Abstract
Pretreatment of mouse L cells with mouse interferon (IFN) inhibits the penetration of vesicular stomatitis virus without affecting viral adsorption. The inhibition of virus uptake by IFN is dose dependent and, at the highest dose tested (1,000 units/ml), reaches 65%; 24 hr of treatment with IFN are required for maximal effect. A similar inhibition of uptake of virus occurs in human diploid fibroblasts and primary chicken embryo fibroblasts treated with homologous IFN. No significant inhibition occurs when cells are treated with heterologous IFN. These results document a previously unrecognized antiviral effect of IFN--namely, inhibition at the level of viral uptake.
Full text
PDF
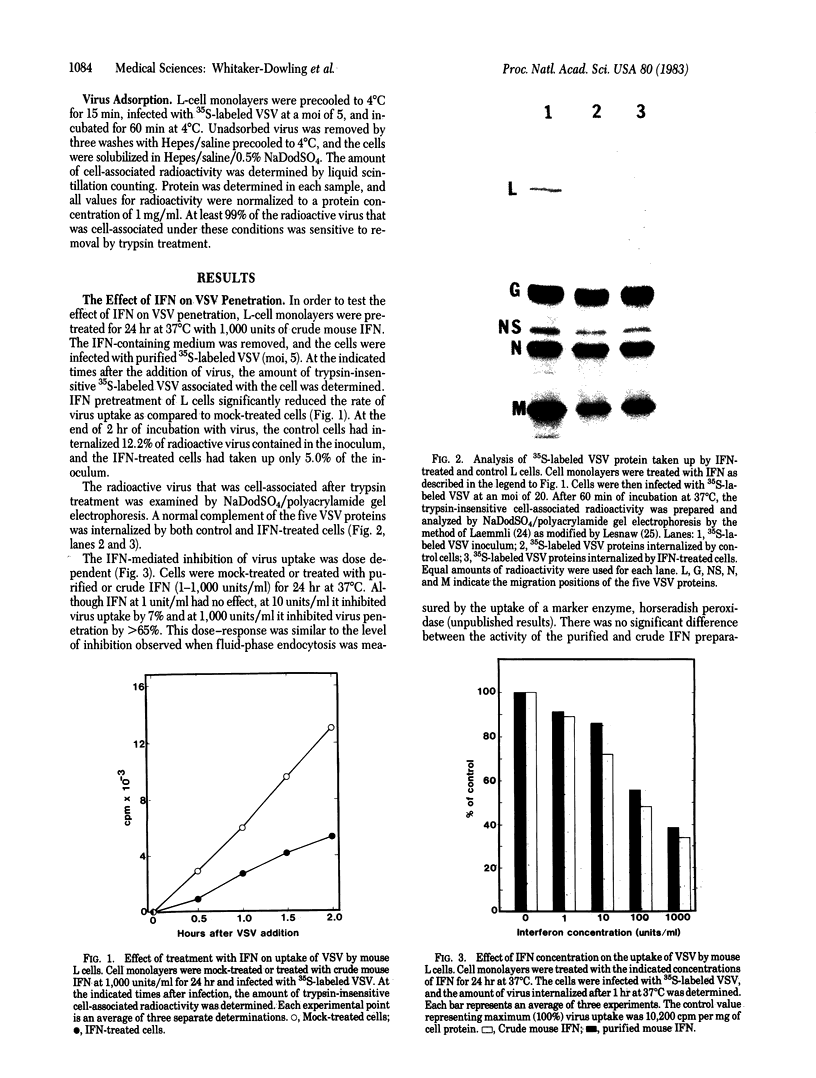
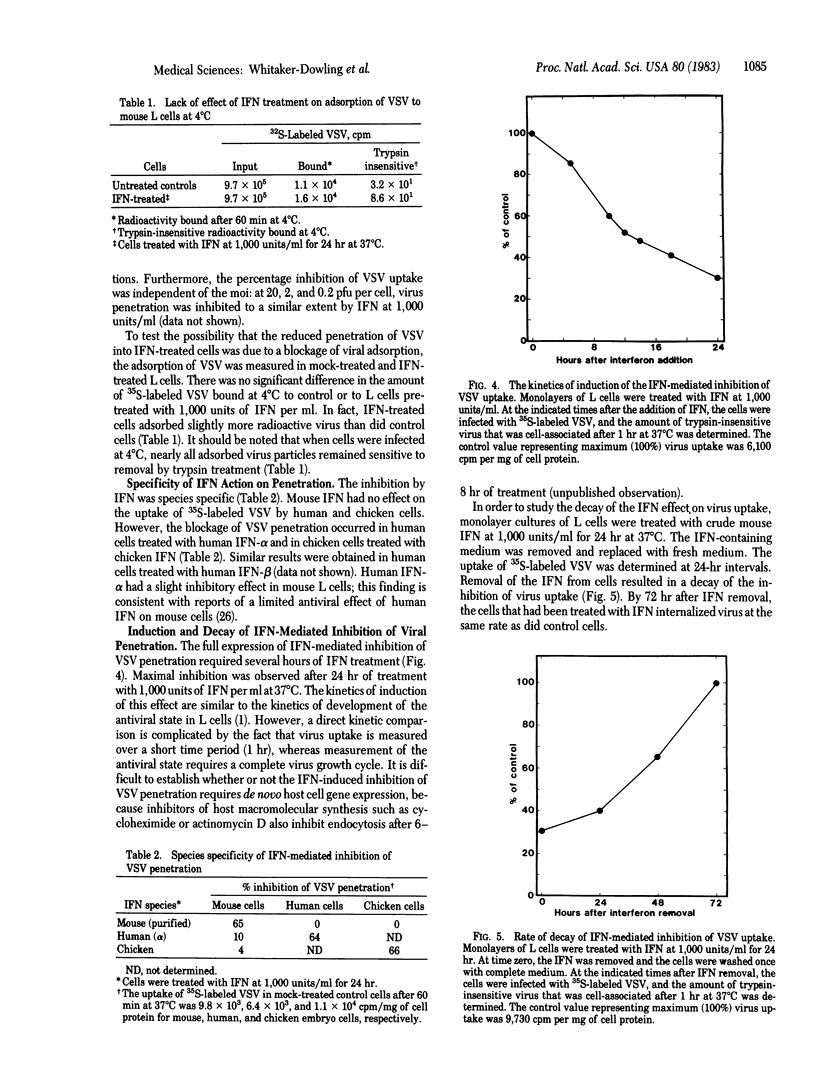

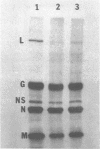
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billiau A., Edy V. G., De Clercq E., Heremans H., De Somer P. Influence of interferon on the synthesis of virus particles in oncornavirus carrier cell lines. III. Survey of effects on A-, B- and C-type oncornaviruses. Int J Cancer. 1975 Jun 15;15(6):947–953. doi: 10.1002/ijc.2910150610. [DOI] [PubMed] [Google Scholar]
- Billiau A., Edy V. G., Sobis H., de Somer P. Influence of interferon on virus-particle synthesis in oncornavirus-carrier lines. II. Evidence for a direct effect on particle release. Int J Cancer. 1974 Sep 15;14(3):335–340. doi: 10.1002/ijc.2910140306. [DOI] [PubMed] [Google Scholar]
- Borecký L., Fuchsberger N., Hajnická V. Electrophoretic profiles and activities of human interferon in heterologous cells. Intervirology. 1974;3(5-6):369–377. doi: 10.1159/000149774. [DOI] [PubMed] [Google Scholar]
- CANTELL K., PAUCKER K. QUANTITATIVE STUDIES ON VIRAL INTERFERENCE IN SUSPENDED L CELLS. IV. PRODUCTION AND ASSAY OF INTERFERON. Virology. 1963 Sep;21:11–21. doi: 10.1016/0042-6822(63)90298-8. [DOI] [PubMed] [Google Scholar]
- Chardonnet Y., Dales S. Early events in the interaction of adenoviruses with HeLa cells. II. Comparative observations on the penetration of types 1, 5, 7, and 12. Virology. 1970 Mar;40(3):478–485. doi: 10.1016/0042-6822(70)90190-x. [DOI] [PubMed] [Google Scholar]
- Dahlberg J. E. Quantitative electron microscopic analysis of the penetration of VSV into L cells. Virology. 1974 Mar;58(1):250–262. doi: 10.1016/0042-6822(74)90159-7. [DOI] [PubMed] [Google Scholar]
- Dianzani F., Baron S. Unexpectedly rapid action of human interferon in physiological conditions. Nature. 1975 Oct 23;257(5528):682–684. doi: 10.1038/257682a0. [DOI] [PubMed] [Google Scholar]
- Emerson S. U., Yu Y. Both NS and L proteins are required for in vitro RNA synthesis by vesicular stomatitis virus. J Virol. 1975 Jun;15(6):1348–1356. doi: 10.1128/jvi.15.6.1348-1356.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M., Ramseur J. M. Inhibition of murine leukemia virus production in chronically infected AKR cells: a novel effect of interferon. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3542–3544. doi: 10.1073/pnas.71.9.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis R. R. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972 May;110(2):706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSSBERG S. E., HOLLAND J. J. Interferon and viral ribonucleic acid. Effect on virus-susceptible and insusceptible cells. J Immunol. 1962 Jun;88:708–714. [PubMed] [Google Scholar]
- HO M. Inhibition of the infectivity of poliovirus ribonucleic acid by an interferon. Proc Soc Exp Biol Med. 1961 Jul;107:639–644. doi: 10.3181/00379727-107-26712. [DOI] [PubMed] [Google Scholar]
- Hallum J. V., Younger J. S. Quantitative aspects of inhibition of virus replication by interferon in chick embryo cell cultures. J Bacteriol. 1966 Oct;92(4):1047–1050. doi: 10.1128/jb.92.4.1047-1050.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna L., Merigan T. C., Jawetz E. Inhibition of TRIC agents by virus-induced interferon. Proc Soc Exp Biol Med. 1966 Jun;122(2):417–421. doi: 10.3181/00379727-122-31150. [DOI] [PubMed] [Google Scholar]
- Ho M., Enders J. F. AN INHIBITOR OF VIRAL ACTIVITY APPEARING IN INFECTED CELL CULTURES. Proc Natl Acad Sci U S A. 1959 Mar;45(3):385–389. doi: 10.1073/pnas.45.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. C., Hirsch J. G. The interaction between Toxoplasma gondii and mammalian cells. II. The absence of lysosomal fusion with phagocytic vacuoles containing living parasites. J Exp Med. 1972 Nov 1;136(5):1173–1194. doi: 10.1084/jem.136.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B., Blanden R. V. Cellular immunity. Prog Allergy. 1967;11:89–140. [PubMed] [Google Scholar]
- Maheshwari R. K., Jay F. T., Friedman R. M. Selective inhibition of glycoprotein and membrane protein of vesicular stomatitis virus from interferon-treated cells. Science. 1980 Feb 1;207(4430):540–541. doi: 10.1126/science.6243416. [DOI] [PubMed] [Google Scholar]
- Manders E. K., Tilles J. G., Huang A. S. Interferon-mediated inhibition of virion-directed transcription. Virology. 1972 Aug;49(2):573–581. doi: 10.1016/0042-6822(72)90508-9. [DOI] [PubMed] [Google Scholar]
- Marcus P. I., Engelhardt D. L., Hunt J. M., Sekellick M. J. Interferon action: inhibition of vesicular stomatitis virus RNA synthesis induced by virion-bound polymerase. Science. 1971 Nov 5;174(4009):593–598. doi: 10.1126/science.174.4009.593. [DOI] [PubMed] [Google Scholar]
- Morgan M. J., Colby C., Jr, Hulse J. L. Isolation and characterization of virus-resistant mouse embryo fibroblasts. J Gen Virol. 1973 Sep;20(3):377–385. doi: 10.1099/0022-1317-20-3-377. [DOI] [PubMed] [Google Scholar]
- Simpson R. W., Hauser R. E., Dales S. Viropexis of vesicular stomatitis virus by L cells. Virology. 1969 Feb;37(2):285–290. doi: 10.1016/0042-6822(69)90209-8. [DOI] [PubMed] [Google Scholar]
- Vilcek J., Jahiel R. I. Action of interferon and its inducers aginst nonviral infectious agents. Arch Intern Med. 1970 Jul;126(1):69–77. doi: 10.1001/archinte.126.1.69. [DOI] [PubMed] [Google Scholar]
- Wagner R. R. VIRAL INTERFERENCE. SOME CONSIDERATIONS OF BASIC MECHANISMS AND THEIR POTENTIAL RELATIONSHIP TO HOST RESISTANCE. Bacteriol Rev. 1960 Mar;24(1):151–166. doi: 10.1128/br.24.1.151-166.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Yamaguchi N., Oda K. Mechanism of interferon-induced inhibition of early simian virus 40(SV40) functions. Virology. 1975 Nov;68(1):58–70. doi: 10.1016/0042-6822(75)90147-6. [DOI] [PubMed] [Google Scholar]
- Youngner J. S., Scott A. W., Hallum J. V., Stinebring W. R. Interferon production by inactivated Newcastle disease virus in cell cultures and in mice. J Bacteriol. 1966 Oct;92(4):862–868. doi: 10.1128/jb.92.4.862-868.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngner J. S., Thacore H. R., Kelly M. E. Sensitivity of ribonucleic acid and deoxyribonucleic acid viruses to different species of interferon in cell cultures. J Virol. 1972 Aug;10(2):171–178. doi: 10.1128/jvi.10.2.171-178.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]