Abstract
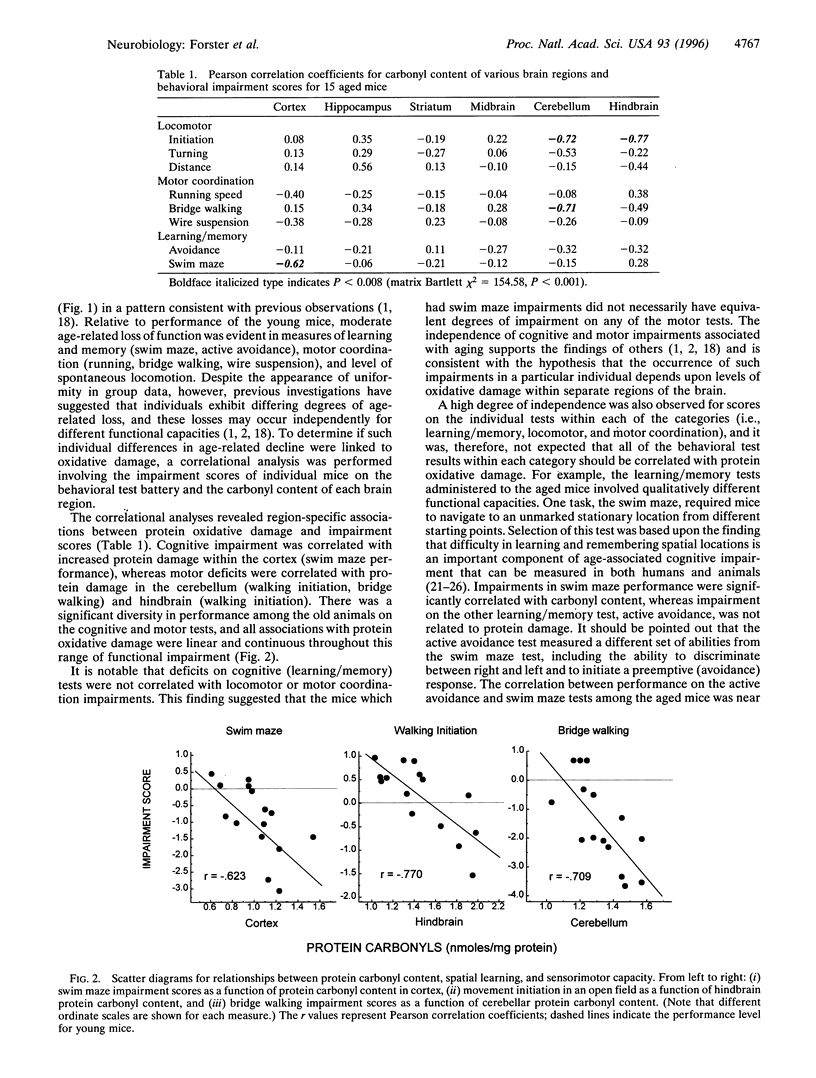
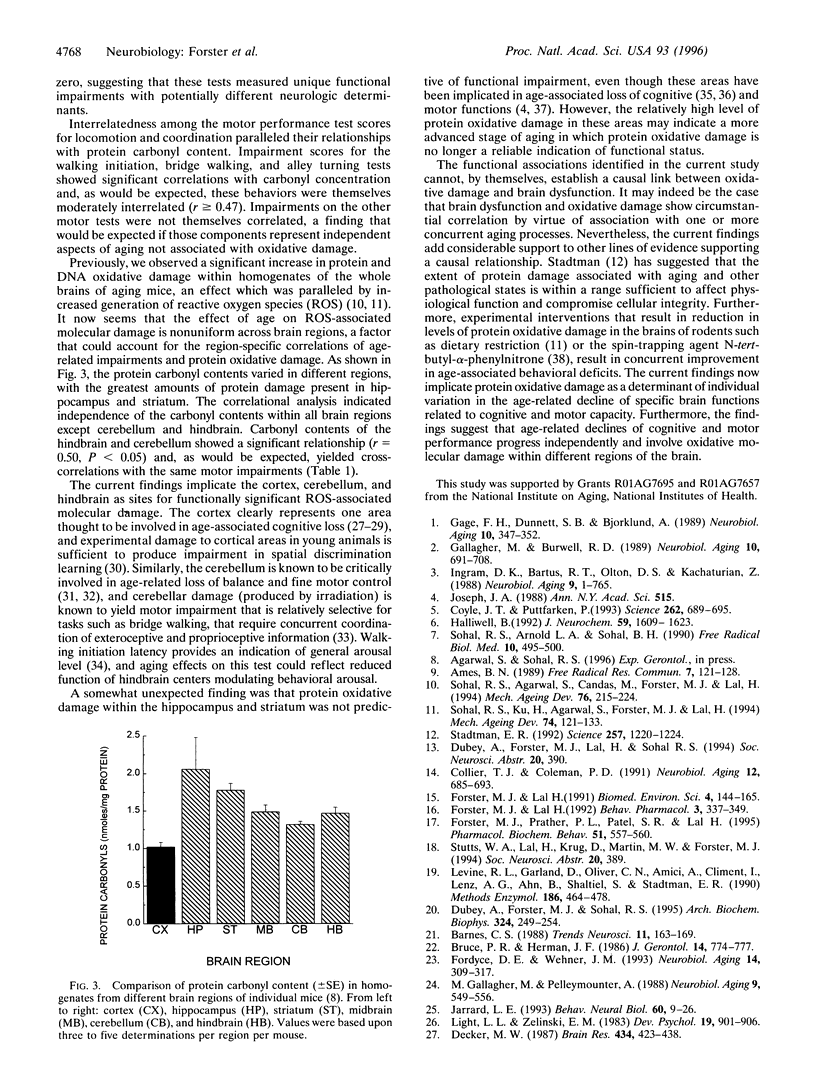
The hypothesis that age-associated impairment of cognitive and motor functions is due to oxidative molecular damage was tested in the mouse. In a blind study, senescent mice (aged 22 months) were subjected to a battery of behavioral tests for motor and cognitive functions and subsequently assayed for oxidative molecular damage as assessed by protein carbonyl concentration in different regions of the brain. The degree of age-related impairment in each mouse was determined by comparison to a reference group of young mice (aged 4 months) tested concurrently on the behavioral battery. The age-related loss of ability to perform a spatial swim maze task was found to be positively correlated with oxidative molecular damage in the cerebral cortex, whereas age-related loss of motor coordination was correlated with oxidative molecular damage within the cerebellum. These results support the view that oxidative stress is a causal factor in brain senescence. Furthermore, the findings suggest that age-related declines of cognitive and motor performance progress independently, and involve oxidative molecular damage within different regions of the brain.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N. Endogenous oxidative DNA damage, aging, and cancer. Free Radic Res Commun. 1989;7(3-6):121–128. doi: 10.3109/10715768909087933. [DOI] [PubMed] [Google Scholar]
- Barnes C. A. Spatial learning and memory processes: the search for their neurobiological mechanisms in the rat. Trends Neurosci. 1988 Apr;11(4):163–169. doi: 10.1016/0166-2236(88)90143-9. [DOI] [PubMed] [Google Scholar]
- Bruce P. R., Herman J. F. Adult age differences in spatial memory: effects of distinctiveness and repeated experience. J Gerontol. 1986 Nov;41(6):774–777. doi: 10.1093/geronj/41.6.774. [DOI] [PubMed] [Google Scholar]
- Brunner R. L., Altman J. Locomotor deficits in adult rats with moderate to massive retardation of cerebellar development during infancy. Behav Biol. 1973 Aug;9(2):169–188. doi: 10.1016/s0091-6773(73)80154-3. [DOI] [PubMed] [Google Scholar]
- Burwell R. D., Lawler C. P., Gallagher M. Mesostriatal dopamine markers in aged Long-Evans rats with sensorimotor impairment. Neurobiol Aging. 1995 Mar-Apr;16(2):175–186. doi: 10.1016/0197-4580(94)00157-x. [DOI] [PubMed] [Google Scholar]
- Carney J. M., Starke-Reed P. E., Oliver C. N., Landum R. W., Cheng M. S., Wu J. F., Floyd R. A. Reversal of age-related increase in brain protein oxidation, decrease in enzyme activity, and loss in temporal and spatial memory by chronic administration of the spin-trapping compound N-tert-butyl-alpha-phenylnitrone. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3633–3636. doi: 10.1073/pnas.88.9.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier T. J., Coleman P. D. Divergence of biological and chronological aging: evidence from rodent studies. Neurobiol Aging. 1991 Nov-Dec;12(6):685–693. doi: 10.1016/0197-4580(91)90122-z. [DOI] [PubMed] [Google Scholar]
- Coyle J. T., Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993 Oct 29;262(5134):689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- Decker M. W. The effects of aging on hippocampal and cortical projections of the forebrain cholinergic system. Brain Res. 1987 Nov;434(4):423–438. doi: 10.1016/0165-0173(87)90007-5. [DOI] [PubMed] [Google Scholar]
- Dubey A., Forster M. J., Sohal R. S. Effect of the spin-trapping compound N-tert-butyl-alpha-phenylnitrone on protein oxidation and life span. Arch Biochem Biophys. 1995 Dec 20;324(2):249–254. doi: 10.1006/abbi.1995.0037. [DOI] [PubMed] [Google Scholar]
- Fordyce D. E., Wehner J. M. Effects of aging on spatial learning and hippocampal protein kinase C in mice. Neurobiol Aging. 1993 Jul-Aug;14(4):309–317. doi: 10.1016/0197-4580(93)90116-s. [DOI] [PubMed] [Google Scholar]
- Forster M. J., Prather P. L., Patel S. R., Lal H. The benzodiazepine receptor inverse agonist RO 15-3505 reverses recent memory deficits in aged mice. Pharmacol Biochem Behav. 1995 Jun-Jul;51(2-3):557–560. doi: 10.1016/0091-3057(95)00063-3. [DOI] [PubMed] [Google Scholar]
- Forster M.J., Lal H. Within-subject behavioral analysis of recent memory in aging mice. Behav Pharmacol. 1992 Aug;3(4):337–349. [PubMed] [Google Scholar]
- Gage F. H., Dunnett S. B., Bjorklund A. Age-related impairments in spatial memory are independent of those in sensorimotor skills. Neurobiol Aging. 1989 Jul-Aug;10(4):347–352. doi: 10.1016/0197-4580(89)90047-x. [DOI] [PubMed] [Google Scholar]
- Gallagher M., Burwell R. D. Relationship of age-related decline across several behavioral domains. Neurobiol Aging. 1989 Nov-Dec;10(6):691–708. doi: 10.1016/0197-4580(89)90006-7. [DOI] [PubMed] [Google Scholar]
- Gallagher M., Pelleymounter M. A. Spatial learning deficits in old rats: a model for memory decline in the aged. Neurobiol Aging. 1988 Sep-Dec;9(5-6):549–556. doi: 10.1016/s0197-4580(88)80112-x. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Reactive oxygen species and the central nervous system. J Neurochem. 1992 Nov;59(5):1609–1623. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- Jarrard L. E. On the role of the hippocampus in learning and memory in the rat. Behav Neural Biol. 1993 Jul;60(1):9–26. doi: 10.1016/0163-1047(93)90664-4. [DOI] [PubMed] [Google Scholar]
- Kolb B., Sutherland R. J., Whishaw I. Q. A comparison of the contributions of the frontal and parietal association cortex to spatial localization in rats. Behav Neurosci. 1983 Feb;97(1):13–27. doi: 10.1037//0735-7044.97.1.13. [DOI] [PubMed] [Google Scholar]
- Landfield P. W. Hippocampal neurobiological mechanisms of age-related memory dysfunction. Neurobiol Aging. 1988 Sep-Dec;9(5-6):571–579. doi: 10.1016/s0197-4580(88)80116-7. [DOI] [PubMed] [Google Scholar]
- Levine R. L., Garland D., Oliver C. N., Amici A., Climent I., Lenz A. G., Ahn B. W., Shaltiel S., Stadtman E. R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- Masliah E., Terry R. D., DeTeresa R. M., Hansen L. A. Immunohistochemical quantification of the synapse-related protein synaptophysin in Alzheimer disease. Neurosci Lett. 1989 Aug 28;103(2):234–239. doi: 10.1016/0304-3940(89)90582-x. [DOI] [PubMed] [Google Scholar]
- Rogers J., Silver M. A., Shoemaker W. J., Bloom F. E. Senescent changes in a neurobiological model system: cerebellar Purkinje cell electrophysiology and correlative anatomy. Neurobiol Aging. 1980 Summer;1(1):3–11. doi: 10.1016/0197-4580(80)90018-4. [DOI] [PubMed] [Google Scholar]
- Roth K. A., Katz R. J. Stress, behavioral arousal, and open field activity--a reexamination of emotionality in the rat. Neurosci Biobehav Rev. 1979 Winter;3(4):247–263. doi: 10.1016/0149-7634(79)90012-5. [DOI] [PubMed] [Google Scholar]
- Sohal R. S., Agarwal S., Candas M., Forster M. J., Lal H. Effect of age and caloric restriction on DNA oxidative damage in different tissues of C57BL/6 mice. Mech Ageing Dev. 1994 Oct 20;76(2-3):215–224. doi: 10.1016/0047-6374(94)91595-4. [DOI] [PubMed] [Google Scholar]
- Sohal R. S., Arnold L. A., Sohal B. H. Age-related changes in antioxidant enzymes and prooxidant generation in tissues of the rat with special reference to parameters in two insect species. Free Radic Biol Med. 1990;9(6):495–500. doi: 10.1016/0891-5849(90)90127-5. [DOI] [PubMed] [Google Scholar]
- Sohal R. S., Ku H. H., Agarwal S., Forster M. J., Lal H. Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech Ageing Dev. 1994 May;74(1-2):121–133. doi: 10.1016/0047-6374(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Special issue in memory of Dr. Arthur Cherkin. Neurobiol Aging. 1988 Jan-Feb;9(1):1–125. [PubMed] [Google Scholar]
- Stadtman E. R. Protein oxidation and aging. Science. 1992 Aug 28;257(5074):1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- Winocur G., Moscovitch M. Hippocampal and prefrontal cortex contributions to learning and memory: analysis of lesion and aging effects on maze learning in rats. Behav Neurosci. 1990 Aug;104(4):544–551. doi: 10.1037//0735-7044.104.4.544. [DOI] [PubMed] [Google Scholar]
- deToledo-Morrell L., Geinisman Y., Morrell F. Age-dependent alterations in hippocampal synaptic plasticity: relation to memory disorders. Neurobiol Aging. 1988 Sep-Dec;9(5-6):581–590. doi: 10.1016/s0197-4580(88)80117-9. [DOI] [PubMed] [Google Scholar]


