Abstract
Objective
This study investigated the effect of verbal prompting on elders’ 10-year longitudinal change in everyday cognition. Differential effects of prompting associated with impaired cognitive status were also examined.
Method
At baseline, 2,802 participants (mean age=73.6 years, mean education=13.5 years) from the ACTIVE clinical trial were classified as unimpaired, having amnestic mild cognitive impairment (MCI) or non-amnestic MCI based on psychometric algorithm. Participants were given the Observed Tasks of Daily Living (OTDL; a behavioral measure with tasks involving medication management/finances/telephone use) at baseline and at 1-, 2-, 3-, 5-, and 10-year follow-ups. When participants said “I don’t know” or did not respond to an item, they received a standardized verbal prompt. At each occasion, Unprompted (sum of items correct without prompting) and Prompted (sum of items correct including both prompted and unprompted) scores were derived for each participant. Multi-level modeling, adjusting for demographics/health/training group, was used to determine the trajectories of OTDL performance.
Results
In general, persons with MCI performed at lower levels than those who were unimpaired (amnestic<non-amnestic<unimpaired), and for all groups, prompted performance exceeded unprompted in all years. There was differential performance of the prompting conditions over time; prompted performance, unlike unprompted, was relatively protected from age-related decline, and persons with MCI experienced greater improvement due to prompting.
Conclusion
Very simple prompting appears to enhance and maintain performance on a task of everyday cognition over 10 years for both unimpaired and mildly-impaired older adults.
Keywords: everyday cognition, verbal prompting, cognitive impairment, cognitive aging, longitudinal follow-up
As rates of cognitive impairment and degenerative neurological diseases increase with population aging, there are concomitant increases in functional disability and healthcare costs (Alzheimer’s Association, 2011). This has spurred a broad gerontological literature on ways to support independent functioning. One method investigated for providing cognitive and functional support is prompting. Prompting, defined here as reminding, persuading, pointing out possible actions or cognitions to an individual, represents one external strategy that may be used when individuals encounter cognitive failures (i.e., unsure what to do next in a sequence of actions; unable to remember a fact or action). When used with individuals with high levels of impairment (e.g., persons with frank dementia, usually in the moderate to severe range), prompting has been shown to be effective in terms of supporting basic activities of daily living (e.g., Mihailidis, Boger, Craig & Hoey, 2008). Prompting has received relatively little attention in less impaired populations, including those who are cognitively normal or who have mild cognitive impairment (MCI).
Providing external cognitive support has been shown to be effective with several older, impaired populations. Some forms of such cognitive support that have been investigated include external automated devices that provide pre-programmed reminders or alarms for complex behaviors such as hand washing (Bharucha et al., 2009; Gillespie, Best, & O’Neill, 2011), social support that provides reminders or spousal support for generating shared problem solutions in the context of collaborative cognition (e.g., Baltes & Staudinger, 1996; Dixon & Gould, 1996; Margrett & Marsiske, 2002), and environmental support through the use of “cues” (e.g., depth-of-processing encoding instructions; cued recall or recognition) to improve memory performance (Craik, 1986; Kirchhoff, Anderson, Barch, & Jacoby, 2012; Park & Shaw, 1992). In the majority of these studies, the additional support through reminders, collaboration, or environmental cues improved the performance on the task. The outcomes measured in studies of cognitive support provision have included performance on basic activities of daily living (e.g., hand washing, Mihailidis et al., 2008), complex problem solving and errand planning (e.g., Berg, Johnson, Meegan, & Strough, 2003), and verbal memory performance (e.g, Johansson, Andersson, & Ronnberg, 2005; Park & Shaw, 1992).
The current study investigated verbal prompting as a form of cognitive support for older adults. In persons with advanced dementia, previous studies of external prompting mostly used step-by-step prompting (e.g., “turn on the water, now wet your hands, get some soap, rub, rinse, etc.”). The current study used a more global prompting approach among elders who were cognitively normal or who met criteria for mild cognitive impairment. In contrast to the previous prompting studies, prompts used in this investigation were relatively “minimal”, and did not offer the step-by-step instructions used in assistive technology dementia studies (e.g., Bewernitz, Mann, Dasler, & Belchior, 2009; LoPresti, Mihailidis & Kirsch, 2004). The current study also sought to compare the effectiveness of these prompts among elders who were generally less impaired than in most previous research. Given that other forms of cognitive support (collaborative cognition, encoding and retrieval support) benefitted cognition even in unimpaired elders, it followed that prompting might also be beneficial for a broad cross-section of older adults. The current study compared older adults with and without mild cognitive impairment on a performance-based measure of everyday cognition; the administration strategy of the task permitted a comparison of performance under prompted and unprompted conditions.
An individual was determined to exhibit Mild Cognitive Impairment (MCI) when they demonstrate cognitive impairment in one or more domains, but do not meet criteria for dementia and have largely intact independent functioning (e.g., Albert et al., 2011; Jak et al., 2009; Petersen, 2004). There is no single classification method for defining MCI, but Cook and colleagues (2013) recently developed an MCI classification method for use in this sample. Several studies have shown that adults with MCI may in fact evince some functional impairment on tasks assessing complex Instrumental Activities of Daily Living (IADL; e.g., medication use, financial management, food preparation; Lawton & Brody, 1969). Specifically, cross-sectional studies have demonstrated that being classified as MCI resulted in more difficulty with IADLs based on self- or informant-report (Perneczky et al., 2006; Teng, Becker, Woo, Cummings, & Lu, 2010; Tuokko, Morris, & Ebert, 2005). In studies of performance-based IADL functioning, persons with MCI or with low levels of executive functioning have also displayed reduced functioning (Cahn-Weiner, Malloy, Bole, Marran, & Salloway, 2000; Goldberg et al., 2010). Reduced performance-based functioning in MCI has also been confirmed longitudinally (e.g., Allaire & Willis, 2006; Gross, Rebok, Unverzagt, Willis, & Brandt, 2011). Wadley and colleagues (2007) demonstrated that having MCI predicted greater three-year decline in self-reported activities of daily living (ADLs; e.g., feeding, hygiene, dressing) and IADL perceived difficulty and performance relative to their unimpaired peers.
The current study sought to determine whether MCI-related functional impairment might extend to measures of functional cognition, or everyday cognition. Everyday cognition has been conceptualized by some investigators as the cognitive performance demonstrated when encountering natural (ecological) stimuli (e.g., read food package labels or official documents; Allaire & Marsiske, 1999). Indeed, measures of everyday cognition and other ways of measuring functioning and everyday task performance have tended to be moderately-to-strongly related. For example, the Observed Tasks of Daily Living (OTDL), which is a performance-based everyday cognition measure (participant behaviorally completes tasks related to medication use, telephone use, and financial management), has demonstrated a strong, positive correlation with IADL scores (r = 0.5; Diehl, Willis, & Schaie, 1995). Domains of the OTDL also demonstrate convergent validity with scales from the Everyday Problems Test (EPT; Diehl, Willis & Schaie, 1995; Willis & Marsiske, 1993) as well as a strong bivariate correlation (r=0.64; Diehl et al., 2005), suggesting that the OTDL is quite similar in scope to other, well-known measures of everyday cognition.
There is a growing body of literature that highlights the predictive utility of measures of everyday cognition. Allaire and colleagues (2009) demonstrated that performance on the Everyday Cognition Battery (ECB; Allaire & Marsiske, 1999) was different between healthy and mild cognitively impaired older adults. The ECB Knowledge Test is also a significant predictor of mortality, above and beyond basic cognitive abilities, self-reported health, and demographic variables (Weatherbee & Allaire, 2008). Additionally, a measure of everyday cognition was found to predict self-reported medication adherence following a kidney transplant (Gelb, Shapiro, & Thornton, 2010).
The current study investigated the effectiveness of verbal prompting during a performance-based task of daily activities (OTDL; e.g., medication, financial management, and telephone use). The performance trajectory of unimpaired elders and those who met criteria for mild impairment was examined, both with and without prompting support, over ten years. Overall, the purpose of the study was to investigate whether responsive verbal prompting (i.e., when participants gave behavioral signs of not knowing what do next in problem solving, or being unwilling to proceed) could improve performance on a measure of everyday cognition for elders with and without MCI. A secondary question was whether such prompting modified the ten-year trajectory of observed everyday cognition performance. Specifically, the primary aims of this investigation were to: (1) to compare prompted and unprompted performance on the Observed Tasks of Daily Living (OTDL) (2) to investigate whether there are MCI-related performance decrements on the OTDL, and (3) to examine whether prompting modified the ten-year trajectory of OTDL performance (compared to unprompted performance), and whether the longitudinal effects of prompting were moderated by participant cognitive status.
Method
All participants (N = 2802) randomized in the Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) clinical trial were considered for inclusion in this investigation. All procedures were approved by the Institutional Review Boards at each collaborating site (University of Alabama-Birmingham, Indiana University, Johns Hopkins University, Hebrew Senior Life, Pennsylvania State University, and Wayne State University/University of Florida) and informed consent was obtained prior to participation. Recruitment procedures, sample characteristics, and study design have been described elsewhere (e.g., Ball et al., 2002; Cook et al., 2013; Willis et al., 2006). The overarching goal of ACTIVE was to investigate the effects of three different cognitive training arms (Memory, Reasoning, Speed). With regard to the dependent measure in this study, the OTDL, there was little evidence that training had any effect on any of the everyday cognition outcomes measured through five years (Willis et al., 2006). Despite this, in the analyses that follow, training group membership was statistically controlled for in the analyses, so that training effects would not influence the trajectories observed in this study.
Participants
In this study, three participants were excluded at baseline for missing OTDL data, making the analytical sample 2,799 participants. ACTIVE study inclusion criteria required participants to have a Mini-Mental State Exam (MMSE; Folstein, Folstein & McHugh, 1975) score of >23, no prior diagnosis of dementia, and no self-reported ADL limitations (e.g., bathing, dressing, and personal hygiene). At baseline, participants had a mean (SD) age of 73.6 (5.9) years, mean education of 13.5 (2.7) years, and 26% were African American. Participants in ACTIVE participated in assessments at baseline (BL), immediately post-intervention (an occasion not included in these analyses), and 1-, 2-, 3-, 5-, and 10-years after baseline. The primary outcome of this study (OTDL) was not administered at the immediate posttest, so the immediate posttest occasion was not included in this investigation. The effective analytical sample for each occasion is determined by the number of participants with OTDL (BL N=2,799; Year 1 N=2,081; Year 2 N=1,969; Year 3 N=1,838; Year 5 N=1,561; Year 10 N=909).
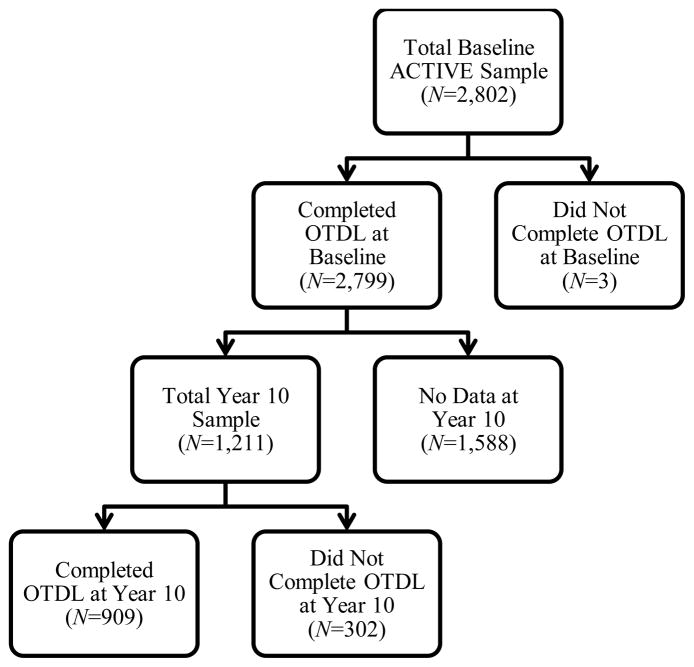
Figure 1 shows the numbers of participants who completed the OTDL at Year 10 and differentiates between the participants who dropped out of the study entirely and participants who had some Year 10 data, but were unable to complete the OTDL at this visit. The number of persons with missing OTDL data at follow-up occasions is somewhat higher than the number of people who dropped out of ACTIVE because some participants who returned for the follow-ups only had “partial visits” (due to health, travel difficulty, etc.) and did not provide OTDL data.
Figure 1.
Participants retained at Year 10 with OTDL data.
To characterize the selectivity of attrition, study participants who had OTDL data at Year 10 (Retained; n= 909) were compared to those for whom OTDL data were not available at this occasion (includes both participants who dropped out and were missing the OTDL at Year 10; Missing; n = 1890). Relative to those who have missing OTDL data, returning participants at Year 10 were younger (p < .001), had more years of education (p < .001), higher MMSE scores (p < .001), and had a higher percentage of females (p = .001). Those who were missing were more likely to be classified as cognitively impaired (p < .001), reported significantly more depression symptoms (p < .001), and self-reported poorer physical functioning (p < .001) at baseline. There were no significant differences in race. In the analyses that follow, these factors associated with attrition are statistically controlled for.
The cognitive status classifications that were used in this study are described by Cook and colleagues (2013). Briefly, participants were considered “unimpaired” when they performed above impairment threshold at BL. The impairment threshold was defined as approximately 1 SD below age/education/race means on normalized cognitive composites (Memory, Attention, Visuospatial Processing Speed, Language, and Complex Cognition). The 1 SD cutoff was implemented because this closely resembles the “Comprehensive criteria” recommended by Jak and colleagues (2009). Our approach differed slightly from Jak and colleagues’, however, in that we used a composite rather than requiring two or more performances within a domain to fall at least 1 SD below the normative mean. Because of low group sizes, no distinction could be made between those with single-domain versus multi-domain impairments. Participants who had one or more impaired cognitive domains at BL that included memory were classified as “amnestic.” Participants who had one or more impairments in domains that did not include memory were “non-amnestic.” Because the ACTIVE sample is a community-based, primarily healthy sample of older adults, there were more unimpaired than cognitive impaired participants. At BL, there were 1,872 unimpaired participants, 225 amnestic participants (58.7% were multidomain amnestic), and 703 non-amnestic participants (32.5% were multidomain non-amnestic). Table 1 displays the demographic and functional measures of the full sample as well as by cognitive status group for the baseline occasion.
Table 1.
Baseline demographic mean (standard deviations) for full analytic sample and by cognitive status group
| Full Sample (N=2,799) | Amnestic (N=225) | Non-Amnestic (N=703) | Unimpaired (N=1872) | F- statistic/χ2§ | df | p- value | ηp2 | |
|---|---|---|---|---|---|---|---|---|
| BL Age | 74.11(5.90) | 75.09 (6.91)a | 74.25 (6.14)ab | 73.95 (5.67)b | 3.93 | 2, 2796 | .020 | .003 |
| Education | 13.53 (2.70) | 13.43 (2.79)a | 13.46 (2.79)a | 13.56 (2.66)a | 0.54 | 2, 2796 | .586 | <.001 |
| BL MMSE | 27.31 (2.00) | 26.10 (1.87)a | 26.53 (2.09)b | 27.75 (1.83)c | 155.45 | 2, 2796 | <.001 | .100 |
| Female, n (%) | 2124 (75.88%) | 130 (57.78%)* | 523 (74.50%) | 1471 (78.58%) | 43.89§ | 2 | <.001 | - |
| AA, n (%) | 727 (25.97%) | 55 (24.44%) | 198 (28.21%) | 474 (25.32%) | 2.48§ | 2 | .289 | - |
Note: Matching superscript letters indicate values are not significantly different, p>.05, using Bonferroni corrected one-way ANOVAs. Chi-squared tests were used to measure dichotomous variables and are denoted with superscript§. Significant overall chi-square statistics were followed up with logistic contrasts, using the Unimpaired group as the reference value; significant differences after adjusting for the 2 comparisons to the reference group are denoted with superscript*. MMSE=Mini Mental State Exam (Folstein et al., 1975); AA=African American.
Measures
The Observed Tasks of Daily Living (OTDL; Diehl et al., 1995) was used to measure everyday cognition and the response to verbal prompts. The OTDL is one of three multi-domain, performance-based measures of functional living skills that were recommended for use in older adults (Moore, Palmer, Patterson & Jeste, 2007). On the OTDL, participants are presented with nine tasks (each with multiple items, for a maximum of 28 points) in the domains of medication use, telephone use, and financial management.
If the participant had trouble responding, a standardized verbal prompt was given. The prompts were not designed to give the answer, but rather, to serve as a reminder, motivation, or to help initiate the first step. Table 2 shows sample items of the OTDL with the corresponding prompts. Prompts were given if the participant did not respond for at least 15 seconds or stated “I don’t know.” The prompt was given only once before the item was marked incorrect. The last item on the OTDL requires the participant to pay a utility bill and has multiple subparts, so the same prompt was used for all subparts (“Please show me all the steps that are involved in paying this bill properly and getting it ready to mail”), but, consistent with the rest of the OTDL, the prompt was only given once per subpart.
Table 2.
Examples of items and corresponding prompts from the Observed Tasks of Daily Living
| Sample #1—Loading a Pill Reminder
| |
| Stimuli | The tester presents a fictitious person’s (named Peggy Wright) medication chart and the medicine bottles for all six medications listed on the chart to the participant. On the chart, the following three medications are marked with an X:
|
| Instructions | The tester then presents a 4 × 6 in. (10.2 × 15.2 cm) index card with the following instructions: “Mrs. Wright uses this pill reminder so that she does not forget to take her pills. Please fill this reminder with the 3 drugs marked on the medication chart.” |
| Correct Steps |
|
| Prompt | “Please look at the medication bottles.” “Is there any information that can help you fill the pill reminder?” |
|
| |
| Sample #2—Checking Itemized Calls on a Phone Bill
| |
| Stimuli | The tester presents a complete monthly phone bill (nine pages) to the participant. All pages are in numerical order and clearly labeled. |
| Instructions | The tester then presents a 4 × 6 in. (10.2 × 15.2 cm) index card with the following instruction: “According to this bill, on which days were the AT&T long-distance calls to Oregon made? Do not include any calling card calls.” |
| Correct Steps |
|
| Prompt | “Can you see any information on this bill that tells you when and where calls were made?” |
|
| |
| Sample #3—Balancing Checking Account
| |
| Stimuli | The tester presents a check ledger and pencil |
| Instructions | The tester presents an index card with the following instructions: “The person who holds this checking account received a check for $100.00 for deposit and also paid a utility bill for the amount of $48.42. Please balance the checkbook for this person.” |
| Correct Steps |
|
| Prompt: | “Which amount is deposited?” “Which amount is paid out?” |
Note: To protect test security, these are example items that are similar to, but not in the current version of, the OTDL.
There were two different OTDL scores calculated for each participant and used in this investigation—an unprompted score and a prompted score. The unprompted score was calculated by summing the number of items correct without a prompt. The prompted score was the unprompted score plus any items where the participant responded correctly after receiving a prompt. At each occasion, if a participant did not receive a prompt, their prompted score was set to missing at that occasion. Given the multi-level modeling approach used in this study (see below), their scores at other occasions were still used.
Testing certification was required of all examiners who administered the OTDL, involving study of a manual, viewing videos and tenured testers, and being observed in testing by trained examiners using a quality control checklist. After certification, subsequent testing was subject to further 5% quality control checks. Scoring was done by certified scorers who first needed to achieve reliability on standardized examples, and then had their scoring checked by a gold standard scorer on both the first five real OTDLs they scored, and then on a subsequent, ongoing random 5%. Quantitatively, reliability (agreement on scoring) on the first three standardized OTDLs (n=131) was 0.987 (range = 0.9 – 1.0, sd = 0.019), and on all subsequent OTDLs checked (n=874) was 0.996 (range = 0.92–1.0, sd = 0.011).
Baseline demographic and health covariates were included in the model to adjust for three factors:
Selective attrition (e.g., age, education, race, vision, general health; Wolinsky et al., 2009) and significant group differences not specifically related to cognition (e.g., depression, gender). See below for a more detailed description of this approach. Measures used were as follows: General health that is non-specific to cognition was measured using the General Health subscale of the MOS Short Form Health Survey (SF-36; Ware & Sherbourne, 1992). Vision in ACTIVE was objectively measured using standard procedures on a GoodLite Model 600A light box with the Early Treatment Diabetic Retinopathy Study chart. Participants stood ten feet from the chart and credit was given for each letter correctly identified. Scores ranged from 0 to 90, with higher scores indicating better vision acuity. The Center for Epidemiological Studies-Depression-12 scale (CES-D) was used to assess frequency of depressive symptoms (Radloff, 1977).
Extraneous covariates associated with multi-site protocol: Consistent with analytic practice in all ACTIVE manuscripts, the ACTIVE Site was included to reduce random variance associated with the arbitrary locations where the study took place (six university/research centers were involved with ACTIVE). Replicate, or cohort, was also included to be consistent with standard ACTIVE practice (e.g., Willis et al. 2006). Replicate refers to the enrollment cohort in which the participant was enrolled; there were six sequential periods of enrollment in ACTIVE to manageably distribute the work across the funded period.
Training effects on OTDL performance: Although no training effects have been detected on OTDL to date, dummy codes representing the intervention groups (1–6 with 7 as reference group) control for any potential and differential cognitive training and booster session gains (coded as 1=Memory training, no booster; 2=Reasoning training, no booster; 3=Speed training, no booster; 4=Memory training, with booster; 5=Reasoning training, with booster; 6=Speed training, with booster; 7=control group, no training).
Statistical Analyses
Statistical Method
The use of a mixed-effects design to answer the study questions allowed for all available data to be utilized without case-wise exclusion for a missing data point (Schaffer & Graham, 2002; Singer & Willet, 2003). First, the data cannot be assumed to be missing completely at random (MCAR) because Little’s MCAR test was significant (p<.001). The use of all available data (i.e., full information maximum likelihood with available cases instead of listwise deletion), as well as control for baseline covariates known to be associated with dropout in ACTIVE (Wolinsky et al., 2009), was expected to reduce bias due to selective attrition (older, more impaired persons were more likely to drop out). The attrition-related covariates explained a large portion of the group differences in attrition (C=.729, p<.001). The use of mixed-effects, full-information maximum likelihood, and the inclusion of attrition covariates represents best practice for estimating longitudinal relationships in a sample with selective attrition (Schaffer & Graham, 2002), in that results will be less biased than older methods (e.g., listwise deletion or mean replacement). Finally, a mixed effects design allowed for both the fixed and random effects to be examined. Fixed effects are the group or “average” effects that include all individuals. Random effects examine whether a particular individual significantly differs from the obtained fixed effect. The random effects of intercept and trajectory were examined in this model. Finally, all multiple group comparisons were Bonferroni-corrected. Model fit was evaluated using -2LL, AIC, and BIC statistics. Lower -2LL, AIC, and BIC indicate improved fit over the previous model. A chi-square test was used to determine if the change in -2LL from a previous model was a significant improvement in fit.
Analysis Plan
Preliminary analyses first investigated descriptive characteristics of the OTDL. Next, the number of OTDL prompts received by group and occasion were examined. For this analysis, the Total Number of Prompts was the dependent variable (DV) and Occasion, Cognitive Status Group, and their interaction were independent variables (IV). Occasion was treated as a factor, rather than a continuous variable for this analysis so the estimated marginal means could be presented. This question asked about the raw numbers of prompts, so only fixed effects were examined and BL covariates were not included.
The remaining aims of this investigation used OTDL Performance as the DV and included the BL covariates as IVs. This model, though similar to the model used above, differed in that the occasion (time) variables were treated as continuous variables rather than fixed factors. Visual inspection of the longitudinal data suggested that there was an initial increase followed by a decrease in performance over time (likely reflecting initial effects of practice or retest). Therefore, a model with linear and quadratic time effects was conducted and was contrasted with a piecewise/multi-phase model (as described below) to determine which one produced the best fitting description of temporal trends (Bollen & Curran, 2006; Singer & Willet, 2003). The piecewise/multi-phase model was determined to be the better fitting model using - 2LL, AIC, and BIC statistics. Thus, this model’s time variables include a 1st linear slope that occurs from Year 0 (BL) through 3. Then there is a 2nd linear slope that represents time from Year 5 through Year 10. Both the fixed effects and random effects that were included in this model are displayed in Table 4. An Autoregressive 1 repeated error structure and an unstructured random error structure were chosen for this model because they resulted in the best-fitting model and are methodologically logical.
Table 4.
Estimates for the full longitudinal model of prompted vs. unprompted OTDL performance by cognitive group.
| Estimate | S.E. | df | F-statistic | p | β | |
|---|---|---|---|---|---|---|
|
|
||||||
| Level 2 (Between Subjects Effects) | ||||||
| Replicate | - | - | 2546.705 | 2.407 | 0.035 | - |
| Site | - | - | 2551.485 | 19.891 | <.001 | - |
| Intervention Group | - | - | 2546.511 | 2.438 | 0.024 | - |
| Memory | −0.334 | 0.179 | 2610.429 | - | 0.063 | −0.073 |
| Reasoning | −0.206 | 0.181 | 2672.474 | - | 0.256 | −0.045 |
| Speed | −0.484 | 0.178 | 2585.594 | - | 0.007 | −0.106 |
| Memory+Booster | −0.089 | 0.170 | 2511.637 | - | 0.602 | −0.019 |
| Reasoning+Booster | 0.078 | 0.170 | 2464.398 | - | 0.645 | 0.017 |
| Speed+Booster | −0.376 | 0.170 | 2502.736 | - | 0.027 | −0.082 |
| Control | - | - | - | - | - | - |
| BL Age | −0.176 | 0.010 | 2622.337 | 335.747 | <.001 | −0.228 |
| BL Education | 0.427 | 0.021 | 2616.598 | 418.882 | <.001 | 0.253 |
| African American | −1.327 | 0.141 | 2583.147 | 88.037 | <.001 | −0.128 |
| Female | 0.531 | 0.124 | 2584.339 | 18.373 | <.001 | 0.050 |
| BL Vision | 0.026 | 0.005 | 2588.524 | 28.208 | <.001 | 0.066 |
| BL CES-D | −0.043 | 0.011 | 2599.200 | 15.029 | <.001 | −0.048 |
| BL SF-36 General Health | 0.008 | 0.003 | 2593.658 | 6.826 | 0.009 | 0.032 |
| Cognitive Group (CG) | - | - | 2678.581 | 224.964 | <.001 | - |
| Amnestic | −3.157 | 0.200 | 2864.490 | - | <.001 | −0.692 |
| Non-Amnestic | −2.255 | 0.131 | 2209.562 | - | <.001 | −0.494 |
| Unimpaired | - | - | - | - | - | - |
| Intercept | 21.101 | 0.984 | 2618.119 | 468.263 | <.001 | −0.261 |
| Level 1 (Within Subjects Effects) | ||||||
| Prompting Status | 3.325 | 0.035 | 9147.098 | 8824.916 | <.001 | 0.365 |
| 1st Linear Slope | 0.357 | 0.026 | 2180.600 | 183.829 | <.001 | 0.090 |
| 2nd Linear Slope | −0.175 | 0.018 | 1036.614 | 91.883 | <.001 | −0.099 |
| Prompting x 1st Linear | −0.282 | 0.037 | 10254.728 | 57.796 | <.001 | −0.036 |
| Prompting x 2nd Linear | 0.245 | 0.023 | 12041.151 | 115.411 | <.001 | 0.069 |
| Prompting x CG | 0.412 | 0.066 | 10059.340 | 38.970 | <.001 | 0.039 |
| CG x 1st Linear | −0.000 | 0.035 | 3653.950 | 0.000 | 0.990 | −0.000 |
| CG x 2nd Linear | −0.078 | 0.025 | 1616.315 | 9.854 | 0.002 | −0.038 |
| CG x Prompting x 1st Linear | −0.027 | 0.036 | 10264.507 | 0.543 | 0.461 | −0.007 |
| CG x Prompting x 2nd Linear | 0.049 | 0.024 | 11632.138 | 4.089 | 0.043 | 0.020 |
| Covariance Parameters | Estimate | S.E. | Wald Z | p |
|---|---|---|---|---|
|
|
||||
| Diagonal (Residual) | 7.614 | 0.120 | 63.579 | <.001 |
| AR1 Rho | 0.213 | 0.011 | 18.787 | <.001 |
| Intercept | 5.727 | 0.283 | 20.260 | <.001 |
| 1st Linear Slope | 0.534 | 0.052 | 10.178 | <.001 |
| 2nd Linear Slope | 0.181 | 0.018 | 10.169 | <.001 |
| Off-diagonal parameters | ||||
| 1st Linear, Intercept | −0.597 | 0.100 | −5.958 | <.001 |
| 2nd Linear, Intercept | 0.078 | 0.061 | 1.291 | 0.197 |
| 1st Linear, 2nd Linear | −0.137 | 0.025 | −5.479 | <.001 |
BL=Baseline occasion; Intervention Group (memory, reasoning, speed, memory+booster, reasoning+booster, speed+booster, or control); CES-D=Center for Epidemiological Studies-Depression-12 item scale; SF-36 General Health=MOS Short Form Health Survey, General Health subscale; CG=Cognitive Group (amnestic, non-amnestic, or unimpaired). AR1 Rho=average between occasion covariance.
Results
Preliminary descriptive analyses were conducted on the OTDL. At baseline, only 6.2% (n=173) of the participants did not receive a prompt. In the baseline sample, the unprompted scores ranged from 1–28 with the mean (SD) score was 17.00 (4.62) and the prompted score ranged from 3–28 with a mean (SD) score of 20.51(3.82), which is a difference in score of 3.51 points. For the prompted score, across all occasions and participants, only 1.7% of participants obtained the maximum score of a 28.
Table 3 displays the raw total number of prompts received both by the whole sample, as well as by Cognitive Status Group. Effect sizes are reported as r-values (small=0.10; medium=0.30; large=0.50, Cohen, 1988). The main effect of Occasion was significant, r=0.12, F(5, 11157)= 32.72, p<.001, such that the Year 10 required the most prompts of all occasions. The main effect of Cognitive Status Group was also significant, r=0.20, F(2, 11157)= 233.86, p<.001, such that, across all occasions, the amnestic group received the highest number of prompts (mean=5.68, s.e.=0.161), followed by the non-amnestic group (mean=5.04, s.e.=.078), with the unimpaired group receiving the fewest number of prompts (mean=3.40, s.e.=.042). The Occasion x Cognitive Status Group interaction had the smallest effect, but was significant, r=0.04, F(10, 11157)= 2.18, p=.016, indicating that there were differences in trajectory of number of prompts received by cognitive status group. Specifically, the amnestic group did not show the same decline in prompts at Years 2 and 3 that the non-amnestic and unimpaired group did. Additionally, the non-amnestic group appeared to have an accelerated need for prompting relative to the amnestic and unimpaired groups between Years 3 to 10.
Table 3.
Mean number of prompts (standard error) at each occasion for total sample and by cognitive status.
| Year from Baseline | Total Sample | Unimpaired | Non-Amnestic | Amnestic |
|---|---|---|---|---|
| 0 | 5.407 (.096) | 4.233 (.083)a | 5.967 (.136)b | 6.022 (.240)b |
| 1 | 4.373 (.121) | 3.357 (.095)a | 4.712 (.162)b | 5.052 (.310)b |
| 2 | 4.052 (.127) | 2.757 (.097)a | 4.118 (.167)b | 5.281 (.328)c |
| 3 | 3.824 (.134) | 2.528 (.099)a | 3.795 (.178)b | 5.148 (.347)c |
| 5 | 4.536 (.154) | 3.190 (.106)a | 4.856 (.198)b | 5.563 (.403)b |
| 10 | 6.042 (.233) | 4.357 (.136)a | 6.768 (.278)b | 7.000 (.628)b |
Note: Letter superscripts that differ represent significant (p<.05) group differences in the number of prompts received after Bonferroni adjustment for multiple comparisons. The effective analytical sample (i.e., had OTDL data) at each occasion: BL N=2,799; Year 1 N=2,081; Year 2 N=1,969; Year 3 N=1,838; Year 5 N=1,561; Year 10 N=909.
Table 4 displays the fixed and random effects of the mixed effects model examining the Prompted versus Unprompted OTDL performance by Cognitive Status Group. The majority of the BL covariates that were included in the model to control for selective attrition, between-subject differences, and between-group differences (e.g., depression) all had significant main effects (p<.05, F-statistics are displayed in Table 4). Specifically, participants who were younger (r=−0.34), were non-African American (r=−0.18), had greater years of education (r=0.37), were female (r=0.08), had better vision (r=0.10), had fewer depressive symptoms (r=−0.08), and generally had better health (r=0.05) performed better on the OTDL. The intervention group main effect was also significant. This effect was driven by an overall difference between the Speed of Processing groups (boosted and unboosted) and controls, where the speed groups performed slightly worse (0.08–0.11 SD) than the control group; this did not interact with either time trend, suggesting the difference reflect pre-existing group differences.
Fixed Effects
There was a significant main effect of Prompting Status, r=0.70, F(1, 9147.10)=8824.92, p<.001, with higher Prompted scores than Unprompted scores, across occasions and participants using the covariate-adjusted estimates (Unprompted mean= 16.81, s.e.=0.08; Prompted mean= 20.14, s.e.=0.08). Both the 1st and 2nd Linear Slope main effects were significant, r=0.28, F(1, 2180.60)=183.83, p<.001 and r=−0.29, F(1, 1036.61)=91.88, p<.001, respectively. The 1st Linear Slope from Year 0 (BL) through Year 3 was positive, indicating improved performance during this time. However, the 2nd Linear trend from Year 3 through Year 10 was negative, indicating a decline in performance during the later occasions. There was also a significant main effect of Cognitive Status Group, r=0.38, F(2,2678.58)=224.96, p<.001, such that the covariate-adjusted estimate of unimpaired participants’ OTDL Performance was the highest (mean=20.15, s.e.=0.07), followed by the non-amnestic (mean=17.90, s.e.=0.11), with amnestic persons having the lowest performance (mean=17.00, s.e.=0.19). These differences between groups were all significantly different (relative to the unimpaired group, the amnestic group has r=−0.28, p<.001 and non-amnestic has r=−0.34, p<.001).
The Prompting Status x Cognitive Status Group interaction was significant [r=0.06, F(1, 10059.34)=38.97, p<.001] such that prompting improved the non-amnestic OTDL Performance the most, followed by the amnestic group’s performance. The unimpaired participants had the smallest change between the Unprompted and Prompted OTDL scores. However, while there was a smaller gap between the unimpaired participants and impaired participants’ Prompted scores than Unprompted, the unimpaired Prompted performance was still better than that of the amnestic and non-amnestic groups’. Both the Prompting Status x 1st Linear Slope and Prompting Status x 2nd Linear Slope interactions were significant, r=−0.07, F(1, 10254.73)=57.80, p<.001 and r=0.10, F(1, 12041.15)=115.41, p<.001, respectively. This result was likely due the greater improvement in Year 0 through 3 (1st Linear Slope) followed by greater decline from Year 3 through 10 (2nd Linear Slope) of the unprompted total. Conversely, the prompted total showed a flatter trajectory over all occasions (less improvement during 1st Linear and less decline during 2nd Linear). The Cognitive Status Group x 1st Linear Slope interaction was non-significant, suggesting that there was not a difference in trajectory of OTDL Performance by Cognitive Status Group for the early occasions. However, there was a significant Cognitive Status Group x 2nd Linear Slope interaction, r=−0.08, F(1, 1616.32)=9.85, p<.01, suggesting that during Years 3 through 10, there were differences in rates of decline by Cognitive Status Group. The three-way Prompting Status x Cognitive Group x 1st Linear Slope interaction was non-significant; however, the Prompting Status x Cognitive Group x 2nd Linear Slope interaction was significant, r=0.02, F(1, 11632.14)=4.09, p<.05. Figure 2 shows the trajectories of the prompted and unprompted OTDL performances by cognitive status group. These graphs suggest that perhaps the unimpaired prompted score was the most resistant to decline at Years 3 through 10. Additionally, it appears as though the non-amnestic groups’ unprompted score showed the greatest decline from Years 3 through 10.
Figure 2.
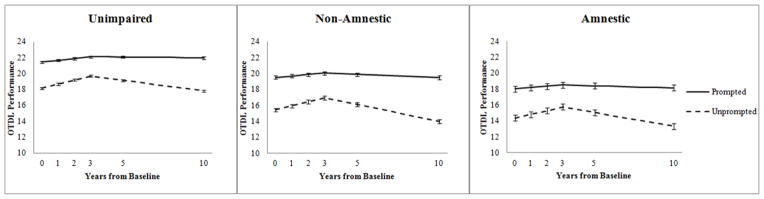
Trajectories of the unprompted vs. prompted performance on the OTDL by cognitive status group. Error bars represent 95% confidence interval.
Random Effects
This model examined between-subject variability in OTDL performance (random intercept) as well as trajectory (1st and 2nd Linear Slopes). The 1st and 2nd Linear Slope variables both had significant random variance [Wald Z=10.178, p<.001 and Wald Z=10.169, p<.001, respectively], suggesting that there was between-person variability in the rate of change of OTDL performance that was not accounted for by the fixed effects. There was additional and significant between- and within-subject variance that was left unexplained by this model. This model had a total R2=0.719, indicating that this model explained 71.9% of the total variance. This model represents a significant improvement over the unconditional growth model that included both fixed and random time (−2LL χ2 difference (df=33) = 9524.056, p<.001, R2 change=0.102).
Discussion
The present study investigated minimally-directive standardized verbal prompts on a measure of everyday cognition (OTDL), and whether this support led to better performance on the OTDL in older adults. The effect of prompting was investigated longitudinally over ten years for both the unprompted (raw score) and prompted (raw score plus correct items after prompting) scores to examine whether the effectiveness of prompting changed over time and by cognitive status. Additionally, a question in the study was whether performance supported via prompting might show more attenuated decline over the ten-year course of study. Participants were defined as unimpaired, amnestic mild cognitive impairment (MCI) or non-amnestic MCI (Cook et al., 2013).
The preliminary analysis revealed that at all occasions, the impaired participants required a greater number of prompts than the unimpaired group. Overall, between Year 0 and 3 there was a decline in the number of prompts received; however, from Year 3 through 10, the need for prompts increased. By Year 10, participants received a greater number of prompts than they did at baseline.
Next, this investigation demonstrated that prompting improved scores on a performance-based measure of everyday cognition. It is important to note that the prompted score was still approximately 7 points below the maximum score of 28, indicating that the prompted score was still relatively far from the ceiling for this measure. The prompted performance was higher across all participants, including those who were classified as cognitively impaired. Interestingly, with the prompting, the non-amnestic and amnestic participant’s performance was very consistent with the unimpaired participants’ unprompted score. While there is no normative data for the OTDL, the unimpaired, unprompted score is likely a good representation of a typical, community dwelling older-adult’s level of everyday cognitive performance. As such, it appears that these minimal verbal prompts brought the performance of impaired participants closer to a normative level.
There were clear mean-level differences on OTDL performance between the cognitive groups. Unimpaired elders performed best; persons with one or more cognitive impairments that did not include memory (non-amnestic) performed more poorly on the OTDL, and persons with one or more cognitive impairments that did include memory (amnestic) performed the worst. Previous work has demonstrated that individuals with amnestic MCI, especially those who were impaired in memory in addition to other domains, generally function more poorly than non-amnestic MCI participants (e.g., Kim et al., 2009; Teng, Becker, Woo, Cummings, & Lu, 2010; Wadley et al., 2007) and were more likely to progress to a dementia (e.g., Palmer, Backman, Winblad, & Fratiglioni, 2008; Yaffe, Petersen, Lindquist, Kramer, & Miller, 2006). This also makes sense given that the majority of the amnestic participants (58.7%) were in fact impaired in multiple domains, as opposed to only 32.5% of non-amnestic participants. Additionally, as described in Cook et al. (2013), a larger proportion of amnestic persons had an incident dementia classification by algorithm compared to non-amnestic and unimpaired participants.
While the intervention group main effect was significant, with the speed-trained groups demonstrating lower performance on the OTDL than the control group, the effect sizes of these contrasts were quite small (all contrast effect sizes < .11). We tested whether the intervention group interacted with time, but these interactions were non-significant, so they were not included in the model. This is consistent with previous work in ACTIVE that has shown that training generally only improved performance within the specific domain that was trained (e.g., speed-trained participants only improved on speed outcomes; Ball et al., 2002), and that training-gains generalized only to improved performance on a composite of everyday cognition tasks (OTDL and EPT) for a subset of participants who received extra booster training, and this was of very small effect sizes (Willis et al., 2006). Thus, it is likely that due to the nature of the interventions in ACTIVE that most participants did not receive dosages sufficient to show generalized effects to the OTDL.
There was a dissociation in the trajectories of prompted and unprompted performance. Unprompted performance showed initial improvement (likely reflecting practice) until year three, followed by decline in subsequent years. In contrast, prompted performance was consistently higher, and showed a relatively stable trajectory over the ten year period. This basic pattern was essentially similar in all three cognitive status groups. Thus, there is an apparent dissociation between the trajectories of prompted and unprompted performance. Moreover, the relative flatness of the prompted trajectory cannot be attributed to ceiling effects because, for all groups at all occasions, the mean was at least six points below the theoretical maximum (28) and only 1.7% of people achieved a score of 28. The surprising finding, then, is that – across all three groups – what participants appear to be capable of (i.e., after prompting) does not decline, even as their usual unprompted performance does. This evokes similar dissociated developmental trajectories seen in the early lifespan, and also discussed theoretically by Kliegl and Baltes (1987). In the early lifespan, linguistics researchers, for example, distinguished between competence and performance. Children could hear that the word “fis” (for fish) was mispronounced, even if they could not say it correctly. Thus, there was a cognitive ability to distinguish the “sh” phoneme before there was an ability to produce it (Berko & Brown, 1960). Kliegl and Baltes extended this idea to lifespan development and suggest that in most cognitive functions, individuals might have a latent performance reserve (i.e., the ability to do better than they normally do) that could be evoked with extra effort, training, etc. It seems likely that the cognitive support effect observed here (i.e., with minimal prompts, performance is improved and maintained) might be an empirical demonstration of the activation of this latent reserve.
What is new and intriguing in this study is the longitudinal evidence that suggests that this baseline performance potential remains relatively stable into advanced old age, even in individuals with low initial cognitive performance. This is consistent with some cognitive training research, which suggests that even into old-old age individuals can continue to benefit from cognitive training (e.g., Buschkuehl et al., 2008; Willis & Nesselroade, 1990); it is also consistent with training research on individuals with and without mild cognitive impairment that shows that both groups can gain equally in memory following a multi-component intervention (Belleville et al., 2006). Thus, taken with other literatures that suggest that simple cognitive support can eliminate or reduce age differences (e.g., in memory, Craik, 1986), the overarching impression is that minimal cognitive support might be useful, if it could be continuously applied, in attenuating age-related cognitive and functional changes into advanced old age.
The slopes of both the 1st and 2nd linear trends differed across individuals, which was evident by the significant random effect. The trajectory of the 1st Linear Slope did not differ by Cognitive Status Group—perhaps indicating that all groups showed comparable learning or practice effects. Conversely, the trajectory of the 2nd Linear Slope did differ by Cognitive Status Group, with the impaired groups showing a faster decline after Year 3.
Few studies have investigated the longitudinal trajectory of everyday cognition (Gross et al., 2011; Tucker-Drob, 2011; Willis et al., 1992; Willis, 1996a). This investigation, however, allowed for the examination of the 10-year trajectory of performance on a measure of everyday cognition and attempted to address the question of whether complex functioning showed relative preservation or decline in older adults over time. The covariate-adjusted trajectory of the OTDL performance was, on average, fairly stable. There was, however, a small net decline in the unprompted scores in that the Year 10 unprompted performance was, on average, about 0.16 SD units lower than the BL performance. The pattern of performance shows that steady improvement from BL through Year 3 was not quite enough to make up for the decline that was observed from Year 3 to Year 10 for the unprompted performance. The initial improvement is likely a result of increasing familiarity and practice with the tasks on the OTDL.
Nevertheless, the decline in unprompted OTDL performance at later occasions (Year 3 through Year 10) of this study suggests that practice and familiarity were challenged by accelerated age-related decline. This decline is consistent with the trajectory that is often seen in more basic cognitive function in that performance starts to decline as people transition into old-old age (e.g., Singer, Verhaeghen, Ghisletta, Lindenberger, & Baltes, 2003). While there was initial gain at the early occasions, as the mean age of participants transitioned from young-old to old-old age, we began to see a more universal or normative decline from Year 3 to Year 10. This is consistent with previous cross-sectional work that demonstrated age-related decline in everyday cognition (Marsiske & Margrett, 2006; Thornton and Dumke, 2005; Willis & Marsiske, 1991). This trajectory of decline also maps onto the longitudinal findings of Willis (1996b) in which the resistance to decline begins to disappear as older adults transition from young-old to old-old (late 70’s to 80’s).
This investigation also offers support to the idea that performance-based measures of instrumental or complex activities of daily living may, in fact, be rather sensitive to decline in everyday functioning that is not yet evident by one’s self-report of functioning. Particularly at BL, for inclusion in the study all participants were deemed “functionally intact,” as they were all living independently and screened out if they had an MMSE ≤ 23 or reported difficulty with basic ADLs such as bathing, dressing, or personal hygiene. Moreover, 3.5% of participants did not endorse any difficulties with IADLs at the BL occasion. This study supports the work that has reported that performance-based measures of everyday functioning were sensitive to the early cognitive decline seen in MCI participants (e.g., Allaire & Willis, 2006; Goldberg et al., 2010). We expected that the non-amnestic group would have the lowest performance based on work that suggested that reasoning explained the most variance in everyday cognitive performance (Burton et al., 2006; Gross et al., 2011; Willis et al., 1992). However, the non-amnestic participants were a very heterogeneous mix of not only participants with reasoning and problem solving impairments (Complex cognition domain), but also Attention, Language, and Visuospatial impairments. Similarly, the amnestic group consists of multidomain amnestic participants. As such, because these were not “pure” impairment groups (i.e., amnestic group has 58.7% multidomain amnestic participants; non-amnestic has 32.5% multidomain non-amnestic participants) and multidomain amnestic participants were generally the lowest functioning MCI subtype, the current finding is reasonable.
The present study has several limitations that will be important to consider when developing future studies to replicate and elaborate on these findings. First, as frequently observed in longitudinal studies of older adults, selective attrition can be a source of significant bias. It is typically seen that participants demonstrating greater impairment (e.g., health-related, dementia) were more likely to drop out of the study prior to conclusion (e.g., Siegler & Botwinick, 1979). This pattern was also observed in this sample with cognitively impaired persons (amnestic and non-amnestic) being more likely to dropout prior to the 5-year follow up than the unimpaired participants (Cook et al., 2013). In this study, variables that were predictive of attrition in this sample (covariate-dependent attrition) were controlled for in the analyses, which could be considered a strength of this investigation. However, this technique likely did not completely control for this selective attrition. Second, the sample used in this investigation was specifically selected for participation in longitudinal cognitive intervention study. As described in the methods section above, participants were generally healthy and able to function independently, as to ensure that they could comply with the demands of the cognitive training intervention. However, this selection bias produced a group of older adults who were less likely to experience physical, cognitive or functional problems compared to the general population. Because this was a sample of generally healthy older adults, the sample size of the unimpaired group to the amnestic and non-amnestic groups is considerably unbalanced. Then, again, due to the attrition bias, impaired participants (particularly amnestic) were even more underrepresented at later occasions.
Lastly, the OTDL is generally a good representation of the construct of everyday cognition in that it includes “real world” activities (Diehl et al, 1995, 1998); however, this measure does have some limitations. Specifically, the measure was delivered in a research context and was not an observation of functioning at home, which limits the generalization to real-world functioning since the environment was unfamiliar. Furthermore, there were components of the delivery of the measure that could introduce additional variance to the measure. For example, the participant was required to read each item off of an index card. Thus, while vision was corrected for in the analysis, literacy may affect the participants’ understanding of the task that they were supposed to complete. While this could be considered a limitation, work by Kirsch and Mosenthal (1990) suggested that “document literacy” (e.g., understanding charts, labels, and forms) may, in fact, be an important component of “real-world” functioning. Also, prompts were delivered if the participant said that they did not know the answer or took too long to respond; however, they were not given if the participants simply gave the wrong answer. Therefore, participants who were impulsive may not have received prompts because they gave an answer quickly, even when they did not know if it was correct. The OTDL has no way of capturing who made these error-types.
The current study found that simple verbal prompts (reminders to look again or try harder) uncovered substantial latent performance potential in older adults of varying level of cognitive ability. Consistent with other work on cognitive support (e.g., Craik, 1986), the results also suggested that there was a growing dissociation between spontaneous versus supported performance. The present study cannot directly address the reasons for normative longitudinal decline of performance that is coincident with underlying stability in performance potential, although past research has suggested that factors like fatigue (e.g., Moreh, Jacobs, & Stessman, 2010), self-efficacy (Bandura, 1993; Hastings & West, 2011), meta-cognitive self-awareness (e.g., Hertzog & Dunlosky, 2011) might all be implicated in the growing dissociation between performance and competence. Given that the current study shows that simple prompts ameliorate performance on important tasks of daily living (e.g. understanding medication side effects, filling out insurance forms, making change), future research should address ways to implement such minimal cognitive support. While machine-based prompting has been effective in more impaired populations (LoPresti, Mihailidis & Kirsch, 2004), the practicalities of implementation are daunting. It may be that the current study lends support to the idea of metacognitive interventions (e.g., Lachman, Weaver, Bandura, Elliott, & Lewkowicz, 1992) that encourage persistence, taking a second look, belief in solvability, and belief in personal problem-solving potential.
Acknowledgments
Supported by grants from the National Institute of Nursing Research (U01 NR04508, U01 NR04507) and the National Institute on Aging (U01 AG14260, U01 AG14282, U01 AG14263, U01 AG14289, U01 AG014276). Ms. Thomas was supported by a grant from the National Institute of Aging (T32 AG020499). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research, National Institute on Aging, or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data. Dr. Marsiske has received research support from Posit Science, Inc., in the form of site licenses for cognitive training programs for different research projects.
References
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allaire JC, Gamaldo A, Ayotte BJ, Sims R, Whitfield K. Mild cognitive impairment and objective instrumental everyday functioning: The Everyday Cognition Battery memory test. Journal of the American Geriatrics Society. 2009;57:120–125. doi: 10.1111/j.1532-5415.2008.02054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allaire JC, Marsiske M. Everyday cognition: Age and intellectual ability correlates. Psychology and Aging. 1999;14(4):627–644. doi: 10.1037/0882-7974.14.4.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allaire JC, Willis SL. Competence in everyday activities as a predictor of cognitive risk and mortality. Aging, Neuropsychology, and Cognition. 2006;13(2):207–224. doi: 10.1080/13825580490904228. [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Association. 2011 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia. 2011;7:208–44. doi: 10.1016/j.jalz.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske, Willis SL for ACTIVE Study Group. Effects of cognitive training interventions with older adults: A randomized controlled trial. JAMA: The Journal of the American Medical Association. 2002;288(18):2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltes PB, Staudinger UM. Interactive minds in a life-span perspective: Prologue. In: Baltes PB, Staudinger UM, editors. Interactive minds: Life-span perspectives on the social foundation of cognition. Melbourne, Australia: Cambridge University Press; 1996. pp. 1–32. [Google Scholar]
- Bandura A. Perceived self-efficacy in cognitive development and functioning. Educational Psychologist. 1993;28(2):117–148. [Google Scholar]
- Berg CA, Johnson MMS, Meegan SP, Strough J. Collaborative problem-solving interactions in young and old married couples. Discourse Processes. 2003;35(1):33–58. doi: 10.1207/S15326950DP3501_2. [DOI] [Google Scholar]
- Belleville S, Gilbert B, Fontaine F, Gagnon L, Ménard E, Gauthier S. Improvement of episodic memory in persons with mild cognitive impairment and healthy older adults: Evidence from a cognitive intervention program. Dementia and Geriatric Cognitive Disorders. 2006;22(5–6):486–499. doi: 10.1159/000096316. [DOI] [PubMed] [Google Scholar]
- Berko J, Brown R. Psycholinguistic research methods. In: Mussen P, editor. Handbook of research methods in child development. New York: John Wiley; 1960. pp. 517–557. [Google Scholar]
- Bewernitz MW, Mann WC, Dasler P, Belchior P. Feasibility of machine-based prompting to assist persons with dementia. Assistive Technology. 2009;21(4):196–207. doi: 10.1080/10400430903246050. [DOI] [PubMed] [Google Scholar]
- Bharucha AJ, Anand V, Forlizzi J, Dew MA, Reynolds CF, Stevens S, Wactlar H. Intelligent assistive technology applications to dementia care: Current capabilities, limitations, and future challenges. The American Journal of Geriatric Psychiatry. 2009;17(2):88–104. doi: 10.1097/JGP.0b013e318187dde5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen KA, Curran PJ. Latent curve models. Hoboken, NJ: John Wily & Sons, Inc; 2006. [Google Scholar]
- Burton CL, Strauss E, Hultsch DF, Hunter MA. Cognitive functioning and everyday problem solving in older adults. The Clinical Neuropsychologist. 2006;20(3):432–452. doi: 10.1080/13854040590967063. [DOI] [PubMed] [Google Scholar]
- Buschkuehl M, Jaeggi SM, Hutchison S, Perrig-Chiello P, Däpp C, Müller M, Perrig WJ. Impact of working memory training on memory performance in old-old adults. Psychology and Aging. 2008;23(4):743. doi: 10.1037/a0014342. [DOI] [PubMed] [Google Scholar]
- Cahn-Weiner DA, Malloy PF, Boyle PA, Marran M, Salloway S. Prediction of functional status from neuropsychological tests in community-dwelling elderly individuals. The Clinical Neuropsychologist. 2000;14(2):187–195. doi: 10.1076/1385-4046(200005)14:2;1-Z;FT187. [DOI] [PubMed] [Google Scholar]
- Cook SE, Marsiske M, Thomas KR, Unverzagt FW, Wadley VG, Langbaum JBS, Crowe M. Identification of mild cognitive impairment in active: algorithmic classification and stability. Journal of the International Neuropsychological Society. 2013;19(01):73–87. doi: 10.1017/S1355617712000938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik FIM. A functional account of age differences in memory. Human memory and cognitive capabilities: Mechanisms and performances. 1986:409–422. [Google Scholar]
- Craik FIM, Jennings JM. Human memory. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. Hillsdale, NJ, England: Lawrence Erlbaum Associates, Inc; 1992. pp. 51–110. [Google Scholar]
- Diehl M, Marsiske M, Horgas AL, Saczynski JS. Psychometric properties of the Revised Observed Tasks of Daily Living (OTDL-R). Paper presented at: Annual Meeting of the Gerontological Society of America; November 20–23, 1998; Philadelphia, PA. 1998. [Google Scholar]
- Diehl M, Willis SL, Schaie KW. Everyday problem solving in older adults: Observational assessment and cognitive correlates. Psychology and Aging. 1995;10(3):478–491. doi: 10.1037/0882-7974.10.3.478. [DOI] [PubMed] [Google Scholar]
- Diehl M, Marsiske M, Horgas AL, Rosenberg A, Saczynski JS, Willis SL. The Revised Observed Tasks of Daily Living: A performance-based assessment of everyday problem solving in older adults. The Journal of Applied Gerontology. 2005;24(3):211–230. doi: 10.1177/0733464804273772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon RA, Gould ON. Adults telling and retelling stories collaboratively. In: Baltes PB, Staudinger UM, editors. Interactive minds: Life-span perspectives on the social foundation of cognition. Melbourne, Australia: Cambridge University Press; 1996. pp. 221–241. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gelb SR, Shapiro RJ, Thornton WJL. Predicting medication adherence and employment status following kidney transplant: The relative utility of traditional and everyday cognitive approaches. Neuropsychology. 2010;24(4):514–526. doi: 10.1037/a0018670. [DOI] [PubMed] [Google Scholar]
- Gillespie A, Best C, O’Neill B. Cognitive function and assistive technology for cognition: A systematic review. Journal of the International Neuropsychological Society. 2011;18(1):1–19. doi: 10.1017/S1355617711001548. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Koppel J, Keehlisen L, Christen E, Dreses-Werringloer U, Conejero-Goldberg C, Gordon ML, et al. Performance-based measures of everyday function in mild cognitive impairment. The American Journal of Psychiatry. 2010;167(7):845–853. doi: 10.1176/appi.ajp.2010.09050692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross AL, Rebok GW, Unverzagt FW, Willis SL, Brandt J. Cognitive predictors of everyday functioning in older adults: Results from the active cognitive intervention trial. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2011;66(5):557–566. doi: 10.1093/geronb/gbr033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings EC, West RL. Goal orientation and self-efficacy in relation to memory in adulthood. Aging, Neuropsychology, and Cognition. 2011;18(4):471–493. doi: 10.1080/13825585.2011.575926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzog C, Dunlosky J. Metacognition in later adulthood: Spared monitoring can benefit older adults’ self-regulation. Current Directions in Psychological Science. 2011;20:167–173. doi: 10.1177/0963721411409026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jak AJ, Bondi MW, Delano-Wood L, Wierenga C, Corey-Bloom J, Salmon DP, et al. Quantification of five neuropsychological approaches to defining mild cognitive impairment. American Journal of Geriatric Psychiatry. 2009;17:368–375. doi: 10.1097/JGP.0b013e31819431d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson NO, Andersson J, Rönnberg J. Compensating strategies in collaborative remembering in very old couples. Scandinavian Journal of Psychology. 2005;46(4):349–359. doi: 10.1111/j.1467-9450.2005.00465.x. [DOI] [PubMed] [Google Scholar]
- Kim KR, Lee KS, Cheong HK, Eom JS, Oh BH, Hong CH. Characteristic profiles of instrumental activities of daily living in different subtypes of mild cognitive impairment. Dementia and Geriatric Cognitive Disorders. 2009;27(3):278–285. doi: 10.1159/000204765. [DOI] [PubMed] [Google Scholar]
- Kirchhoff BA, Anderson BA, Barch DM, Jacoby LL. Cognitive and neural effects of semantic encoding strategy training in older adults. Cerebral Cortex. 2012;22(4):788–799. doi: 10.1093/cercor/bhr129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch IS, Mosenthal PB. Exploring document literacy: Variables underlying the performance of young adults. Reading Research Quarterly. 1990;25:5–30. [Google Scholar]
- Kliegl R, Baltes PB. Theory-guided analysis of mechanisms of development and aging through testing-the-limits and research on expertise. In: Schooler C, Schaie KW, editors. Cognitive functioning and social structure over the life course. Norwood, NJ: Ablex; 1987. pp. 95–119. [Google Scholar]
- Lachman ME, Weaver SL, Bandura M, Elliott E, Lewkowitz CJ. Improving memory and control beliefs through cognitive restructuring and self-generated strategies. Journal of Gerontology: Psychological Sciences. 1992;47:293–299. doi: 10.1093/geronj/47.5.p293. [DOI] [PubMed] [Google Scholar]
- Lawton MP, Brody EM. Assessment of older people: Self-maintaining and instrumental activities of daily living. The Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- Lopresti FE, Mihailidis A, Kirsch N. Assistive technology for cognitive rehabilitation: State of the art. Neuropsychological Rehabilitation. 2004;14(1–2):5–39. doi: 10.1080/09602010343000101. [DOI] [Google Scholar]
- Margrett JA, Marsiske M. Gender differences in older adults’ everyday cognitive collaboration. International Journal of Behavioral Development. 2002;26(1):45–59. doi: 10.1080/01650250143000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsiske M, Margrett JA. Everyday problem solving and decision making. In: Birren JE, Schaie KW, editors. Handbook of the Psychology of Aging. 6. New York: Academic Press; 2006. pp. 315–342. [Google Scholar]
- Mihailidis A, Boger JN, Craig T, Hoey J. The COACH prompting system to assist older adults with dementia through handwashing: An efficacy study. BMC Geriatrics. 2008;8(1):28. doi: 10.1186/1471-2318-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DJ, Palmer BW, Patterson TL, Jeste DV. A review of performance-based measures of functional living skills. Journal of Psychiatric Research. 2007;41:97–118. doi: 10.1016/j.jpsychires.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Moreh E, Jacobs JM, Stessman J. Fatigue, function, and mortality in older adults. The Journal of Gerontology: Medical Sciences. 2010;65A:887–895. doi: 10.1093/gerona/glq064. [DOI] [PubMed] [Google Scholar]
- Palmer K, Backman L, Winblad B, Fratiglioni L. Mild cognitive impairment in the general population: Occurrence and progression to Alzheimer disease. American Journal of Geriatric Psychiatry. 2008;16(7):603–611. doi: 10.1097/JGP.0b013e3181753a64. [DOI] [PubMed] [Google Scholar]
- Park DC, Shaw RJ. Effect of environmental support on implicit and explicit memory in younger and older adults. Psychology and Aging. 1992;7(4):632–642. doi: 10.1037/0882-7974.7.4.632. [DOI] [PubMed] [Google Scholar]
- Perneczky R, Pohl C, Sorg C, Hartmann J, Tosic N, Grimmer T, Heitele S, et al. Impairment of activities of daily living requiring memory or complex reasoning as part of the MCI syndrome. International Journal of Geriatric Psychiatry. 2006;21(2):158–162. doi: 10.1002/gps.1444. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychological Methods. 2002;7(2):147–177. doi: 10.1037/1082-989X.7.2.147. [DOI] [PubMed] [Google Scholar]
- Siegler IC, Botwinick J. A long-term longitudinal study of intellectual ability of older adults: The matter of selective subject attrition. Journal of Gerontology. 1979;34(2):242–245. doi: 10.1093/geronj/34.2.242. [DOI] [PubMed] [Google Scholar]
- Singer T, Verhaeghen P, Ghisletta P, Lindenberger U, Baltes PB. The fate of cognition in very old age: Six-year longitudinal findings in the Berlin Aging Study (BASE) Psychology and Aging. 2003;18(2):318–331. doi: 10.1037/0882-7974.18.2.318. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: modeling change and event occurrence. New York, NY: Oxford University Press; 2003. [Google Scholar]
- Teng E, Becker BW, Woo E, Cummings JL, Lu PH. Subtle deficits in instrumental activities of daily living in subtypes of mild cognitive impairment. Dementia and Geriatric Cognitive Disorders. 2010;30(3):189–197. doi: 10.1159/000313540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton WL, Dumke HA. Age differences in everyday problem-solving and decision-making effectiveness: A meta-analytic review. Psychology and Aging. 2005;20(1):85–99. doi: 10.1037/0882-7974.20.1.85. [DOI] [PubMed] [Google Scholar]
- Tucker-Drob EM. Neurocognitive functions and everyday functions change together in old age. Neuropsychology. 2011;25(3):368–377. doi: 10.1037/a0022348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuokko H, Morris C, Ebert P. Mild cognitive impairment and everyday functioning in older adults. Neurocase. 2005;11(1):40–47. doi: 10.1080/13554790490896802. [DOI] [PubMed] [Google Scholar]
- Wadley VG, Crowe M, Marsiske M, Cook SE, Unverzagt FW, Rosenberg AL, Rexroth D. Changes in everyday function in individuals with psychometrically defined mild cognitive impairment in the Advanced Cognitive Training for Independent and Vital Elderly study. Journal of the American Geriatrics Society. 2007;55(8):1192–1198. doi: 10.1111/j.1532-5415.2007.01245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Medical Care. 1992;30:473–483. [PubMed] [Google Scholar]
- Weatherbee SR, Allaire JC. Everyday cognition and mortality: Performance differences and predictive utility of the everyday cognition battery. Psychology and Aging. 2008;23(1):216–221. doi: 10.1037/0882-7974.23.1.216. [DOI] [PubMed] [Google Scholar]
- Willis SL. Everyday problem solving. In: Birren JE, Schaie KW, Abeles RP, Gatz M, Salthouse TA, editors. Handbook of the psychology of aging. 4. San Diego, CA: Academic Press; 1996a. pp. 287–307. The handbooks of aging. [Google Scholar]
- Willis SL. Everyday cognitive competence in elderly persons: Conceptual issues and empirical findings. The Gerontologist. 1996b;36(5):595–601. doi: 10.1093/geront/36.5.595. [DOI] [PubMed] [Google Scholar]
- Willis SL, Jay GM, Diehl M, Marsiske M. Longitudinal change and prediction of everyday task competence in the elderly. Research on Aging. 1992;14(1):68–91. doi: 10.1177/0164027592141004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SL, Marsiske M. Life-span perspective on practical intelligence. In: Tupper DE, Cicerone KD, editors. The neuropsychology of everyday life: Issues in development and rehabilitation. Boston: Kluwer; 1991. pp. 183–198. [Google Scholar]
- Willis SL, Nesselroade CS. Long-term effects of fluid ability training in old-old age. Developmental Psychology. 1990;26(6):905–910. [Google Scholar]
- Willis SL, Tennstedt SL, Marsiske M, Ball K, Elias J, Koepke KM, Wright E. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA: The Journal of the American Medical Association. 2006;296(23):2805–2814. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky FD, Vander Weg MW, Martin R, Unverzagt FW, Willis SL, Marsiske M, Tennstedt SL. Does cognitive training improve internal locus of control among older adults? The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2009;65B(5):591–598. doi: 10.1093/geronb/gbp117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Petersen RC, Lindquist K, Kramer J, Miller B. Subtype of mild cognitive impairment and progression to dementia and death. (2006) Dementia and Geriatric Cognitive Disorders. 2006;22(4):312–319. doi: 10.1159/000095427. [DOI] [PubMed] [Google Scholar]