Abstract
Background
Chronic periodontitis is an inflammatory disease in which cytokines play a major role in the progression of disease. Anti-inflammatory cytokines (IL-4 and IL-10) were reported to be absent or reduced in diseased periodontal tissues, suggesting an imbalance between the pro- and anti-inflammatory mediators. We have tested the hypothesis that there is cellular cross-talk mediated by pro- and anti-inflammatory cytokines and that blocking pro-inflammatory cytokine (TNF-α and IL-1) production will enhance anti-inflammatory cytokine (IL-4 and IL-10) production from peripheral blood mononuclear cells (PBMC) in response to P. gingivalis.
Methods
PBMC were isolated from individuals diagnosed with chronic periodontitis or healthy individuals and cultured for 24 hours. Concanavalin-A (ConA) was used as an activator of lymphocyte function. Live and heat-killed P .gingivalis or lipopolysaccharide from P. gingivalis was used as the bacterial stimulants. TNF-α and IL-1 production was neutralized by specific antibodies against TNF-α and IL-1α or β. Culture supernatants were evaluated by ELISA for TNF-α, IL-1β, IL-4, and IL-10 production.
Results
Live P. gingivalis did not result in any significant IL-10 or IL-4 release while heat-killed P. gingivalis led to a significant increase in IL-10 levels compared to unstimulated or live P. gingivalis-stimulated cells from both healthy and periodontitis individuals. Overall, PBMC from patients with chronic periodontitis produced significantly lower IL-10 in response to ConA and P. gingivalis suggesting chronic suppression of the anti-inflammatory cytokine production. Blocking the pro-inflammatory cytokine response did not result in any substantial change in IL-10 or IL-4 response to live P. gingivalis. Blocking the pro-inflammatory cytokine response restored IL-10 production by cells from chronic periodontitis in response to P. gingivalis LPS.
Conclusion
These findings suggest that PBMC from patients with chronic periodontitis have suppressed anti-inflammatory cytokine production that can, in part, be restored by neutralizing pro-inflammatory cytokines. Monocytes are an important source of IL-10 production and monocyte-derived IL-10 might play a regulatory role in the pathogenesis of chronic periodontitis.
Keywords: IL-4, IL-10, monocytes, Porphyromonas gingivalis, Periodontitis
Chronic inflammatory periodontal diseases are associated with Porphyromonas gingivalis as a major pathogen with a large array of virulence factors1–4. Complex immune responses to P. gingivalis play an important role in the progression of tissue destruction in chronic periodontitis4–7. Lymphocytes (B and T cells) as well as mononuclear phagocytes are present in diseased tissues and participate in host defense by actively producing cytokines.7, 8. Cytokine balance is considered to play an important role in the initiation and progression and host modulation of periodontal disease9–10. T cells can be categorized into various subgroups with different functions11. T-helper (Th) 1 clones produce interleukin (IL)-2, interferon (IFN)-γ and tumor necrosis factor (TNF)-α, while Th2 clones produce IL-4, IL-5, IL-6, and IL-1311. IL-10 was originally described as a product of Th2 clones, but it is now known that Th1 cells and activated monocytes/macrophages also secrete IL-10 in humans suggesting a critical role for IL-10-mediated regulation of the inflammatory response12. Various studies have reported decreased Th1 and increased Th2 responses in chronic periodontitis with gingival mononuclear cells producing higher levels of IL-5 and IL-6, but not IL-213. Memory T cells from the peripheral blood of adult periodontitis patients stimulated in vitro with P. gingivalis were shown to produce significantly more IL-4 than cells from healthy individuals14. It is however, not clear how the interactions between T cell clones and monocytes/macrophages might modulate disease activity and chronicity and at what stage IL-10 is involved.
Evidence suggests that stimulation of peripheral blood mononuclear cells (PBMC) from individuals with periodontitis and gingivitis results in upregulation of IFN-γ and IL-13, while IL-4 and IL-10 are downregulated15. An imbalance of cytokine production may induce bone and collagen destruction in periodontal disease as demonstrated by cell infiltration and elevated levels of pro-inflammatory cytokines (IL-1, TNF and IL-6) associated with active tissue destruction in periodontitis and other chronic inflammatory diseases such as rheumatoid arthritis16–19. One theory suggests that a lack of, or insufficient, response in anti-inflammatory cytokines is associated with the up-regulation of pro-inflammatory cytokines20, 21. Therefore, we hypothesized that the release of anti-inflammatory cytokines will be restored when pro-inflammatory cytokines are neutralized after triggering the host-response with P. gingivalis. The purpose of this study was to analyze P. gingivalis–mediated IL-4 and IL-10 production from PBMC after blocking of TNF-α and IL-1 in the presence and absence of donor periodontitis.
MATERIAL AND METHODS
Selection of Participants
Twelve individuals were included in the study. Six of these participants were diagnosed with moderate to severe generalized chronic periodontitis as defined by the accepted criteria22 while 6 healthy donors were used as matched controls with respect to age, gender, and race. The study was approved by the Institutional Review Board at Boston University Medical Center and informed consent was obtained from all individuals prior to evaluation. Study was conducted between 2004 and 2005. The majority of individuals were nonsmokers (9/12). Mean age was 39.3 ± 9.8 years. None of the individuals had any known systemic disorders or used antibiotics, anti-inflammatory medications within 3 months of the experiment. Participants with active infectious diseases such as hepatitis, HIV, and tuberculosis or were chronically treated with medications (phenytoin, cyclosporin-A, or calcium channel blockers), as well as females who were lactating or pregnant, were excluded.
Porphyromonas Gingivalis Culture and LPS Production
P. gingivalis strain A7436 was cultured as previously described23,24. After 24 hours of anaerobic growth in Schaedler’s broth‡, bacteria were harvested by centrifugation, washed with sterile pyrogen-free saline, and adjusted to an OD660 of 1.0 (approximately 1×109 CFU/ml). Bacterial cell counts were determined in all bacterial cultures to confirm P. gingivalis viability prior to cell culture experiments. A Gram stain kit§ was used for assessing the purity of bacterial cell cultures. Three different P. gingivalis preparations were used; live P. gingivalis was prepared as described above and used at and multiplicity of infection (M.O.I.) of 100. Heat-killed P. gingivalis was used after adjusting the bacterial cell counts and incubating the P. gingivalis colonies in water bath heated to 60°C for 20 minutes. M.O.I. for heat killed bacterial cultures was also set at 100. LPS from P. gingivalis A7436 was isolated by the technique described by Westphall and Jann25. Briefly, after 48–72 hours of growth, bacteria were washed, pelleted and resuspended in distilled water. Phenol was melted and slowly added to an equal volume of bacterial suspension at 68°C. The emulsion was then chilled on ice for 5 min and phases were separated by centrifugation at 10,000 RPM for 30 minutes at 4°C. The aqueous phase (containing the LPS) was removed and dialyzed against distilled water for 72 hours at 4°C. Phenol-water LPS extract was then lyophilized, purified on cesium chloride isopycnic gradient, and analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). For cell stimulation, 100 ng/ml of P. gingivalis LPS was used.
Cell Isolation and Culture
Ninety milliliters of peripheral venous blood was obtained from each individual into heparinized (10U/ml) tubes and mononuclear cells (PBMC) were isolated using a discontinuous gradient system as described before26, 27. Briefly, peripheral blood was layered on a mixture of MonoPoly**, Histopaque 1119, and Histopaque 1077†† and the tubes were centrifuged at 500×g for 30 minutes. The PBMC-rich layer was collected and washed twice with phosphate buffered saline (PBS, pH 7.4), counted, and suspended in RPMI 1640‡‡ with 5% AB serum. PBMC (1×106 cells/ml) were cultured for 24 hours at 37°C in a 5% CO2 atmosphere. After incubation, the cell supernatants were aspirated and saved at −80°C for cytokine analysis. Viability of cells was assessed by Trypan blue exclusion§§ under a light microscope.
In parallel experiments, peripheral blood mononuclear cells were isolated from the same individuals using a discontinuous gradient system (0.25×106/ml). Contaminating non-monocytic cells were removed and monocyte populations were purified by magnetic cell sorting (MACS) column in the magnetic field of a separator with monocyte isolation kit II using an indirect magnetic labeling system for the isolation of untouched monocytes from human peripheral blood mononuclear cells***. Contaminating non-monocytes, i.e. T cells, NK cells, B cells, dendritic cells and basophils, were magnetically labeled using a cocktail of biotin-conjugated antibodies against CD3, CD7, CD16, CD19, CD56, CD123 and Glycophorin A, and anti-biotin microbeads and depleted by retaining them on a MACS Column in the magnetic field of a MACS Separator, while the unlabeled monocytes passed through the column. Pure monocyte preparations were washed and cultured for 24, 48, or 72 hours in the same way as PBMC as outlined above. Cells were stimulated with various agents. In addition to live and heat-killed P. gingivalis, Concanavalin A (ConA; 500µg/ml; Sigma, St. Louis, MO) was used to stimulate T-lymphocyte-mediated cytokine release in PBMC cultures. E. coli LPS (100ng/ml; strain O55:B5†††) was used to activate monocytes.
Neutralization of Pro-Inflammatory Cytokines (TNF-α, IL-1α, and IL-1β)
Activity of TNF-α, IL-1α, and IL-1β secreted by monocytes in cultures was blocked by mono-specific antibodies‡‡‡. Neutralization bioactivity doses (ND50) of anti-human TNF-α, IL-1α, and IL-1β antibodies were 0.02–0.04 µg/ml, 0.05–0.15 µg/ml, and 0.001–0.003 µg/ml, respectively. Dose-response experiment performed to determine the optimal concentrations for neutralizing cytokines indicated that 0.25µg/ml (anti-TNF-α), 0.31µg/ml (anti-IL-1α), and 0.031µg/ml (anti-IL-1β) were needed to block the cytokine activity from 1×106 cells. The inhibition ranged between 87–94% for TNF-α, 62–93% for IL-1α, and 93–94% for IL-1β production. TNF-α, IL-1α, and IL-1β are not produced in significant amounts from resting, Con-A-stimulated or live P. gingivalis stimulated cell cultures. Neutralization achieved at 24 hours stayed at the same level throughout 48 and 72 hours of culture; therefore, the PBMC data is presented over 24 hours. No major variation between different periods in terms of inhibition was noted (data not shown); antibodies were used in combination to ensure complete neutralization of TNF-α, IL-1α, and IL-1β.
Enzyme-Linked Immunosorbent Assay
The levels of TNF-α, IL-1α, IL-1β, IL-4, and IL-10 production were measured by commercially available Enzyme-Linked Immunosorbent Assay (ELISA). The assays were conducted according to the manufacturer’s instructions. For IL-4 and IL-10 assays, high-sensitive kits§§§ were used to detect low levels, while DuoSet ELISA development systems**** were utilized to monitor TNF-α, IL-1α and IL-1β levels. Briefly, diluted standards and standard cytokine dilutions were added to 96-well microplates coated with mouse antihuman antibodies. Biotinylated anti-human antibodies were used as the detection antibody and streptavidin-HRP was added as the conjugate. Hydrogen peroxide and tetramethylbenzidine were used as the substrate solution and the reaction was stopped by adding 2N sulfuric acid. All samples and standards were run in duplicate and optical density was determined with a microplate reader†††† at 450nm wavelength. Samples above the standard determination range for optical density readings were assayed again and read at an appropriate dilution in order to ensure the levels were within the linear slope of the standard curve.
Statistical Analysis and Data Presentation
Each experiment was repeated in triplicate and data are expressed as the mean of three separate values for each individual (± standard deviation). Data analysis was performed by Mann-Whitney U test and significance was set at p<0.05.
RESULTS
IL-10 and IL-4 Release by PBMCs From Healthy Donors
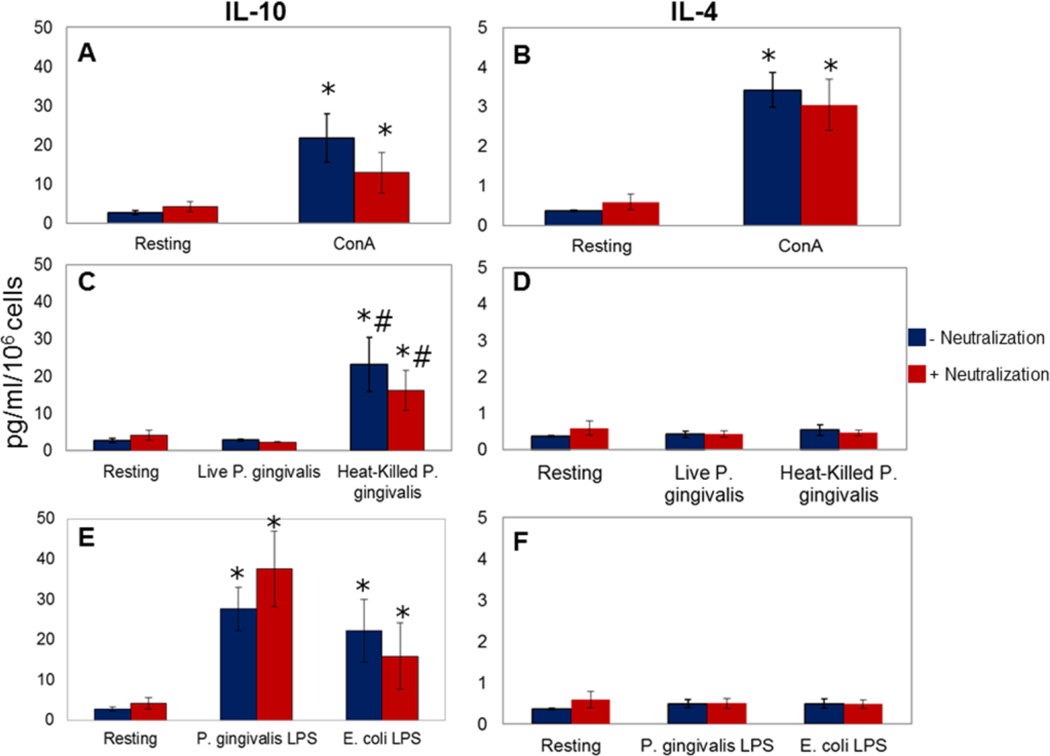
ConA induced reproducibly significant IL-10 and IL-4 production in healthy cells compared to unstimulated (resting) cells (21.69± 6.20 vs. 2.70±0.53 pg/ml of IL-10 and 3.42±0.44 vs 0.37±0.02 pg/ml of IL-4; **p<0.05; Figure 1, Panels A and B). Blocking pro-inflammatory cytokine release did not significantly change ConA-induced IL-10 and IL-4 release by healthy cells. Live P. gingivalis did not result in any significant production of IL-10 or IL-4 (2.80±0.24 pg/ml of IL-10 and 0.42±0.09 pg/ml of IL-4), while heat-killed P. gingivalis led to a significant increase in IL-10 levels compared to unstimulated or live P. gingivalis-stimulated cells (23.11±7.27 pg/ml; p<0.05; Figure 1, Panels C and D). Blocking the pro-inflammatory cytokine response did not result in any substantial change in IL-10 or IL-4 response in healthy cells. P. gingivalis LPS induced a significant increase in IL-10 (27.66±5.37 pg/ml) by healthy PBMC with no effect on IL-4 levels. This response was comparable to LPS from E. coli (22.16±7.77 pg/ml; Figure 1, Panels E and F).
Figure 1. IL-10 and IL-4 release by PBMCs from healthy donors.
PBMC were stimulated in the presence and absence of a combination of neutralizing antibodies to the pro-inflammatory cytokines TNF-α, IL-1α, and IL-1β [0.25µg/ml (anti-TNF-α), 0.31µg/ml (anti-IL-1α), and 0.031µg/ml (anti-IL-1β)]. Each experiment was repeated in duplicate; results represent mean and standard deviations. Panels A and B: A specific T cell-activator (ConA; 500µg/ml) significantly increased cytokine release compared to unstimulated (resting) cells (*p<0.05). Blocking pro-inflammatory cytokine release did not significantly change the ConA-induced IL-10 and IL-4 release by healthy cells (p>0.05). Panels C and D: Live P. gingivalis did not significantly induce IL-10 or IL-4 production by the PBMC (p>0.05); heat-killed P. gingivalis led to a significant increase in IL-10 levels compared to unstimulated (*p<0.05) or live P. gingivalis-stimulated cells (#p<0.05). Blocking pro-inflammatory cytokine response did not result in any substantial change in IL-10 or IL-4 response (p>0.05). Panels E and F: P. gingivalis LPS (100ng/ml) induced a significant increase in IL-10 (*p<0.05) with no impact on IL-4 levels (p>0.05). E. coli LPS (100ng/ml) induced significant IL-10 production (*p<0.05) with no substantial change in IL-4 levels (p>0.05).
IL-10 and IL-4 Release by PBMC From Patients With Chronic Periodontitis
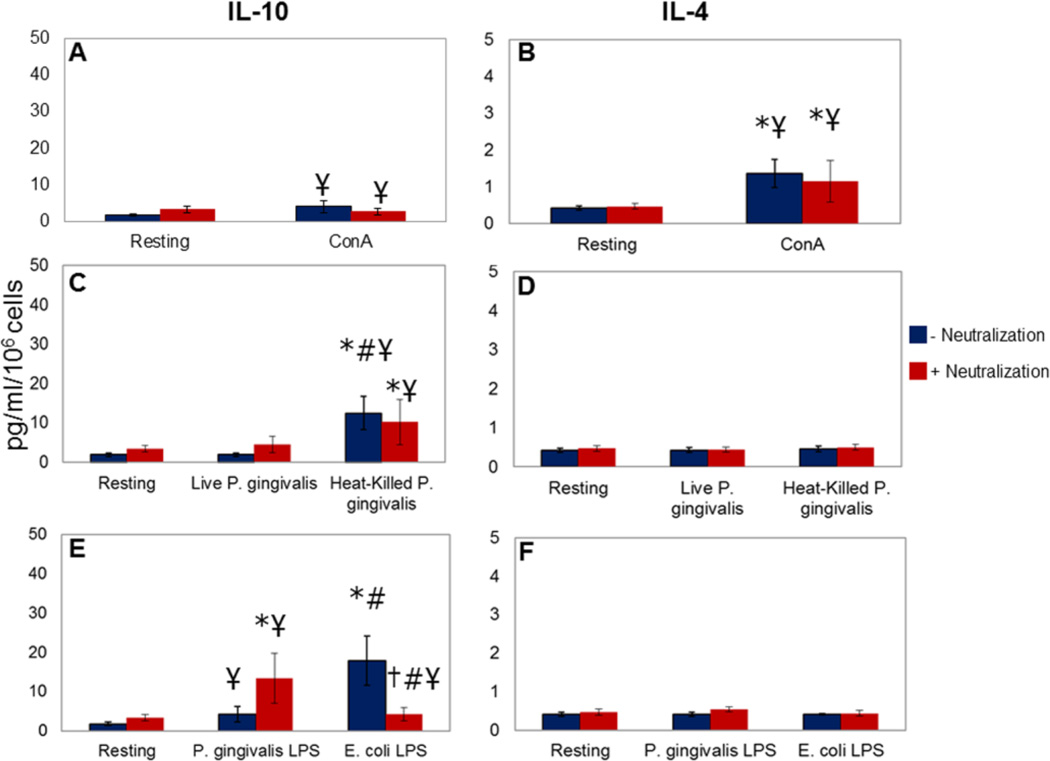
PBMC obtained from patients with chronic periodontitis failed to produce significant levels of IL-10 in response to ConA compared to those from healthy donors, while IL-4 production was also significantly less (4.17±1.65 vs 1.81 vs 0.38 pg/ml of IL-10 and 1.36±0.39 vs 0.42±0.06 pg/ml of IL-4; p<0.05; Figure 2, Panels A and B). Likewise, there was a significant loss of IL-10 production in response to heat-killed P. gingivalis (12.36±4.25 pg/ml) and LPS from P. gingivalis (4.25±1.98 pg/ml) compared to healthy cells with no impact on IL-4 production (Figure 2, Panels C–F). E. coli LPS generated comparable levels of IL-10 by PBMC from periodontitis patients (17.90±6.30 pg/ml). Blocking the pro-inflammatory cytokine response in part restored the IL-10 production by cells from chronic periodontitis in response to P. gingivalis LPS (13.38±6.29 pg/ml), while reducing E. coli LPS-induced IL-10 production (4.27±1.76 pg/ml).
Figure 2. IL-10 and IL-4 release by PBMC from patients with chronic periodontitis.
PBMC were stimulated in the presence and absence of a combination of neutralizing antibodies to the pro-inflammatory cytokines TNF-α, IL-1α, and IL-1β [0.25µg/ml (anti-TNF-α), 0.31µg/ml (anti-IL-1α), and 0.031µg/ml (anti-IL-1β)]. Each experiment was repeated in duplicate; results represent mean and standard deviations. Panels A and B: Con A (500µg/ml) stimulation did not produce significant IL-10 in PBMC from chronic periodontitis patients compared to unstimulated cells (p>0.05). The difference was significant compared to cells from healthy donors (¥p<0.05). IL-4 production was significantly increased compared to unstimulated cells (*p<0.05), while significantly less than healthy cells (¥p<0.05). Panels C and D: Live P. gingivalis did not significantly induce IL-10 or IL-4 production (p>0.05); heat-killed P. gingivalis led to a significant increase in IL-10 levels compared to unstimulated (*p<0.05) or live P. gingivalis-stimulated cells (#p<0.05). Blocking the pro-inflammatory cytokine response did not result in any substantial change in IL-10 or IL-4 response (p>0.05). IL-10 production by PBMC from periodontitis patients in response to heat-killed P. gingivalis was significantly less than the cells from healthy donors (¥p<0.05). Panels E and F: P. gingivalis LPS (100ng/ml) did not induce a significant increase in IL-10 or in IL-4 levels (p>0.05) compared to unstimulated cells. When IL-1 and TNF-α were blocked, P. gingivalis LPS-induced IL-10 production was significantly higher than unstimulated cells (*p<0.05) with no change in Il-4 release. P. gingivalis LPS-induced IL-10 production by the PBMC from periodontitis patients was significantly less than the healthy cells with or without neutralization of pro-inflammatory cytokines (¥p<0.05). E. coli (100ng/ml) LPS stimulated IL-10 by the PBMC significantly compared to resting (*p<0.05) and P. gingivalis-LPS-stimulated (#p<0.05) cells. When the TNF-α and IL-1 responses were blocked, E. coli LPS-induced IL-10 production was significantly reduced (†p<0.05), significantly lower than the P. gingivalis LPS (#p<0.05) or healthy cells (¥p<0.05).
IL-10 Production From Monocytes in Response to P. Gingivalis
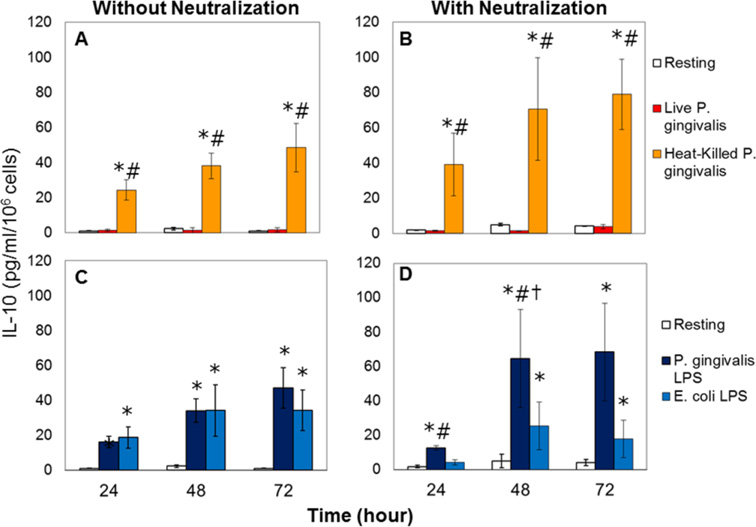
In order to identify the contribution of mononuclear phagocytes (monocytes) to overall IL-10 production by the PBMC, pure monocyte cultures were next obtained by negative selection through magnetic cell sorting and treated the same way as the PBMC. Figure 3 demonstrates the time-course of IL-10 production in response to P. gingivalis over 72 hours. As in the case of PBMC, monocytes failed to produce any detectable IL-10 in response to live P. gingivalis while heat-killed P. gingivalis led to a statistically significant increase in IL-10 production (Figure 3; Panel A); blocking modestly restored this response (Figure 3; Panel B). Cells were then challenged with LPS from E. coli or P. gingivalis (Figure 3; Panel C). Both LPS preparations caused significant and parallel IL-10 production from monocytes (p<0.05). Blocking pro-inflammatory cytokines did not lead to any significant change in IL-10 production in response to E. coli LPS, while the IL-10 response to LPS from P. gingivalis was significantly restored after 48 hours of neutralization (Figure 3; Panel D).
Figure 3. IL-10 production from monocytes in response to P. gingivalis.
Each experiment was repeated in duplicate; results represent mean and standard deviations. Panels A and B: Live P. gingivalis did not generate any significant IL-10 production by the monocytes; heat-killed P. gingivalis led to a statistically significant increase in IL-10 production compared to resting (*p<0.05) or live bacteria-stimulated (#p<0.05) cells. Blocking the pro-inflammatory cytokine activity did not result any significant change compared to cells that were not exposed to neutralization, while statistically significant compared to resting (*p<0.05) or live- bacteria-stimulated (#p<0.05) cells with neutralization. Panels C and D: LPS (100ng/ml) from both E. coli and P. gingivalis caused significant IL-10 production from monocytes compared to resting cells (*p<0.05). Blocking pro-inflammatory cytokines did not lead to any significant change in IL-10 production in response to E. coli LPS (p>0.05), while there was a significant increase in IL-10 response to LPS from P. gingivalis after 48 hours of neutralization (†p<0.05). At both 24 and 48 hours, P. gingivalis LPS-stimulated IL-10 production by the monocytes was significantly higher than the E. coli LPS-treated cells under neutralization (#p<0.05).
DISCUSSION
P. gingivalis can modulate the innate immune response rendering the host susceptible to disease by its virulence factors, such as LPS, capsule, and gingipains1, 2, 28–31. P. gingivalis can perturb the cytokine network, not only by stimulating the release of cytokines from host cells, but also by removing cytokines from local environment in periodontal lesions29–34. “Pro-inflammatory” cytokines initiate a profound immune response, while IL-4, IL-10 act as “anti-inflammatory” cytokines and regulate the immune response by controlling the pro-inflammatory cytokine response35–39. The net outcome of this cross-talk between the “pro-“ and “anti-inflammatory” arms of the immune response is the down-regulation of the excessive and harmful inflammation in infections preventing host mediated tissue destruction and controlling the resolution of inflammation. Based on the assumption that chronic periodontitis is due to an imbalance between pro- and anti-inflammatory cytokines8, 9, 15, 16, 18, 19, 40 we studied the regulation of anti-inflammatory cytokines in P. gingivalis–stimulated PBMC cultures and the role of pro-inflammatory cytokines in this process in healthy people and patients with chronic periodontitis. The results demonstrate that heat-killed P. gingivalis had a profound impact on IL-10 production in PBMC with reduced production by the cells from donors with chronic periodontitis suggesting an aberrant IL-10-mediated host response. There was also differential regulation of cytokine release when cells were stimulated with LPS from P. gingivalis compared to E. coli suggesting different TLR pathways. Blocking pro-inflammatory cytokine discharge restored P. gingivalis LPS-elicited IL-10 production suggesting that the host response is, at least in part, directed to resolution in the absence of pro-inflammatory stimuli. The lack of response to live P. gingivalis is likely due to in vitro degradation of mediators by proteases as reported in similar systems. Overall, these findings indicate that the resolution pathways mediated by IL-10 are downregulated in chronic periodontal inflammation and can only be partially restored when acute pro-inflammatory cytokine release has been blocked.
P. gingivalis synthesizes and secretes high levels of proteolytic enzymes to degrade peptides to essential amino acids as a food source. These bacterial proteases (gingipains) have been shown to cleave and inactivate released cytokines and, as a consequence, are thought to impair the inflammatory response34, 41. Gingipains cleave monocyte CD14, result in attenuation of the cellular recognition of bacteria, and sustain chronic inflammation32. In this study, we tested both live P. gingivalis and heat-killed P. gingivalis. Live cultures did not result in significant IL-10 and IL-4 from PBMC, while heat-killed bacterial preparations led to a substantial anti-inflammatory response indicating that P. gingivalis surface molecules affect the host’s immune mechanisms when the bacterium is not viable. The inhibition of the production of IL-4 and IL-10 by PBMC from healthy individuals and patients with periodontitis elicited by live P. gingivalis is interpreted as an in vitro artifact of high gingipain concentration in the absence of serum protease inhibitors. Within the limits of this study, it is not clear if the inhibition is due to an active impact on the transcription or the secretion of IL-4 and IL-10, or post-release proteolytic degradation. The most likely mechanism is proteolytic degradation, which is supported by the previous findings for IL-4 release that demonstrate that gingipain complexes inactivated the T helper 2 cytokines IL-4 and IL-542. It is possible that the same action is being observed for IL-10 release accounting for broader immunosuppression.
LPS is among the possible virulence factors, which can still be highly active after the bacteria lose its viability. Therefore, we next tested LPS preparation from P. gingivalis. LPS from neither P. gingivalis nor E. coli stimulated the IL-4 response suggesting the specificity of the LPS activation of monocytic cells. IL-10, on the other hand, was produced by both monocytes and PBMC in response to P. gingivalis LPS and E. coli LPS supporting the notion that monocytes are an important source of IL-10. Blocking the pro-inflammatory cytokine response restored IL-10 production by cells from chronic periodontitis in response to P. gingivalis LPS, while reduced E. coli LPS-induced IL-10 production suggesting different TLR-mediated responses are involved in LPS-mediated IL-10 production.
Monocytes are assumed to be initially in a quiescent state, and they are stimulated to produce IL-1 by an external stimulus such as the LPS. This in turn invokes an autocrine IL-1 response and induces the production of the anti-inflammatory cytokine IL-10, which acts to down-regulate IL-1 production. In order to identify the role of mononuclear phagocytes (monocytes) in overall IL-10 production, pure monocyte cultures were obtained by negative selection through magnetic cell sorting and cells were treated in the same way as the PBMC at 24, 48, and 72 hours. As in the case of PBMC, pure monocytes failed to produce any detectable IL-10 in response to live P. gingivalis, while heat-killed P. gingivalis led to statistically significant increases in IL-10 production. Both LPS preparations caused significant and parallel IL-10 production from monocytes (p<0.05). Blocking the pro-inflammatory cytokines did not lead to any significant change in IL-10 production in response to E. coli LPS, while IL-10 responses to LPS from P. gingivalis were significantly restored after 48 hours of neutralization. These findings suggest that blocking IL-1 and TNF-α may only provide a limited benefit for treatment of IL-10 deficiency in inflammatory periodontal lesions.
Cross-talk between pro- and anti-inflammatory cytokines during different stages of inflammation may determine the shift to chronicity. In order to prevent an uncontrolled inflammatory response and rampant tissue destruction, the activity of IL-1 and TNF-α must be regulated. This is done naturally by the elaboration of the anti-inflammatory cytokines or cytokine antagonists. When the soluble receptors to IL-1 or TNF-α are applied in vivo, pathologic processes can be inhibited in arthritis, septic shock, autoimmune diseases and periodontitis43, 44. Based on these observations, we hypothesized that neutralization of pro-inflammatory cytokine release in cell cultures will restore anti-inflammatory cytokine production. More studies are needed to confirm these findings in larger cohorts. Individual variation and various other factors (e.g. smoking) known to play a role in the pathogenesis of periodontal disease can certainly impact the cell response. Yet, the statistically significant differences obtained in this study suggest that the imbalance between pro- and anti-inflammatory regulatory molecules is impaired in chronic periodontitis and P. gingivalis, as well as a cross-talk between cytokines, plays a major role in the process. IL-10 production is suppressed in monocytes from periodontitis patients, which raises the question of temporality on how the IL-10 release is associated with the colonization of P. gingivalis. This is a challenging question where the answers to whether the changes in the immune response allow colonization or is the result of bacterial invasion has not been clear in vivo. It is possible that both can be true where an initial decrease/insufficiency of IL-10 could predispose the P. gingivalis colonization, while chronic periodontitis may be associated with an extended IL-10 function. In primary cell cultures from both health and disease, the phenotypic characteristics of the cells do not change over the study period (24–72 hours). However, this report does not directly address whether depressed levels of IL-10 predispose to chronic periodontitis in humans where P. gingivalis is a dominant pathogen, or if P. gingivalis directly suppresses the protective and anti-inflammatory arm of the immune response. The reported observations may imply that IL-10 has pleiotropic actions at different stages of infection and inflammation depending upon the cellular source.
CONCLUSION
In summary, our study suggests that in chronic periodontitis, anti-inflammatory cytokine production is suppressed. The response can, in part, be restored by neutralizing pro-inflammatory cytokines. The data are also consistent with the suggestion that P. gingivalis can contribute to progression of periodontitis by inducing high levels of inflammatory cytokines and by inhibition of regulator cytokines IL-10 and IL-4.
ACKNOWLEDGEMENTS
Supported in part by NIH USPHS Grants DE15566, DE19938 to TVD, HH, and AK and The Scientific and Technical Research Council of Turkey (TUBITAK) to EB.
Footnotes
None of the authors have any conflict of interest.
Difco, Detroit, MI
Fisher Scientific, Fairlawn, NJ
Flow Laboratories, McLean, VA
Sigma, St. Louis, MO
ATCC, Manassas, VA
Gibco, Invitrogen, Carlsbad, CA
Miltenyi Biotec, Auburn, CA
Sigma, St. Louis, MO
R&D Systems, Minneapolis, MN
R&D Systems, Minneapolis, MN
R&D Systems, Minneapolis, MN
Molecular Devices, Sunnyvale, CA
REFERENCES
- 1.Grenier D, La VD. Proteases of Porphyromonas gingivalis as important virulence factors in periodontal disease and potential targets for plant-derived compounds: a review article. Curr Drug Targets. 2011;12:322–331. doi: 10.2174/138945011794815310. [DOI] [PubMed] [Google Scholar]
- 2.Lin L, Li C, Liu J, et al. Virulence genes of Porphyromonas gingivalis W83 in chronic periodontitis. Acta Odontol Scand. 2009;67:258–264. doi: 10.1080/00016350902841890. [DOI] [PubMed] [Google Scholar]
- 3.Huang PA, Roth GA, Cheng B, et al. Enhanced monocyte migration and pro-inflammatory cytokine production by Porphyromonas gingivalis infection. J Periodont Res. 2010;45:239–245. doi: 10.1111/j.1600-0765.2009.01225.x. [DOI] [PubMed] [Google Scholar]
- 4.Wang PL, Ohura K. 2002. Porphyromonas gingivalis lipopolysaccharide signaling in gingival fibroblasts-CD14 and toll-like receptors. Crit Rev Oral Med. 2002;13:132–142. doi: 10.1177/154411130201300204. [DOI] [PubMed] [Google Scholar]
- 5.Kornman KS, Page RC, Tonetti MS. The host response to the microbial challenge in periodontitis: assembling the players. Periodontology 2000. 1997;14:33–53. doi: 10.1111/j.1600-0757.1997.tb00191.x. [DOI] [PubMed] [Google Scholar]
- 6.Bostanci N, Allaker RP, Belibasakis GN, et al. Porphyromonas gingivalis antagonizes Campylobacter rectus induced cytokine production by human monocytes. Cytokine. 2007;39:147–156. doi: 10.1016/j.cyto.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Teng YT. Protective and destructive immunity in the periodontium. 1. Innate and humoral immunity and the periodontium. J Dent Res. 2006;85:198–208. doi: 10.1177/154405910608500301. [DOI] [PubMed] [Google Scholar]
- 8.Gemmell E, Carter CL, Grieco DA, Sugerman PB, Seymour GJ. P. gingivalis-specific T-cell lines produce Th1 and Th2 cytokines. J Dent Res. 2002;81:303–307. doi: 10.1177/154405910208100503. [DOI] [PubMed] [Google Scholar]
- 9.Pradeep AR, Roopa Y, Swati PP. Interleukin-4, a T-helper 2 cell cytokine, is associated with the remission of periodontal disease. J Periodontal Res. 2008;43:712–716. doi: 10.1111/j.1600-0765.2007.01079.x. [DOI] [PubMed] [Google Scholar]
- 10.Zaric S, Shelburne C, Darveau R, et al. Impaired immune tolerance to Porphyromonas gingivalis Lipopolysaccharide promotes neutrophil migration and decreased apoptosis. Infect Immun. 2010;78:4151–4156. doi: 10.1128/IAI.00600-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mossman TR, Cherwinski H, Bond MV, Giedlen MA, Coffman RL. Two types of helper T cells clones. J Immunol. 1986;136:2348–2356. [PubMed] [Google Scholar]
- 12.Opal SM, DePalo VA. Anti-Inflammatory cytokines. Chest. 2000;117:1162–1172. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
- 13.Fujihashi K, Beagley KW, Kono Y, et al. Gingival mononuclear cells from chronic inflammatory periodontal tissues produce interleukin (IL)-5 and IL-6 but not IL-2 and IL-4. Am J Pathol. 1993;142:1239–1250. [PMC free article] [PubMed] [Google Scholar]
- 14.Aoyagi T, Sugawara-Aoyagi M, Yamazaki K, Hara K. Interleukin-4 (IL-4) and Interleukin-6 -producing memory T-cells in peripheral blood and gingival tissues in periodontitis patients with high serum antibody titers to Porphyromonas gingivalis. Oral Microbiol Immunol. 1995;10:304–310. doi: 10.1111/j.1399-302x.1995.tb00159.x. [DOI] [PubMed] [Google Scholar]
- 15.Nakajima T, Yamazaki K, Cullinan MP, Gemmell E, Seymour GJ. T-cell antigen specificity in humans following stimulation with Porpyhromonas gingivalis. Arch Oral Biol. 1999;44:1045–1053. doi: 10.1016/s0003-9969(99)00094-1. [DOI] [PubMed] [Google Scholar]
- 16.Bozkurt FY, Berker E, Akkus S, Bulut S. Relationship between interleukin-6 levels in gingival crevicular fluid and periodontal status in patients with rheumatoid arthritis and adult periodontitis. J Periodontol. 2000;71:1756–1760. doi: 10.1902/jop.2000.71.11.1756. [DOI] [PubMed] [Google Scholar]
- 17.Walmsley M, Katsikis PD, Abney E, et al. Intrleukin-10 inhibition of the progression established collagen-induced arthritis. Arthritis & Rheumatism. 1996;39:495–503. doi: 10.1002/art.1780390318. [DOI] [PubMed] [Google Scholar]
- 18.Han JY, Reynolds MA. Effect of anti-rheumatic agents on periodontal parameters and biomarkers of inflammation: a systematic review and meta-analysis. J Periodontal Implant Sci. 2012;42:3–12. doi: 10.5051/jpis.2012.42.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartold PM, Marshall RI, Haynes DR. Periodontitis and rheumatoid arthritis: a review. J Periodontol. 2005;76:2066–2074. doi: 10.1902/jop.2005.76.11-S.2066. [DOI] [PubMed] [Google Scholar]
- 20.Katsikis PD, Chu CQ, Brennan FM, Maini RN, Feldman M. Immunoregulatory role of Interleukin-10 in rheumatoid arthiritis. J Exp Med. 1994;179:1517–1527. doi: 10.1084/jem.179.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Roon JAG, Van Roy JL, Gmelig-Meyling FHJ, Lafeber FPJG, Bijlsma JWJ. Prevention and reversal of cartilage degradation in rheumatoid arthritis by interleukin-10 and interleukin-4. Arthritis & Rheumatism. 1996;39:829–835. doi: 10.1002/art.1780390516. [DOI] [PubMed] [Google Scholar]
- 22.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 23.Genco CA, Cutler CW, Kapczynski D, Maloney K, Arnold RR. A novel mouse model to study the virulence of and host response to Porphyromonas (Bacteroides) gingivalis. Infect Immun. 1991;59:1255–1263. doi: 10.1128/iai.59.4.1255-1263.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Genco CA, Schifferle RE, Njorage T, Forng RY, Cutler CW. Resistance of a Tn4351- generated polysaccharide mutant of Porphyromonas gingivalis to polymorphonuclear leukocyte killing. Infect Immun. 1995;63:393–401. doi: 10.1128/iai.63.2.393-401.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Westphal O, Jann K. Extraction with phenol and further applications of the procedure. Methods Carbohydr Chem. 1965;5:83–91. [Google Scholar]
- 26.Kalmar JR, Arnold RR, Warbington ML, Gardner MK. Superior leukocyte separation with a discontinous one-step Ficoll-Hypaque gradient for the isolation of human neutrophils. J Immunol Methods. 1988;110:275–280. doi: 10.1016/0022-1759(88)90115-9. [DOI] [PubMed] [Google Scholar]
- 27.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Scan J Clin Lab Invest. 1965;21(Suppl. 97):77–89. [PubMed] [Google Scholar]
- 28.Zadeh HH, Nichols FC, Miyasaki KT. The role of the cell-mediated immune response to Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in periodontitis. Periodontology 2000. 1999;20:39–288. doi: 10.1111/j.1600-0757.1999.tb00163.x. [DOI] [PubMed] [Google Scholar]
- 29.Jotwani R, Cutler CW. Fimbriated Porphyromonas gingivalis is more efficient than fimbria-deficient P. gingivalis in entering human dendritic cells in vitro and induces an inflammatory Th1 effector response. Infect Immun. 2004;72:1725–1732. doi: 10.1128/IAI.72.3.1725-1732.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suwatanapongched P, Surarit R, Srisatjaluk R, Offenbacher S. Translocation of Porphyromonas gingivalis infected monocytes and associated cellular responses. Asian Pac J Allergy Immunol. 2010;28:192–199. [PubMed] [Google Scholar]
- 31.Yun PL, Decarlo AA, Collyer C, Hunter N. Hydrolysis of IL-12 by Porphyromonas gingivalis major cysteine proteinases may affect local gamma interferon accumulation and the Th1 or Th2 T-cell phenotype in periodontitis. Infect Immun. 2001;69:5650–5660. doi: 10.1128/IAI.69.9.5650-5660.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sugawara S, Nemoto E, Tada H, Miyake K, Imamura T, Takada H. Proteolysis of human monocyte CD14 by cysteine proteinases (gingipains) from Porphyromonas gingivalis leading to lipopolysaccaride hyporesponsiveness. J Immunol. 2000;165:411–418. doi: 10.4049/jimmunol.165.1.411. [DOI] [PubMed] [Google Scholar]
- 33.Shimauchi H, Ogawa T, Okuda K, Kusumoto Y, Okada H. Autoregulatory effect of interleukin-10 on proinflammatory cytokine production by Porphyromonas gingivalis lipopolysaccharide-tolerant human monocytes. Infect Immun. 1999;67:2153–2159. doi: 10.1128/iai.67.5.2153-2159.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamedi M, Belibasakis GN, Cruchley AT, et al. Porphyromonas gingivalis culture supernatants differentially regulate interleukin-1beta and interleukin-18 in human monocytic cells. Cytokine. 2009;45:99–104. doi: 10.1016/j.cyto.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Kinane DF, Lappin DF. Clinical, pathological and immunological aspects of periodontal disease. Acta Odontol. 2001;59:154–160. doi: 10.1080/000163501750266747. [DOI] [PubMed] [Google Scholar]
- 36.Page RC. The pathobiology of periodontal diseases may affect systemic diseases: Inversion of a paradigm. Ann Periodontol. 1998;3:108–120. doi: 10.1902/annals.1998.3.1.108. [DOI] [PubMed] [Google Scholar]
- 37.Paul WE. Interleukin-4: a prototypic immunoregulatory lymhokine. Blood. 1991:1859–1870. [PubMed] [Google Scholar]
- 38.Howard M, O’Garra A. Biological properties of IL-10. Immunol Today. 1992;13:198–200. doi: 10.1016/0167-5699(92)90153-X. [DOI] [PubMed] [Google Scholar]
- 39.Champaiboon C, Yongvanitchit K, Pichyangkul S, Mahanonda R. The immune modulation of B-cell responses by Porphyromonas gingivalis and IL-10. J Periodontol. 2000;71:468–475. doi: 10.1902/jop.2000.71.3.468. [DOI] [PubMed] [Google Scholar]
- 40.Yamamato M, Fujihashi K, Hiroi T, et al. Molecular and cellular mechanisms for periodontal disease: role of Th1 and Th2 type cytokines. J Periodontal Res. 1997;32:115–119. doi: 10.1111/j.1600-0765.1997.tb01391.x. [DOI] [PubMed] [Google Scholar]
- 41.Rudney JD, Chen R, Sedgewick GJ. Intracellular Actinobacillus actinomycetemcomitants and Porphyromonas gingivalis in buccal epithelial cells collected from human subjects. Infect Immun. 2001;69:2700–2707. doi: 10.1128/IAI.69.4.2700-2707.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tam V, O’Brien-Simpson NM, Chen YY, et al. The RgpA-Kgp proteinase-adhesin complexes of Porphyromonas gingivalis inactivate the Th2 cytokines interleukin-4 and interleukin-5. Infect Immun. 2009;77:1451–1458. doi: 10.1128/IAI.01377-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marcelletti JF. IL-10 inhibits lipopolysaccharide-induced murine B-cell proliferation and cross-linking of surface antigen receptors or ligation of CD40 restores the response. J Immunol. 1996;157:3323–3333. [PubMed] [Google Scholar]
- 44.Miossec P, Naviliat M, D’Angeac AD, Sany J, Banchereau J. Low levels of IL-4 and high levels of transforming growth factor β in rheumatoid synovitis. Arth Rheum. 1990;33:1180–1187. doi: 10.1002/art.1780330819. [DOI] [PubMed] [Google Scholar]