Abstract
In vertebrates, the Müllerian duct elongates along the Wolffian duct, a mesonephric structure that is required for Müllerian duct formation. Recently, several genes required for initial Müllerian duct formation have been identified. However, the precise mechanism of Müllerian duct elongation remains to be elucidated. In this study, we investigated dynamic morphological changes in the elongating Müllerian duct in rat urogenital ridges in organ culture manipulated by microincision and/or chemical inhibitors. Mechanical division of the developing Müllerian duct showed that epithelial cells of the Müllerian duct actively migrate along the anterior–posterior axis independent of the proliferative expansion of the anterior portion of the duct. We found that the PI3K/AKT signaling pathway is activated in the Müllerian duct epithelium and is required for elongation of the tip of the duct; however, migration of Müllerian duct epithelial cells proximal to the tip remains intact when PI3K/AKT is inactivated. Although much is known about the molecular and cellular mechanisms leading to Müllerian duct regression, the present findings provide a fuller understanding of the mechanisms contributing to Müllerian duct formation and to the general process of early tubulogenesis.
Keywords: Müllerian duct, Cell migration, Duct elongation, PI3K/AKT pathway, LY294002, Phosphorylated AKT, Wolffian duct, Coelomic epithelium, Tubulerization
Introduction
The Müllerian duct is the anlage of the female reproductive tract in mammals giving rise to the oviduct, uterus, and the upper third of the vagina (Hunter, 1929; Cunha, 1975). During embryonic development, both sexes form the Müllerian (paramesonephric) duct in the mesonephros. Thereafter, the now sexually dimorphic Müllerian duct continues to grow in the female while it regresses completely in males in response to Müllerian Inhibiting Substance (MIS) secreted from the testes (Jost, 1947; Picon, 1969; Hayashi et al., 1982; Tsuji et al., 1992; Allard et al., 2000; Zhan et al., 2006). Müllerian duct formation is divided into three phases: initiation, invagination, and elongation (Orvis and Behringer, 2007). During the first two phases, a group of Lim1 positive coelomic epithelial cells, at the most anterior part of the mesonephros, invaginate under the influence of Wnt4 to commence Müllerian duct formation. The third phase begins when the Müllerian duct elongates between the already formed Wolffian duct and the coelomic epithelium along the anterior–posterior (A–P) axis (Gruenwald, 1941; Dyche, 1979; Orvis and Behringer, 2007). The origin of Müllerian duct epithelial (MDE) cells contributing to the elongation of the duct has been controversial (Gruenwald, 1941; Frutiger,1969; Del Vecchio,1982; Inomata et al.,1989). However, electron microscopy and genetic fate mapping have demonstrated that the MDE originates exclusively from the most anterior part of the mesonephric coelomic epithelium (Jacob et al., 1999; Guioli et al., 2007; Orvis and Behringer, 2007). It has also been demonstrated, by extirpation or blockage of the Wolffian duct in the chick and by mouse mutations, that the Wolffian duct is required for Müllerian duct elongation (Gruenwald, 1937; Bishop-Calame, 1966; Didier, 1971, 1973; Kobayashi et al., 2005; Pedersen et al., 2005). Mice mutant for either of the transcription factors Lim1, Emx2, and Pax2, all of which are needed for Wolffian duct development, are devoid of Müllerian ducts (Torres et al., 1995; Miyamoto et al., 1997; Kobayashi and Behringer, 2003; Kobayashi et al., 2004). In addition, mutations in Wnt9b, which is expressed in the Wolffian duct but is not required for its formation, also lead to Müllerian duct dysgenesis. Although Wnt9b null mice exhibit normal Wolffian duct development, Müllerian duct elongation, the third phase of its formation, is absent, suggesting that Wnt9b acts as a diffusible signal required for the duct elongation (Carroll et al., 2005). Thus, Müllerian duct development is not only dependent on the physical presence of the Wolffian duct, but also on signals emanating from it.
The highly proliferative state of MDE cells along the entire elongating Müllerian duct is thought to be a major contributor to its extension along the A–P axis (Jacob et al., 1999; Guioli et al., 2007). Although passive propulsion driven by intense proliferation is likely a major mechanism required for rapid elongation of the Müllerian duct, it is also possible that a strong stimulus from additional guidance signals is needed to move and direct the MDE cells.
While the genes involved in Müllerian duct formation have been well studied, the signaling pathways responsible for Müllerian duct development are less well understood. The phosphatidylinositol 3-kinase (PI3K)/AKT pathway is known to play major roles in cell proliferation, inhibition of apoptosis, cell adherence, and migration, in normal development and in many malignant neoplasms (reviewed by Krasilnikov, 2000; Vivanco and Sawyers, 2002). Although the PI3K/AKT pathway is known to be activated in the epithelial cells of developing ureter, lung, and submandibular gland, and is essential for proper tube formation and branching, especially budding of the epithelium into surrounding mesenchyme (Tang et al., 2002; Larsen et al., 2003; Steinberg et al., 2005; Wang et al., 2005), the status of the PI3K activity in the Müllerian duct has not been investigated. The basement membrane of the Müllerian duct epithelium can be detected early in the formation of the duct (Gruenwald, 1941; Jacob et al., 1999). Components of the basement membranes are known to stimulate receptor tyrosine kinases (Panayotou et al., 1989; Vogel et al., 1997; reviewed by Tran et al., 2004), and to activate the PI3K/AKT pathway. Hurst et al., (2002) showed that, at a later embryonic stage, the MDE cells express epidermal growth factors receptor (EGFR) (Okano et al., 2000), which also can activate the PI3K/AKT pathways. These reports suggested the PI3K/AKT pathway might be activated in the Müllerian duct.
In the present study, we investigated cell movement in the developing Müllerian duct epithelium in the rat urogenital ridge by mechanically dividing the Müllerian duct into segments by microincisions into the mesonephros. This dissection isolated a group of distal MDE cells (see methods and Fig. 1) from the anterior portion of the duct, following which we observed the effects on elongation and progression of the Müllerian duct over a specified time course in organ culture (Donahoe et al., 1977; Zhan et al., 2006). During this process, we investigated the status of the PI3K/AKT pathway in the MDE, and by using chemical PI3K inhibitors, examined the dependence of Müllerian duct development, particularly its elongation, on this pathway. Our results suggest that, in addition to active proliferation, cell migration is also an important mechanism for Müllerian duct development. This MDE cell migration is PI3K/AKT independent while Müllerian duct tip elongation and subsequent mesonephric development are PI3K/AKT dependent.
Fig. 1.
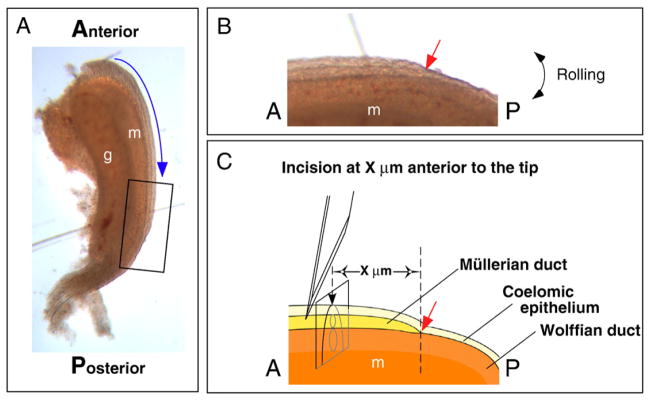
The rat urogenital ridge and the method for making incisions in the mesonephros to divide the Müllerian duct. (A) Rat urogenital ridges were harvested at E14.5. Blue arrow indicates the direction of Müllerian duct elongation along the A–P axis. (B) Higher magnification of the whole mount image of the distal tip of the Müllerian duct (box in A). (C) A schematic defining of the image in (B). The lateral edge of the mesonephros was incised with a scalpel blade, deep enough to sever both the Müllerian duct and the Wolffian duct. The site of the incision is determined by a set distance from the Müllerian duct tip (X μm anterior to the tip). Red arrows in (B) and (C) indicate the tip of the Müllerian duct. A, anterior; P, posterior; g, gonad; m, mesonephros.
Materials and methods
Animals, harvesting of urogenital ridges, micromanipulation, and organ culture
Urogenital ridges (Fig. 1A) were obtained from the embryos of timed pregnant Sprague–Dawley rats (Harlan) and studied at developmental stages from embryonic day E13.5 to E16.5. With the aid of a dissecting microscope, transverse incisions were made with a #11 scalpel blade in the lateral edge of the urogenital ridges deep enough to sever the Wolffian duct (Fig. 1C); the growing Müllerian duct tip was detected by rolling the specimen (Fig. 1B), and incisions were made at a set distance anterior or posterior to the Müllerian duct tip (Red Arrow, Figs. 1B and C).
Tissues were cultured at the air/fluid interface on 2% agarose coated stainless steel grids for up to 48 h or submerged for up to 16 h in CMRL1066 medium (Life Technologies) supplemented with 10% female fetal bovine serum, penicillin, and streptomycin at 37 °C in 5% CO2 in a humid atmosphere. In all experiments, at least two urogenital ridges were treated per condition, and each experiment was repeated at least 3 times.
All animal protocols were approved by the Massachusetts General Hospital Internal Review Committee on the use of animals and experiments (IACUC approval #2005N000281/2).
Fixation of tissues and preparation of slides
Tissues were fixed in 4% paraformardehyde in phosphate buffered saline overnight at 4 °C. For preparing permanent sections, tissues were treated with increasing concentrations of alcohol and xylene, embedded in paraffin, and sectioned at 6 μm. For frozen sections, fixed tissues were treated with increasing concentrations of sucrose up to 35%, then embedded in Tissue Freezing Medium (Triangle Biomedical Sciences), frozen at −20 °C, and 8 μm (BrdU staining) or 10 μm sections thenprepared.
Whole mount in situ hybridization
Whole mount in situ hybridization was used for visualizing the Müllerian duct (Zhan et al., 2006). Fixed tissues were dehydrated, rehydrated, treated with 6% hydrogen peroxide and proteinase K, re-fixed, pre-hybridized, and then hybridized with mouse Wnt7a anti-sense digoxigenin labeled riboprobe (1 μg/ml) overnight at 70 °C. After hybridization, tissues were rinsed thoroughly, blocked with 10% sheep serum, then incubated with anti-digoxigenin-AP antibody (1:1,500) (Roche Pharmaceuticals, # 1093274) overnight at 4 °C. BM-Purple AP substrate (Roche, # 1442074) was used to detect the hybridized probe colorimetrically. Samples were subsequently cryosectioned at 10 μm. Mouse Wnt7a anti-sense riboprobe was synthesized from the IMAGE consortium clone (GenBank Accession Number BC049093) as previously described (Zhan et al., 2006).
5-bromo-2′-deoxyuridine (BrdU) labeling
Tissues were incubated in the complete medium described above supplemented with 50 μM BrdU (Sigma, #B-5002) for 30 min to 8 h, fixed, and then processed for paraffin sections, frozen sections, or whole mount in situ hybridization.
Phosphatidylinositol 3-phosphate (PI3K) inhibition
Urogenital ridges were submerged and incubated in complete medium with the PI3K inhibitors, LY294002 (Cell Signaling Technologies, #9901) at 1–50 μM and wortmannin (Fluka, #95455) at 10–500 nM, for 30 min to 24 h. Tissues were then fixed and processed for paraffin sections, frozen sections, or whole mount in situ hybridization as described above.
Histology, immunofluorescence, and immunohistochemistry
For antigen retrieval, sections were microwaved in 0.01 M sodium citrate (pH 6.0) for 1 min at maximum power then allowed to cool to room temperature. Prior to staining for BrdU, slides were incubated in 6% hydrogen peroxide at room temperature for 10 min, in 20 μg/ml of proteinase K at 37 °C for 20 min, and then in 2 N hydrochloric acid at 37 °C for 30 min. Slides were blocked with 5% bovine serum albumin in Tris-buffered saline with 0.1% Tween-20 (TBST) for 1 h at room temperature. Primary antibodies were diluted in 5% BSA in TBST and applied to the slides for 1 h at room temperature; primary antibodies include rabbit monoclonal anti-phosphorylated AKT (Ser 473) (1:100 dilution; Cell Signaling, #4058), rabbit polyclonal anti-human PAX8 (1:500 dilution; a kind gift from Dr. R. Drapkin of the Dana Faber Cancer Institute, Boston), and mouse monoclonal anti-BrdU (1:400 dilution; Sigma, #B2531). Slides were incubated with secondary antibodies diluted in TBST for 1 h at room temperature; secondary antibodies included AlexaFluor 488 goat anti-rabbit IgG or AlexaFluor 555 goat anti-mouse IgG (1:1000 dilution; Invitrogen), or biotinylated goat anti-mouse IgG (1:250 dilution; Vector Laboratories, #BA-9200). After washing, slides were coverslipped with Vectashield with DAPI (Vector, #H-1200) for immunofluorescence. For BrdU detection, slides were treated with the Vectastain Elite® ABC kit (Vector, #PK-6100) and color developed in 3,3-diaminobenzidine in 0.01% hydrogen peroxide in TBST, then counterstained with eosin. The 6 μm paraffin sections were stained with hematoxylin and eosin (H and E).
Terminal deoxynucleotidyl transferase mediated dUTP-X nick end labeling (TUNEL)
To detect apoptotic cells by TUNEL staining, either paraffin embedded 6 μm or 8 μm frozen sections were prepared using the In Situ Cell Death Detection Kit (Roche, #1684795) according to the manufacturer’s instructions. Slides were incubated in 10 μg/ml proteinase K at 37 °C for 20 min to permit antigen retrieval.
Results
Müllerian duct epithelial (MDE) cells actively migrate along the A–P axis
To understand the precise mechanisms of Müllerian duct elongation, we divided rat mesonephroi into segments by mechanical incisions and studied Müllerian duct movement in organ culture. At E14.5 in the developing urogenital ridge, the Müllerian duct, which can be visualized by whole mount in situ hybridization with Wnt7a (Miller and Sassoon, 1998: Parr and McMahon,1998; Zhan et al. 2006), elongates alongside the previously formed Wolffian duct (Figs. 2A, D, and G). When a horizontal incision was made into the mesonephros 200 μm posterior to the tip of the elongating Müllerian duct (black arrow in Fig. 2B), the leading tip of the Müllerian duct migrated to the incision site in organ culture (black arrow in Fig. 2E) and then enlarged proportionate to the time of incubation; the Müllerian duct never extended posterior beyond the point of incision (Figs. 2E and H). In contrast to the bulbous change at the tip (Fig. 2H), the diameter of the anterior region of the Müllerian duct remained the same as that observed along the length of the intact control ridge (Figs. 2D, E and G, H). Cross sections anterior to the bulbous tip (Fig. 2H, red line) showed normal MDE morphology (Fig. 2L). Cross sections at the bulbous tip of the Müllerian duct (solid black line in Fig. 2H) showed an increased number of the MDE cells, aligned with epithelial polarity (Fig. 2M). Cross sections at the dashed line in Fig. 2H show small packed cells at the very tip (Fig. 2N).
Fig. 2.
Divided Müllerian duct segments (E14.5–15.5) move caudally without propulsion from a more anterior segment. The Müllerian duct is visualized (A–K) over time by whole mount in situ hybridization for Wnt7a. Intact urogenital ridges harvested at E14.5 (A), and after 10 h (D) or 20 h (G) in organ culture, show normal elongation of the Müllerian duct. Mesonephroi were incised at 200 μm posterior to the Müllerian duct tip (arrow) at E14.5 (B), and incubated for 10 h (E) or 20 h (H). Elongation was blocked at the site of incision (arrow) after 10 h and the tip enlarged relative to the time of incubation. The mesonephroi were incised at 200 μm and 700 μm anterior to the Müllerian duct tip (arrows) at E14.5 (C), and incubated 10 h (F) or 20 h (I). The bulge of the Müllerian duct anterior to the incisions and the Müllerian duct gap posterior to the incisions were seen at each incision site after 10 h. The tip of the most distal segment (red arrowhead) moved at the same rate as did the tip of the intact Müllerian duct (compare D and F; G and I). E 15.5 female rat urogenital ridges incised at 2 sites (J) and incubated for 20 h (K) similarly showed the bulging tip of the Müllerian duct anterior to the incisions and the gap of the Müllerian duct posterior to the incisions (J, K) after 20 h. Transverse sections (L–N) of the mesonephros at the levels of the red line (L), black line (M), and dashed line (N) in (H) stained with H and E, show cell accumulation and enlargement of diameter at (M) and cell accumulation at the distal blocked tip (N) of the Müllerian duct. Arrow, site of incision; red arrowhead, posterior tip of the Müllerian duct. Scale bar: 500 μm in (A)–(K), 100 μm in (L)–(N).
When the developing Müllerian duct was divided at two sites (Fig. 2C, black arrows), 200 μm and 700 μm anterior to the tip (red arrowhead), thereby creating three segments, Wnt7a positive MDE cells appeared to proceed towards and accumulate at the posterior end of each segment during incubation, leaving no MDE cell on the immediate posterior side of each incision site (Figs. 2F and I). The leading tip of the isolated most posterior segment of the Müllerian duct shifted towards the caudal end of the mesonephroi at the same rate as occurred in intact ridges (compare the position of the tips indicated by the red arrowheads in Figs. 2D and G with F and I). Bulging of the Müllerian duct just anterior to incision sites and absence of Wnt7a positive MDE cells just posterior to incision sites, except for the most anterior segment, were replicated regardless of the level of incision (data not shown).
At E15.5, the Müllerian duct has reached the urogenital sinus and is separated from the Wolffian duct by mesenchymal cells (Figs. 2J and L). When urogenital ridges at E15.5 were incubated after mesonephric incision, the same findings, the gap of the Müllerian duct posterior to the site of incision and the bulge, just anterior to incision were observed after 20 h of incubation as in E14.5 ridges (compare Figs. 2J and K with C and I). These results indicate that the MDE cells, during duct elongation and even after reaching the urogenital sinus, move actively unless the route is blocked along the A–P axis.
Isolated Müllerian duct tip migrates along the A–P axis without apoptosis or lateral migration
To understand further the details of MDE cell movement, we studied a time course of histological changes at a single section level in the posterior urogenital ridge during elongation of the duct. At E14.5, the Müllerian duct was divided at 250 μm anterior to the tip (black arrow in Figs. 3A–D) and transverse sections at 500 μm posterior to the initial incision site were studied at two-hour intervals for 24 h (dashed line in Figs. 3A–D at 8 hour intervals).
Fig. 3.
The Müllerian duct cell migrates along the A–P axis without lateral migration or apoptosis. Time course of the E14.5 urogenital ridge whose Müllerian duct was divided at 250 μm (arrow) anterior to the tip (red arrowhead). Transverse sections at 500 μm posterior to the initial incision site (dashed line) were studied for 24 h. E14.5 urogenital ridge was incised in the mesonephros (A) and incubated for 8 h (B), 16 h (C), and 24 h (D), followed by whole mount in situ hybridization for Wnt7a. Transverse sections observed at 0 h (E, I and M) at the level of the dashed line in (A), after 8 h (F, J, N and Q) in (B), after 16 h (G, K and O) in (C), and after 24 h (H, L and P), of incubation in (D). (E–H) H and E staining. (I–L) PAX8 staining, green; DAPI staining, blue. (M–P) TUNEL staining, green; DAPI, blue. (Q and R) BudU staining of section made at the dashed line in (B) after incubation with 50 μM of BrdU for 8 h following Müllerian duct division. Migrating tip of the Müllerian duct contains dividing (BrdU) as well as non-dividing (eosin) cells. (R) is a higher magnification of the dashed square in (Q). BrdU, brown; eosin, pink. Arrow, initial incision site; red arrowhead, tip of the isolated posterior Müllerian duct. Autofluorescence in the blood vessel was observed in the mesenchymal area in (I)–(P). Scale bar: 500 μm in (A)–(D), 100 μm, 100 μm in (Q) and 50 μm in (R).
At initiation of the culture, before the Müllerian duct reached the observation level (dashed line Fig. 3A), the Wolffian duct, which expresses PAX8 (Fig. 3I), and the coelomic epithelium were closely approximated (Figs. 3E and I). Wnt7a was not detected at the observation level at this time (data not shown). After 8 h, the isolated posterior portion of the Müllerian duct reached the observation level (Fig. 3B) and insinuated itself between the Wolffian duct and the coelomic epithelium (Fig. 3F). PAX8 marked the nuclei of both the Wolffian duct and the Müllerian duct epithelial cells (Fig. 3J). After 16 h, when, for the most part, the isolated Müllerian duct has crossed the observation level (Fig. 3C), the diameter of the Müllerian duct has decreased (Fig. 3G). The number of PAX8 stained nuclei in the Müllerian duct decreased (Fig. 3K) compared to that seen at 8 h (Fig. 3J), and remained along the original line of migration without scattering. After 24 h, when the isolated MDE cells have passed the observation level (Fig. 3D), only mesehchymal cells were found between the Wolffian duct and the coelomic epithelium (Fig. 3H), as determined by the absence of either PAX8 (Fig. 3L) or Wnt7a staining (data not shown) The lack of Pax8 or Wnt7a positive cells outside the line of migration suggested that the MDE cells retained their epithelial integrity as they migrated and did not move laterally off the A–P course.
To determine whether apoptosis leads to the disappearance of the Wnt7a positive MDE cells, we conducted TUNEL staining. MDE cells exhibited little to no apoptosis as they crossed the observation level (Figs. 3N and O), nor were they seen in the area between the Wolffian duct and the coelomic epithelium after they have passed that area (Fig. 3P), indicating that apoptosis does not play a prominent role in the disappearance of the MDE cells after incision.
Taken together, the caudal shift of the isolated posterior portion of the Müllerian duct epithelium seems the result of cell migration along the A–P axis without significant apoptosis or lateral migration.
Non-dividing as well as dividing cells contribute to Müllerian duct elongation
We followed the contribution of dividing, as well as non-dividing MDE cells to Müllerian duct elongation with BrdU. Urogenital ridges were incised as described and then incubated with 50 μM of BrdU continuously. After 8-h of incubation, when the isolated Müllerian duct tip has shifted posteriorly (Fig. 3B), BrdU negative MDE cells were still present at the observation level, as visualized by immunohistochemistry (Figs. 3Q and R), indicating that BrdU negative cells contributed to Müllerian duct elongation by migration, as did BrdU positive dividing cells by proliferation. Thus both migration and proliferation contributed to elongation.
PI3K/AKT pathway is highly activated in the Müllerian ducts
PI3K/AKT pathway has been shown to play an important role during tubulogenesis in other organs (Tang et al., 2002; Larsen et al., 2003; Steinberg et al., 2005; Wang et al., 2005). Given the cell migration and rare apoptosis of MDE cells that we observed (Figs. 2 and 3), and the proximal active proliferation that we and others (Fig. 4B; Jacob et al., 1999; Guioli et al., 2007) observed, we examined and found that the PI3K/AKT pathway was activated in the developing Müllerian duct as measured by phosphorylation of AKT with the anti-Phosphorylated-AKT (P-AKT) (Ser473) antibody.
Fig. 4.
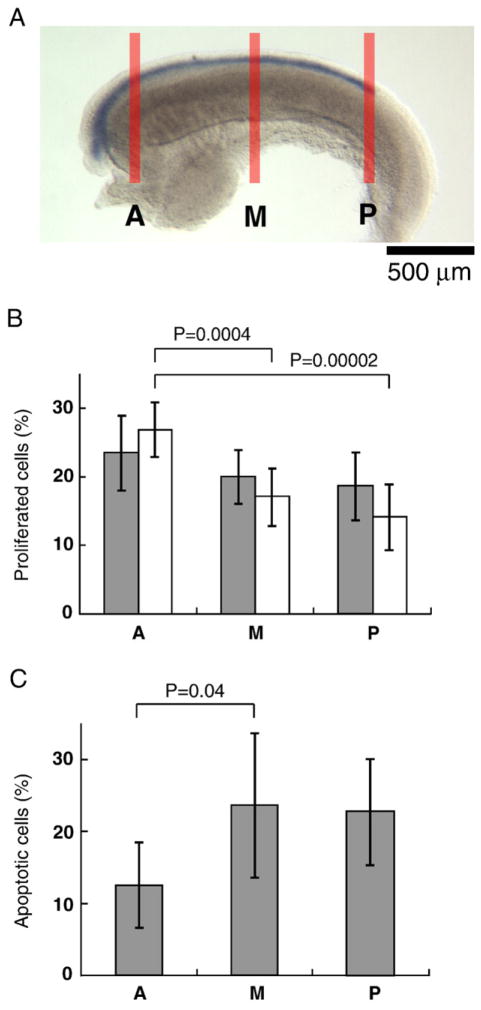
Proliferation and apoptosis of the MDE cells under PI3K inhibition. (A) E14.5 rat female urogenital ridge stained with whole mount in situ hybridization for Wnt7a with thick transverse lines indicating the positions examined. After incubation with or without 25 μM of LY294002 for 2 or 4 h, ridges were fixed, sectioned through and stained. Five serial sections were selected from the posterior tip of the Müllerian duct (P), anterior portion where the most anterior portion of the ovary is included (A) and the mid level (M). (B) Percentages of divided MDE cells were determined in the different portions of the Müllerian duct after 2-h incubation with or without 25 μM of LY294002. Divided cells were detected by BrdU, PAX8, and DAPI staining. Six urogenital ridges were examined (N=6). Columns represent mean percentages ± SD of BrdU positive cells in the MDE cells with LY294002 (gray columns) and nontreatment (white columns). Significant differences between the proliferating proportion in A, M, and P were determined by single-factor analysis of variance (ANOVA) and multiple comparison (p<0.05). A gradual decrease in the proportion of proliferating cells was observed along the A-P axis, with position A showing a statistically higher proliferation than M and P (p=0.0004 and 0.00002, respectively). Student’s t-test indicated that LY294002 did not affect the rate of proliferation of the MDE at any position. (C) The percentage of apoptotic MDE cells was examined in different portions of the Müllerian duct after 4 h of incubation with 25 μM of LY294002. Apoptotic cells were detected by TUNEL, PAX8, and DAPI staining. Six urogenital ridges were examined (N=6). Columns represent mean percentages ± SD of TUNEL positive cells in the Müllerian duct. Statistical analysis using multiple comparison showed that apoptosis in A was significantly less than M (p=0.04), while P (PL) did not differ significantly from M and A.
P-AKT was consistently detected in the Wolffian duct epithelium, mesonephric tubules and the gonad from E14.5 to E16.5 in both sexes (Figs. 5A–G). In the Müllerian duct, P-AKT was detected by late E13.5 when Müllerian duct formation has just commenced at the anterior end of the mesonephros (data not shown). As the Müllerian duct elongates, P-AKT was detected on the Müllerian duct epithelium from the funnel shaped anterior end to the posterior leading tip at an intensity higher than that observed in the adjacent Wolffian duct (Figs. 4A and B, female; and E, male). Before the Müllerian duct had fully elongated and reached the urogenital sinus, there was no sexual dimorphism in P-AKT level, consistent with the concept that Müllerian duct formation is identical until male specific regression begins (Orvis and Behringer, 2007). However, in synchrony with regression of the Müllerian duct, P-AKT expression decreased (E15.5: Fig. 5F) and eventually disappeared (E16.5: Fig. 5G) in the male Müllerian duct, but remained intense in the female Müllerian duct (Figs. 5C and D). Consistent with these observations in the rat, we also detected P-AKT in the Müllerian duct in the mouse from E12 to E14 (data not shown).
Fig. 5.
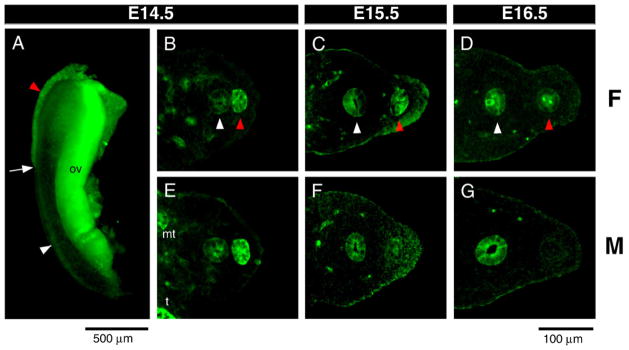
Activated PI3K/AKT pathway is in the Müllerian duct. (A) Phosphorylated-AKT (P-AKT) was detected by whole mount immunofluorescence of E14.5 female urogenital ridge stained with anti-P-AKT antibody. Transverse sections of urogenital ridges at the level of the center of gonads (B–G) stained with anti-P-AKT antibody. E14.5 female (B) and male (E) show that expression in the Müllerian duct is higher than in the Wolffian duct. As the male Müllerian duct undergoes regression (F, G), the intensity of P-AKT progressively decreases to become undetectable. Autofluorescence of blood vessels was observed in the mesenchymal area in B–G. White arrow, tip of the Müllerian duct; red arrowhead, Müllerian duct; white arrowhead, Wolffian duct; t, testis; mt, mesonephric tubules; ov, ovary. Scale bar: 500 μm (A), 100 μm (B–G).
PI3K inhibitors blocked elongation of the Müllerian ducts
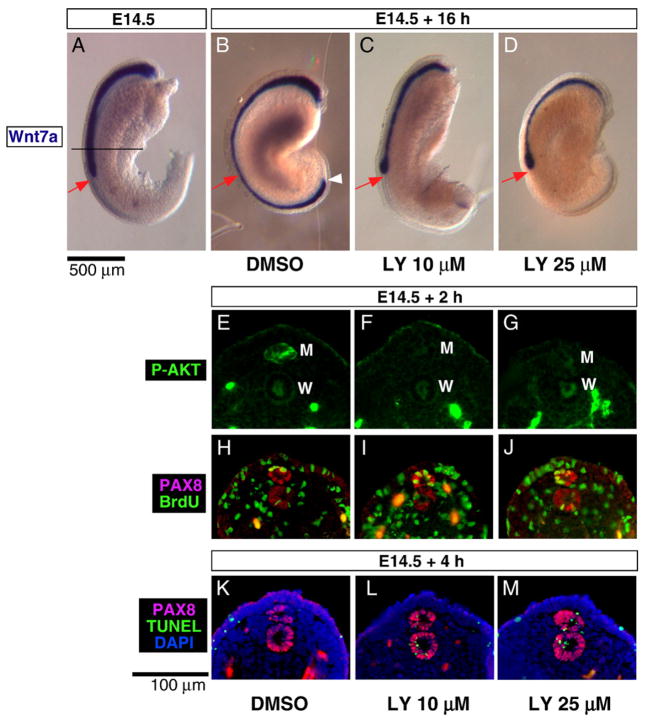
While examining the role of the PI3K/AKT pathway in the development of the Müllerian duct, we noted in E14.5 rat urogenital ridges in organ culture under the influence of the chemical PI3K inhibitors, 0–50 μM LY294002 (Figs. 6B–D) and 10–500 nM wortmannin (data not shown), that Müllerian duct elongation was inhibited after 16 h of incubation in a dose dependent manner. LY294002 is known to block PI3K and subsequent PI3K-dependent AKT phosphorylation and kinase activity with high specificity (Vlahos et al., 1994), while wortmannin, has an irreversible inhibitory effect on a relatively broad spectrum of substrates (Wymann et al. 1996). Ridges incubated with vehicle (DMSO) showed normal Müllerian duct elongation, which approached the posterior end of the mesonephroi of the explants (white arrowhead in Fig. 6B). However, at concentrations of 10 or 25 μM LY294002, elongation of the Müllerian duct tip failed to occur beyond the level observed at the start of the incubation (red arrows in Figs. 6A, C, and D). In doses lower than 10 μM, inhibition of elongation was not observed (data not shown). When the elongation was blocked by LY294002, the posterior tip of the Müllerian duct showed bulging, similar to that observed when Müllerian duct elongation was blocked by mechanical interruption (compare Figs. 6C and D with 2E and H). The Müllerian duct anterior to the bulge became thinner (Figs. 6C and D) compared to that observed after treatment with DMSO (Fig. 6B). The effect observed with LY294002 was recapitulated with 50 and 100 nM of wortmannin in both sexes (data not shown).
Fig. 6.
PI3K inhibition blocks Müllerian duct elongation. The E14.5 urogenital ridge (A) was incubated for 16 h with DMSO (B), or with 10 μM (C) or 25 μM (D) LY294002, followed by whole mount in situ hybridization for Wnt7a. The tip of the Müllerian duct remained at the initial position (red arrow) showing bulging after 16 h of incubation with LY294002 (C and D), while the Müllerian duct incubated with vehicle (DMSO) showed normal elongation (B). Frozen sections taken at the center of E14.5 urogenital ridge (black line in A) after 2 h (E–J) or 4 h (K–M) of incubation with DMSO (E, H and K), 10 μM (F, I and L) LY294002, or 25 μM (G, J and M) LY294002 were stained by immunofluorescence. Phosphorylated-AKT was remarkably reduced in the Müllerian ducts treated with LY294002 (E–G). BrdU (green) and PAX8 (red) doublestaining showed no significant difference in proliferation of the MDE cells with LY294002 treatment (H–J). However, TUNEL (green) and PAX8 (red) doublestaining revealed that apoptosis was induced in the Müllerian duct with LY294002 treatment (K–M). Autofluorescence was observed in blood vessels in the mesenchymal area in (E)–(M). Red arrow, initial position of the Müllerian duct tip before incubation; white arrowhead, tip of the Müllerian duct after incubation; M, Müllerian duct; W, Wolffian duct. Scale bar: 500 μm (A–C) and 100 μm (E–M).
When urogenital ridges were incubated with 0–50 μM LY294002 for 2 h, P-AKT staining by immunofluorescence was clearly reduced in the Müllerian duct at 10 and 25 μM LY294002 (Figs. 6E, F and G), concentrations previously reported in other tissue (Larsen et al., 2003; Steinberg et al., 2005; Wang et al., 2005), indicating that LY294002 targeted PI3K and inhibited AKT phosphorylation.
Urogenital ridges incubated for 4 h with 10–25 μM LY294002 and visualized at the black line in Fig. 6A showed increased apoptosis in the Müllerian and the Wolffian duct epithelium (Figs. 6L and M) compared to the vehicle control in DMSO with no apoptosis (Fig. 6K) which may explain the smaller diameter of the Müllerian duct after incubation with PI3K inhibitors for 16 h (compare Figs. 6B, 7B and E, with 6C, D, 7C and F). When viewed along the entire length of the duct, the proportion of apoptotic cells in the Müllerian duct was 20–30% at all levels, after PI3K inhibition (Fig. 4C). Although apoptosis was induced in both the Wolffian and the Müllerian duct, the effect on the Müllerian duct seemed stronger than in the Wolffian duct, which was better maintained than the Müllerian duct after 16 hour incubation with PI3K inhibitors (data not shown), suggesting that the Müllerian duct is more susceptible to PI3K inhibition than the Wolffian duct.
Fig. 7.
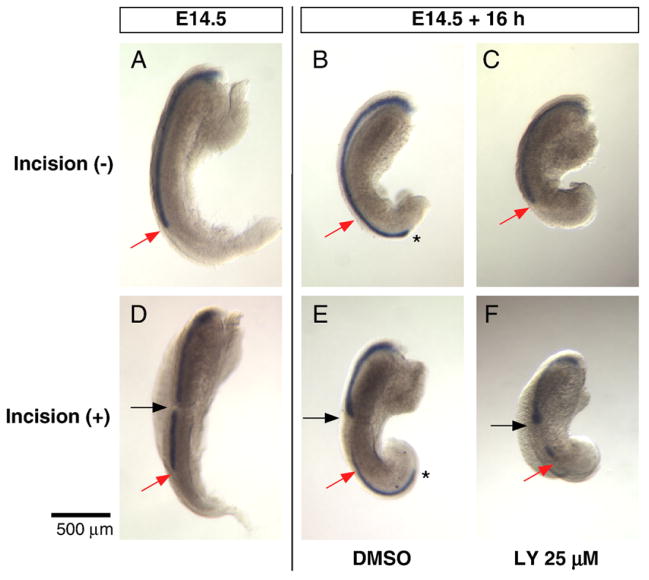
PI3K inhibition blocks elongation of the Müllerian duct but not migration of the MDE cells within the incised duct. The Müllerian duct was visualized by whole mount in situ hybridization for Wnt7a (A–F). E14.5 female urogenital ridge before (A) and after 16 h of incubation with DMSO (B) or 25 μM LY294002 (C). The tip of the DMSO treated Müllerian duct reached the posterior end of the urogenital bulge (asterisk in B), but elongation was inhibited as the tip remained at the initial position (red arrow) in the ridge treated with LY294002 (C). E14.5 female Müllerian duct was divided (black arrow in D), and then incubated for 16 h with DMSO (E) or 25 μM LY294002 (F). The tip of the Müllerian duct reached the posterior end (asterisk in E) of the DMSO treated and incised explant; however, elongation was inhibited by the PI3K inhibitor and the tip remained at the initial position (red arrow in F). Despite failure of elongation, the MDE cells have migrated posteriorly (F) and accumulated at the incision site (black arrow in F) and at the initial site of the tip (red arrow in F), creating a Wnt7a blank region. Black asterisks mark the elongating tip of the Müllerian duct. Scale bar: 500 μm.
In contrast to the significant effects on apoptosis, proliferation in the Müllerian duct did not appear to be affected by PI3K inhibition when BrdU incorporation was measured (Figs. 6H, I, and J). After incubation of the urogenital ridges for 2 h with 0–25 μM of LY294002 in 50 μM of BrdU, transverse frozen sections of the treated urogenital ridges (10–25 μM) showed that MDE cells actively incorporated BrdU at all levels of the elongating Müllerian duct (Fig. 4B), as was observed in DMSO controls (Figs. 6H, I and J). These results suggest that although proliferation of the MDE cell may not be dependent on the PI3K pathway, the Müllerian duct requires PI3K activation to inhibit apoptosis and to stimulate its elongation along the A–P axis.
The migratory movement of the MDE cell is independent of the PI3K/AKT pathway
When the E14.5 elongating Müllerian duct was divided at a site 500 μm anterior to the tip (black arrow in Figs. 7D–F) and incubated with 25 μM of LY294002 for 16 h, the Wnt7a positive MDE cells anterior to the site of incision migrated posteriorly and accumulated at the site of incision (Fig. 7F), where it bulged, as observed in DMSO controls with incision (Fig. 7E). In LY294002 treated ridges, the leading posterior tip of the Müllerian duct remained at the initial site (compare red arrows in Fig. 7D with F), as observed in ridges without incision (red arrows in Figs. 7A and C), while it moved posteriorly in DMSO controls (black asterisk in Figs. 7B and E). However, the MDE cells in the distal segment accumulated at the posterior tip and generated a blank area between the bulged tip at the incision site and the trailing edge of the distal segment (Figs. 7E and F), indicating that cells in the isolated posterior Müllerian duct segment migrated posteriorly, even in the presence of apoptosis (Fig. 6L). These results suggest that the migration of each MDE cell is independent of the PI3K/AKT pathway, while elongation of the tip of the Müllerian duct requires activation of this pathway.
Müllerian duct insinuation is required for separation of the Wolffian duct from the coelomic epithelium, and for subsequent mesenchymal migration between the Wolffian and Müllerian ducts
When the Müllerian duct is elongating along the A–P axis in the rat mesonephros at E14.5 (Fig. 8A), the Wolffian duct approximates the coelomic epithelium (Fig. 8I) until the Müllerian duct tip insinuates between them (Fig. 8E). After 24 h of organ culture (Fig. 8B), the tip of the Müllerian duct has reached the posterior end of the ridge separating the Wolffian duct from the coelomic epithelium. Meanwhile, mesenchymal cells migrate in and separate the Wolffian duct and the Müllerian duct along the length of the A–P axis (red arrow in Figs. 8F and J).
Fig. 8.
Müllerian duct insinuation is required for separation of the Wolffian duct from the coelomic epithelium, and for subsequent mesenchymal migration between the Wolffian and Müllerian ducts. The Müllerian duct is visualized by whole mount in situ hybridization for Wnt7a. The urogenital ridges at E14.5 (A) were incubated intact (B), incised posterior to Müllerian duct tip and incubated, (C) or incubated with 25 μM LY294002 without incision (D) for 24 h. White arrowhead indicates Müllerian duct tip at the start of incubation. Asterisk in (C) indicates the site of incision. Solid line and dashed line indicate sites of histological sections 500 μm anterior and posterior to the tip at E14.5, respectively. Transverse section histology (E–H) of the mesonephros is observed at the anterior black line in (A)–(D) respectively. Transverse section histology (I–L) is observed at the posterior black dashed line in (A)–(D) respectively. Mesenchyme separated the Wolffian duct and the Müllerian duct (red arrow in F, G and J), but not in (H). The Wolffian duct stayed attached to the coelomic epithelium (K and L). W, Wolffian duct; M, Müllerian duct; CE, coelomic epithelium. Scale bar: 500 μm (A–D) and 100 μm (E–L).
After 24 h of incubation following mesonephric incision at a site posterior to the tip, Müllerian duct elongation was interrupted at the site of incision (asterisk in Fig. 8C). The Wolffian duct failed to detach from the coelomic epithelium in the area posterior to the incision (Fig. 8K, compare with J), while normal separation occurs in the area anterior to the incision (Fig. 8G, compare with F). The Wolffian duct also remains attached to the coelomic epithelium distally after 48 h of incubation in the same experiments (data not shown).
When E14.5 urogenital ridges were incubated with LY294002 for 24 h, elongation of the Müllerian duct was blocked immediately after adding LY294002, leaving the tip at the initial site (white arrowhead in Fig. 8D), while the Wolffian duct remained attached to the coelomic epithelium in the area posterior to the Müllerian duct tip (Fig. 8L), as observed in the incised ridges (Fig. 8K). In the region anterior to the tip, mesenchymal cell migration between the Wolffian and the Müllerian ducts did not occur (Fig. 8H, compare with F and G). These results suggest that the Müllerian duct insinuation is required for the separation of the Wolffian duct and the coelomic epithelium. In addition, separation between the Wolffian and the Müllerian duct did not occur in urogenital ridges treated with LY294002, indicating that PI3K activation is required for the mesenchymal separation of these ducts.
Discussion
Müllerian duct elongation and epithelial cell migration
During Müllerian duct elongation, MDE cells manifest a high rate of proliferation (Jacob et al., 1999; Guioli et al., 2007), which contributes significantly to the elongation of the duct itself. In this study, we show that in addition to proliferation, migration of MDE cells plays an integral role in the formation of the Müllerian duct. Migration of each MDE cell implies a driving force, which differs from simple mechanical pressure caused by volume expansion due to cell proliferation. Chemotaxic attractants produced at the end of, or as a gradient along, the A–P axis of the urogenital ridge could cause the MDE cells to move, as observed in the trachea or lung during branching morphogenesis (Sutherland et al., 1996; Weaver et al., 2000), or, conversely, there could be a negative signal from the anterior end of the urogenital ridge, release of which could propel MDE cells caudally. The fact that the Müllerian duct elongates normally in the agonadal ridge in organ culture (data not shown) excludes the gonads and most caudal tissue as a major source of chemoattractants. It is also possible that individual MDE cells are preprogrammed to migrate cell-autonomously along the A–P axis independently of chemotaxic signaling. Given that Müllerian duct formation requires the Wolffian duct (Gruenwald,1937; Bishop-Calame, 1966; Didier, 1971, 1973), it may influence migratory movement of the MDE cells by producing attractants or by signaling through cell–cell adhesion (Jacob et al., 1999). For example, the Wolffian duct product, WNT9B, was recently identified as a possible candidate for the induction of Müllerian duct elongation, since the Wnt9b null mice have truncated Müllerian ducts (Carroll et al., 2005). However, migration of MDE cells along the A–P axis in the E15.5 mesonephroi (Figs. 2J and K) occurs without direct attachment either to the coelomic epithelium or the Wolffian duct (Figs. 5C, D and F). Stimulation and guidance for MDE cell migration could also come from some component of the extracellular matrix (ECM) rich region between the coelomic epithelium and the Wolffian duct (Jacob et al., 1999), such as laminin and collagen (Ikawa et al., 1984), as observed in tracheogenesis and nephrogenesis (reviewed by Schock and Perrimon, 2002; Tran et al., 2004).
Migration and division of MDE cells contribute to the ultimate size of the Müllerian duct diameter. Once the route is interrupted, migrated MDE cells accumulate and enlarge the duct at the site of interruption (Figs. 2E, F, H, I, and M), while the diameter becomes smaller in the isolated posterior tip (Figs. 3F and G). Although apoptosis is one of the most important mechanisms for determining shape and volume of tissues or organs (reviewed by Jacobson et al., 1997) and is major phenomenon in Müllerian duct regression in the male (Price et al.,1977; Roberts et al.,1999), its role in initial Müllerian duct development is less significant, as few apoptotic cells were observed during (Figs. 3N–P) and after Müllerian duct elongation in the female (Allard et al. 2000). Taken together, both proliferation and MDE cell migration are the important contributing factors to the diameter of the Müllerian duct.
Although MDE cells at all levels seemed to migrate along the A–P axis (data not shown), depletion of Wnt7a positive cells never occurred at the most anterior portion where proliferation is highest (Fig. 4B), suggesting presence of a steadysource of cells feeding the entire length of the ductora niche where the environment is uniquely suited for maintenance of the Müllerian duct epithelial stem cells.
PI3K dependent Müllerian duct tip elongation and PI3K independent MDE migration
The high rate of proliferation, rare apoptosis, and the considerable degree of cell migration, observed during Müllerian duct development prompted us to examine the activity of PI3K/AKT pathway in the urogenital ridge (Krasilnikov, 2000). We first confirmed that the PI3K/AKT pathway was activated in the MDE in the rat (Figs. 5A–G) and the mouse (data not shown) embryo, during Müllerian duct formation as previously observed in the epithelium of other developing tubular organs (Tang et al., 2002; Wang et al., 2005; Larsen et al., 2003).
Using PI3K inhibitors and mechanical microincision, we examined the roles of PI3K/AKT pathway and showed that the migratory movement of MDE cells and the elongation of the duct tip are governed by different mechanisms (Fig. 7), elongation being PI3K/AKT pathway dependent and migration of the MDE cells along A–P axis being PI3K/AKT independent (Figs. 6C, D, and 7). Since PI3K inhibitors induce Müllerian duct apoptosis in less than 25% of MDE in 4 h (Figs. 6K–M, Fig. 4C), and its susceptibility is not limited to the elongating tip, the apoptotic mechanism alone seems insufficient to explain the early cessation of elongation seen with LY294002. Inhibition of enzymatic reactions might be a reasonable alternative mechanism.
Müllerian duct elongation and modeling of the mesonephros
The Müllerian duct separates the Wolffian duct from the coelomic epithelium as it elongates in the mesonephros, following which mesenchymal cells migrate between to separate the Müllerian and Wolffian ducts (Gruenwald, 1941; Figs. 8E, I and J) via movements which are perpendicular to the A–P axis (Guioli et al., 2007). This lateral migration of the mesenchymal cells, however, does not occur when Müllerian duct elongation was blocked by either mechanical incision (Fig. 8K) or PI3K inhibition (Fig. 8L) and is not observed in Wnt4 knockout mice that fail to form the Müllerian duct, at E13.5 (data not shown), during the interval in which the Müllerian duct of wild type animals has reached the urogenital sinus. Furthermore, PI3K inhibition in the tissue results in failure to separate the Wolffian duct from the Müllerian duct by mesenchymal cell migration (Fig. 8H) suggesting that the PI3K/AKT pathway may be required for this lateral mesenchymal migration between these epithelial structures that are early attached to each other via a common basement mambrane (Jacob et al., 1999). Although the PI3K/AKT pathway is activated in both the Müllerian duct and the Wolffian duct, given that the Wolffian duct alone does not induce mesenchymal cell migration (Fig. 8K), it is probable that the Müllerian duct may have a key role in remodeling the basement membrane and inducing mesenchymal cell migration.
The Müllerian duct basement membrane and extracellular matrix
While possibly playing a critical role in a plane perpendicular to the A–P axis, the Müllerian duct elongates in an ECM rich area between the Wolffian duct and the coelomic epithelium along the A–P axis (Jacob et al., 1999). Although the Müllerian duct may use the ECM as a guide, it may also need to digest or separate the matrices in order to elongate, as is required of some cancer cells (Giannelli et al., 1997). In Wnt9b null mice, the Müllerian duct undergoes initial normal invagination, but does not thereafter elongate along the Wolffian duct (Carroll et al., 2005), suggesting that MDE cells in these mice lack the direction or the ability to migrate or to break into and through an ECM rich path.
An active enzymatic reaction may be required for ECM digestion or basement membrane remodeling adjacent to the Müllerian duct during its elongation. Our experiments using PI3K inhibitors suggest that activation of the PI3K/AKT pathway in the Müllerian duct is required for these processes, as observed in some endothelial cells and cancer cells which require this pathway for activation of matrix metalloproteinases (MMPs) and for migration (Zahradka et al. 2004; Park et al., 2007). For example, in branching and tubularization in submandibular gland epithelium in organ culture under FGF7 stimulation, activities of MMPs are increased (Steinberg et al., 2005). Although we examined the effect of the general MMP inhibitor GM6001 (Roberts et al., 2002; Steinberg et al., 2005) on Müllerian duct elongation in organ culture, we observed no effects on this process (data not shown), probably signifying redundancy of other proteinases.
Müllerian duct regression and the PI3K/AKT pathway
Although we focused mainly on the development of the Müllerian duct, our results with PI3K inhibitors suggest that the sexually dimorphic fate of the Müllerian duct may depend on the PI3K/AKT pathway. The differential intensity of P-AKT staining in the MDE between the sexes at the start of regression (Figs. 5C, F and D, G) suggests that one of the roles of PI3K/AKT pathway in the developing Müllerian duct may be as a survival factor to prevent apoptosis (Price et al., 1977; Roberts et al., 1999) and the epithelial–mesenchymal transitions (Dyche, 1979; Trelstad et al., 1982; Allard et al., 2000, Zhan et al., 2006), that are major events in Müllerian duct regression. It is not clear, however, whether suppression of the PI3K pathway in the male Müllerian duct initiates regression or simply that P-AKT in the male MDE decreases as a result of regression.
Müllerian Inhibiting Substance (MIS), the initiator of Müllerian duct regression, acts through the MIS type II receptor (reviewed by Teixeira et al., 2001), which is expressed in the mesenchymal cells surrounding the MDE in the male after E15.5 in the rat (Catlin et al., 1992; Teixeira et al., 1996; Zhan et al., 2006; Arango et al., 2008), resulting in subsequent apoptosis or epithelial–mesenchymal transformation of MDE cells (Dyche, 1979; Trelstad et al., 1982; Allard et al., 2000). To elicit MIS signaling, some mesenchymal–epithelial interaction must occur (Roberts et al., 1999), which may block the PI3K pathway in the MDE. In fact, it has been known that MIS blocks early autophosphorylation of the survival factor, EGF receptor, in the Müllerian duct epithelium (Hutson et al., 1984; Hurst et al., 2002), by inhibiting tyrosine kinase (Coughlin et al.,1987), which could result in PI3K pathway inactivation as a subsequent event.
Conclusion
Our investigations of Müllerian duct formation using PI3K inhibitors and mechanical incision demonstrate that the MDE cells actively migrate along the A–P axis and suggest that activation of the PI3K pathway is required for elongation of Müllerian duct tip, although MDE cell migration is independent of the PI3K/AKT pathway. Furthermore, Müllerian duct formation and the subsequent developmental events in the surrounding mesenchyme likely occur via PI3K/AKT activation of the MDE cells. Further investigation is required for a fuller understanding of the detailed molecular mechanisms. Signaling or interaction between the Müllerian duct epithelium and surrounding ECM may be the key. Understanding precise molecular mechanisms contributing to elongation of the Müllerian duct may shed light on the etiology of anomalies of the female reproductive system, such as the 46XX Mayer–Rokintansky–Kuster–Hauser syndrome (reviewed by Morcel et al., 2007), characterized by Müllerian duct agenesis, and further, may reveal therapeutics to act in concordance with MIS in the treatment of Müllerian derived cancers if inhibition of the PI3K/AKT system plays a role in the initiation of Müllerian duct regression.
Acknowledgments
We would like to thank Dr. Ronny Drapkin for providing the anti-PAX8 antibody, Drs. Jose Teixeira, Allan Goldstein and Liz Perkins for suggestions and critical review of the manuscript, Drs. Maria Loscertales, Henry L. Chang, Rafael V. Pieretti, and Paul P. Szotek for sharing techniques and reagents, and members of the Donahoe laboratory for helpful discussions. This work was supported by a grant from the NIH (NICHD-HD-32112 and NCI R01CA 137393 to P. K. D. and D. T. M. and NCI C1105009 Ovarian Cancer SPORE grant, Principal Investigator, Daniel Cramer).
References
- Allard S, Adin P, Gouedard L, di Clemente N, Josso N, Orgebin-Crist MC, Picard JY, Xavier F. Molecular mechanisms of hormone-mediated Müllerian duct regression: involvement of beta-catenin. Development. 2000;127:3349–3360. doi: 10.1242/dev.127.15.3349. [DOI] [PubMed] [Google Scholar]
- Arango NA, Kobayashi A, Wang Y, Jamin SP, Lee H, Orvis GD, Behringer RR. A mesenchymal perspective of Müllerian duct differentiation and regression in Amhr2-lacz mice. Mol Reprod Dev. 2008;75:1154–1162. doi: 10.1002/mrd.20858. [DOI] [PubMed] [Google Scholar]
- Bishop-Calame S. Experimental study of the organogenesis of the urogenital system of the chicken embryo. Arch Anat Microsc Morphol Exp. 1966;55:215–309. [PubMed] [Google Scholar]
- Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP. Wnt9b plays a central role in the regulation of mesenchymal to epitehelial transitions underlying organogenesis of the mammarian urogenital system. Dev Cell. 2005;9:283–292. doi: 10.1016/j.devcel.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Catlin EA, Ezzell RM, Donahoe PK, Manganaro TF, Ebb RG, MacLaughlin DT. Müllerian inhibiting substance binding and uptake. Dev Dyn. 1992 Apr;193 (4):295–299. doi: 10.1002/aja.1001930402. [DOI] [PubMed] [Google Scholar]
- Coughlin JP, Donahoe PK, Budzik GP, MacLaughlin DT. Müllerian inhibiting substance blocks autophosphorylation of the EGF receptor by inhibiting tyrosine kinase. Mol Cell Endocrinol. 1987;49:75–86. doi: 10.1016/0303-7207(87)90065-7. [DOI] [PubMed] [Google Scholar]
- Cunha GR. The dual origin of vaginal epithelium. Am J Anat. 1975;143:387–392. doi: 10.1002/aja.1001430309. [DOI] [PubMed] [Google Scholar]
- Del Vecchio FR. Development of the caudal portions of the Müllerian ducts in the rat (Rattus norgegicus) Acta Anat (Basel) 1982;113:235–245. [PubMed] [Google Scholar]
- Didier E. The Wolffian duct induces the formation of the ostium of the Müllerian duct: demonstration in the chick embryo. J Embryol Exp Morphol. 1971;25:115–129. [PubMed] [Google Scholar]
- Didier E. Recherches sur la morphogenese du canal de Muller chez les oiseaux, Etude experimental. Wilhelm Roux’ Arch. 1973;172:287–302. doi: 10.1007/BF00577882. [DOI] [PubMed] [Google Scholar]
- Donahoe PK, Ito Y, Hendren WH., III A graded organ culture assay for the detection of Müllerian inhibiting substance. J Surg Res. 1977;23:141–148. doi: 10.1016/0022-4804(77)90202-5. [DOI] [PubMed] [Google Scholar]
- Dyche WJ. A comparative study of the differentiation and involution of the Müllerian duct and Wolffian duct in the male and female fetal mouse. J Morphol. 1979;162:175–209. doi: 10.1002/jmor.1051620203. [DOI] [PubMed] [Google Scholar]
- Frutiger P. On the early development of the ductus paramesonephricus and the Müllerian capsule in man. Acta Anat (Basel) 1969;72:233–245. [PubMed] [Google Scholar]
- Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 1997;277:225–228. doi: 10.1126/science.277.5323.225. [DOI] [PubMed] [Google Scholar]
- Gruenwald P. Zur Entwicklungsmechanik des Urogenital systems beim Huhn. Arch Entwicklungsmech. 1937;136:786–813. doi: 10.1007/BF00582219. [DOI] [PubMed] [Google Scholar]
- Gruenwald P. The relation of the growing Müllerian duct to the Wolffian duct and its importance for the genesis of malformations. Anat Rec. 1941;81:1–19. [Google Scholar]
- Guioli S, Sekido R, Lovell-Badge R. The origin of the Müllerian duct in chick and mouse. Dev Biol. 2007;302:389–398. doi: 10.1016/j.ydbio.2006.09.046. [DOI] [PubMed] [Google Scholar]
- Hayashi A, Donahoe PK, Budzik GP, Trelstad RL. Periductal and matrix glycosaminoglycans in rat Müllerian duct development and regression. Dev Biol. 1982;92:16–26. doi: 10.1016/0012-1606(82)90146-4. [DOI] [PubMed] [Google Scholar]
- Hunter R. Observations on the development of the human female genital tract. Anat Rec. 1929;42:22–23. [Google Scholar]
- Hurst CH, Abbott B, Schmid JE, Birnbaum LS. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) disrupts early morphogenetic events that form the lower reproductive tract in female rat fetuses. Toxicol Sci. 2002;65:87–98. doi: 10.1093/toxsci/65.1.87. [DOI] [PubMed] [Google Scholar]
- Hutson JM, Fallat ME, Kamagata S, Donahoe PK, Budzik GP. Phosphorylation events during Müllerian duct regression. Science. 1984;223:586–589. doi: 10.1126/science.6607531. [DOI] [PubMed] [Google Scholar]
- Ikawa H, Trelstad RL, Hutson JM, Manganaro TF, Donahoe PK. Changing patterns of fibronectin, laminin, type IV collagen, and a basement membrane proteoglycan during rat Müllerian duct regression. Dev Biol. 1984;102:260–263. doi: 10.1016/0012-1606(84)90190-8. [DOI] [PubMed] [Google Scholar]
- Inomata T, Eguchi Y, Nakamura T. Origin of Müllerian duct and its later developmental changes in relation to Wolffian duct in bovine fetuses. Nippon Juigaku Zasshi. 1989;51:693–701. doi: 10.1292/jvms1939.51.693. [DOI] [PubMed] [Google Scholar]
- Jacob M, Konrad K, Jacob HJ. Early development of the Müllerian duct in avian embryos with reference to the human. An ultrastructural and immunohistochemical study. Cells Tissues Organs. 1999;164:63–81. doi: 10.1159/000016644. [DOI] [PubMed] [Google Scholar]
- Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell. 1997;88:347–354. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- Jost A. Recherches sue la differentiation sesulle de l’embryonde lapen. 1947;8:379–418. [Google Scholar]
- Kobayashi A, Behringer RR. Developmental genetics of the female reproductive tract in mammals. Nat Rev Genet. 2003;4:969–980. doi: 10.1038/nrg1225. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Shawlot W, Kania A, Behringer RR. Requirement of Lim1 for female reproductive tract development. Development. 2004;131:539–549. doi: 10.1242/dev.00951. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Kwan KM, Carroll TJ, McMahon AP, Mendelsohn CL, Behringer RR. Distinct and sequential tissue-specific activities of the LIM-class homeobox gene Lim1 for tubular morphogenesis during kidney development. Development. 2005;132:2809–2823. doi: 10.1242/dev.01858. [DOI] [PubMed] [Google Scholar]
- Krasilnikov MA. Phosphatidylinositol-3 kinase dependent pathways: the role in control of cell growth, survival, and malignant transformation. Biochemistry. 2000;65:59–67. [PubMed] [Google Scholar]
- Larsen M, Hoffman MP, Sakai T, Neibaur JC, Mitchell JM, Yamada KM. Role of PI 3-kinase and PIP3 in submandibular gland branching morphogenesis. Dev Biol. 2003;255:178–191. doi: 10.1016/S0012-1606(02)00047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C, Sassoon DA. Wnt-7a maintains appropriate uterine patterning during the development of the mouse female reproductive tract. Development. 1998;125:3201–3211. doi: 10.1242/dev.125.16.3201. [DOI] [PubMed] [Google Scholar]
- Miyamoto N, Yoshida M, Kuratani S, Matsuo I, Aizawa S. Defects of urogenital development in mice lacking Emx2. Development. 1997;124:1644–1653. doi: 10.1242/dev.124.9.1653. [DOI] [PubMed] [Google Scholar]
- Morcel K, Camborieux L, Guerrier D Programme de Recherches sur les Aplasies Müllériennes. Mayer–Rokitansky–Küster–Hauser (MRKH) syndrome. Orphanet J Rare Dis. 2007;2:13. doi: 10.1186/1750-1172-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano J, Gaslightwala I, Birnbaum MJ, Rustgi AK, Nakagawa H. Akt/protein kinase B isoforms are differentially regulated by epidermal growth factor stimulation. J Biol Chem. 2000;275:30934–30942. doi: 10.1074/jbc.M004112200. [DOI] [PubMed] [Google Scholar]
- Orvis GD, Behringer RR. Cellular mechanisms of Müllerian duct formation in the mouse. Dev Biol. 2007;306:493–504. doi: 10.1016/j.ydbio.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panayotou G, End P, Aumailley M, Timpl R, Engel J. Domains of laminin with growth-factor activity. Cell. 1989;56:93–101. doi: 10.1016/0092-8674(89)90987-2. [DOI] [PubMed] [Google Scholar]
- Parr BA, McMahon AP. Sexually dimorphic development of the mammalian reproductive tract requires Wnt-7a. Nature. 1998;395:707–710. doi: 10.1038/27221. [DOI] [PubMed] [Google Scholar]
- Park MJ, Kwak HJ, Lee HC, Yoo DH, Park IC, Kim MS, Lee SH, Rhee CH, Hong SI. Nerve growth factor induces endothelial cell invasion and cord formation by promoting matrix metalloproteinase-2 expression through the phosphatidylinositol-3 kinase/AKT signaling pathway and AP-2 transcription factor. J Biol Chem. 2007;282:30485–30496. doi: 10.1074/jbc.M701081200. [DOI] [PubMed] [Google Scholar]
- Pedersen A, Skijong C, Shawlot W. Lim1 is required for nephric duct extension and ureteric bud morphogenesis. Dev Biol. 2005;288:571–581. doi: 10.1016/j.ydbio.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Picon R. Action of the fetal testis on the development in vitro of the Müllerian ducts in the rat. Arch Anat Microsc Morphol Exp. 1969;58:1–19. [PubMed] [Google Scholar]
- Price JM, Donahoe PK, Ito Y, Hendren WH., III Programmed cell death in the Müllerian duct induced by Müllerian inhibiting substance. Am J Anat. 1977;156:265–284. doi: 10.1002/aja.1001490304. [DOI] [PubMed] [Google Scholar]
- Roberts LM, Hirokawa Y, Nachtigal MW, Ingraham HA. Paracrine-mediated apoptosis in reproductive tract development. Dev Biol. 1999;208:110–122. doi: 10.1006/dbio.1998.9190. [DOI] [PubMed] [Google Scholar]
- Roberts LM, Visser JA, Ingraham HA. Involvement of a matrix metalloproteinase in MIS-induced cell death during urogenital development. Development. 2002;129:1487–1496. doi: 10.1242/dev.129.6.1487. [DOI] [PubMed] [Google Scholar]
- Schock F, Perrimon N. Molecular mechanisms of epithelial morphogenesis. Annu Rev Cell Dev Biol. 2002;18:463–493. doi: 10.1146/annurev.cellbio.18.022602.131838. [DOI] [PubMed] [Google Scholar]
- Steinberg Z, Myers C, Heim VM, Lathrop CA, Rebustini IT, Stewart JS, Larsen M, Hoffman MP. FGFR2b signaling regulates ex vivo submandibular gland epithelial cell proliferation and branching morphogenesis. Development. 2005;132:1223–1234. doi: 10.1242/dev.01690. [DOI] [PubMed] [Google Scholar]
- Sutherland D, Samakovlis C, Krasnow MA. Branchless encodes a Drosophila FGF homolog that controls tracheal cell migration and the pattern of branching. Cell. 1996;87:1091–1102. doi: 10.1016/s0092-8674(00)81803-6. [DOI] [PubMed] [Google Scholar]
- Tang MJ, Cai Y, Tsai SJ, Wang YK, Dressler GR. Ureteric bud outgrowth in response to RET activation is mediated by phosphatidylinositol 3-kinase. Dev Biol. 2002;243:128–136. doi: 10.1006/dbio.2001.0557. [DOI] [PubMed] [Google Scholar]
- Teixeira J, He WW, Shah PC, Morikawa N, Lee MM, Catlin EA, Hudson PL, Wing J, Maclaughlin DT, Donahoe PK. Developmental expression of a candidate müllerian inhibiting substance type II receptor. Endocrinology. 1996;137:160–165. doi: 10.1210/endo.137.1.8536608. [DOI] [PubMed] [Google Scholar]
- Teixeira J, Maheswaran S, Donahoe PK. Müllerian inhibiting substance: an instructive developmental hormone with diagnostic and possible therapeutic applications. Endocr Rev. 2001;22:657–674. doi: 10.1210/edrv.22.5.0445. [DOI] [PubMed] [Google Scholar]
- Torres M, Gomez-Pardo E, Dressler GR, Gruss P. Pax-2 controls multiple steps of urogenital development. Development. 1995;121:4057–4065. doi: 10.1242/dev.121.12.4057. [DOI] [PubMed] [Google Scholar]
- Tran KT, Griffith L, Wells A. Extracellular matrix signaling through growth factor receptors during wound healing. Wound Repair Regen. 2004;12:262–268. doi: 10.1111/j.1067-1927.2004.012302.x. [DOI] [PubMed] [Google Scholar]
- Trelstad RL, Hayashi A, Hayashi K, Donahoe PK. The epithelial–mesenchymalinterface of the rat Müllerian duct: loss of basement membrane integrity and ductal regression. Dev Biol. 1982;92:27–40. doi: 10.1016/0012-1606(82)90147-6. [DOI] [PubMed] [Google Scholar]
- Tsuji M, Shima H, Yonemura CY, Brody J, Donahoe PK, Cunha GR. Effect of human recombinant Müllerian inhibiting substance on isolated epithelial and mesenchymal cells during Müllerian duct regression in the rat. Endocrinology. 1992;131:1481–1488. doi: 10.1210/endo.131.3.1505479. [DOI] [PubMed] [Google Scholar]
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase-AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- Vogel W, Gish GD, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. 1997;1:13–23. doi: 10.1016/s1097-2765(00)80003-9. [DOI] [PubMed] [Google Scholar]
- Wang J, Ito T, Udaka N, Okudela K, Yazawa T, Kitamura H. PI3K-AKT pathway mediates growth and survival signals during development of fetal mouse lung. Tissue Cell. 2005;37:25–35. doi: 10.1016/j.tice.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Weaver M, Dunn NR, Hogan BLM. Bmp4 and Fgf10 play opposing roles during lung bud morphogenesis. Development. 2000;127:2695–2704. doi: 10.1242/dev.127.12.2695. [DOI] [PubMed] [Google Scholar]
- Wymann MP, Bulgarelli-Leva G, Zvelebil MJ, Pirola L, Vanhaesebroeck B, Waterfield MD, Panayotou G. Wortmannin inactivates phosphoinositide 3-kinase by covalent modification of Lys-802, a residue involved in the phosphate transfer reaction. Mol Cell Biol. 1996;16:1722–1733. doi: 10.1128/mcb.16.4.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahradka P, Harding G, Litchie B, Thomas S, Werner JP, Wilson DP, Yurkova N. Activation of MMP-2 in response to vascular injury is mediated by phosphatidylinositol 3-kinase-dependent expression of MT1-MMP. Am J Physiol Heart Circ Physiol. 2004;287:H2861–2870. doi: 10.1152/ajpheart.00230.2004. [DOI] [PubMed] [Google Scholar]
- Zhan Y, Fujino A, MacLaughlin DT, Manganaro TF, Szotek PP, Arango NA, Teixeira J, Donahoe PK. Müllerian inhibiting substance regulates its receptor/SMAD signaling and causes mesenchymal transition of the coelomic epithelial cells early in Müllerian duct regression. Development. 2006;133:2359–2369. doi: 10.1242/dev.02383. [DOI] [PubMed] [Google Scholar]