish to thank Mary Jo Rucker and Judy A. Peterson for assistance with patient recruitment and Susan M. Schlichter for their assistance in the preparation of the manuscript. Declaration of personal interests: N. J. Talley has received research support from Merck, Darmstadt and Tioga Pharma; and is also supported by u01 DK065713. Declaration of funding interests: This study was supported by Merck, Darmstadt, Germany and Tioga Pharma.
REFERENCES
- 1.Greydanus MP, Vassallo M, Camilleri M, Nelson DK, Hanson RB, Thomforde GM. Neurohormonal factors in functional dyspepsia: insights on pathophysiological mechanisms. Gastroenterology. 1991;100(5 Pt 1):1311–1318. [PubMed] [Google Scholar]
- 2.Stanghellini V, Ghidini C, Maccarini MR, Paparo GF, Corinaldesi R, Barbara L. Fasting and postprandial gastrointestinal motility in ulcer and non-ulcer dyspepsia. Gut. 1992;33:184–190. doi: 10.1136/gut.33.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilja OH, Hausken T, Wilhelmsen I, Bers-tad A. Impaired accommodation of proximal stomach to a meal in functional dyspepsia. Dig Dis Sci. 1996;41:689–696. doi: 10.1007/BF02213124. [DOI] [PubMed] [Google Scholar]
- 4.Thumshirn M, Camilleri M, Saslow SB, Williams DE, Burton DD, Hanson RB. Gastric accommodation in non-ulcer dyspepsia and the roles of Helicobacter pylori infection and vagal function. Gut. 1999;44:55–64. doi: 10.1136/gut.44.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samsom M, Verhagen MA, vanBerge Henegouwen GP, Smout AJ. Abnormal clearance of exogenous acid and increased acid sensitivity of the proximal duodenum in dyspeptic patients. Gastroenterology. 1999;116:515–520. doi: 10.1016/s0016-5085(99)70171-x. [DOI] [PubMed] [Google Scholar]
- 6.Feinle-Bisset C, Vozzo R, Horowitz M, Talley NJ. Diet, food intake, and disturbed physiology in the pathogenesis of symptoms in functional dyspepsia. Am J Gastroenterol. 2004;99:170–181. doi: 10.1111/j.1572-0241.2004.04003.x. [DOI] [PubMed] [Google Scholar]
- 7.Lemann M, Dederding JP, Flourie B, Franchisseur C, Rambaud JC, Jian R. Abnormal perception of visceral pain in response to gastric distension in chronic idiopathic dyspepsia. The irritable stomach syndrome. Dig Dis Sci. 1991;36:1249–1254. doi: 10.1007/BF01307517. [DOI] [PubMed] [Google Scholar]
- 8.Tack J, Caenepeel P, Fischler B, Piessevaux H, Janssens J. Symptoms associated with hypersensitivity to gastric distention in functional dyspepsia. Gastro-enterology. 2001;121:526–535. doi: 10.1053/gast.2001.27180. [DOI] [PubMed] [Google Scholar]
- 9.Mertz H, Fullerton S, Naliboff B, Mayer EA. Symptoms and visceral perception in severe functional and organic dyspepsia. Gut. 1998;42:814–822. doi: 10.1136/gut.42.6.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouin M, Lupien F, Riberdy M, Boivin M, Plourde V, Poitras P. Intolerance to visceral distension in functional dyspepsia or irritable bowel syndrome: an organ specific defect or a pan intestinal dysregulation? Neurogastroenterol Motil. 2004;16:311–314. doi: 10.1111/j.1365-2982.2004.00511.x. [DOI] [PubMed] [Google Scholar]
- 11.Holtmann G, Gschossmann J, Neufang-Huber J, Gerken G, Talley NJ. Differences in gastric mechanosensory function after repeated ramp distensions in non-consulters with dyspepsia and healthy controls. Gut. 2000;47:332–336. doi: 10.1136/gut.47.3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holtmann G, Gschossmann J, Buenger L, Gerken G, Talley NJ. Do changes in visceral sensory function determine the development of dyspepsia during treatment with aspirin? Gastroenterology. 2002;123:1451–1458. doi: 10.1053/gast.2002.36556. [DOI] [PubMed] [Google Scholar]
- 13.Wood JD, Galligan JJ. Function of opioids in the enteric nervous system. Neurogastroenterol Motil. 2004;16(Suppl. 2):17–28. doi: 10.1111/j.1743-3150.2004.00554.x. [DOI] [PubMed] [Google Scholar]
- 14.Langlois A, Diop L, Friese N, et al. Fedotozine blocks hypersensitve visceral pain in conscious rats: action at peripheral kappa-opioid receptors. Eur J Pharmacol. 1997;324:211–217. doi: 10.1016/s0014-2999(97)00089-7. [DOI] [PubMed] [Google Scholar]
- 15.Sengupta JN, Su X, Gebhart GF. Kappa, but not mu or delta, opioids attenuate responses to distention of afferent fibers innervating the rat colon. Gastroenterology. 1996;111:968–980. doi: 10.1016/s0016-5085(96)70064-1. [DOI] [PubMed] [Google Scholar]
- 16.Bonaz B, Riviere PJ, Sinniger V, et al. Fedotozine, a kappa-opioid agonist, prevents spinal and supra-spinal Fos expression induced by a noxious visceral stimulus in the rat. Neurogastroenterol Motil. 2000;12:135–147. doi: 10.1046/j.1365-2982.2000.00188.x. [DOI] [PubMed] [Google Scholar]
- 17.Coffin B, Bouhassira D, Chollet R, et al. Effect of the kappa agonist fedotozine on perception of gastric distension in healthy humans. Aliment Pharmacol Ther. 1996;10:919–925. doi: 10.1046/j.1365-2036.1996.109280000.x. [DOI] [PubMed] [Google Scholar]
- 18.Fraitag B, Homerin M, Hecketsweiler P. Double-blind dose-response multicenter comparison of fedotozine and placebo in treatment of nonulcer dyspepsia. Dig Dis Sci. 1994;39:1072–1077. doi: 10.1007/BF02087560. [DOI] [PubMed] [Google Scholar]
- 19.Read NW, Abitbol JL, Bardhan KD, Whorwell PJ, Fraitag B. Efficacy and safety of the peripheral kappa agonist fedotozine versus placebo in the treatment of functional dyspepsia. Gut. 1997;41:664–668. doi: 10.1136/gut.41.5.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Callahan MJ. Irritable bowel syndrome neuropharmacology. A review of approved and investigational compounds. J Clin Gastroenterol. 2002;35(1 Suppl.):S58–S67. doi: 10.1097/00004836-200207001-00011. [DOI] [PubMed] [Google Scholar]
- 21.Gottschlich R, Krug M, Barber A, Devant RM. kappa-Opioid activity of the four stereoisomers of the peripherally selective kappa-agonists, EMD 60,400 and EMD 61,753. Chirality. 1994;6:685–689. doi: 10.1002/chir.530060814. [DOI] [PubMed] [Google Scholar]
- 22.Barber A, Bartoszyk GD, Bender HM, et al. A pharmacological profile of the novel, peripherally-selective kappa-opioid receptor agonist, EMD 61753. Br J Pharmacol. 1994;113:1317–1327. doi: 10.1111/j.1476-5381.1994.tb17142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bender HM, Dasenbrock J. Brain concentrations of asimadoline in mice: the influence of coadministration of various P-glycoprotein substrates. Int J Clin Pharmacol Ther. 1998;36:76–79. [PubMed] [Google Scholar]
- 24.Sengupta JN, Snider A, Su X, Gebhart GF. Effects of kappa opioids in the inflamed rat colon. Pain. 1999;79:175–185. doi: 10.1016/s0304-3959(98)00175-4. [DOI] [PubMed] [Google Scholar]
- 25.Lembo A. Peripheral opioids for functional GI disease: a reappraisal. Dig Dis. 2006;24:91–98. doi: 10.1159/000090312. [DOI] [PubMed] [Google Scholar]
- 26.Burton MB, Gebhart GF. Effects of kappa-opioid receptor agonists on responses to colorectal distension in rats with and without acute colonic inflammation. J Pharmacol Exp Ther. 1998;285:707–715. [PubMed] [Google Scholar]
- 27.Delvaux M, Beck A, Jacob J, Bouzamondo H, Weber FT, Frexinos J. Effect of asimadoline, a kappa opioid agonist, on pain induced by colonic distension in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2004;20:237–246. doi: 10.1111/j.1365-2036.2004.01922.x. [DOI] [PubMed] [Google Scholar]
- 28.Delgado-Aros S, Chial HJ, Camilleri M, et al. Effects of a kappa-opioid agonist, asimadoline, on satiation and GI motor and sensory functions in humans. Am J Physiol Gastrointest Liver Physiol. 2003;284:G558–G566. doi: 10.1152/ajpgi.00360.2002. [DOI] [PubMed] [Google Scholar]
- 29.O’Keefe EA, Talley NJ, Tangalos EG, Zinsmeister AR. A bowel symptom questionnaire for the elderly. J Gerontol. 1992;47:M116–M121. doi: 10.1093/geronj/47.4.m116. [DOI] [PubMed] [Google Scholar]
- 30.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psy-chiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 31.Barber A, Gottschlich R. Novel developments with selective, non-peptidic kappa-opioid receptor agonists. Expert Opin Investig Drugs. 1997;6:1351–1368. doi: 10.1517/13543784.6.10.1351. [DOI] [PubMed] [Google Scholar]
- 32.Delgado-Aros S, Chial HJ, Cremonini F, et al. Effects of asimadoline, a kappa-opioid agonist, on satiation and postprandial symptoms in health. Aliment Pharmacol Ther. 2003;18:507–514. doi: 10.1046/j.1365-2036.2003.01670.x. [DOI] [PubMed] [Google Scholar]
- 33.Dimenas E, Glise H, Hallerback B, Hernqvist H, Svedlund J, Wiklund I. Quality of life in patients with upper gastrointestinal symptoms. An improved evaluation of treatment regimens? Scand J Gastroenterol. 1993;28:681–687. doi: 10.3109/00365529309098272. [DOI] [PubMed] [Google Scholar]
- 34.Talley NJ, Verlinden M, Jones M. Quality of life in functional dyspepsia: responsiveness of the Nepean Dyspepsia Index and development of a new 10-item short form. Aliment Pharmacol Ther. 2001;15:207–216. doi: 10.1046/j.1365-2036.2001.00900.x. [DOI] [PubMed] [Google Scholar]
- 35.Tack J, Piessevaux H, Coulie B, Caenepeel P, Janssens J. Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology. 1998;115:1346–1352. doi: 10.1016/s0016-5085(98)70012-5. [DOI] [PubMed] [Google Scholar]
- 36.Chial HJ, Camilleri M, Ferber I, et al. Effects of venlafaxine, buspirone, and placebo on colonic sensorimotor functions in healthy humans. Clin Gastroenterol Hepatol. 2003;1:211–218. doi: 10.1053/jcgh.2003.50031. [DOI] [PubMed] [Google Scholar]
- 37.Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466–1479. doi: 10.1053/j.gastro.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 38.Bhargava HN, Gulati A, Ramarao P. Effect of chronic administration of U-50,488H on tolerance to its pharmacological actions and on multiple opioid receptors in rat brain regions and spinal cord. J Pharmacol Exp Ther. 1989;251:21–26. [PubMed] [Google Scholar]
- 39.Liu-Chen LY. Agonist-induced regulation and trafficking of kappa opioid receptors. Life Sci. 2004;75:511–536. doi: 10.1016/j.lfs.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 40.Chen Y, Chen C, Wang Y, Liu-Chen LY. Ligands regulate cell surface level of the human kappa opioid receptor by activation-induced down-regulation and pharmacological chaperone-mediated enhancement: differential effects of nonpeptide and peptide agonists. J Pharmacol Exp Ther. 2006;319:765–775. doi: 10.1124/jpet.106.107987. [DOI] [PubMed] [Google Scholar]
- 41.Szarka LA, Camilleri M, Burton D, et al. Efficacy of on-demand asimadoline, a peripheral kappa-opioid agonist, in females with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2007;5:1268–1275. doi: 10.1016/j.cgh.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karamanolis G, Caenepeel P, Arts J, Tack J. Association of the predominant symptom with clinical characteristics and pathophysiological mechanisms in functional dyspepsia. Gastroenterology. 2006;130:296–303. doi: 10.1053/j.gastro.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 43.Kramer HJ, Uhl W, Ladstetter B, Backer A. Influence of asimadoline, a new kappa-opioid receptor agonist, on tubular water absorption and vasopressin secretion in man. Br J Clin Pharmacol. 2000;50:227–235. doi: 10.1046/j.1365-2125.2000.00256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Haaren F, Scott S, Tucker LB. kappa-opioid receptor-mediated analgesia: hotplate temperature and sex differences. Eur J Pharmacol. 2000;408:153–159. doi: 10.1016/s0014-2999(00)00769-x. [DOI] [PubMed] [Google Scholar]
- 45.Gear RW, Miaskowski C, Gordon NC, Paul SM, Heller PH, Levine JD. Kappa-opioids produce significantly greater analgesia in women than in men. Nat Med. 1996;2:1248–1250. doi: 10.1038/nm1196-1248. [DOI] [PubMed] [Google Scholar]
- 46.Binder W, Carmody J, Walker J. Effect of gender on anti-inflammatory and analgesic actions of two kappa-opioids. J Pharmacol Exp Ther. 2000;292:303–309. [PubMed] [Google Scholar]