Abstract
Objective
We hypothesized that 1) neointimal formation in a rat carotid balloon injury model could be reduced in vivo following targeted ultrasound delivery of rapamycin-loaded microbubbles (RMBs), and 2) the addition of dual mode ultrasound decreases the total amount of drug needed to reduce neointima formation.
Methods and Results
Balloon injury was performed in rat carotids to induce neointima formation. High or low doses of RMBs were injected I.V. and ruptured at the site of injury with ultrasound. Compared to non-treated injured arteries, neointima formation was reduced by 0% and 35.9% with 108 RMBs, and by 28.7% and 34.9% in arteries treated with 109 RMBs with and without ultrasound respectively.
Conclusion
Without ultrasound, 10-fold higher concentrations of RMBs were needed to reduce neointima formation by at least 28%, whereas 108 RMBs combined with ultrasound were sufficient to achieve the same therapeutic effect demonstrating that this technology may have promise for localized potent drug therapy.
Keywords: Balloon Injury, Ultrasound, Microbubbles, Drug Delivery, Rats
Non-systemic (i.e focal) drug delivery is desired in many disease conditions such as cancerous tumors and vascular disease in which many drugs are too potent to be delivered systemically. Rapamycin is an anti-proliferative drug shown to decrease neointima formation, but is consistent with complications at high doses 1, 2 such as decreased immune function and increased risk of cancers.
Microbubbles (MBs), ultrasound contrast agents, have been shown to increase reagent delivery to cells/tissues in the presence of low frequency ultrasound3. By increasing the duration of the ultrasound pulse bursts, microbubbles can be pushed to vessel walls4 prior to rupture and drug delivery. We attempted to target rapamycin delivery, and thereby reduce neointimal proliferation, by focusing ultrasound to the site of arterial injury. We validated the hypothesis that focused ultrasound-mediated drug delivery from microbubbles can effectively reduce the drug dose necessary for therapeutic effect on neointima formation.
Materials and Methods
All animal experiments were approved by ACUC at the University of Virginia. Sprague-Dawley rats underwent carotid balloon injury by inserting a 2F balloon catheter 1.5 cm past the bifurcation of the right common carotid. The balloon was inflated with 0.04ml of saline, pulled toward the bifurcation, deflated, and then withdrawn back into the carotid a total of 3 times. After injury, two ultrasound transducers were aligned with the injured vessel – one for imaging and bursting of microbubbles, and one for pushing of microbubbles (see Fig. 1A). Radiation force ultrasound was applied to push microbubbles via 135 kPa, 1.2MHz continuous wave ultrasound, and burst ultrasound was applied from a linear array (Sequoia 15L8, Siemens Medical Solutions, Malvern, PA) by emitting 5MHz, 1.5 MPa pulses.
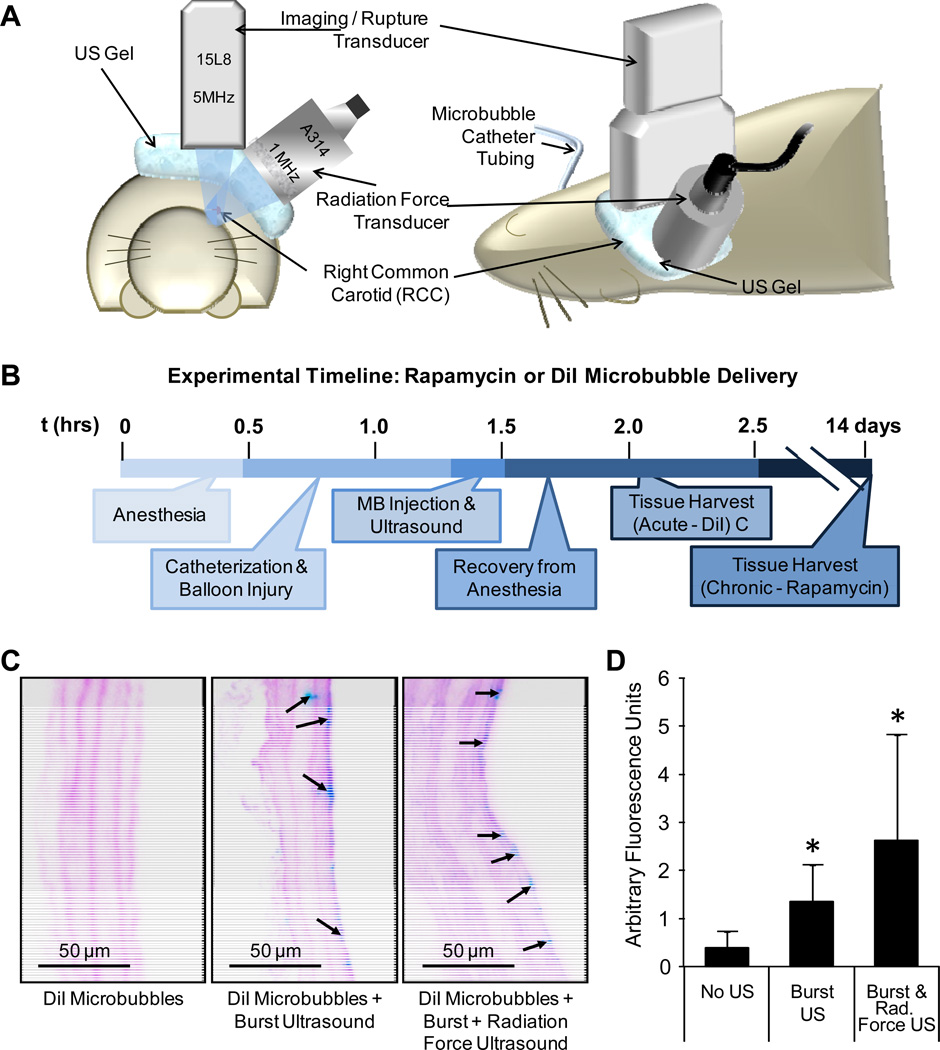
Figure 1.
A) Coincident radiation force and imaging/burst ultrasound transducers were aligned with the injured common carotid during microbubble (MB) infusion. B) Full timeline of the DiI-MB or rapamycin-MB delivery procedure. Control rats received either no microbubbles without ultrasound, or rapamycin-loaded microbubbles without ultrasound. C) Representative images of DiI fluorescence (inverted as blue) and auto-fluorescence of the elastic laminae (inverted as pink) in balloon injured carotid arteries of rats injected with DiI microbubbles. No DiI fluorescence was observed in non-injured or non-insonated controls. D) Quantified fluorescence intensity of DiI delivered to arteries (n=2, 4 to 12 images per artery, mean ± S.E., *indicates p≤0.05 compared to no ultrasound.)
Rapamycin-loaded and fluorescent DiI-loaded microbubbles were prepared as described previously5. DiI microbubbles were injected (109) in acute studies to visualize delivery along the vessel walls following no ultrasound (n=4), burst ultrasound (n=2), or dual burst and radiation force ultrasound applications (n=4). Microbubbles were infused through a left jugular vein catheter over 5 minutes. Ultrasound was applied concurrently with injection, and for another 3 minutes for a total of 8 minutes. For chronic studies, rapamycin microbubbles (RMBs) at high (109 RMBs total, n=7) or low (108 RMBs total, n=6) dose were infused through a left jugular vein catheter over 5 minutes. Both ultrasound modes were applied during infusion plus an additional 3 minutes. Control rats received high (n=7) or low dose RMBs (n=6) without US. Rats which received RMBs were euthanized 2 weeks after insonation. In each set of animals, left carotids served as contralateral uninjured, non-insonated controls. Arteries were excised and processed for sectioning and histology. Images were traced to find the area of the lumen, neointima, and media. Statistical analysis between groups was performed with a Student’s t-test.
Results
DiI delivery was observed in the carotids of injured rats where ultrasound was applied (Fig. 1C). DiI delivery was enhanced 3.4-fold in arteries which received burst ultrasound (n=4, p<0.001) compared to arteries not treated with ultrasound (n=2). The addition of radiation force ultrasound (n=2) enhanced delivery 1.9-fold over burst ultrasound alone, but not significantly (p=0.09).
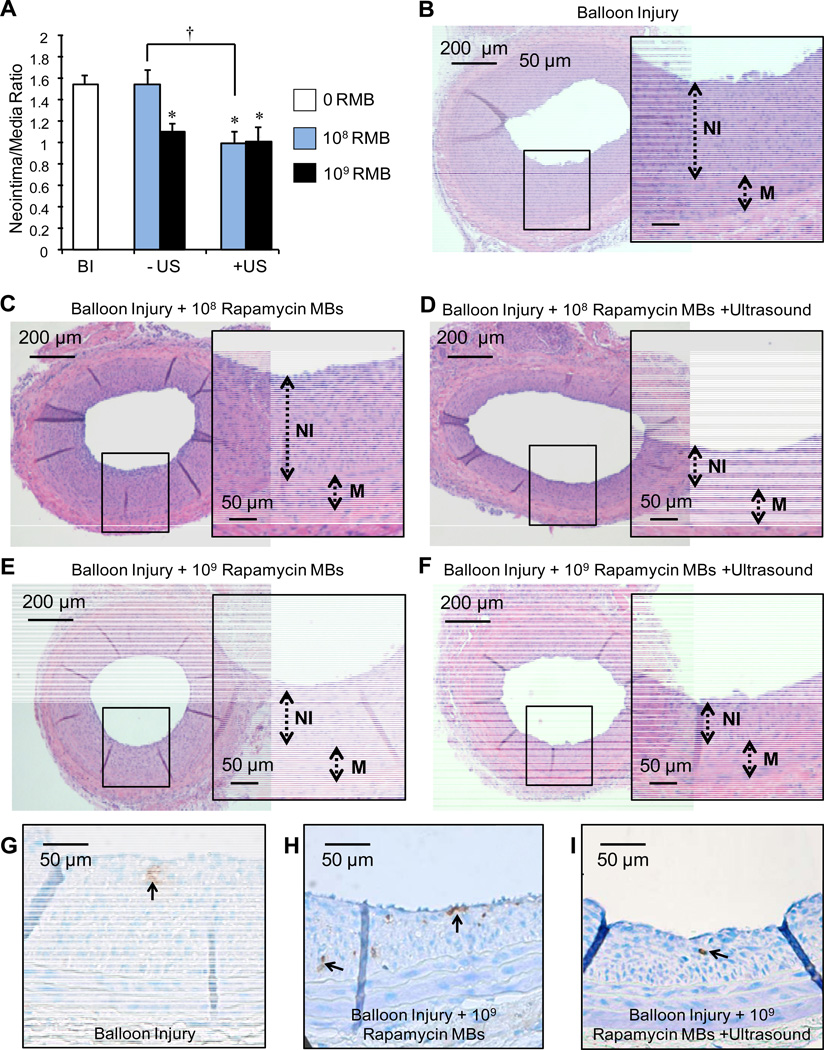
The neointima to media ratio (NI/M) of rats which underwent only balloon injury was 1.54±0.25 (n=11) compared to 0.0 for uninjured controls (n=5). The NI/M of injured carotids treated with high dose (109) RMBs alone was 1.10 ± 0.16 (n=7) as compared to 1.01 ± 0.33 (n=7) for carotids treated with 109 RMBs and ultrasound (Fig. 2A). These results correspond to reductions in NI/M ratios of 34.9% and 28.7% (p<0.001) with and without dual US application respectively. Among injured arteries exposed to the low dose (108) of RMBs, NI/M was significantly reduced by 35.9% (n=6, p<0.001) only in those treated with ultrasound. NI/M ratios of 1.54 ± 0.24 (n=6) were observed in injured arteries treated with the low dose of rapamycin microbubbles (108) without ultrasound. Representative images of arteries from each treatment group are shown in Fig. 2B–F. TUNEL staining revealed no significant difference in apoptosis rates between arteries treated with or without ultrasound, or RMBs (Fig. 2G–I).
Figure 2.
A) Quantitative comparison of carotids at two weeks post balloon injury (BI) following treatment with low (108) or high (109) dose of rapamycin microbubbles (RMB), with or without ultrasound (n≥6, mean ± S.E., *p<0.05 compared to BI alone, †p<0.008 between +/−US with 108 RMB). Representative images of H&E stained injured carotid artery sections. Balloon injured (BI) arteries received either B) no treatment, C) treatment with low dose (108) RMBs, D) both low dose (108) RMBs and ultrasound (both radiation force and burst pulses), E) treatment with high dose (109) RMBs or F) both high dose (109) RMBs and ultrasound (both radiation force and burst pulses). Inlays are ~3× zoom of black box regions of full cross sectional images. G, H, I) Representative images of TUNEL-positive cells (brown) in the neointima and media of rat carotids from the three treatment groups. Ultrasound mediated rupture of microbubbles in injured arteries does not affect apoptosis.
Discussion and Conclusions
The results demonstrate that dual frequency US combined with RMBs significantly reduces neointima formation with 1/10 of the dose necessary to achieve an equal therapeutic effect without ultrasound. While the high dose of microbubbles may have been sufficient to reduce proliferation on its own, it corresponds to a rapamycin concentration of 7.3µg/kg - 3.5 times lower than the dose associated with side effects in humans (2mg 1). The low dose of RMBs is approximately the concentration suggested for contrast imaging with Optison® or Definity® contrast agents. Not only does this therapeutic tool have promise for reducing neointima formation using only a fraction of the drug dose, but it can also be applied to drug delivery models in which localized drug delivery is desired, particularly with drugs that are very toxic in high levels systemically. This is advantageous particularly in experimental animal models of vascular injury and disease whereby pharmacological intervention (e.g. small molecules, antibody, plasmid DNA, RNAi) to reduce pathology is often achieved by IP or IV injection of a particular agent and thus the therapeutic mechanism of action at the vessel wall is difficult to deduce. Currently, the technology is limited to pre-clinical small animal restenosis models but translation to an intravascular catheter will allow for larger animal studies in swine, as we showed similarly for IVUS-mediated microbubble delivery of DNA to swine coronary arteries in vivo6.
Supplementary Material
Acknowledgements
This study was supported by the NIH NIBIB grant EB002185 and HL090700, and UVA Coulter Translational Research Grant. We thank Bobi Thornhill for her surgical expertise.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Mahalati K, Kahan BD. Clinical pharmacokinetics of sirolimus. Clinical Pharmacokinetics. 2001;40:573–585. doi: 10.2165/00003088-200140080-00002. [DOI] [PubMed] [Google Scholar]
- 2.Stojkovic S, Ostojic M, Nedeljkovic M, Stankovic G, Beleslin B, Vukcevic V, Orlic D, Arandjelovic A, Kostic J, Dikic M, Tomasevic M. Systemic rapamycin without loading dose for restenosis prevention after coronary bare metal stent implantation. Catheterization and Cardiovascular Interventions. 2010;75:317–325. doi: 10.1002/ccd.22301. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara K, Pollard R, Bordeni M. Ultrasound microbubble contrast agents: Fundamentals and application to gene and drug delivery. Annual Review of Biomedical Engineering. 2007;9:415–447. doi: 10.1146/annurev.bioeng.8.061505.095852. [DOI] [PubMed] [Google Scholar]
- 4.Dayton P, Klibanov A, Brandenburger G, Ferrara K. Acoustic radiation force in vivo: A mechanism to assist targeting of microbubbles. Ultrasound in Medicine and Biology. 1999;25:1195–1201. doi: 10.1016/s0301-5629(99)00062-9. [DOI] [PubMed] [Google Scholar]
- 5.Phillips LC, Klibanov AL, Wamhoff BR, Hossack JA. Localized ultrasound enhances delivery of rapamycin from microbubbles to prevent smooth muscle proliferation. J. Control. Release. 2011 doi: 10.1016/j.jconrel.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phillips LC, Klibanov AL, Bowles DK, Ragosta M, Hossack JA, Wamhoff BR. Focused in vivo delivery of plasmid dna to the porcine vascular wall via intravascular ultrasound destruction of microbubbles. Journal of Vascular Research. 2010;47:270–274. doi: 10.1159/000258905. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.