Abstract
The study of blood ex vivo can occur in closed or open systems, with or without flow. Microfluidic devices facilitate measurements of platelet function, coagulation biology, cellular biorheology, adhesion dynamics, pharmacology, and clinical diagnostics. An experimental session can accommodate 100s to 1000s of unique clotting events. Using microfluidics, thrombotic events can be studied on defined surfaces of biopolymers, matrix proteins, and tissue factor under constant flow rate or constant pressure drop conditions. Distinct shear rates can be created on a device with a single perfusion pump. Microfluidic devices facilitated the determination of intraluminal thrombus permeability and the discovery that platelet contractility can be activated by a sudden decrease in flow. Microfluidics are ideal for multicolor imaging of platelets, fibrin, and phosphatidylserine and provide a human blood analog to the mouse injury models. Overall, microfluidic advances offer many opportunities for research, drug testing under relevant hemodynamic conditions, and clinical diagnostics.
Keywords: Thrombosis, Hemostasis, Platelet Function, von Willebrand Factor, Collagen, Fibrin
1. INTRODUCTION: Coagulation biochemistry and platelet function under flow
Plasma and platelets
Human blood is composed of plasma, platelets, white blood cells and red blood cells. While central to diseases like heart attacks, strokes or hemophilia, the regulated function of blood is also critically important during acute events such as surgery or trauma. Within plasma, major protein based pathways include: extrinsic coagulation (triggered by tissue factor), contact-activated coagulation (triggered by generation of Factor XIIa), fibrin polymerization, complement activation, and fibrinolysis. Platelets, neutrophils, and monocytes can undergo homotypic and heterotypic aggregation under flow conditions. Formation of aggregates on surfaces represents a spatially-dependent deposition process and is distinguished, both kinetically and mechanically, from bulk aggregation within a flowing suspension.
The generation of thrombin is central to the study of thrombosis and hemostasis. Thrombin activates platelets and triggers the cleavage of fibrinogen to a fibrin monomer that undergoes polymerization to form fibrin via protofibril extension followed by lateral aggregation. Tissue factor (TF), a membrane protein that binds Factor VIIa to activate Factor X to Xa and Factor IX to IXa, is the fundamental trigger of the extrinsic pathway. Central to biomaterial thrombosis is the generation of Factor XIIa, which is the trigger of the contact (also commonly called intrinsic) pathway. The role of the contact pathway Factor XIIa and Factor XIa during in vivo thrombosis is supported by studies of the XIIa knockout mouse and the role of thrombin mediated feedback activation of Factor XIa (Fig. 1A)
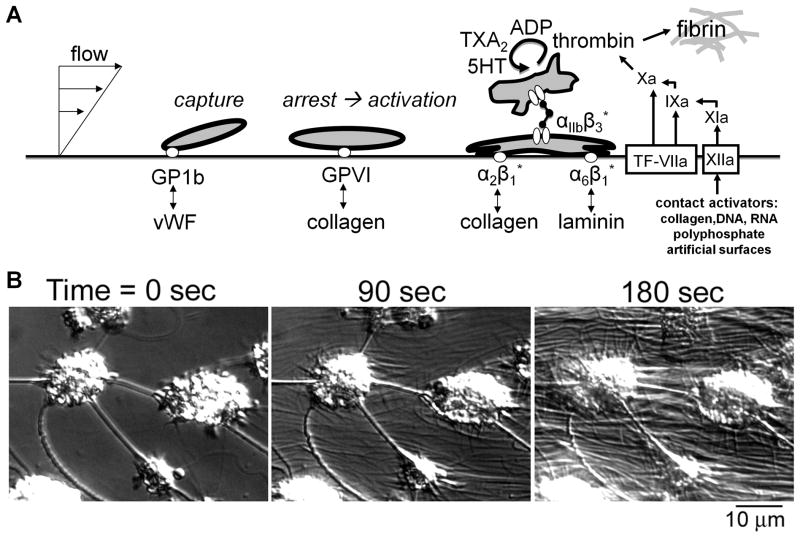
Fig. 1. Autocatalytic deposition of platelets on an injured vascular wall and generation of coagulation proteases.
A, Adhesion of platelet to vWF mediates capture under arterial flow conditions, followed by platelet activation via GPVI. Once activated, the platelet integrins can bind collagen, laminin, and fibrinogen. Platelet activation is also associated with release of ADP and serotonin (5-HT), synthesis of thromboxane (TXA2) and exposure of phosphatidylserine which facilitates thrombin generation. Thrombin production is triggered primarily by tissue factor with contact activation via Factor XIIa having a secondary role in thrombosis. Thrombin also triggers the polymerization of fibrinogen to fibrin. B, Video microscopy of platelet aggregates forming on a surface with generation of fibrin strands.
Platelets contain numerous receptors and are highly responsive to collagen, thrombin, ADP, thromboxane, serotonin, histamine, and epinephrine. Platelets are also inhibited by endothelial production of prostacyclin and nitric oxide. Additionally, platelets can bind collagen via α2β1, laminin via α6β1, von Willebrand factor via GPIbα and αIIbβ3 (ie. GPIIb/IIIa), and fibrinogen via αIIbβ3 (Fig. 1A) (1,2). Additionally, FDA approved drugs or drugs used in clinical trials include: aspirin (targeting COX1), P2Y12 inhibitors (metabolized and nonmetabolized forms), thrombin inhibitors (heparin, dabigatran), direct FXa inhibitors (apixaban, rivoraxaban), and anti-αIIbβ3 inhibitor (Reopro). Warfarin has broad activity on vitamin K-dependent coagulation factors. Since the transport rate of reactive molecular and cellular species to and from a developing thrombus or site of bleeding as well as the shear forces controlling adhesion and embolization are controlled by prevailing hemodynamics, flow devices are central to the in vitro study of blood biology.
Blood Systems Biology
Complementary to controlled perfusion and clotting experiments, mathematical modeling efforts often include reaction kinetics, flow, and transport physics: Homotypic and heterotypic aggregation/fragmentation in linear shear fields or complex flows (1–4), coagulation as a pseudo homogeneous cascade [TF-triggered (5) or TF/XIIa-triggered (6)] or platelet surface-dependent coagulation cascade under no-flow or flow conditions (7–10), fibrin polymerization under flow (11), fibrinolysis under flow conditions (12), reactive platelet deposition with or without coagulation (7,9,13,14), and shear-induced changes in vWF conformation (15). These physics-based models seek to identify, quantify, or deconvolute kinetic or mechanical sub-processes that occur within complex reactive blood flows.
Adhesion Biology & Receptor Mechanics
The requirement for cells to bind to subendothelial matrix proteins while they are entrained in the bloodstream places stringent physical characteristics on the receptors involved in adhesion and arrest of blood cells and platelets. Specifically, the on-rate of a platelet receptor binding to a subendothelial matrix protein must be fast enough to allow efficient adhesive interactions within the time frame that a free flowing platelet is in the proximity of the exposed subendothelial matrix. In humans, blood circulates at different flow velocities depending on anatomical location and presence in the arterial versus venous circulation. Flow velocity is a direct determinant of shear rate for fully developed, Newtonian flow and directly influences the residence time of a platelet above a subendothelial matrix protein. In mammals, shear rates span two orders of magnitude from 50–60 s−1 in the vena cava to 1000–5000 s−1 in the arterioles (16,17). In humans, the only receptor-ligand interaction with a high enough on-rate to cause platelet adhesion at shear rates above 500 s−1 is vWF/GPIbα). The adhesion of bound platelets in the presence of blood flow places a hydrodynamic shear stress on the receptor-matrix protein bond which may determine the duration that the formed bond persists (i.e. off-rate). Subsequently, native vWF only binds GPIbα when subjected to high fluid shear rates, or if vWF is first bound to collagen (19), suggesting that other receptor-ligand interactions mediate platelet adhesion at lower shear rates. This possibility is further supported by the observation that platelets roll on vWF surfaces at higher velocity under lower shear rates (20). These characteristics are identical to “catch-slip” bonds previously described for leukocyte expressed selectins (22), and sustained arrest of platelets requires additional contributions from other receptor-matrix protein interactions. Stable adhesion to collagen includes contributions from collagen receptors α2β1 and GPVI (23). In addition, fibrin(ogen) deposition has been observed at sites of subendothelial matrix exposure, and platelets can bind to fibrin(ogen) through GPIIbIIIa, which can also form homotypic bonds with other platelets to facilitate the aggregation of platelets, a requirement to form a hemostatic plug in vivo.
Anticoagulation for In Vitro Research
The deployment of microfluidic devices requires careful consideration of anticoagulation, which depends on the intent of the investigation. Use of phosphate buffers is always avoided due to formation of calcium phosphate precipitate. The first 5 to 10 mL of drawn blood is often discarded to avoid tissue factor contamination during venipuncture. The following approaches are most commonly used and have advantages and disadvantages:
EDTA or citrate with and without recalcification. For purely biorheological studies (i.e. velocity profile blunting, platelet drift, or Fahraeus-Lindqvist effect) without coagulation or platelet function, EDTA is an ideal inhibitor of thrombin production and platelet adhesion. Addition of excess calcium will overwhelm calcium chelators like EDTA or citrate, however EDTA destroys αIIbβ3 function and citrate significantly impairs αIIbβ3 even after recalcification. Citrate also chelates Mg2+ and Zn2+ ions, which are not restored during recalcification. For studies of TF-triggered or FXIIa-triggered coagulation, citrate has some relatively modest effects following recalcification (6) despite its wide spread clinical use. Importantly, thrombin once formed does not require calcium, and Factor XIIa can be generated without calcium.
Corn trypsin inhibitor (CTI). CTI, when used at 10 to 50 μg/ml, can inhibit the activity of Factor βXIIa, the soluble cleavage product derived from surface bound Factor αXIIa. Since CTI does not inhibit αFXIIa, it provides about 40 to 60 min of inhibition of the contact pathway without interfering with physiological divalent cation levels required for TF-mediated clotting. CTI is a useful inhibitor for study of blood function (albeit with an attenuated βFXIIa pathway) under flow if data is collected within about 30 min of phlebotomy. Other less commonly used routes of controlling in vitro contact pathway activation include: antibodies against FXIIa and FXIa (21); FXIIa antagonism with infestin-4 (24,25); FXIa antagonism with domains from protease nexin-2 (26). A low level of CTI (<5 μg/ml) can be used to partially delay contact activation which allows the study of FXIIa ex vivo if blood is perfused within 20 to 30 min of phlebotomy.
PPACK (100 μM), direct FXa inhibitor (apixaban, 0.25 to 1 μM), or heparin (>1- 10 U/mL) provides strong and irreversible inhibition of prothrombinase or thrombin. This approach is useful for the study of platelet activation, adhesion, or aggregation in the absence of thrombin and fibrin. Heparin has some interference of P-selectin pathways.
Replacing the endothelium. The endothelium is often difficult to recreate within microfluidic systems, and so the addition of a nitric oxide (NO) donor, platelet IP activators like PGE1, PGE2, iloprost, or soluble thrombomodulin (to activate protein C) is often utilized in order to account for the contribution of the endothelium. Fewer studies have perfused whole blood over cultured endothelium under conditions of coagulation and thrombin production, whereas blood flow studies with endothelium in the absence of thrombin production are not uncommon (27–29).
Thrombosis and Hemodynamics
The biorheology of reactive blood flow has presented numerous challenges and opportunties for discovery. In recreating the relevant hemodynamics in a laboratory device, whole blood perfusion is required to create an enriched platelet-plasma layer (~ 5 μm thick) near the wall. Platelet concentrations near the wall are enhanced several fold relative to that of whole blood. Also, thrombosis in vivo occurs under constant pressure drop conditions where a growing thrombus, once nearly occlusive, results in a significant resistance to flow that diverts flow to other vessels. Often experiments are conducted at a constant flow rate using syringe pumps, which is appropriate when the thrombus is thin relative to the flow geometry. However, flows become non-physiological (> 10,000 s−1 and >300 dyne/cm2) when the clot obstructs the flow channel. Most laboratory flow devices recreate the shear rate or shear stress at the wall; they do not recreate the in vivo Reynolds number (Re = Dvρ/μ for D = pipe diameter, v = average velocity, ρ = density and μ = viscosity) and transitions to turbulence that occur in vivo with severe stenosis and pulsatile flow (Re > 400); these flow conditions are not typically possible in microfluidic devices where Re < 1. Additionally, there are differences between tube flows and flows in the rectilinear channels of laboratory devices, as phenomena in the corner of the flow are completely non-physiological.
Parallel-Plate/Capillary Devices for Platelet Adhesion Research
Parallel plate flow chambers used from early 1970s to present have a characteristic gap separation of about 100 to 200 microns and can be operated with 1 to 10 mL of blood or fluid, limiting studies to 2 or 3 conditions per 20–60 mL blood draw. Parallel-plate studies in thrombosis have revealed the critical role of von Willebrand factor for platelet adhesion under arterial flow conditions (30).
Von Willebrand factor (vWF) circulates in blood plasma in a protein complex with coagulation factor VIII. The VIII/vWF complex mediates platelet adhesion to denuded arteries placed in an annular flow chamber under conditions of flow (31). In turn, the adhesion of platelets to subendothelial matrix proteins depends upon red blood cell size and deformability, the presence of divalent calcium ions, and specific multimeric vWF distribution (32–35). Patients with Bernard-Soulier disease have platelets that do not bind to collagen under flow even in the presence of VIII/vWF, indicating that platelets from these patients lacked a vWF receptor (36), now identified as the 155 kDA glycoprotein platelet glycoprotein Ib (37–41).
Without the use of flow chambers, the correct physiological scenario by which platelet adhesion to arterial vessel walls would not have been evident. vWF is not necessary for platelet binding to collagen under conditions of stasis or venous flow (100 s−1 wall shear rate). Subsequent studies identified other subendothelial matrix proteins capable of binding platelets in venous flow conditions. Fibronectin facilitates the adhesion of vWF to collagen (42–44). Additionally, laminin, the second most abundant subendothelial matrix protein, binds platelets under flow through glycoprotein α6β1 (45,46) and vWF/GPIb (47). Studies have demonstrated that αIIbβ3 on platelets could bind to fibrinogen under flow (48). Adherent platelets can also bind to collagen through α2β1 (49,50) as well as glycoprotein VI (51). In addition to collagen, fibronectin and laminin, the major constituents of normal subendothelial matrix, other proteins have been identified in pathological portions of blood vessels. Tenascin-C is enriched in atheromas, and perfusion of platelets over prepared surfaces of Tenascin-C resulted in platelet adhesion and activation, independent of collagen and laminin, suggesting a possible pathological role for Tenascin-C in atherosclerosis (52).
Parallel-Plate/Microcapillary Devices for Thrombosis Research
Coagulation is a surface-bound enzymatic reaction whose initiation is isolated to locations of exposed tissue factor and propagation requires coagulation factors to transport from bulk plasma to the vessel wall. To be procoagulant, TF requires a phospholipid surface to initiate coagulation (53). In the case of a vessel injury, where the blood flows past the site of TF-exposure, convective mass transport supplies coagulation factors from the blood to the lipid membrane containing TF.
The effects of flow on blood coagulation and fibrin formation can be studied ex vivo with the use of flow chambers and microfluidics. Flow chambers typically require a syringe pump to perfuse liquid through the capillary. Control of the infusion rate, coupled with measurements of the capillary geometry allow for control of shear rate at the capillary wall. Flow rate can potentiate convective mass transport, and flow is a major regulator of coagulation kinetics (54). In addition, larger regions of exposed TF or lower shear rate increase the probability for a soluble coagulation factor to bind to the surface and subsequently participate in coagulation (55). Conversely, the presence of flow can wash formed enzyme complex or activated enzymes from the vicinity of the TF-exposed site. This effectively dilutes the concentration of enzyme, mostly thrombin, and can be prohibitive for the formation of fibrin perpendicular to flow (56). Using printed microarrays mounted to a parallel-plate flow chamber, Okorie et al. (56) demonstrated a threshold surface requirement of 1 to 10 molecules of lipidated TF per μm2 to initiate fibrin formation under venous and arterial flow conditions. One potential drawback to pump-based perfusion chamber is the increase in pressure at the forming thrombus due to occlusion of the chamber lumen which would not be present in vivo, due to collateral circulation. This may be overcome with a gravity driven capillary perfusion assay that was developed to maintain a constant pressure head with which to drive blood flow through a capillary tube (57).
Flow chambers that combine platelet adhesion with coagulation under flow have shown that the arrest of platelets at the injury site effectively seals off the initial coagulation stimulus of the surface (58). The procoagulant stimulus that maintains the growth of a thrombus following initial platelet deposition is not certain. Thrombin was discovered to activate Factor XI (FXI), leading to the discovery of the thrombin-FXI feedback loop by which thrombin can propagate itself, in the absence of TF, by activating FXI to FXIa and thereby initiating the intrinsic coagulation cascade (59). Supporting this mechanism, inhibition of FXIa was shown to reduce the formation of fibrin over collagen coated surfaces, supporting a role for the FXIa to contribute to thrombus formation (60,61). Blood-borne TF may bind to platelet aggregates and contribute to thrombus formation under flow (62,63, 64), a pathway that may be pronounced in certain cancers (65). Furthermore, platelet-derived polyphosphates can activate factor XIIa (albeit very weakly compared to long chain bacterial polyphosphate), which may represent an additional route for activated platelets to sustain thrombin production in a growing thrombus (66).
Ex vivo coagulation experiments must use precaution to limit the confounding effects of FXII activation leading to the intrinsic pathway of blood coagulation. Surfaces can be treated chemically with low surface tension polymers to limit the charged surface activation of FXII. Further, high energy surfaces may be blocked with native blood proteins, such as albumin, to limit the activation of FXII. However, there is mounting evidence that subendothelial matrix proteins, such as collagen and laminin, also have the ability to activate FXII, suggesting a possible contribution to blood coagulation in vivo (67). In contrast to flow chambers that require many centimeters of tubing to connect a blood reservoir, microfluidic devices minimize the travel distance from the blood reservoir to the site of the thrombotic event to only a few millimeters.
The adhesion of white blood cells, such as neutrophils, and their role in coagulation has also been considered in square glass microcapillaries. Goel et al. demonstrated enhancement of coagulation through neutrophil release of Cathepsin G on platelet monolayers, where fibrin generation was visualized in real time (68,69). Capillaries have been functionalized with P-selectin, as in the visualization of neutrophil tether formation performed by Schmidtke et al. (70) or P-selectin coated beads, used to assess neutrophil deformation and bond lifetimes after collision. Furthermore, microcapillaries have been used to culture endothelial cells to confluence to mimic microvasculature. Nash et al., who described this technique (71), used these surfaces to describe a mechanism by which neutrophils can be transferred to the surface of intact endothelium when coated with activated platelets (72).
2. MICROFLUIDICS AND COAGULATION BIOLOGY
2a. CLOSED SYSTEMS
Microfluidic systems may be as simple as plates of small μL or sub-μL volume wells. Closed blood systems do not undergo change in volume and, once initiated, do not exchange components with the external environment. While the test tube represents the classical mL-scale basic batch reactor format, advances in robotic liquid handling allow the use of 96, 384, and 1536-well plate formats for investigations of clotting and platelet function at 1 to 200 μL scale with the absence of significant fluid flow or mixing. These methods offer the advantage of high throughput and high replicate number and dynamic readouts of kinetic processes. Such reactions are generally isotropic initially but may develop gradients due to cell settling, wall-dependent phenomena, or evaporation over the course of an hour long experiment.
Important analytical techniques to monitor thrombin include the use of fluorogenic substrates in 96-well plates (73) and 384-well plates (6). Fibrin generation is a dual measure of thrombin production and fibrinogen level and can be measured in closed systems by clot rigidity as measured by oscillatory rheometry such as TEG or ROTEM (74), magnetorheometry, ultrasound (75), or turbidity in clotting plasma. Platelet function can be measured by aggregometry or by calcium mobilization in 384-well plate assays (76). Beyond the well plate approach, the use of microfluidic platforms for nanoliter-scale reactions include printed arrays of fluorogenic substrates (77–83) or fluorogenic substrates deployed in micropattenered wells or channels. Label-free reactions of thrombin function have been also conducted on MALDI-plates for MS interrogation (84).
Cone-and-plate viscometry, a closed system that includes a defined bulk shear rate and linear shear flow, is mostly used to study processes in bulk suspensions such as platelet aggregation, vWF aggregation, or shear induced platelet aggregation (85,86). Aggregometry measures platelet aggregation rates in the presence of a chaotic stir-bar induced flow. Despite its poorly defined hemodynamics, aggregometry is very useful for monitoring receptor function and platelet signaling.
2b. OPEN SYSTEMS
Microfluidic devices for the study of platelet aggregation and coagulation offer the ability to spatially and temporally control: flow, surface, and perfused blood chemistry (Fig. 2). They allow for high number of replicates and use low volumes of blood. Microfluidic devices allow control of the local flow environment by providing well defined geometries and command of sample flow rates. Physiological venous (100–200 s−1) or arterial forces (1000–2000 s−1) may be achieved in microchannels, as well as pathological shear environments (10,000 to 100,000 s−1) (Fig. 3). Because most microfluidic models support only single pass perfusion, the transport and kinetic properties of these systems are preferable to closed system methodologies. For straight rectangular channels, the x-directed velocity field for steady flow over a domain of –w/2 ≤ y ≤ w/2 and 0 ≤ z ≤ h is given by:
| Eqn. 1 |
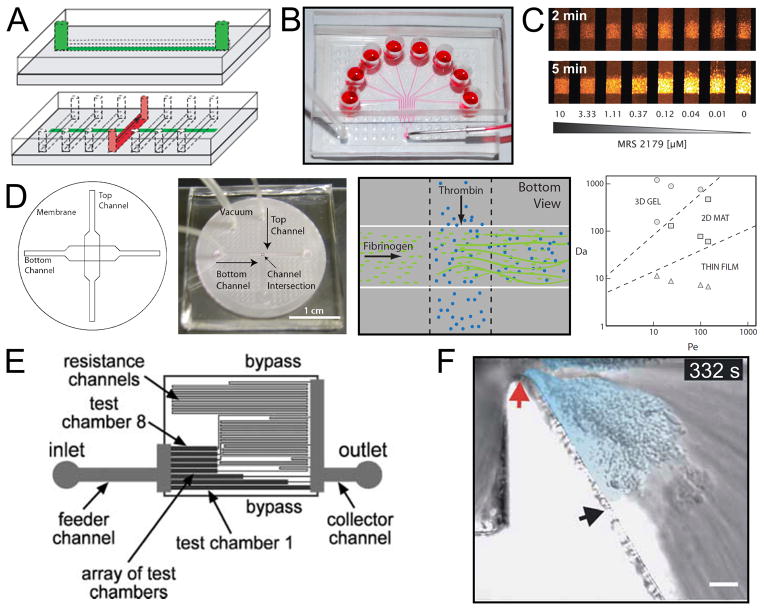
Fig. 2. Microfluidic devices for blood biology.
A (top), A schematic drawing of a microfluidic channel casted in PDMS intended for patterning procoagulant proteins onto the surface of glass slides. (Bottom), The flow device, also in PDMS, is affixed onto the protein stripe and samples are perfused via syringe pump. Reproduced with permission from Reference 88. Copyright International Society on Thrombosis and Hemostasis. B, The 8-channel microfluidic device consists of 8 inlets perfused via syringe pump from one outlet. The device is adhered reversibly to a glass substrate via vacuum (bottom left tube) with a protein stripe patterned perpendicular to the region in which the 8 channels run parallel. Reproduced with permission from Reference 98. Copyright American Heart Association, Inc. C, Platelet adhesion is monitored in the 8-channel microfluidic device using epi-fluorescence microscopy. Here, fluorescently labeled platelets adhere to a collagen surface at venous shear rate in the presence of 8 different concentration of the P2Y1 antagonist, MRS 2179. [90] – Reproduced by permission of The Royal Society of Chemistry. D (left), A schematic representation of a 3 dimensional microfluidic device which allowed for the localized release of soluble agonists from one channel (bottom channel) to another channel (top channel) through a porous membrane. (Middle left), An image of this device. (Middle right), A drawing of the site of release of thrombin into a channel of flowing fibrinogen. The illustration indicates the generation of fibrin, which adhered to the membrane. (Right), In the thrombin release experiment it was found that the fibrin deposit formed had a morphology which was dependent on the ratios of reaction rate to convection (Da) and convection to diffusion (Pe). Reprinted from Reference 95 with permission from the Biophysical Society. E, A schematic representation of a microfluidic device designed to study the role of shear stress in platelet adhesion. The image demonstrates that each of the test chambers is connected to a downstream channel of varying length which provides varying resistance and defines the flow rate (and therefore shear rate) in each of the chambers. Reproduced from Reference 91 with permission of The Royal Society of Chemistry. F, In this microfluidic model a severe stenosis has been generated. Blood flow, from left to right, experiences extreme accelerating and decelerating flows as it traverses the stenosis apex (red arrow). As the platelets exit the high shear stress region they form a thrombus in the deceleration region (light blue). Reprinted by permission from Macmillan Publishers Ltd: Nature Biotechnology (Reference 99), copyright 2009.
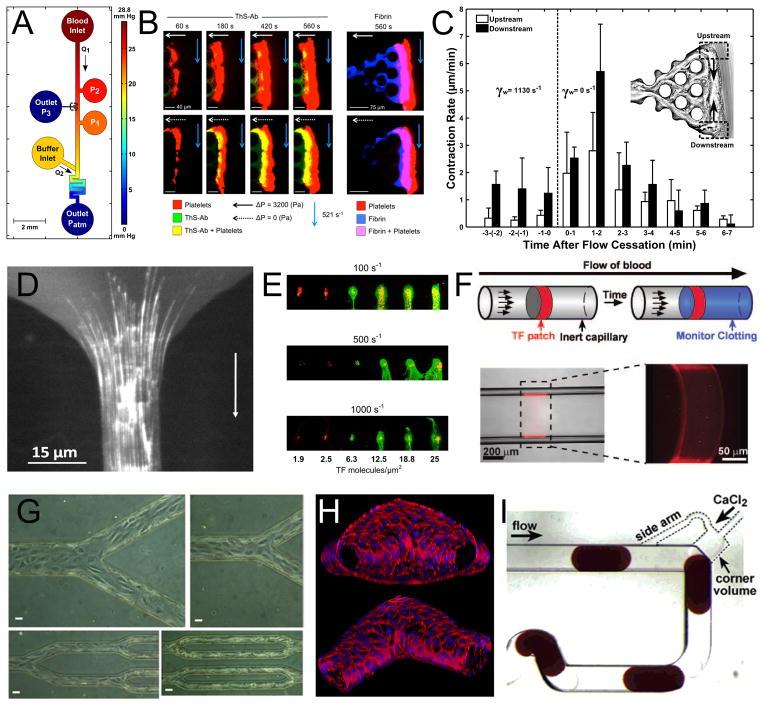
Fig. 3. Microfluidic Devices of Coagulation Research.
A, A microfluidic model of bleeding designed for the study of thrombus permeability under flow. The device has integrated ports for localization of collagen and TF to the channel wall, pressure sensors, and buffer flow for precise pressure control. Blood flow (from top to bottom) will seep through the collagen scaffold connected to Outlet P3 until a platelet plug is formed. Reproduced with permission from Reference 97. Copyright American Heart Association, Inc. B, Epi-fluorescence microscopy performed at the collagen/TF scaffold of the device pictured in A reveals a large platelet aggregate (red) formed during perfusion which also stains positive for thrombin using a novel thrombin bio-sensor. Comparison of the top to bottom rows in which blood flow is allowed (top) or not allowed (bottom) to seep through Outlet P3 reveals that thrombin permeation into the clot is enhanced when it is not convected away. These clots also stain positive for fibrin (right). Reproduced with permission from Reference 96. Copyright International Society on Thrombosis and Hemostasis. C, Thrombus contraction was also observed in the microfluidic bleeding model. Here it is demonstrated that ~1 min after flow cessation the rate of clot retraction, measured by tracking the movement of trapped fluorescent beads, was 3-fold enhanced. Reproduced with permission from Reference 97. Copyright American Heart Association, Inc. D, A microfluidic model of stenosis reveals long fiber bundles of von Willebrand factor forming on a collagen type 1 surface under plasma flow at shear rates >30,000 s−1. Reproduced with permission from Reference 101. Copyright American Heart Association, Inc. E, Microcontact printing of collagen and varying amounts of tissue factor reveals a steep threshold for fibrin generation (green) under whole blood flow at varying shear rates in a parallel plate flow chamber construct. Platelets are labeled in red. This research was originally published in Reference 56. Copyright the American Society of Hematology. F, TF has also been patterned into microcapillary flow models using photolithographic techniques. The drawing illustrates that a soluble fluorescent reporter of thrombin will be activated downstream of the procoagulant patch. Reproduced with permission from Reference 55. Copyright Wolters Kluwer Health. G, The culture of confluent monolayers of endothelium has been achieved in microfluidic devices of PDMS (Republished with permission of the American Society for Clinical Investigation, from Reference 27) as well as on 3-dimensional collagen scaffolds (H). The in vitro vessels pictured in H were designed for studies involving permeability, angiogenesis, as well as thrombus formation after endothelial activation. Reproduced with permission from Reference 29. I, The microfluidic droplet reactor pictured here was designed to study clotting time in the presence of various inhibitors. Plugs of citrated whole blood enter at the top to which CaCl2 is added. The plug at the bottom left is entering the mixing region after which clotting will be assessed in a downstream region of the channel. Reprinted with permission from Reference 92. Copyright 2006 American Chemical Society.
Rectangular capillaries with high aspect ratio are ideal for low volume and high quality visualization and create a quasi-2D flow in the central region away from the side walls. Cylindrical microcapillaries, while more physiological (no corners), present challenges for microscopy based imaging. The advent of poly(dimethylsiloxane) (PDMS) allows the creation of rectangular flow channels with feature sizes down to about 5 microns. Furthermore, microstamp technology and cantilever technologies allow for microprinting of proteins or lipids on substrates with spatial resolution below 1 micron.
PDMS and microprinting approaches are well reviewed in ref. (87). Generally, microfluidic devices made of PDMS are cured over master molds fabricated using photolithographic techniques. Briefly, a photoresistive substrate, such as SU-8 or KMPR-1050, is spin coated onto a silicon wafer to a height which will represent the channel depth. The coated wafer is then brought into close contact with a high-resolution transparency of the desired channel design using a mask-aligner. The sample is exposed to ultraviolet light which initiates cross linking in the photoresistive substrate. Unexposed photoresist is then removed with a development solution. The resulting master mold can produce 100s of PDMS casts.
Microfluidic Devices : Focal Thrombosis and High Replicates
In 2008, Neeves et al. demonstrated the use of a micropatterned surface of collagen in a PDMS microchannel (88). Protein was patterned onto a glass slide using a single channel device which was removed to allow the placement of the flow device which had 13 channels running perpendicular to the collagen stripe (Fig. 2A). The resulting device consisted of 13 individual flow paths, each with a well defined “injury site” where collagen was presented to the flow. Murine platelets only adhered to this focal collagen region and were visualized in real-time using epi-fluorescence microscopy. This design proved particularly useful in the ex-vivo study of mouse blood because of its low blood volume (<50 μL) per channel requirement. Mouse platelets deficient in integrin α2 adhered very poorly in this assay. Activation of PAR4 receptor with a peptide agonist enhanced platelet deposit stability when challenged with a step change in shear rate to 8000 s−1. Human blood samples behave in a similar manner to murine samples in that they only form platelet aggregates on the collagen surfaces. Furthermore, these aggregates grow in response to the local hemodynamic conditions. Platelet aggregates formed 10 to 50-micron mound- or dune-like structures which had a circular shape at venous shear and ellipsoidal shape at higher shear rates, approximately 2-fold elongated in the direction of flow (89).
In a more recent generation of the focal thrombosis model, Maloney et al. developed an 8-channel design (Fig. 2B), perfused by withdrawl through a single outlet (90). Using this device they reported on 8-point dose-response curves for multiple anti-platelet agents using less than 0.5 mL of blood (Fig. 2C). Small molecular inhibitors of P2Y1 and P2Y12 had similar potency under flow as measured by equilibrium binding, however apyrase potentiated platelet deposition under flow due to its rapid conversion of released ATP to ADP. This 8-channel device was recently used to phenotype individual donors and their responses to COX-1 inhibitors (aspirin and indomethacin), P2Y1 inhibitor (MRS-2179), and IP activators (iloprost) (12), leading to the discovery of a novel V241G mutation in the thromboxane receptor. In this study, a full simulation of platelet deposition on collagen (no thrombin) was possible using patient-specific model of platelet response to combinatorial stimulation (14, 76).
Gutierrez et al. also considered the multiple channel/single outlet design, but used length-varying flowpaths for each channel (91) to create different shear rates on the same device (Fig. 2E). This allowed the simultaneous investigation of platelet adhesion under multiple shear rate environments via a single withdrawal. This design also required small blood volumes (<100 μL) per test which proved very useful in the study of genetically modified mouse blood.
Microfluidic devices have also been purposed for high throughput studies of coagulation; The plug-based system developed by Song et al. was designed such that a train of separated blood/reagent droplets could be formed, mixed and analyzed in a serpentine channel (Fig 3I). This technique has been used to measure APTT in high replicate (92) as well as to study the role of mixing in coagulation (93).
In addition to surface chemistry, microfluidic constructs have been used to localize the release of soluble agonists (ADP and thrombin) into flowing samples, a technique described by Neeves et al (94–95). Briefly, a porous membrane was used to separate two stacked PDMS channels oriented in a perpendicular cross formation (Fig. 2D). Agonists were perfused in the bottom channel and their flux into the top channel was controlled by their flow rate relative to the flow rate of the sample. Depending on device operation, solute flux into blood flow stream could be diffusive, convective, or both. This technique led to the discovery of critical thrombin concentrations at which polymerization of fibrin occurred under flow (95). Recently, a thrombin sensor was developed to show in real-time the formation of thrombin in thrombi formed over collagen and lipidated TF (96). In addition, permeability of formed thrombi is an area of active research. The development of a device to measure intraluminal clot retraction showed that this only occurred in the absence of flow when platelet releasates were allowed to collect (Fig. 3A,B). This permits platelet aggregates to contract centrally and conveys a flow sensing ability to formed aggregates (Fig. 3C) (97).
Control of Hemodyamic Conditions
In contrast with in vivo mouse models of thrombosis, the flow environment in microchannels is well defined and can be controlled through the use of syringe pumps or pressure heads to generate a wide range of shear conditions that mimic normal physiologic or pathologic conditions. It is important to note that the use of syringe pumps generates a constant flow rate environment in which a growing thrombus only serves to develop large pressure drops rather than to perturb the flow rate. With this in mind, Colace et al. used the 8-channel microfluidic device described earlier (8 inlets, 1 outlet) to create a constant pressure drop setting in which growing thrombi in 4 of the 8 channels diverted flow to 4 channels feeding from EDTA treated samples (97). Combining this technique with computational fluid dynamics they demonstrated that a growing clot experiences a wide range of shear rate conditions (100–100,000 s−1) as it progresses to full occlusion or embolus in a microfluidic channel. They used this platform to study the strength of clots under flow and described a 28-fold increase in clot shear-resistance attributable to the fibrin network.
While defining the flow conditions around a thrombus are important to understand its growth and stability, understanding its dissolution, both by physiologic and therapeutic mechanisms, requires characterization of transport within the clot. Recently, Muthard et al. described a microfluidic bleeding model which allows for the loss of flowing sample through a permeable collagen scaffold to be measured via epifluorescence microscopy (Fig. 3A) (97). If the sample is whole blood, then clot formed on the scaffold will perturb sample loss offering the first measure of clot permeability under flow (Fig. 3B). The microfluidic design allowed for precise measurement and control of pressure drop across the collagen network as the thrombus evolved under flow. Incorporating tissue factor bearing liposomes into the collagen scaffold led to a dense fibrin network which further perturbed transport across the thrombus.
Microfluidic platforms have also been used to study the role of pathologic shear, such as that through a severe stenosis (>10,000 s−1). Although the turbulent flow that can be generated as a result of these environments is not captured in these models, the low flow rates necessary to create these large shear rates make them valuable. In 2006, Ruggeri et al. demonstrated that platelet rolling and platelet-platelet adhesion in microfluidic flow chambers could be achieved in high shear rate environments (>15,000 s−1), and that this interaction was dependent on soluble vWF (48). More recently, Nesbitt et al. reported exacerbated thrombosis of discoid platelets in the outlet regions of severe stenosis (>20,000 s−1), where strong decelerating flows are present (99). A microfluidic model of this geometry was generated to test their hypothesis and allowed for high resolution imaging of the process (Fig. 2F) (100). A novel stenosis microfluidic channel has also been described by Colace et al. who used the device to demonstrate the formation of long vWF fibers (>100 um) composed of many stretched molecules (Fig. 3D) (101). In the future, microfluidics will be instrumental in defining a role for pathological shear in diseases such as thrombosis, acquired vWF syndrome, and TTP.
Patterned Surfaces – Techniques
Micropatterned surfaces of hemostatically active proteins such as fibrinogen, collagen, von Willebrand factor (vWF), and lipidated tissue factor (TF) have been described in the literature using a variety of techniques and geometries. Often, microchannels are filled with a protein of interest and rinsed before sample perfusion, but this presents a variety of issues. More sophisticated techniques can ensure that samples and patterned proteins do not come into contact in regions of the channel with undefined flows, which can prevent clogging. Microcontact printing, a practice which uses a small pin to dispense samples into discrete arrays onto a glass substrate was used by Okorie et al. to generate a surface of collagen and TF to support both platelet aggregation and coagulation in a flow chamber (Fig. 3E) (56). This technique helped to define a threshold level of TF to support coagulation under flow. Ismagilov et al. generated arrays of TF “patches” using photolithographic techniques to study the role of diffusion in coagulation under static conditions (102). They extended this work to coagulation under flow using a technique to localize TF into a lipid membrane inside a cylindrical microcapillary (103). Using a fluorescent reporter of thrombin generation, this system elegantly demonstrated the shear-rate dependent nature of coagulation over well defined tissue factor coatings (Fig. 3F). Microchannel patterned stripes of protein which generate distinct regions of thrombosis in microfluidic channels ensure that samples do not come into contact with hemostatically active surfaces prior to the viewing window. Colace et al. used this technique to generate surfaces of collagen and immobilized tissue factor in PDMS microchannels (104). This technique is important in the study of coagulation as TF patch size has been described as a very sensitive variable. Corum et al. have used PDMS as a means to stamp fibrinogen onto glass substrates to create well defined patterns of variable surface coverage (105). These surfaces were used to study platelet adhesion and spreading dynamics.
In addition to surfaces of proteins, endothelial cell coatings have also been described in the literature. As described earlier, Nash et al. have generated confluent monolayers in microcapillaries. Tsai et al. have had success culturing endothelial cells in microfluidic chambers on fibronectin-coated surfaces (Fig. 3G) (27). Recently, Stroock et al. reported in vitro microvasculature consisting of endothelial monolayers grown on 3-dimensional micropatterned collagen scaffolds (Fig. 3H) (29). The group described their potential use in thrombosis models as well as angiogenesis models.
The discovery of specific matrix protein-platelet receptor interactions as well as coagulation initiation reactions has demanded the development of novel surface coatings with which to characterize these interactions under flow. For platelet adhesion and aggregation studies, preparations of purified subendothelial matrix proteins are often used to coat glass or plastic surfaces. The adherence of these proteins is often non-specific, making the preparation of these surfaces variable between protein preparations and between laboratories. To standardize these assays, specific fragments of the matrix proteins may be utilized. This negates any confounding effects of protein structure in influencing platelet-matrix protein binding site interactions. This approach has been used for collagen, the most abundant subendothelial matrix protein, with the use of collagen-related-peptide or CRP (106). CRP has been shown to bind to platelet GPVI and subsequently lead to platelet activation. Further, platelet adhesion to CRP was found to potentiate GPVI signaling as compared to platelets in suspension, outlining the importance for flow chambers in elucidating physiological roles of platelets in hemostasis. A novel approach to coat surface with thin films of soluble collagen demonstrated that a uniform coating of reconstituted fibers can be deposited on glass, and this approach may lead to better control of collagen surface preparations (107). Along these lines, the tripeptide arginine-glycine-aspartic acid (RGD) is present in many extracellular matrix proteins including collagen and fibronectin and can be utilized to simulate an adhesive cell surface for platelets (108).
Clinical Applications
Generation of microfluidic channels in PDMS requires access to a microfabrication facility. For interested users without this access, commercial products are available for microfluidic experiments. Specifically, the Bioflux system has been adapted to a well plate format (109) offering high throughput analysis. Clinically, a variety of diagnostic tests have been developed on microfluidic platforms because the small blood volumes required do not exclude neonatal patients. The Platelet Function Analyzer 100 (PFA-100), for example, perfuses small volumes of blood through a small annulus made of collagen coated with a platelet agonist such as ADP or epinephrine. Pressure sensors measure the time to annulus occlusion. The test results are strongly related to the traditional variables in platelet function testing, such as platelet count, hematocrit, antiplatelet therapy, and congenital platelet disorders (110). However, the high shear rate perfusion used by this device makes it acutely sensitive to disorders of vWF. Furthermore, the use of citrated whole blood excludes its use in coagulation testing and raises questions about αIIbβ3 function. Other tests, such as the VerifyNow test, an aggregometry based platelet function test, and TEG-5000, a viscoelasticity based coagulation test, are both closed-system designs, which, in all likelihood, do not reflect physiologic kinetics of platelet aggregation and thrombin formation, despite proven utility (110–112). Each of the tests described have niches in platelet function, vWF function, coagulation, or fibrinolysis, which suggests the need for an integrated test of global hemostasis.
3. CONCLUSIONS
Microfluidic devices are finding increasing use in the study of blood function. These devices create controlled hemodynamic conditions that mimic in vivo flows while using a minimal volume of blood. When combined with micropatterning techniques, the experimentalist can precisely control the prevailing shear rate, duration of exposure, and prevailing pharmacological background. Such devices are fully compatible with multicolor imaging of in situ thrombus generation and embolism and proteomics techniques of the device effluent. Future work to create technologies for the clinical lab will require: reliable manufacturing of stable devices and patterned surfaces, hands-free automated operation, and validation of meaningful diagnostic information to inform clinical decisions.
Acknowledgments
This work was supported by NIH R01 HL-103419 to S.L.D. and R01 HL-101972 to O.J.T.M and the AHA 12PRE11930019 to G.T. G.T. is an ARCS scholar.
Contributor Information
Owen J. T. McCarty, Email: mccartyo@ohsu.edu.
Scott L. Diamond, Email: sld@seas.upenn.edu.
References
- 1.Watson SP, Auger JM, McCarty OJ, Pearce AC. GPVI and integrin αIIbβ3 signaling in platelets. J Thromb Haemost. 2005;3:1752–62. doi: 10.1111/j.1538-7836.2005.01429.x. [DOI] [PubMed] [Google Scholar]
- 2.Ruggeri ZM, Mendolicchio GL. Adhesion mechanisms in platelet function. Circ Res. 2007;100:1673–85. doi: 10.1161/01.RES.0000267878.97021.ab. [DOI] [PubMed] [Google Scholar]
- 3.Flamm MH, Sinno T, Diamond SL. Simulation of aggregating particles in complex flows by the lattice kinetic Monte Carlo method. J Chem Phys. 2011;134:034905. doi: 10.1063/1.3521395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laurenzi IJ, Diamond SL. Monte Carlo simulation of the heterotypic aggregation kinetics of platelets and neutrophils. Biophys J. 1999;77:1733–46. doi: 10.1016/S0006-3495(99)77019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hockin MF, Jones KC, Everse SJ, Mann KG. A model for the stoichiometric regulation of blood coagulation. J Biol Chem. 2002;277:18322–33. doi: 10.1074/jbc.M201173200. [DOI] [PubMed] [Google Scholar]
- 6.Chatterjee MS, Denney WS, Jing H, Diamond SL. Systems biology of coagulation initiation: kinetics of thrombin generation in resting and activated human blood. PLoS Comput Biol. 2010;6 doi: 10.1371/journal.pcbi.1000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fogelson AL, Hussain YH, Leiderman K. Blood clot formation under flow: the importance of factor XI depends strongly on platelet count. Biophys J. 2012;102:10–8. doi: 10.1016/j.bpj.2011.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fogelson AL, Kuharsky AL. Membrane binding-site density can modulate activation thresholds in enzyme systems. J Theor Biol. 1998;193:1–18. doi: 10.1006/jtbi.1998.0670. [DOI] [PubMed] [Google Scholar]
- 9.Fogelson AL, Tania N. Coagulation under flow: the influence of flow-mediated transport on the initiation and inhibition of coagulation. Pathophysiol Haemost Thromb. 2005;34:91–108. doi: 10.1159/000089930. [DOI] [PubMed] [Google Scholar]
- 10.Kuharsky AL, Fogelson AL. Surface-mediated control of blood coagulation: the role of binding site densities and platelet deposition. Biophys J. 2001;80:1050–74. doi: 10.1016/S0006-3495(01)76085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guy RD, Fogelson AL, Keener JP. Fibrin gel formation in a shear flow. Math Med Biol. 2007;24:111–30. doi: 10.1093/imammb/dql022. [DOI] [PubMed] [Google Scholar]
- 12.Diamond SL. Engineering design of optimal strategies for blood clot dissolution. Annu Rev Biomed Eng. 1999;1:427–62. doi: 10.1146/annurev.bioeng.1.1.427. [DOI] [PubMed] [Google Scholar]
- 13.Pivkin IV, Richardson PD, Karniadakis G. Blood flow velocity effects and role of activation delay time on growth and form of platelet thrombi. Proc Natl Acad Sci U S A. 2006;103:17164–9. doi: 10.1073/pnas.0608546103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flamm MH, Colace TV, Chatterjee MS, Jing H, Zhou S, Jaeger D, Brass LF, Sinno T, Diamond SL. Multiscale prediction of patient-specific platelet function under flow. Blood. 120:190–8. doi: 10.1182/blood-2011-10-388140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneider SW, Nuschele S, Wixforth A, Gorzelanny C, Alexander-Katz A, Netz RR, Schneider MF. Shear-induced unfolding triggers adhesion of von Willebrand factor fibers. Proc Natl Acad Sci U S A. 2007;104:7899–903. doi: 10.1073/pnas.0608422104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitmore RL. Rheology of the circulation. Oxford: Pergamon Press; 1968. [Google Scholar]
- 17.Lipowsky HH, Zweifach BW. Methods for the simultaneous measurement of pressure differentials and flow in single unbranched vessels of the microcirculation for rheological studies. Microvasc Res. 1977;14:345–61. doi: 10.1016/0026-2862(77)90030-9. [DOI] [PubMed] [Google Scholar]
- 18.Savage B, Saldivar E, Ruggeri ZM. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell. 1996;84:289–97. doi: 10.1016/s0092-8674(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 19.Dong JF, Berndt MC, Schade A, McIntire LV, Andrews RK, Lopez JA. Ristocetin-dependent, but not botrocetin-dependent, binding of von Willebrand factor to the platelet glycoprotein Ib-IX-V complex correlates with shear-dependent interactions. Blood. 2001;97:162–8. doi: 10.1182/blood.v97.1.162. [DOI] [PubMed] [Google Scholar]
- 20.Yago T, Lou J, Wu T, Yang J, Miner JJ, Coburn L, Lopez JA, Cruz MA, Dong JF, McIntire LV, McEver RP, Zhu C. Platelet glycoprotein Ibalpha forms catch bonds with human WT vWF but not with type 2B von Willebrand disease vWF. J Clin Invest. 2008;118:3195–207. doi: 10.1172/JCI35754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White-Adams TC, Berny MA, Patel IA, Tucker EI, Gailani D, Gruber A, McCarty OJ. Laminin promotes coagulation and thrombus formation in a factor XII-dependent manner. J Thromb Haemost. 8:1295–301. doi: 10.1111/j.1538-7836.2010.03850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yago T, Wu J, Wey CD, Klopocki AG, Zhu C, McEver RP. Catch bonds govern adhesion through L-selectin at threshold shear. J Cell Biol. 2004;166:913–23. doi: 10.1083/jcb.200403144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Auger JM, Kuijpers MJ, Senis YA, Watson SP, Heemskerk JW. Adhesion of human and mouse platelets to collagen under shear: a unifying model. Faseb J. 2005;19:825–7. doi: 10.1096/fj.04-1940fje. [DOI] [PubMed] [Google Scholar]
- 24.Campos IT, Guimaraes BG, Medrano FJ, Tanaka AS, Barbosa JA. Crystallization, data collection and phasing of infestin 4, a factor XIIa inhibitor. Acta Crystallogr D Biol Crystallogr. 2004;60:2051–3. doi: 10.1107/S0907444904021596. [DOI] [PubMed] [Google Scholar]
- 25.Campos IT, Tanaka-Azevedo AM, Tanaka AS. Identification and characterization of a novel factor XIIa inhibitor in the hematophagous insect, Triatoma infestans (Hemiptera: Reduviidae) FEBS Lett. 2004;577:512–6. doi: 10.1016/j.febslet.2004.10.052. [DOI] [PubMed] [Google Scholar]
- 26.Navaneetham D, Sinha D, Walsh PN. Mechanisms and specificity of factor XIa and trypsin inhibition by protease nexin 2 and basic pancreatic trypsin inhibitor. J Biochem. 2010;148:467–79. doi: 10.1093/jb/mvq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsai M, Kita A, Leach J, Rounsevell R, Huang JN, Moake J, Ware RE, Fletcher DA, Lam WA. In vitro modeling of the microvascular occlusion and thrombosis that occur in hematologic diseases using microfluidic technology. J Clin Invest. 122:408–18. doi: 10.1172/JCI58753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wick TM, Moake JL, Udden MM, Eskin SG, Sears DA, McIntire LV. Unusually large von Willebrand factor multimers increase adhesion of sickle erythrocytes to human endothelial cells under controlled flow. J Clin Invest. 1987;80:905–10. doi: 10.1172/JCI113151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng Y, Chen J, Craven M, Choi NW, Totorica S, Diaz-Santana A, Kermani P, Hempstead B, Fischbach-Teschl C, Lopez JA, Stroock AD. In vitro microvessels for the study of angiogenesis and thrombosis. Proc Natl Acad Sci U S A. 2012;109:9342–7. doi: 10.1073/pnas.1201240109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alevriadou BR, Moake JL, Turner NA, Ruggeri ZM, Folie BJ, Phillips MD, Schreiber AB, Hrinda ME, McIntire LV. Real-time analysis of shear-dependent thrombus formation and its blockade by inhibitors of von Willebrand factor binding to platelets. Blood. 1993;81:1263–76. [PubMed] [Google Scholar]
- 31.Sakariassen KS, Bolhuis PA, Sixma JJ. Human blood platelet adhesion to artery subendothelium is mediated by factor VIII-Von Willebrand factor bound to the subendothelium. Nature. 1979;279:636–8. doi: 10.1038/279636a0. [DOI] [PubMed] [Google Scholar]
- 32.Aarts PA, Bolhuis PA, Sakariassen KS, Heethaar RM, Sixma JJ. Red blood cell size is important for adherence of blood platelets to artery subendothelium. Blood. 1983;62:214–7. [PubMed] [Google Scholar]
- 33.Aarts PA, Heethaar RM, Sixma JJ. Red blood cell deformability influences platelets--vessel wall interaction in flowing blood. Blood. 1984;64:1228–33. [PubMed] [Google Scholar]
- 34.Sakariassen KS, Ottenhof-Rovers M, Sixma JJ. Factor VIII-von Willebrand factor requires calcium for facilitation of platelet adherence. Blood. 1984;63:996–103. [PubMed] [Google Scholar]
- 35.Sixma JJ, Sakariassen KS, Beeser-Visser NH, Ottenhof-Rovers M, Bolhuis PA. Adhesion of platelets to human artery subendothelium: effect of factor VIII-von Willebrand factor of various multimeric composition. Blood. 1984;63:128–39. [PubMed] [Google Scholar]
- 36.Weiss HJ, Tschopp TB, Baumgartner HR, Sussman, Johnson MM, Egan JJ. Decreased adhesion of giant (Bernard-Soulier) platelets to subendothelium. Further implications on the role of the von Willebrand factor in hemostasis. Am J Med. 1974;57:920–5. doi: 10.1016/0002-9343(74)90170-3. [DOI] [PubMed] [Google Scholar]
- 37.Nurden AT, Caen JP. Specific roles for platelet surface glycoproteins in platelet function. Nature. 1975;255:720–2. doi: 10.1038/255720a0. [DOI] [PubMed] [Google Scholar]
- 38.Jenkins CS, Phillips DR, Clemetson KJ, Meyer D, Larrieu MJ, Luscher EF. Platelet membrane glycoproteins implicated in ristocetin-induced aggregation. Studies of the proteins on platelets from patients with Bernard-Soulier syndrome and von Willebrand's disease. J Clin Invest. 1976;57:112–24. doi: 10.1172/JCI108251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nachman RL, Jaffe EA, Weksler BB. Immunoinhibition of ristocetin-induced platelet aggregation. J Clin Invest. 1977;59:143–8. doi: 10.1172/JCI108612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naim HY, Clemetson KJ, Luscher EF. Effects of galactose-binding lectins on human blood platelets: identity of the peanut agglutinin receptor with the von Willebrand factor receptor. Thromb Res. 1982;26:431–41. doi: 10.1016/0049-3848(82)90315-2. [DOI] [PubMed] [Google Scholar]
- 41.Nieuwenhuis HK, Akkerman JW, Houdijk WP, Sixma JJ. Human blood platelets showing no response to collagen fail to express surface glycoprotein Ia. Nature. 1985;318:470–2. doi: 10.1038/318470a0. [DOI] [PubMed] [Google Scholar]
- 42.Houdijk WP, Sakariassen KS, Nievelstein PF, Sixma JJ. Role of factor VIII-von Willebrand factor and fibronectin in the interaction of platelets in flowing blood with monomeric and fibrillar human collagen types I and III. J Clin Invest. 1985;75:531–40. doi: 10.1172/JCI111729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Houdijk WP, Sixma JJ. Fibronectin in artery subendothelium is important for platelet adhesion. Blood. 1985;65:598–604. [PubMed] [Google Scholar]
- 44.de Groot PG, Ottenhof-Rovers M, van Mourik JA, Sixma JJ. Evidence that the primary binding site of von Willebrand factor that mediates platelet adhesion on subendothelium is not collagen. J Clin Invest. 1988;82:65–73. doi: 10.1172/JCI113602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hindriks G, Ijsseldijk MJ, Sonnenberg A, Sixma JJ, de Groot PG. Platelet adhesion to laminin: role of Ca2+ and Mg2+ ions, shear rate, and platelet membrane glycoproteins. Blood. 1992;79:928–35. [PubMed] [Google Scholar]
- 46.Inoue O, Suzuki-Inoue K, McCarty OJ, Moroi M, Ruggeri ZM, Kunicki TJ, Ozaki Y, Watson SP. Laminin stimulates spreading of platelets through integrin alpha6beta1-dependent activation of GPVI. Blood. 2006;107:1405–12. doi: 10.1182/blood-2005-06-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Inoue O, Suzuki-Inoue K, Ozaki Y. Redundant mechanism of platelet adhesion to laminin and collagen under flow: involvement of von Willebrand factor and glycoprotein Ib-IX-V. J Biol Chem. 2008;283:16279–82. doi: 10.1074/jbc.C700241200. [DOI] [PubMed] [Google Scholar]
- 48.Ruggeri ZM, Orje JN, Habermann R, Federici AB, Reininger AJ. Activation-independent platelet adhesion and aggregation under elevated shear stress. Blood. 1996;108:1903–10. doi: 10.1182/blood-2006-04-011551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vandenberg P, Kern A, Ries A, Luckenbill-Edds L, Mann K, Kuhn K. Characterization of a type IV collagen major cell binding site with affinity to the alpha 1 beta 1 and the alpha 2 beta 1 integrins. J Cell Biol. 1991;113:1475–83. doi: 10.1083/jcb.113.6.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Staatz WD, Rajpara SM, Wayner EA, Carter WG, Santoro SA. The membrane glycoprotein Ia-IIa (VLA-2) complex mediates the Mg++-dependent adhesion of platelets to collagen. J Cell Biol. 1989;108:1917–24. doi: 10.1083/jcb.108.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Asselin J, Gibbins JM, Achison M, Lee YH, Morton LF, Farndale RW, Barnes MJ, Watson SP. A collagen-like peptide stimulates tyrosine phosphorylation of syk and phospholipase C gamma2 in platelets independent of the integrin alpha2beta1. Blood. 1997;89:1235–42. [PubMed] [Google Scholar]
- 52.Schaff M, Receveur N, Bourdon C, Wurtz V, Denis CV, Orend G, Gachet C, Lanza F, Mangin PH. Novel function of tenascin-C, a matrix protein relevant to atherosclerosis, in platelet recruitment and activation under flow. Arterioscler Thromb Vasc Biol. 2011;31:117–24. doi: 10.1161/ATVBAHA.110.206375. [DOI] [PubMed] [Google Scholar]
- 53.Nemerson Y. The phospholipid requirement of tissue factor in blood coagulation. J Clin Invest. 1968;47:72–80. doi: 10.1172/JCI105716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gemmell CH, Turitto VT, Nemerson Y. Flow as a regulator of the activation of factor X by tissue factor. Blood. 1988;72:1404–6. [PubMed] [Google Scholar]
- 55.Shen F, Kastrup CJ, Liu Y, Ismagilov RF. Threshold response of initiation of blood coagulation by tissue factor in patterned microfluidic capillaries is controlled by shear rate. Arterioscler Thromb Vasc Biol. 2008;28:2035–41. doi: 10.1161/ATVBAHA.108.173930. [DOI] [PubMed] [Google Scholar]
- 56.Okorie UM, Denney WS, Chatterjee MS, Neeves KB, Diamond SL. Determination of surface tissue factor thresholds that trigger coagulation at venous and arterial shear rates: amplification of 100 fM circulating tissue factor requires flow. Blood. 2008;111:3507–13. doi: 10.1182/blood-2007-08-106229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berny M, Patel I, White-Adams T, Simonson P, Gruber A, Rugonyi SO, McCarty J. Rational Design of an Ex Vivo Model of Thrombosis. Cellular and Molecular Bioengineering. 2010;3:187–189. [Google Scholar]
- 58.Hathcock JJ, Nemerson Y. Platelet deposition inhibits tissue factor activity: in vitro clots are impermeable to factor Xa. Blood. 2004;104:123–7. doi: 10.1182/blood-2003-12-4352. [DOI] [PubMed] [Google Scholar]
- 59.Gailani D, Broze GJ., Jr Factor XI activation in a revised model of blood coagulation. Science. 1991;253:909–12. doi: 10.1126/science.1652157. [DOI] [PubMed] [Google Scholar]
- 60.Tucker EI, Marzec UM, White TC, Hurst S, Rugonyi S, McCarty OJ, Gailani D, Gruber A, Hanson SR. Prevention of vascular graft occlusion and thrombus-associated thrombin generation by inhibition of factor XI. Blood. 2009;113:936–44. doi: 10.1182/blood-2008-06-163675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cheng Q, Tucker EI, Pine MS, Sisler I, Matafonov A, Sun MF, White-Adams TC, Smith SA, Hanson SR, McCarty OJ, Renne T, Gruber A, Gailani D. A role for factor XIIa-mediated factor XI activation in thrombus formation in vivo. Blood. 116:3981–9. doi: 10.1182/blood-2010-02-270918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Giesen PL, Rauch U, Bohrmann B, Kling D, Roque M, Fallon JT, Badimon JJ, Himber J, Riederer MA, Nemerson Y. Blood-borne tissue factor: another view of thrombosis. Proc Natl Acad Sci U S A. 1999;96:2311–5. doi: 10.1073/pnas.96.5.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chou J, Mackman N, Merrill-Skoloff G, Pedersen B, Furie BC, Furie B. Hematopoietic cell-derived microparticle tissue factor contributes to fibrin formation during thrombus propagation. Blood. 2004;104:3190–7. doi: 10.1182/blood-2004-03-0935. [DOI] [PubMed] [Google Scholar]
- 64.Tormoen GW, Rugonyi S, Gruber A, McCarty OJ. The role of carrier number on the procoagulant activity of tissue factor in blood and plasma. Phys Biol. 8:066005. doi: 10.1088/1478-3975/8/6/066005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tormoen GW, Haley KM, Levine RL, McCarty OJ. Do circulating tumor cells play a role in coagulation and thrombosis? Front Oncol. 2:115. doi: 10.3389/fonc.2012.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Muller F, Mutch NJ, Schenk WA, Smith SA, Esterl L, Spronk HM, Schmidbauer S, Gahl WA, Morrissey JH, Renne T. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009;139:1143–56. doi: 10.1016/j.cell.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.White-Adams TC, Berny MA, Patel IA, Tucker EI, Gailani D, Gruber A, McCarty OJ. Laminin promotes coagulation and thrombus formation in a factor XII-dependent manner. J Thromb Haemost. 2010;8:1295–301. doi: 10.1111/j.1538-7836.2010.03850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goel MS, Diamond SL. Neutrophil Cathepsin G promotes prothrombinase and fibrin formation under flow conditions by activating fibrinogen-adherent platelets. J Biol Chem. 2003;278:9458–9463. doi: 10.1074/jbc.M211956200. [DOI] [PubMed] [Google Scholar]
- 69.Goel MS, Diamond SL. Neutrophil enhancement of fibrin deposition under flow through platelet-dependent and independent mechanisms. Arterioscler Thromb Vasc Biol. 2001:2093–2098. doi: 10.1161/hq1201.100255. [DOI] [PubMed] [Google Scholar]
- 70.Schmidtke DW, Diamond SL. Direct observation of membrane tethers formed during neutrophil attachment to platelets or P-selectin under physiological flow. J Cell Biol. 2000;149:719–729. doi: 10.1083/jcb.149.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cooke BM, Usami S, Perry I, Nash GB. A simplified method for culture of endothelial cells and analysis of blood cells under conditions of flow. Microvasc Res. 1993;45:33–45. doi: 10.1006/mvre.1993.1004. [DOI] [PubMed] [Google Scholar]
- 72.Kirton DM, Nash GB. Activated platelets adherent to an intact endothelial cell monolayer bind flowing neutrophils and enable them to transfer to the endothelial surface. J Lab Clin Med. 2000;136:304–313. doi: 10.1067/mlc.2000.109406. [DOI] [PubMed] [Google Scholar]
- 73.van Berkel SS, van der Lee B, van Delft FL, Wagenvoord R, Hemker HC, Rutjes FP. Fluorogenic peptide-based substrates for monitoring thrombin activity. ChemMedChem. 2012;7:606–17. doi: 10.1002/cmdc.201100560. [DOI] [PubMed] [Google Scholar]
- 74.Jackson GN, Ashpole KJ, Yentis SM. The TEG vs the ROTEM thromboelastography/thromboelastometry systems. Anaesthesia. 2009;64:212–5. doi: 10.1111/j.1365-2044.2008.05752.x. [DOI] [PubMed] [Google Scholar]
- 75.Viola F, Mauldin FW, Jr, Lin-Schmidt X, Haverstick DM, Lawrence MB, Walker WF. A novel ultrasound-based method to evaluate hemostatic function of whole blood. Clin Chim Acta. 411:106–13. doi: 10.1016/j.cca.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chatterjee MS, Purvis JE, Brass LF, Diamond SL. Pairwise agonist scanning predicts cellular signaling responses to combinatorial stimuli. Nat Biotechnol. 2010;28:727–32. doi: 10.1038/nbt.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gosalia DN, Denney WS, Salisbury CM, Ellman JA, Diamond SL. Functional phenotyping of human plasma using a 361-fluorogenic substrate biosensing microarray. Biotechnol Bioeng. 2006;94:1099–110. doi: 10.1002/bit.20927. [DOI] [PubMed] [Google Scholar]
- 78.Gosalia DN, Salisbury CM, Ellman JA, Diamond SL. High throughput substrate specificity profiling of serine and cysteine proteases using solution-phase fluorogenic peptide microarrays. Mol Cell Proteomics. 2005;4:626–36. doi: 10.1074/mcp.M500004-MCP200. [DOI] [PubMed] [Google Scholar]
- 79.Gosalia DN, Salisbury CM, Maly DJ, Ellman JA, Diamond SL. Profiling serine protease substrate specificity with solution phase fluorogenic peptide microarrays. Proteomics. 2005;5:1292–8. doi: 10.1002/pmic.200401011. [DOI] [PubMed] [Google Scholar]
- 80.Salisbury CM, Maly DJ, Ellman JA. Peptide microarrays for the determination of protease substrate specificity. J Am Chem Soc. 2002;124:14868–70. doi: 10.1021/ja027477q. [DOI] [PubMed] [Google Scholar]
- 81.Maly DJ, Leonetti F, Backes BJ, Dauber DS, Harris JL, Craik CS, Ellman JA. Expedient solid-phase synthesis of fluorogenic protease substrates using the 7-amino-4-carbamoylmethylcoumarin (ACC) fluorophore. J Org Chem. 2002;67:910–5. doi: 10.1021/jo016140o. [DOI] [PubMed] [Google Scholar]
- 82.Harris JL, Backes BJ, Leonetti F, Mahrus S, Ellman JA, Craik CS. Rapid and general profiling of protease specificity by using combinatorial fluorogenic substrate libraries. Proc Natl Acad Sci U S A. 2000;97:7754–9. doi: 10.1073/pnas.140132697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Backes BJ, Harris JL, Leonetti F, Craik CS, Ellman JA. Synthesis of positional-scanning libraries of fluorogenic peptide substrates to define the extended substrate specificity of plasmin and thrombin. Nat Biotechnol. 2000;18:187–93. doi: 10.1038/72642. [DOI] [PubMed] [Google Scholar]
- 84.Gosalia D, Diamond SL. Nanodroplet chemical microarrays and label-free assays. Methods Mol Biol. 2010;669:69–78. doi: 10.1007/978-1-60761-845-4_6. [DOI] [PubMed] [Google Scholar]
- 85.Moake JL, Turner NA, Stathopoulos NA, Nolasco L, Hellums JD. Shear-induced platelet aggregation can be mediated by vWF released from platelets, as well as by exogenous large or unusually large vWF multimers, requires adenosine diphosphate, and is resistant to aspirin. Blood. 1988;71:1366–74. [PubMed] [Google Scholar]
- 86.McCarty OJ, Abulencia JP, Mousa SA, Konstantopoulos K. Evaluation of platelet antagonists in in vitro flow models of thrombosis. Methods Mol Med. 2004;93:21–34. doi: 10.1385/1-59259-658-4:21. [DOI] [PubMed] [Google Scholar]
- 87.McDonald JC, Duffy DC, Anderson JR, Chiu DT, Wu H, Schueller OJA, Whitesides GM. Fabrication of microfluidic systems in poly(dimethylsiloxane) Electrophoresis. 2000;21:27–40. doi: 10.1002/(SICI)1522-2683(20000101)21:1<27::AID-ELPS27>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 88.Neeves KB, Maloney SF, Fong KP, Schmaier AA, Kahn ML, Brass LF, Diamodn SL. Microfluidic focal thrombosis model for measuring murine platelet deposition and stability: PAR4 signaling enhances shear-resistance of platelet aggregates. J Thromb and Haemost. 2008;6:2193–2301. doi: 10.1111/j.1538-7836.2008.03188.x. [DOI] [PubMed] [Google Scholar]
- 89.Colace T, Falls E, Zheng XL, Diamond SL. Analysis of morphology of platelet aggregates formed on collagen under laminar blood flow. Ann Biomed Eng. 39:922–9. doi: 10.1007/s10439-010-0182-4. [DOI] [PubMed] [Google Scholar]
- 90.Maloney SF, Brass LF, Diamond SL. P2Y12 or P2Y1 inhibitor reduce platelet deposition in a microfluidic model of thrombosis while apyrase lacks efficacy under flow conditions. Lab Chip. 2010;2:153–220. doi: 10.1039/b919728a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gutierrez E, Petrich BG, Shattil SJ, Ginsberg MH, Groisman A, Kasierer-Friede Ana. Microfluidic devices for the studies of shear-dependent platelet adhesion. Lab Chip. 2008;8:1486–1495. doi: 10.1039/b804795b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Song H, Li HW, Munson MS, Van Ha TG, Ismagilov RF. On-chip titration of an anticoagulant argatroban and determination of the clotting time within whole blood or plasma using a plug-based microfluidic system. Anal Chem. 2006;78:4839–49. doi: 10.1021/ac0601718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pompano RR, Li HW, Ismagilov RF. Rate of mixing controls rate and outcome of autocatalytic processes: theory and microfluidic experiments with chemical reactions and blood coagulation. Biophys J. 2008;95:1531–43. doi: 10.1529/biophysj.108.129486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Neeves KB, Diamond SL. A membrane-based microfluidic device for controlling the flux of platelet agonists into flowing blood. Lab Chip. 2008;8:701–709. doi: 10.1039/b717824g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Neeves KB, Illing DA, Diamond SL. Thrombin flux and wall shear rate regulate fibrin fiber deposition state during polymerization under flow. Biophys J. 2010;98:1344–52. doi: 10.1016/j.bpj.2009.12.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Welsh JD, Colace TV, Muthard RW, Stalker TJ, Brass LF, Diamond SL. Platelet-targeting sensor reveals thrombin gradients within blood clots forming in microfluidic assays and in mouse. J Thromb Haemost. 2012 doi: 10.1111/j.1538-7836.2012.04928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Muthard RW, Diamond SL. Blood Clots Are Rapidly Assembled Hemodynamic Sensors: Flow Arrest Triggers Intraluminal Thrombus Contraction. Arterioscler Thromb Vasc Biol. 2012 doi: 10.1161/ATVBAHA.112.300312. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Colace TV, Muthard RW, Diamond SL. Thrombus growth and embolism on tissue factor-bearing collagen surfaces under flow: Role of thrombin with and without fibrin. Arteriocl Thromb Vasc Biol. 2012;32:1466–1476. doi: 10.1161/ATVBAHA.112.249789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nesbitt WS, Westein E, Tovar-Lopez JF, Tolouei E, Mitchell A, Fu J, Carberry J, Fouras A, Jackson SP. A shear gradient-dependent platelet aggregation mechanism drives thrombus formation. Nature Med. 2009;15:665–75. doi: 10.1038/nm.1955. [DOI] [PubMed] [Google Scholar]
- 100.Tovar-Lopez FJ, Rosengarten G, Westein E, Khoshmanesh K, Jackson SP, Mitchell A, Nesbitt WS. A microfluidics device to monitor platelet aggregation dynamics in response to strain rate micro-gradients in flowing blood. Lab Chip. 2010;10:291–302. doi: 10.1039/b916757a. [DOI] [PubMed] [Google Scholar]
- 101.Colace TV, Diamond SL. Direct Observation of von Willebrand Factor Elongation and Fiber Formation on Collagen During Acute Whole Blood Exposure to Pathological Flow. Arterioscler Thromb Vasc Biol. 2012 doi: 10.1161/ATVBAHA.112.300522. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kastrup CJ, Shen F, Runyon MK, Ismagilov RF. Characterization of the threshold response of initiation of blood clotting to stimulus patch size. Biophys J. 2007;93:2969–2977. doi: 10.1529/biophysj.107.109009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shen F, Kastrup CJ, Liu Y, Ismagilov RF. Threshold response of initiation of blood coagulation by tissue factor in patterned microfluidic capillaries is controlled by shear rate. Arterioscler Thromb Vasc Biol. 2008;28:2035–2041. doi: 10.1161/ATVBAHA.108.173930. [DOI] [PubMed] [Google Scholar]
- 104.Colace TV, Jannielle J, Diamond SL. Relipidated tissue actor linked to collagen surfaces potentiates platelet adhesion and fibrin formation in a microfluidic model of vessel injury. Bioconj Chem. 2011;22:2104–2109. doi: 10.1021/bc200326v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Corum LE, Eichinger CD, Hsiao TW, Hlady V. Using microcontact printing of fibrinogen to control surface-induced platelet adhesion and activation. Langmuir. 2011;27:8316–8322. doi: 10.1021/la201064d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Morton LF, Hargreaves PG, Farndale RW, Young RD, Barnes MJ. Integrin alpha 2 beta 1-independent activation of platelets by simple collagen-like peptides: collagen tertiary (triple-helical) and quaternary (polymeric) structures are sufficient alone for alpha 2 beta 1-independent platelet reactivity. Biochem J. 1995;306(Pt 2):337–44. doi: 10.1042/bj3060337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hansen RR, Tipnis AA, White-Adams TC, Di Paola JA, Neeves KB. Characterization of collagen thin films for von Willebrand factor binding and platelet adhesion. Langmuir. 2011;27:13648–58. doi: 10.1021/la2023727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Plow EF, Pierschbacher MD, Ruoslahti E, Marguerie G, Ginsberg MH. Arginyl-glycyl-aspartic acid sequences and fibrinogen binding to platelets. Blood. 1987;70:110–5. [PubMed] [Google Scholar]
- 109.Conant CG, Schwartz MC, Beecher JE, Rudoff RC, Iomesco-Zanetti C, Nevill JT. Well plate microfluidic system for investigation of dynamic platelet behavior under variable shear loads. Biotechnol Bioeng. 2011;108:2978–2987. doi: 10.1002/bit.23243. [DOI] [PubMed] [Google Scholar]
- 110.Hayward CPM, Harrison P, Cattaneo M, Ortel TL, Rao AK. Platelet function analyzer (PFA)-100 closure time in the evaluation of platelet disorders and platelet function. J Thromb Haemost. 2006;4:312–319. doi: 10.1111/j.1538-7836.2006.01771.x. [DOI] [PubMed] [Google Scholar]
- 111.Malinin A, Pokov A, Spergling M, Defranco A, Schwartz K, Schwartz D, Mahmud E, Atar D, Serebruany V. Monitoring platelet inhibition after clopidogrel with the VerifyNow-P2Y12 rapid analyzer: The VERify Thrombosis risk Assessment (Veritas) Study. Thrombosis Res. 2007;119:277–284. doi: 10.1016/j.thromres.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 112.Johansson PI, Stissing T, Bochsen L, Ostrowski SR. Thrombelestography and tromboelastometry in assessing coagulopathy in trauma. Scand J Trauma Resusc Emerg Med. 2009:17. doi: 10.1186/1757-7241-17-45. [DOI] [PMC free article] [PubMed] [Google Scholar]