Abstract
Chlorpyrifos (CPF) is a widely used organophosphorus (OP) pesticide. CPF is bioactivated by cytochrome P450s (CYPs) to the potent cholinesterase inhibitor chlorpyrifos oxon (CPF-O) or detoxified to 3,5,6-trichloro-2-pyridinol (TCPy). Human CYP2B6 has the highest reported Vmax/Km (intrinsic clearance - CLint) for bioactivation while CYP2C19 has the highest reported CLint for detoxification of CPF. In this study, 22 human liver microsomes (HLMs) genotyped for common variants of these enzymes (CYP2B6*6 and CYP2C19*2) were incubated with 10μM and 0.5μM CPF and assayed for metabolite production. While no differences in metabolite production were observed in homozygous CYP2C19*2 HLMs, homozygous CYP2B6*6 specimens produced significantly less CPF-O than wild-type specimens at 10μM (mean 144 and 446 pmol/min/mg, respectively). This correlated with reduced expression of CYP2B6 protein (mean 4.86 and 30.1 pmol/mg, for CYP2B6*6 and *1, respectively). Additionally, CYP2B6*1 and CYP2B6*6 were over-expressed in mammalian COS-1 cells to assess for the first time the impact of the CYP2B6*6 variant on the kinetic parameters of CPF bioactivation. The Vmax for CYP2B6*6 (1.05 × 105 pmol/min/nmol CYP2B6) was significantly higher than that of CYP2B6*1 (4.13 × 104 pmol/min/nmol CYP2B6) but the Km values did not differ (1.97 μM for CYP2B6*6 and 1.84 μM for CYP2B6*1) resulting in CLint rates of 53.5 and 22.5 nL/min/nmol CYP2B6 for *6 and *1, respectively. These data suggest that CYP2B6*6 has increased specific activity but reduced capacity to bioactivate CPF in HLMs compared to wild-type due to reduced hepatic protein expression, indicating that individuals with this genotype may be less susceptible to CPF toxicity.
Keywords: CYP2B6, CYP2C19, chlorpyrifos, biotransformation
1.1 Introduction
Chlorpyrifos (CPF) is a widely used organophosphorus pesticide and is thought to primarily produce neurotoxic effects via inhibition of acetylcholinesterase (AChE). While acute exposure to CPF is well-known to cause neurotoxicity, less is known about chronic low-level exposure, though there is mounting evidence that neurotoxicity is also the primary endpoint for repeated OP exposures (Jiang et al. 2010; Middlemore-Risher et al. 2010; Rohlman et al. 2011; Salvi et al. 2003; Speed et al. 2011). Although residential use of CPF has been banned in the U.S. since 2001 (U.S. EPA 2000), inhalation and dermal exposures in agricultural communities remain a public health concern due to occupational exposures of pesticide workers and environmental exposures of the residents (Eaton et al. 2008). In addition, CPF is used worldwide with the highest exposures reported in an Egyptian agricultural population (Farahat et al. 2011; Farahat et al. 2010). This population was found to have neurobehavioral deficits when compared to a reference population (Abdel Rasoul et al. 2008; Farahat et al. 2003).
CPF is itself a weak inhibitor of AChE and must be bioactivated to the more potent metabolite, chlorpyrifos oxon (CPF-O) (Figure 1). CPF can also be detoxified to the nontoxic metabolite, 3,5,6-trichloro-2-pyridinol (TCPy). CYP2B6 has the highest reported Vmax/Km (intrinsic clearance CLint) (15.6 nL/min/nmol P450) for the bioactivation of CPF to CPF-O, approximately five-fold higher than CYP1A2, the next most active CYP for this reaction (Foxenberg et al. 2007). For the detoxification of CPF to TCPy, CYP2C19 has the highest intrinsic clearance (8.1 nL/min/nmol P450), approximately six-fold higher than the next most active CYP (Foxenberg et al. 2007). However, it should be noted that all CYP enzymes which catalyze CPF biotransformation are capable of both the bioactivation and detoxification reaction at some level though CYP2B6 highly favors the bioactivation pathway while CYP2C19 favors detoxification. The predominant role of these CYP enzymes in CPF metabolism has been consistently demonstrated by both metabolism and inhibitor studies (Buratti et al. 2003; Croom et al. 2010; Foxenberg et al. 2007; Tang et al. 2001). Both enzymes have also been detected in human brain tissue, the target organ of toxicity for CPF (Miksys et al. 2003; Miksys and Tyndale 2002). The other main CYP involved in CPF metabolism is CYP3A4 which, while highly abundant in the liver, has a low affinity for the biotransformation of CPF (Km 27.2μM, Vmax 1.20 × 104 pmol/min/nmol P450 for the bioactivation reaction and Km 33.4μM, Vmax 1.27 × 104 for the detoxification reaction) and is thought to be the predominant enzyme of the low-affinity component of CPF metabolism, (Croom et al. 2010; Foxenberg et al. 2007).
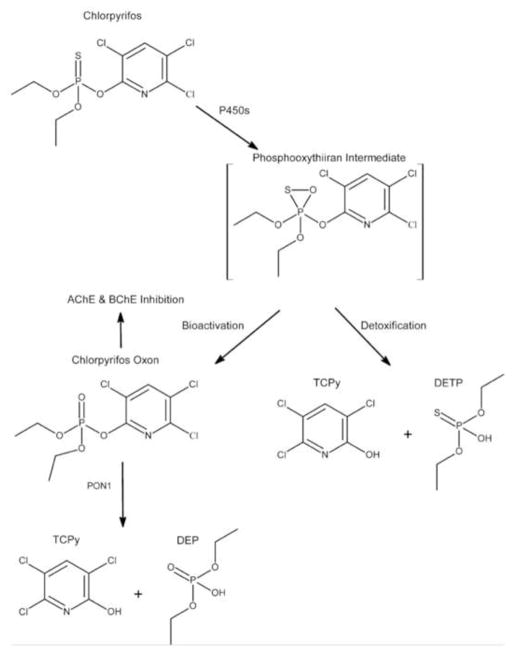
Figure 1.
Illustrates the major pathways of metabolism for chlorpyrifos (CPF). CYP2B6 has the highest CLint for the bioactivation pathway while CYP2C19 has the highest CLint for the detoxification pathway. TCPy (3,5,6-trichloro-2-pyridinol) is a metabolite specific to CPF. DETP (o,o-diethylphosphorothionate) and DEP (o,o-diethylphosphate) are non-specific metabolites of CPF. PON1 (paraoxonase1) is an alpha esterase.
CYP2B6 is a highly inducible and polymorphic enzyme which metabolizes not only clinically important drugs (e.g., bupropion, cyclophosphamide, efavirenz, propofol, selegiline) but also many environmental chemicals (Hodgson and Rose 2007; Turpeinen et al. 2006; Zanger et al. 2007). Variants of this enzyme have the potential to modify metabolism of CPF in vivo. The most prevalent allelic isoform of CYP2B6 is CYP2B6*6 (15631G>T and 18053 A>G) at a frequency of 20–31% in Caucasian populations and up to 60% in African and other populations (Zanger et al. 2007). The 15631G>T SNP in this variant codes for amino acid change Q172H in exon 4 but the important functional consequence is thought to result from erroneous splicing leading to decreased hepatic expression and activity (Hofmann et al. 2008; Lang et al. 2001). However, the 18053A>G SNP [K262R] also found in CYP2B6*6 and other variants appears to confer increased substrate turnover and some substrate-dependent alterations in metabolism (Bumpus et al. 2005). Indeed, CYP2B6*6 has been found to confer decreased metabolism of bupropion (Hesse et al. 2004) and efavirenz (for review see (Roca 2008) but not cyclophosphamide (Nakajima et al. 2007; Xie et al. 2006). CYP2C19 is also a polymorphic enzyme with the most common variant being the null variant CYP2C19*2 at a frequency of 12–13% in Caucasians and up to 30% in Asians (Goldstein et al. 1997; Mizutani 2003). Examining the kinetics for CPF metabolism by common CYP variants has the potential to enhance our understanding of variability in human susceptibility to this common pesticide. Kinetic parameters for CPF metabolism can also be used in physiologically based pharmacokinetic/pharmacodynamic (PBPK/PD) models which attempt to better quantify exposure, transportation, activation, detoxification, and clearance of OP pesticides (Foxenberg et al. 2011; Knaak et al. 2004; Timchalk et al. 2002). PBPK/PD models are dependent on the available kinetic parameters for metabolism, which are often not available and/or consistent and often based on data from studies with rat liver microsomes (for review, see (Knaak et al. 2004). Use of these data can under represent the interindividual variability present in humans. Kinetic data on the CYP-specific metabolism of CPF will inform current CYP specific CPF models by addressing interindividual variability and the impact of CYP2B6 and CYP2C19 genotype on the inhibition of AChE and butyrylcholinesterase (BChE), which serve as biomarkers of neurotoxicity (Foxenberg et al. 2011). A pharmacokinetic/pharmacogenetic model has recently been developed for the optimization of efavirenz therapy in caucasians with the inclusion of known CYP2B6*1/*6 genotype - successfully reducing the initial interindividual variability of the model (Sanchez et al, 2011).
Herein we report the effect of CYP2B6 and CYP2C19 genotype on CPF metabolism in 22 individual human liver microsomes (HLMs) and the effect of CYP2B6 genotype on CPF metabolism in recombinant enzymes. Also reported, for the first time, are the Km and Vmax values for bioactivation by CYP2B6*6, the most common genetic variant of CYP2B6. Together with estimates of human hepatic CYP2B6 content, these data can enhance current risk assessment efforts for CPF through assessing the potential impact of CYP2B6*6 and CYP2C19*2 genotype on relative susceptibility to CPF.
2.2 Materials and Methods
2.2 Chemicals
Chlorpyrifos (CAS 2921-88-2), chlorpyrifos-oxon (CAS 5598-15-2), and 3,5,6-trichloro-2-pyridinol (CAS 6515-38-4) were purchased from ChemService Inc. (West Chester, PA). Tetraisopropyl pyrophosphoramide (iso-OMPA; CAS 513-00-8) was of reagent grade and purchased from Sigma-Aldrich (St. Louis, MO). EDTA and MgCl2, methanol, and acetonitrile were purchased from J. T. Baker (Phillipsburg, NJ) and were of at least reagent grade quality. Recombinant human CYP2B6 was purchased from BD Gentest (Woburn, MA).
2.3 HLM Characterization
22 individual human liver microsome specimens (HLMs), were selected according to CYP2B6 and CYP2C19 genotype from a collection of liver tissue specimens and corresponding blood samples (n=250) from patients of Caucasian ethnicity undergoing liver surgery at the Campus Virchow, University Medical Center Charité, Humboldt University, Berlin, Germany. The study was approved by the ethics committees of the medical faculties of the Charité, Humboldt University, and of the University of Tuebingen and conducted in accordance with the Declaration of Helsinki. The CYP2B6/CYP2C19 genotype groups (*1*1/*1*1; *6*6/*1*1; *6*6/*1*2; *1*1/*2*2) did not differ significantly in age (56.3y; 56.8y; 62y; 47.3y, respectively). Gender and medication classification is shown in Table 1. Genotyping methods have been described before (Blievernicht et al. 2007; Lang et al. 2001; Lang et al. 2004).
Table 1.
Genotype and Phenotype Characteristics of Human Liver Microsome Specimens
| HLM ID | Drug exposure | Gender | CYP2B6 Genotype | CYP2C19 Genotype | CYP2B6 (pmol/mg) | Bupropion Hydroxylation Activity (pmol/min/mg OH-Bupropion formation) |
|---|---|---|---|---|---|---|
| 1 | Yes | female | *1/*1 | *1/*1 | 6.21 | 86.22 |
| 2 | P450 inducer | male | *1/*1 | *1/*1 | 6.59 | 115.66 |
| 3 | Yes | female | *1/*1 | *1/*1 | 8.00 | 194.13 |
| 4 | Yes | male | *1/*1 | *1/*1 | 8.30 | 152.30 |
| 5 | Yes | female | *1/*1 | *1/*1 | 8.40 | 218.73 |
| 6 | No | female | *1/*1 | *1/*1 | 17.36 | 119.60 |
| 7 | Yes | male | *1/*1 | *1/*1 | 22.94 | 232.46 |
| 8 | Yes | male | *1/*1 | *1/*1 | 25.43 | 148.65 |
| 9 | Yes | male | *1/*1 | *1/*1 | 44.26 | 158.33 |
| 10 | No | male | *1/*1 | *1/*1 | 75.91 | 1204.93 |
| 11 | N/A | male | *1/*1 | *1/*1 | 100.76 | 752.41 |
| 12 | P450 inducer | male | *6/*6 | *1/*1 | 1.50 | 14.70 |
| 13 | No | female | *6/*6 | *1/*2 | 2.11 | 10.53 |
| 14 | Yes | male | *6/*6 | *1/*2 | 2.56 | 41.36 |
| 15 | No | female | *6/*6 | *1/*1 | 4.51 | 71.66 |
| 16 | P450 inducer | female | *6/*6 | *1/*1 | 5.00 | 56.18 |
| 17 | No | male | *6/*6 | *1/*1 | 7.30 | 124.26 |
| 18 | No | female | *6/*6 | *1/*1 | 11.04 | 57.76 |
| 19 | Yes | male | *1/*1 | *2/*2 | 19.05 | 76.40 |
| 20 | Yes | female | *1/*1 | *2/*2 | 24.45 | 105.88 |
| 21 | P450 inducer | male | *1/*1 | *2/*2 | 28.27 | 280.67 |
| 22 | No | male | *1/*1 | *2/*2 | 54.95 | 578.60 |
Genotype and phenotype characteristics of the 22 individual HLMs used. Original ID numbers were changed to a simple numbering system. Drug exposure indicates whether liver donors were exposed to known P450 inducers (omeprazole, pantoprazole, budenosid); the other patients received either no drugs or drugs not known to induce P450 prior to surgery; N/A., not available.
2.4 Bupropion hydroxylase activity
HLMs were assayed for the ability to metabolize bupropion to the metabolite OH-Bupropion (bupropion hydroxylase activity), a specific marker of CYP2B6 activity, as previously described (Hofmann et al. 2008). Briefly, 50μg of protein was incubated in a final volume of 0.1mL with a substrate concentration of 50μM. The reaction was initiated with 10μL of a 10-fold concentrated NADPH-regenerating system and terminated after 15 minutes with 20μL 1 N HCl. OH-Bupropion concentration was determined by liquid chromatography-mass spectroscopy with a detection limit of 1pmol as previously described (Richter et al. 2004). Samples were analyzed in duplicate.
2.5 CYP2B6 Content
HLMs were assayed for CYP and total protein content as described previously (Hofmann et al. 2008). Briefly, recombinant CYP2B6 lymphoblast microsomes (BD Biosciences, San Jose, CA) were coanalyzed as a standard on each 10% gel for quantification. CYP2B6 protein was immunodetected with a specific monoclonal mouse anti-human CYP2B6 antibody (BD Biosciences) and quantified by secondary IR Dye 800 labeled goat anti-mouse antibody using the infrared imaging system Odyssey (LI-COR Biosciences GmbH, Bad Homburg, Germany).
2.6 CPF Metabolism Assay
HLMs were incubated with CPF at concentrations greater than (10μM) and less than (0.5μM) the reported Kms for bioactivation and detoxification of CPF by CYP2B6 (0.8 and 2.1μM) and CYP2C19 (1.2 and 1.6μM), respectively. Incubations with 0.5 mg of protein/mL were carried out in buffer consisting of 100mM Tris-HCL, and 5mM MgCl2, 1mM EDTA to inhibit A-esterases, and 50μM iso-OMPA to inhibit B-esterases at a pH of 7.4. Reactions were initiated with 1mM NADPH and incubated at 37°C for a period of two minutes at a final volume of 200μL and quenched with an equal volume of ice cold methanol with 0.1% phosphoric acid. Reactions were then centrifuged and transferred to HPLC vials for analysis. Production of metabolites chlorpyrifos oxon (CPF-O) and 3,5,6-trichloro-2-pyridinol (TCPy) was measured by reverse phase HPLC with diode array detection as described previously (Foxenberg et al. 2007). Chemical detection was determined at the UV wavelengths of 290nm for CPF and CPF-O and 300nm for TCPy. The minimum level of detection of these compounds was 2.5ng. Assays were conducted in duplicate except where noted as done in singlet due to insufficient total protein content for two assays.
2.7 Transfection
Studies were conducted in COS-1 cells (ATCC, Washington DC). 3 × 106 COS-1 cells were plated onto 10cm dishes two days prior to transfection with cDNA vectors (Lang et al. 2004) for CYP2B6.1 and CYP2B6.6. The sequence of each plasmid was confirmed after transport and storage as well as after amplification for experiments by direct sequencing (Roswell Park Cancer Institute DNA Sequencing Laboratory, Buffalo, NY). One plate was transfected each time with a renilla luciferase reporter plasmid pRL-CMV (Promega, Madison, WI) to monitor relative transfection efficiency between experiments. 24μg of DNA was transfected using 72μL per plate Lipofectamine 2000 (Life Technologies, Carlsbad, CA). Two days post-transfection, cells were washed with phosphate-buffered saline and scraped off the plate. Cells were centrifuged and resuspended in 5mM HEPES buffer (pH 7.4). Cell lysates were homogenized with two strokes of a glass/glass homogenizer.
2.8 Recombinant CYP2B6 Content
The lysate fraction was analyzed for CYP2B6 content via western blot with a rabbit anti-human polyclonal antibody from Enzo Life Sciences (Farmingdale, NY). Recombinant CYP2B6 (BD Gentest, Woburn, AM) was coanalyzed on each gel to generate a standard curve. Horseradish peroxidase-conjugated donkey anti-rabbit immunoglobulin was used as the secondary antibody. Immunoreactive proteins were visualized with an enhanced chemiluminescence detection system from Amersham Biosciences (Piscataway, NJ). Relative protein concentration was determined by densitometry and is expressed as pmol/mg.
2.9 CPF Metabolism Assay
Assay was conducted as stated in section 2.6 for HLM specimens with the following changes: cell lysates were incubated with 0.1–100μM CPF and the final protein concentration was 2.0 mg of protein/mL.
2.10 Kinetic and Statistical Analysis
Data were fitted to the Michaelis-Menten equation by non-linear regression analysis using SigmaPlot 11 software (SyStat Software Inc, Chicago, IL) to derive Km and Vmax values. The mean and standard error of the mean (SEM) was determined for the Km and Vmax value for each allelic isoform. Km and Vmax values for CYP2B6*6 were analyzed for statistically significant differences compared to wild type protein using independent samples t-test using SigmaPlot. Genotyped groups of HLMs were compared using independent samples t-test using SPSS (Chicago, IL). Pearson’s correlation coefficient was calculated for linear regression analysis (SPSS). In all cases, p < 0.05 was considered significant.
3.1 Results
3.2 Characterization of HLMs
Of 22 HLM specimens, 11 were homozygous wild-type CYP2B6 and CYP2C19, five were CYP2B6*6/*6 and homozygous wild-type CYP2C19, two were CYP2B6*6/*6 and CYP2C19*1/*2 and four were homozygous wild-type CYP2B6 and CYP2C19*2/*2. CYP2B6 content ranged from 1.50–100.8 pmol/mg and bupropion hydroxylase activity ranged from 10.5–1205 pmol/min/mg (Table 1). Four specimens were from patients on drugs known to be P450 inducers as noted in Table 1.
3.3 Correlation of CYP2B6 content and bupropion hydroxylation activity
Single donor HLM specimens (n=22) were assayed for CYP2B6 content and bupropion hydroxylation activity. Bupropion hydroxylation is specific for CYP2B6 and is used as an in vitro probe for CYP2B6 catalytic activity (Faucette et al. 2000; Loboz et al. 2006). To test the reliability of this assay in our sample set, bupropion hydroxylation activity (measured as pmol/min/mg OH-Bupropion formation) was compared to total CYP2B6 content (pmol/mg). Bupropion hydroxylation activity and CYP2B6 content were well-correlated (r2 = 0.86, p<0.01) in HLM samples (Figure 2).
Figure 2.
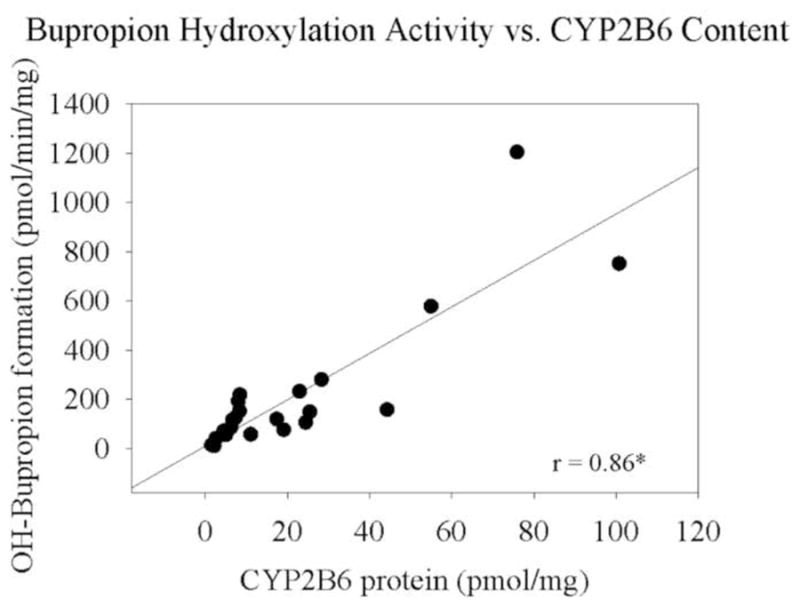
Relationship between bupropion metabolism (OH-bupropion formation, pmol/min/mg) and CYP2B6 content (pmol/mg) in HLMs. Each point represents an individual specimen.
* indicates statistical significance at p< 0.01.
3.4 Correlation of CYP2B6 content with CPF metabolism by HLMs
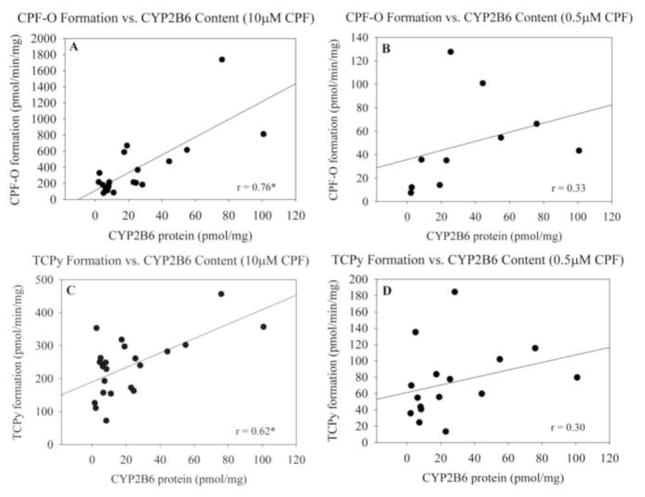
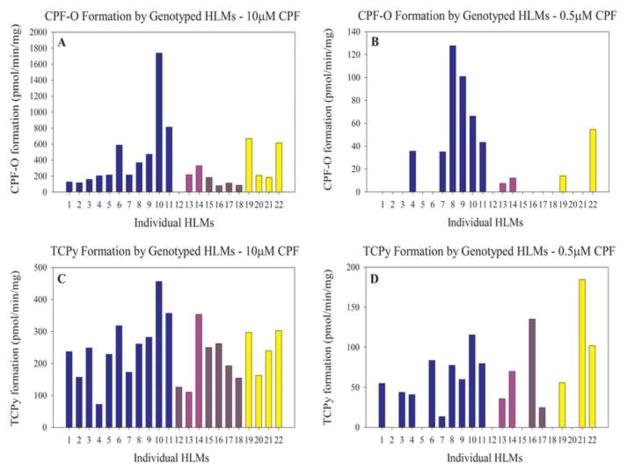
Production of toxic metabolite CPF-O and nontoxic metabolite TCPy was measured at 10μM CPF and at 0.5μM CPF for 22 characterized HLM specimens. The rate of CPF-O formation from CPF (10μM) varied widely in these specimens from 0 (below the limit of detection) to 1738 pmol/min/mg, while TCPy formation was less variable with a range of 72.5 to 456.4 pmol/min/mg. The range of CPF-O formation at 0.5μM CPF was 0 to 127.7 pmol/min/mg while for TCPy it was 0 to 184.5 pmol/min/mg. At 10μM CPF, CYP2B6 content was correlated with the rate of CPF-O formation (r = 0.76, p<0.01) and also correlated to a lesser extent with TCPy formation (r = 0.62, p<0.01) (Figure 3A&3C). At 0.5μM CPF, CYP2B6 content was neither correlated with CPF-O formation (r2 = 0.33) nor with TCPy formation (r2 = 0.30) (Figure 3B&3D). Specimens with undetectable metabolite formation were removed from analyses. Every sample except one produced detectable levels of CPF-O at 10μM CPF and 10/22 produced detectable levels of CPF-O at 0.5μM CPF. All samples produced detectable levels of TCPy at 10μM CPF while 16/22 produced detectable levels of TCPy at 0.5μM CPF (Figure 4).
Figure 3.
Biotransformation of CPF to CPF-O (A&B) and TCPy (C&D) (pmol/min/mg) by HLMs relative to CYP2B6 content (pmol/mg) at 10μM CPF and at 0.5μM CPF. Specimens producing non-detectable levels of metabolite were not included in the analysis. * indicates statistical significance at p< 0.01.
Figure 4.
Biotransformation of CPF to CPF-O (A&B) and TCPy (C&D) (pmol/min/mg) by individual, genotyped HLMs at 10μM CPF and at 0.5μM CPF. Each bar represents an individual HLM assayed in duplicate except samples 3, 6, 7, 17, and 19, which were done in singlet. Specimens 1–11 are CYP2B6*1/*1 and CYP2C19*1/*1, specimens 12 & 15–18 are CYP2B6*6/*6 and CYP2C19*1/*1, specimens 13 & 14 are CYP2B6*6/*6 and CYP2C19*1/*2, and specimens 19–22 are CYP2B6*1/*1 and CYP2C19*2/*2. A color version of this figure is available in the online version of this manuscript.
3.5 Impact of genotype on CPF metabolism by HLMs
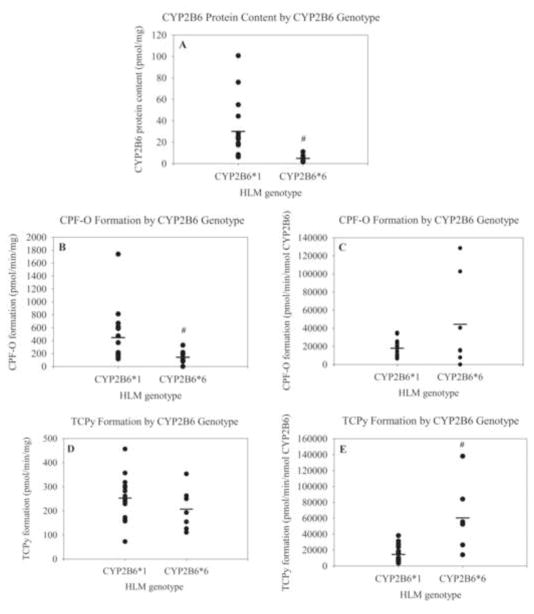
CYP2B6 protein content in CYP2B6*6/*6 (4.86 ± 1.28 pmol/mg - mean ± SEM) HLM specimens was significantly lower than in CYP2B6*1/*1 wild type specimens (30.1 ± 7.19 pmol/mg) (Figure 5A). Similarly, CYP2B6*6/*6 specimens had a significant, albeit less dramatic decrease in the rate of formation of CPF-O at 10μM CPF relative to CYP2B6*1/*1 wild type specimens with means and SEMs of 144 ± 40.9 and 446 ± 109 pmol/min/mg, respectively (Figure 5B). Differences between genotypes remained significant when the analysis was repeated with omission of the potential outlier wild-type point (specimen with the highest CPF-O activity). The mean with this point removed for wild type CYP2B6 specimens was 353 ± 62.2 pmol/min/mg. In addition, the specimen that produced undetectable levels of CPF-O was a CYP2B6*6/*6 specimen. When production of CPF-O was standardized to nmol of CYP2B6 protein at 10μM CPF the mean rate was 1.79 × 104 ± 2.38 × 103 pmol/min/nmol CYP2B6 for CYP2B6 homozygous wild-type specimens and 4.44 × 104 ± 1.92 × 104 pmol/min/nmol CYP2B6 for CYP2B6*6/*6 specimens with no statistically significant difference between the two (Figure 5C). Mean rates of TCPy formation at 10μM CPF for homozygous wild-type CYP2B6 were 253 ± 23.8 pmol/min per mg total protein or 1.45 × 104 ± 2.79 × 103 pmol/min per nmol of CYP2B6 and for CYP2B6*6/*6 were 207 ± 32.8 pmol/min/mg or 6.04 × 104 ± 1.55 × 104 pmol/min/nmol CYP2B6 (Figures 5D&E). When the data is expressed per mg of total protein, CYP2B6*6/*6 variant specimens produce significantly less CPF-O metabolite. However, there is no significant difference between the two groups on a per nmol CYP2B6 basis, indicating that differences in CPF-O production are likely attributable to the lower overall CYP2B6 protein content of CYP2B6*6/*6 HLMs. Similar results were found at 0.5μM CPF, with the formation of CPF-O being significantly less for CYP2B6*6/*6 variants than CYP2B6*1/*1 specimens when expressed as pmol/min/mg (data not shown).
Figure 5.
(A) CYP2B6 protein content (pmol/mg) of individual HLM specimens by CYP2B6 genotype. Each dark circle represents an individual HLM and the mean is represented by a black line. CPF-O formation by CYP2B6 genotype groups expressed as pmol/min/mg (B) or in pmol/min/nmol CYP2B6 (C). TCPy formation by CYP2B6 genotype groups expressed as pmol/min/mg (D) or in pmol/min/nmol CYP2B6 (E). # indicates statistical significance at p< 0.05.
Of the 15 homozygous wild-type CYP2B6 HLMs, 10/15 produced more CPF-O metabolite than TCPy (expressed as pmol/min/mg) while only 1/6 CYP2B6*6/*6 HLMs produced more CPF-O than TCPy at 10μM CPF. At 0.5 μM CPF, of the 12 wild-type specimens that produced detectable levels of both CPF metabolites, only three produced greater amounts of CPF-O than TCPy while all four CY2B6*6/*6 HLMs with detectable metabolite production produced more TCPy.
No significant differences in the formation of CPF-O or TCPy were found between homozygous CYP2C19 wild type specimens and CYP2C19*2/*2 specimens at 10μM CPF (Figure 6A&B). The two heterozygous CYP2C19 samples were not included in this analysis. Means and SEM for homozygous wild-type CYP2C19 were 342 ± 107 pmol CPF-O/min/mg and 236 ± 23.4 pmol TCPy/min/mg. Means for CYP2C19*2 specimens were 419 ± 130 pmol CPF-O/min/mg and 251 ± 32.5 pmol TCPy/min/mg. No significant differences in metabolite formation were found between the two genotype groups at 0.5μM CPF (data not shown).
Figure 6.
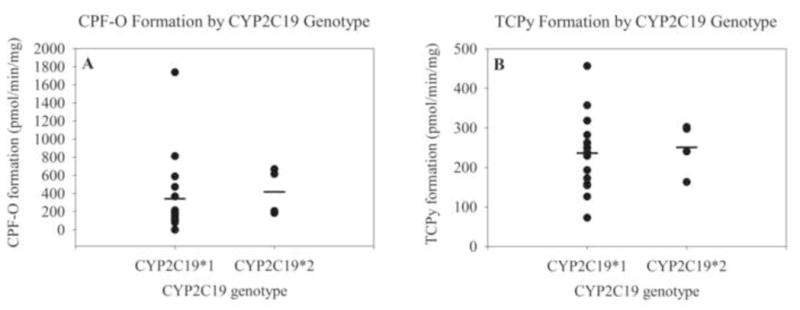
CPF-O formation (A) or TCPy formation (B) by HLMs according to CYP2C19 genotype expressed as pmol/min/mg. Each dark circle represents an individual HLM and the mean is represented by a black line. The two heterozygous CYP2C19 samples were not included in this analysis.
3.6 CPF metabolism by recombinant CYP2B6*1 and CYP2B6*6
CYP2B6*1 and CYP2B6*6 were over-expressed in mammalian COS-1 cells. Three different cell lysate preparations for each isoform were incubated with 0.1–100μM CPF to assess the kinetics for CPF metabolism for each genotype. CYP2B6 content of each preparation was determined by western blot. The rate of CPF metabolism exhibited Michaelis-Menten kinetics with the *1 and *6 genotypes exhibiting similar Km values of 1.84 ± 0.80 and 1.97 ± 0.97μM, respectively (Figure 7). The Vmax for CYP2B6*1 was found to be 4.13 × 104± 0.38 × 104 pmol/min/nmol CYP2B6 (Figure 7A). The Vmax for CYP2B6*6 was determined to be 1.05 × 105 ± 0.12 × 105 pmol/min/nmol CYP2B6 (Figure 7B). While Km values were not significantly different between the two recombinant CYP2B6 isoforms the Vmax of CYP2B6*6 was significantly higher compared to that of CYP2B6*1. CLint rates (53.5 and 22.5 nL/min/nmol for CYP2B6*6 and *1, respectively), were not significantly different.
Figure 7.
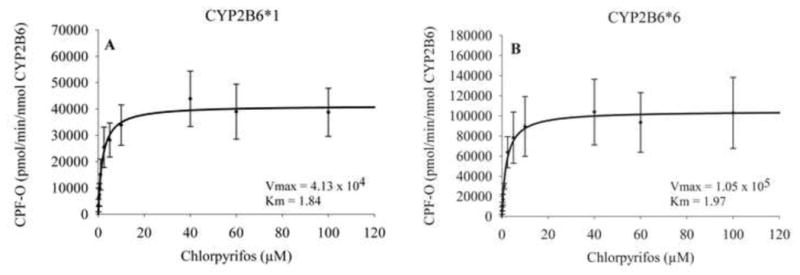
Michaelis-Menton plot for the biotransformation of CPF to CPF-O by recombinant CYP2B6*1 (A) and CYP2B6*6 (B). Formation of metabolite is expressed as pmol/min/nmol CYP2B6. Graphs are the composite of three separate experiments and error bars represent standard error of the mean.
4.1 Discussion
Current efforts in risk assessment often rely heavily on animal data which does not accurately reflect human metabolism or human interindividual variability. The understanding of human variability is critical to accurate models of risk assessment, including PBPK/PD models. Using 22 single donor HLMs, we determined that 21/22 samples formed detectable levels of CPF-O and all formed detectable levels of TCPy at a concentration of 10μM CPF. Roughly half (10/22) formed detectable levels of CPF-O at 0.5μM CPF and most (16/22) formed detectable levels of TCPy at the lower concentration. The high correlation of the rate of CPF-O production with HLM CYP2B6 content at 10μM CPF suggests that CYP2B6 is the dominant P450 enzyme at relatively low concentrations for bioactivation of CPF which agrees with previous studies indicating that the metabolism of CPF is mediated by both low and high-affinity enzymes with CYP2B6 and CYP2C19 being the most likely high-affinity candidates for the biotransformation of CPF (Croom et al. 2010; Foxenberg et al. 2007; Mutch and Williams 2006; Sams et al. 2004; Tang et al. 2001). More TCPy than CPF-O production and less correlation between CPF-O and CYP2B6 content was found in previous studies using CPF concentrations of 20μM and 100μM which also suggests that CPF may be metabolized by lower affinity enzymes such as CYP3A4 at higher concentrations (Croom et al. 2010). Importantly, our data also suggest that bioactivation of CPF to CPF-O may outpace detoxification to TCPy at low exposure levels that are relevant to occupational and environmental exposures (Farahat et al. 2011). The majority of CYP2B6*1/*1 HLM specimens (10/15) favored the bioactivation reaction over the detoxification reaction at 10μM CPF, though not at 0.5μM CPF (3/12). Unlike at higher concentrations of CPF (> 20μM), low concentrations of CPF that are above the Km of CYP2B6 may result in the domination of the bioactivation pathway and production of the toxic metabolite. CYP2B6*6/*6 HLM specimens, on the other hand, favored the detoxification reaction at both 0.5μM and 10μM of CPF. This is an indication of the potential for CYP2B6 genotype to alter the balance between the two major pathways of CPF metabolism.
When comparing CYP2B6*1/*1 and CYP2B6*6/*6 HLM specimens, statistical differences were found in both total CYP2B6 protein content as well as formation of the toxic metabolite CPF-O at both 10μM and 0.5μM CPF. This is consistent with the proposed mechanism of the CYP2B6*6 polymorphism which is thought to result in decreased hepatic expression due to erroneous splicing (Hofmann et al. 2008). No statistical differences in metabolite formation were found when comparing CYP2C19*1/*1 and CYP2C19*2/*2 HLM specimens. It is surprising that CYP2C19*2 homozygous samples did not produce less TCPy on average when compared to wild type specimens given that CYP2C19*2 is a null phenotype although the availability of CYP2C19*2 homozygous sample group was very small (n=4). It is likely that other CYPs in HLMs such as CYP3A4, 3A5, and 1A2 can compensate in the detoxification of CPF in the absence of CYP2C19, even at low concentrations of CPF. Despite the small sample size, an advantage of this study was the use of mostly homozygous samples, allowing us to more easily compare genotype effects which are more difficult to detect in a mix of heterozygous and homozygous samples.
The Kms found here for recombinant CYP2B6*1 and *6 (1.84 vs. 1.97μM, respectively) are very close to that of 0.8μM determined for commercially available recombinant CYP2B6*1 by Foxenberg et al., (2007). While the Kms did not differ, CYP2B6*6 had a significantly higher Vmax than the wild-type enzyme. Despite the differences in Vmax values, the in vitro data generated in this study as well as previous literature reports for the metabolism of other substrates by CYP2B6*6 suggest that this slightly higher Vmax does not compensate for the decreased hepatic expression of CYP2B6*6. Results from characterized HLMs and these data indicate that the reduced rate of bioactivation of CPF in HLMs with the CYP2B6*6 genotype is due to reduced protein expression and not in different kinetic properties of the two isoforms.
4.2 Conclusions
Bioactivation of CPF in HLMs is decreased in CYP2B6*6/*6 specimens as compared to CYP2B6*1/*1 specimens and this decrease in metabolism corresponds to a decrease in protein expression in CYP2B6*6 HLMs. No change in the detoxification of CPF was seen in CYP2C19*2/*2 specimens, although the N was small (4). Kinetics of human recombinant CYP2B6*1 and CYP2B6*6 indicate that while CYP2B6*6 has a higher Vmax than wild-type, there is no significant difference in Km or CLint. Together, these data suggest that the CYP2B6*6 allele is a protein with similar kinetics but decreased expression, leading to decreased bioactivation of CPF to CPF-O and possibly conferring decreased susceptibility to CPF.
Acknowledgments
Funding: This work was supported by the National Institute of Environmental Health Sciences at the National Institutes of Health [R01 ES016308] and the U. S. Environmental Protection Agency Science to Achieve Results [R-833454].
Nonstandard abbreviations used in this manuscript
- CPF
chlorpyrifos
- CYP
cytochrome P450
- OP
organophosphorus
- CPF-O
chlorpyrifos oxon
- TCPy
3,5,6-trichloro-2-pyridinol
- HLM
human liver microsome
- AChE
acetylcholinesterase
- BChE
butyrylcholinesterase
Footnotes
Conflict of Interest Statement:
Dr. Zanger is named as co-inventor on a patent application directed to the detection of specific CYP2B6 polymorphisms for diagnostic purposes; this entitles him to share in any net income derived from licensing these patent rights under standard academic institutional policies. Drs. Klein and Olson and Ms. Crane declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Alice L. Crane, Email: alcrane@buffalo.edu.
Kathrin Klein, Email: kathrin.klein@ikp-stuttgart.de.
Ulrich M. Zanger, Email: uli.zanger@ikp-stuttgart.de.
James R. Olson, Email: jolson@buffalo.edu.
References
- Abdel Rasoul GM, Abou Salem ME, Mechael AA, Hendy OM, Rohlman DS, Ismail AA. Effects of occupational pesticide exposure on children applying pesticides. Neurotoxicology. 2008;29:833–838. doi: 10.1016/j.neuro.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Blievernicht JK, Schaeffeler E, Klein K, Eichelbaum M, Schwab M, Zanger UM. MALDI-TOF mass spectrometry for multiplex genotyping of CYP2B6 single-nucleotide polymorphisms. Clinical chemistry. 2007;53:24–33. doi: 10.1373/clinchem.2006.074856. [DOI] [PubMed] [Google Scholar]
- Bumpus NN, Sridar C, Kent UM, Hollenberg PF. The naturally occurring cytochrome P450 (P450) 2B6 K262R mutant of P450 2B6 exhibits alterations in substrate metabolism and inactivation. Drug Metab Dispos. 2005;33:795–802. doi: 10.1124/dmd.105.003749. [DOI] [PubMed] [Google Scholar]
- Buratti FM, Volpe MT, Meneguz A, Vittozzi L, Testai E. CYP-specific bioactivation of four organophosphorothioate pesticides by human liver microsomes. Toxicol Appl Pharmacol. 2003;186:143–154. doi: 10.1016/s0041-008x(02)00027-3. [DOI] [PubMed] [Google Scholar]
- Costa LG. Current issues in organophosphate toxicology. Clin Chim Acta. 2006;366:1–13. doi: 10.1016/j.cca.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Croom EL, Wallace AD, Hodgson E. Human variation in CYP-specific chlorpyrifos metabolism. Toxicology. 2010;276:184–191. doi: 10.1016/j.tox.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Eaton DL, Daroff RB, Autrup H, Bridges J, Buffler P, Costa LG, Coyle J, McKhann G, Mobley WC, Nadel L, Neubert D, Schulte-Hermann R, Spencer PS. Review of the toxicology of chlorpyrifos with an emphasis on human exposure and neurodevelopment. Crit Rev Toxicol. 2008;38(Suppl 2):1–125. doi: 10.1080/10408440802272158. [DOI] [PubMed] [Google Scholar]
- Ellison CA, Tian Y, Knaak JB, Kostyniak PJ, Olson JR. Human Hepatic Cytochrome P450-specific Metabolism of the Organophosphorus Pesticides Methyl Parathion and Diazinon. Drug metabolism and disposition: the biological fate of chemicals. 2011 doi: 10.1124/dmd.111.042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahat FM, Ellison CA, Bonner MR, McGarrigle BP, Crane AL, Fenske RA, Lasarev MR, Rohlman DS, Anger WK, Lein PJ, Olson JR. Biomarkers of Chlorpyrifos Exposure and Effect in Egyptian Cotton Field Workers. Environ Health Perspect. 2011 doi: 10.1289/ehp.1002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahat FM, Fenske RA, Olson JR, Galvin K, Bonner MR, Rohlman DS, Farahat TM, Lein PJ, Anger WK. Chlorpyrifos exposures in Egyptian cotton field workers. Neurotoxicology. 2010;31:297–304. doi: 10.1016/j.neuro.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahat TM, Abdelrasoul GM, Amr MM, Shebl MM, Farahat FM, Anger WK. Neurobehavioural effects among workers occupationally exposed to organophosphorous pesticides. Occup Environ Med. 2003;60:279–286. doi: 10.1136/oem.60.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faucette SR, Hawke RL, Lecluyse EL, Shord SS, Yan B, Laethem RM, Lindley CM. Validation of bupropion hydroxylation as a selective marker of human cytochrome P450 2B6 catalytic activity. Drug Metab Dispos. 2000;28:1222–1230. [PubMed] [Google Scholar]
- Foxenberg RJ, Ellison CA, Knaak JB, Ma C, Olson JR. Cytochrome P450-specific human PBPK/PD models for the organophosphorus pesticides: chlorpyrifos and parathion. Toxicology. 2011;285:57–66. doi: 10.1016/j.tox.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxenberg RJ, McGarrigle BP, Knaak JB, Kostyniak PJ, Olson JR. Human hepatic cytochrome p450-specific metabolism of parathion and chlorpyrifos. Drug Metab Dispos. 2007;35:189–193. doi: 10.1124/dmd.106.012427. [DOI] [PubMed] [Google Scholar]
- Gatanaga H, Hayashida T, Tsuchiya K, Yoshino M, Kuwahara T, Tsukada H, Fujimoto K, Sato I, Ueda M, Horiba M, Hamaguchi M, Yamamoto M, Takata N, Kimura A, Koike T, Gejyo F, Matsushita S, Shirasaka T, Kimura S, Oka S. Successful efavirenz dose reduction in HIV type 1-infected individuals with cytochrome P450 2B6 *6 and *26. Clin Infect Dis. 2007;45:1230–1237. doi: 10.1086/522175. [DOI] [PubMed] [Google Scholar]
- Goldstein JA, Ishizaki T, Chiba K, de Morais SM, Bell D, Krahn PM, Evans DA. Frequencies of the defective CYP2C19 alleles responsible for the mephenytoin poor metabolizer phenotype in various Oriental, Caucasian, Saudi Arabian and American black populations. Pharmacogenetics. 1997;7:59–64. doi: 10.1097/00008571-199702000-00008. [DOI] [PubMed] [Google Scholar]
- Hesse LM, He P, Krishnaswamy S, Hao Q, Hogan K, von Moltke LL, Greenblatt DJ, Court MH. Pharmacogenetic determinants of interindividual variability in bupropion hydroxylation by cytochrome P450 2B6 in human liver microsomes. Pharmacogenetics. 2004;14:225–238. doi: 10.1097/00008571-200404000-00002. [DOI] [PubMed] [Google Scholar]
- Hodgson E, Rose RL. The importance of cytochrome P450 2B6 in the human metabolism of environmental chemicals. Pharmacology & therapeutics. 2007;113:420–428. doi: 10.1016/j.pharmthera.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Hofmann MH, Blievernicht JK, Klein K, Saussele T, Schaeffeler E, Schwab M, Zanger UM. Aberrant splicing caused by single nucleotide polymorphism c.516G>T [Q172H], a marker of CYP2B6*6, is responsible for decreased expression and activity of CYP2B6 in liver. J Pharmacol Exp Ther. 2008;325:284–292. doi: 10.1124/jpet.107.133306. [DOI] [PubMed] [Google Scholar]
- Jiang W, Duysen EG, Hansen H, Shlyakhtenko L, Schopfer LM, Lockridge O. Mice treated with chlorpyrifos or chlorpyrifos oxon have organophosphorylated tubulin in the brain and disrupted microtubule structures, suggesting a role for tubulin in neurotoxicity associated with exposure to organophosphorus agents. Toxicological sciences: an official journal of the Society of Toxicology. 2010;115:183–193. doi: 10.1093/toxsci/kfq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaak JB, Dary CC, Power F, Thompson CB, Blancato JN. Physicochemical and biological data for the development of predictive organophosphorus pesticide QSARs and PBPK/PD models for human risk assessment. Crit Rev Toxicol. 2004;34:143–207. doi: 10.1080/10408440490432250. [DOI] [PubMed] [Google Scholar]
- Lang T, Klein K, Fischer J, Nussler AK, Neuhaus P, Hofmann U, Eichelbaum M, Schwab M, Zanger UM. Extensive genetic polymorphism in the human CYP2B6 gene with impact on expression and function in human liver. Pharmacogenetics. 2001;11:399–415. doi: 10.1097/00008571-200107000-00004. [DOI] [PubMed] [Google Scholar]
- Lang T, Klein K, Richter T, Zibat A, Kerb R, Eichelbaum M, Schwab M, Zanger UM. Multiple novel nonsynonymous CYP2B6 gene polymorphisms in Caucasians: demonstration of phenotypic null alleles. J Pharmacol Exp Ther. 2004;311:34–43. doi: 10.1124/jpet.104.068973. [DOI] [PubMed] [Google Scholar]
- Loboz KK, Gross AS, Williams KM, Liauw WS, Day RO, Blievernicht JK, Zanger UM, McLachlan AJ. Cytochrome P450 2B6 activity as measured by bupropion hydroxylation: effect of induction by rifampin and ethnicity. Clin Pharmacol Ther. 2006;80:75–84. doi: 10.1016/j.clpt.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Middlemore-Risher ML, Buccafusco JJ, Terry AV., Jr Repeated exposures to low-level chlorpyrifos results in impairments in sustained attention and increased impulsivity in rats. Neurotoxicology and teratology. 2010;32:415–424. doi: 10.1016/j.ntt.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miksys S, Lerman C, Shields PG, Mash DC, Tyndale RF. Smoking, alcoholism and genetic polymorphisms alter CYP2B6 levels in human brain. Neuropharmacology. 2003;45:122–132. doi: 10.1016/s0028-3908(03)00136-9. [DOI] [PubMed] [Google Scholar]
- Miksys SL, Tyndale RF. Drug-metabolizing cytochrome P450s in the brain. Journal of psychiatry & neuroscience: JPN. 2002;27:406–415. [PMC free article] [PubMed] [Google Scholar]
- Mileson BE, Chambers JE, Chen WL, Dettbarn W, Ehrich M, Eldefrawi AT, Gaylor DW, Hamernik K, Hodgson E, Karczmar AG, Padilla S, Pope CN, Richardson RJ, Saunders DR, Sheets LP, Sultatos LG, Wallace KB. Common mechanism of toxicity: a case study of organophosphorus pesticides. Toxicol Sci. 1998;41:8–20. doi: 10.1006/toxs.1997.2431. [DOI] [PubMed] [Google Scholar]
- Mizutani T. PM frequencies of major CYPs in Asians and Caucasians. Drug metabolism reviews. 2003;35:99–106. doi: 10.1081/dmr-120023681. [DOI] [PubMed] [Google Scholar]
- Mutch E, Williams FM. Diazinon, chlorpyrifos and parathion are metabolised by multiple cytochromes P450 in human liver. Toxicology. 2006;224:22–32. doi: 10.1016/j.tox.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Komagata S, Fujiki Y, Kanada Y, Ebi H, Itoh K, Mukai H, Yokoi T, Minami H. Genetic polymorphisms of CYP2B6 affect the pharmacokinetics/pharmacodynamics of cyclophosphamide in Japanese cancer patients. Pharmacogenet Genomics. 2007;17:431–445. doi: 10.1097/FPC.0b013e328045c4fb. [DOI] [PubMed] [Google Scholar]
- Richter T, Murdter TE, Heinkele G, Pleiss J, Tatzel S, Schwab M, Eichelbaum M, Zanger UM. Potent mechanism-based inhibition of human CYP2B6 by clopidogrel and ticlopidine. J Pharmacol Exp Ther. 2004;308:189–197. doi: 10.1124/jpet.103.056127. [DOI] [PubMed] [Google Scholar]
- Roca B. Pharmacogenomics of antiretrovirals. Recent patents on anti-infective drug discovery. 2008;3:132–135. doi: 10.2174/157489108784746650. [DOI] [PubMed] [Google Scholar]
- Rohlman DS, Anger WK, Lein PJ. Correlating neurobehavioral performance with biomarkers of organophosphorous pesticide exposure. Neurotoxicology. 2011;32:268–276. doi: 10.1016/j.neuro.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi RM, Lara DR, Ghisolfi ES, Portela LV, Dias RD, Souza DO. Neuropsychiatric evaluation in subjects chronically exposed to organophosphate pesticides. Toxicological sciences: an official journal of the Society of Toxicology. 2003;72:267–271. doi: 10.1093/toxsci/kfg034. [DOI] [PubMed] [Google Scholar]
- Sams C, Cocker J, Lennard MS. Biotransformation of chlorpyrifos and diazinon by human liver microsomes and recombinant human cytochrome P450s (CYP) Xenobiotica. 2004;34:861–873. doi: 10.1080/00498250400017273. [DOI] [PubMed] [Google Scholar]
- Speed HE, Blaiss CA, Kim A, Haws ME, Melvin NR, Jennings M, Eisch AJ, Powell CM. Delayed reduction of hippocampal synaptic transmission and spines following exposure to repeated, subclinical doses of organophosphorus pesticide in adult mice. Toxicological sciences: an official journal of the Society of Toxicology. 2011 doi: 10.1093/toxsci/kfr253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Cao Y, Rose RL, Brimfield AA, Dai D, Goldstein JA, Hodgson E. Metabolism of chlorpyrifos by human cytochrome P450 isoforms and human, mouse, and rat liver microsomes. Drug Metab Dispos. 2001;29:1201–1204. [PubMed] [Google Scholar]
- Timchalk C, Nolan RJ, Mendrala AL, Dittenber DA, Brzak KA, Mattsson JL. A Physiologically based pharmacokinetic and pharmacodynamic (PBPK/PD) model for the organophosphate insecticide chlorpyrifos in rats and humans. Toxicol Sci. 2002;66:34–53. doi: 10.1093/toxsci/66.1.34. [DOI] [PubMed] [Google Scholar]
- Turpeinen M, Raunio H, Pelkonen O. The functional role of CYP2B6 in human drug metabolism: substrates and inhibitors in vitro, in vivo and in silico. Curr Drug Metab. 2006;7:705–714. doi: 10.2174/138920006778520633. [DOI] [PubMed] [Google Scholar]
- U.S. EPA. Chlorpyrifos Revised Risk Assessment and Agreement with Registrants. Washington, DC: U.S. Environmental Protection Agency; 2000. [Google Scholar]
- Xie H, Griskevicius L, Stahle L, Hassan Z, Yasar U, Rane A, Broberg U, Kimby E, Hassan M. Pharmacogenetics of cyclophosphamide in patients with hematological malignancies. Eur J Pharm Sci. 2006;27:54–61. doi: 10.1016/j.ejps.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Zanger UM, Klein K, Saussele T, Blievernicht J, Hofmann MH, Schwab M. Polymorphic CYP2B6: molecular mechanisms and emerging clinical significance. Pharmacogenomics. 2007;8:743–759. doi: 10.2217/14622416.8.7.743. [DOI] [PubMed] [Google Scholar]