Abstract
Background
Many studies have shown that exposures to air pollution are associated with cardiovascular events although the mechanism remains to be clarified. To identify whether exposures to ambient particles act on autonomic function via the lipid/endothelial metabolism pathway, we evaluated whether the effects of particulate matter < 2.5 µm in aerodynamic diameter (PM2.5) on heart rate variability (HRV) were modified by gene polymorphisms related to those pathways.
Methods
We used HRV and gene data from the Normative Aging Study and PM2.5 from a monitor located a kilometer from the examination site. We fitted a mixed effect model to investigate the associations between PM2.5 and repeated measurements of HRV by gene polymorphisms of apolipoprotein E (APOE), lipoprotein lipase (LPL) and vascular endothelial growth factor (VEGF) adjusting for potential confounders chosen a priori.
Results
A 10-µg/m3 increase of PM2.5 in the two days before the examination was associated with 3.8% [95% confidence interval (CI): 0.2%, 7.4%], 7.8% [95 CI: 0.4%, 15.3%] and 10.6% [95% CI: 1.8 %, 19.4%] decreases of the standard deviation of normal-to-normal intervals, low frequency and high frequency, respectively. In general, carriers of wild type APOE, LPL and VEGF genes had stronger effects of particles on HRV compared to those with hetero- or homozygous types. Variations of LPL-N291S, LPL-D9N and APOE-G113C significantly modified effects of PM2.5 on HRV.
Conclusion
Associations between PM2.5 and HRV were modified by gene polymorphisms of APOE, LPL and VEGF and biological metabolism remains to be identified.
Keywords: air pollution, heart rate variability, effect modification, apolipoprotein E, lipoprotein lipase, vascular endothelial growth factor
Introduction
A large number of studies have shown that long- and short-term exposures to ambient particulate matter are associated with cardiovascular diseases.[1–6] Most of the deaths associated with particle exposure are sudden deaths,[7] suggesting that arrhythmia and myocardial infarctions are involved. A number of single city studies have examined the associations of myocardial infarctions with airborne particles,[8–10] and one large multicity study has demonstrated a robust association.[11] Other studies have found that ambient particles are associated with arrhythmias.[12–13] The mechanisms by which particles produce these effects are not fully understood.
Decreased heart rate variability (HRV), a noninvasive measure of cardiac autonomic dysfunction,[14] is predictive of sudden deaths and arrhythmias,[15–17] and have been associated with short-term exposure to particulate matter, particularly to fine particles of <2.5 µm in aerodynamic diameter (PM2.5).[18–22]
Suggested mechanisms linking air pollution to cardiovascular diseases (CVD) include changes in the response of the autonomic nervous system through direct pulmonary reflexes from airways, chemical effects on ion channel function in myocardial cells, ischemic response in the myocardium, and pulmonary and systemic oxidative stress and inflammatory responses that trigger endothelial dysfunction, atherosclerosis, and thrombosis.[23–25] However, the exact mechanisms underlying such effects remain to be clarified. Identifying these potential pathways is one of the most important research priorities in air pollution epidemiology.
One of the suggested mechanistic pathways is related to the formation of atheromatous plaques, in which metabolisms of lipid and cholesterol play an important role. Formations of atheromatous plaques result in atherosclerosis, including coronary atherosclerosis. Many studies have shown that atherosclerosis is significantly associated with decreased HRV.[26–31] Particle exposure has been shown to influence plaque formation and stability.[32] Atherosclerosis is associated with lipid metabolism.[33–34]
The metabolism of cholesterol and lipids are regulated by many enzymes and proteins, including lipoprotein lipase (LPL), apolipoprotein E (APOE) and vascular endothelial growth factor (VEGF), etc. Genetic polymorphisms in these genes influence the functions of the relative enzymes and proteins, and therefore are related to the metabolisms of lipid and cholesterol. Concentrations of blood cholesterol and lipid are related to cardiovascular diseases in the circulation system and their genetic variants are associated with cardiovascular diseases.[35–38] For example, a meta-analysis conducted by Bennet et al.[35] shows that APOE genotypes are associated with low-density lipoprotein (LDL) cholesterol and coronary risk. VEGF genotypes have also been associated with atherosclerosis.[39–41]
Whether differences in gene polymorphisms related to lipid or cholesterol metabolisms modify the effects of the exposure to PM2.5 on HRV has not been reported to our knowledge. The present study examined how the associations of PM2.5 with HRV in the Normative Aging Study population, a repeated-measurement investigation of elderly subjects from the Boston metropolitan area, was modified by selected APOE, LPL and VEGF gene variants.
Materials and Methods
Study population
The Normative Aging Study (NAS) is a longitudinal study of aging established in 1963 by the US Veterans Administration. A total of 2,280 men 21 to 80 years of age from the greater Boston area free of known chronic medical conditions were enrolled.[42] All participants provided written informed consent. The present investigation was approved by the Institutional Review Boards of all participating institutions. Since 1963, participants have undergone detailed examination every 3 to 5 years, including routine physical examinations, laboratory tests, collection of medical history information, and completion of questionnaires on smoking history, education level, food intake, and other factors that may influence health.
Between November 2000 and December 2007, 686 participants still presenting for examination were evaluated for HRV one or more times for a total of 1196 visits. Of those, 357 visits from 103 subjects were excluded because of heart arrhythmia, HRV measurement time <3.5 minutes, missing potential confounders or failed gene tests. The remaining participants (n = 583) had either one (n = 378), two (n = 154) or three (n = 51) repeated HRV measurements, for a total of 839 visits.
HRV Measurement
After a 5-minute rest, HRV was measured for 7 minutes in a sitting position with a 2-channel (5-lead) ECG monitor (Trillium 3000 model, Forest Medical, East Syracuse, NY, sampling rate of 256 Hz per channel). The ECG was digitally recorded and the best 4-consecutive-minute interval was used for the HRV calculations. The standard deviation of normal-to-normal intervals (SDNN), high frequency (HF; 0.15 to 0.4 Hz), and low frequency (LF; 0.04 to 0.15 Hz) were calculated with a fast Fourier transform using software (Trillium-3000, PC-Companion Software, Forest Medical), which conforms to established guidelines (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology 1996).
Air pollution and weather data
Continuous ambient PM2.5 was measured at a stationary monitoring site 1 km from the examination site with a tapered-element oscillating microbalance (model 1400A, Rupprecht & Pataschnick Co, East Greenbush, NY), aethalometer (Magee Scientific, Berkeley, Calif), and Harvard-EPA annular denuder system sampler, respectively. The 48-hour moving average of PM2.5 was used as the exposure index in this analyses because the previous Normative Aging study has shown the strongest associations of the 48-hour moving average of PM2.5 with HRV compared to other period moving averages of PM2.5 such as 4-hour or 24-hour moving averages.[43] To control for weather variables, we used apparent temperature, defined as a person’s perceived air temperature and calculated from ambient temperature, humidity and wind. [43] The weather data were recorded at Logan Airport monitoring site, about 5 miles from the physical examination site. The hourly weather measures were recorded through the whole study period. Room temperature was measured at the physical examination site.
Genotyping
Genetic polymorphism measurements in this study included APOE-T530C (rs7412), APOE-G219T (rs405509), APOE-T392C (rs429358), APOE-G113C (rs440446), APOE-A491T (rs449647), APOE-T427C (rs769446), VEGF-G634C (rs2010963), LPL-N291S (G→A at position 280, rs268), LPL-S447X (C→G at position 1127, rs328) and LPL-D9N (G→A at position 1595, rs1801177).
Multiplex PCR assays were designed using Sequenom SpectroDESIGNER software by inputting sequence containing the single nucleotide polymorphism (SNP) site and 100 bp of flanking sequence on either side of the SNP. Most assays were genotyped using the Sequenom MassArray MALDI-TOF mass spectrometer (Sequenom, CA, USA) with semiautomated primer design (SpectroDESIGNER, Sequenom) and implementation of the very short extension method.[44] Assays which failed to multiplex were genotyped using the TaqMan 5’ exonuclease [Applied Biosystems (ABI), Foster City, CA, USA] with primers from ABI using radioactive labeled probes detected using ABI PRISM 7900 Sequence Detector System.[45]
Statistical analysis
Log10 transformations of HRV measurements were used to improve normality and stabilize the variance. The following covariates were chosen a priori in the analysis: age, body mass index, mean arterial pressure, fasting blood glucose, cigarette smoking (never/former/current), alcohol consumption (≥2 drinks per day, yes/no), use of β-blockers, calcium channel blockers, angiotensin-converting enzyme inhibitors, room temperature, season, and 48-hour moving average of outdoor apparent temperature.[18] In order to control for a potential nonlinear relationship between temperature and HRV, we adjusted for apparent temperature with a quadratic term and a term indicating temperature over 20 °C.
Because of repeated measures of HRV for many participants, the assumption that the errors in the regression are independent is likely to be violated. Hence, we fit a mixed-effects model (PROC MIXED in SAS version 9.1, SAS Institute Inc, Cary, NC). The model could be described as: Yit = β0 + μi + β1X1it + … + βnitXnit + βpollit + εit, where Yit is the logarithm of HRV in subject i at time t, β0 is the overall intercept, and μi is the separate random intercept for subject i. X1it to Xnit denote the covariates.
We first assessed the main effects of PM2.5 on HRV and then examined the effect modification by the individual genotypes related to lipid metabolism and endothelial function. To evaluate the effect modification by a genotype, we added an interaction term for the genotype variable (a dummy variable) and PM2.5 and a genotype variable. Due to the small proportions for homozygous and heterozygous genotypes for APOE, LPL and VEGF, we combined the two types comparing wild type to any variant (dominant model). As a sensitive analysis, we also examined whether the use of statin confounded the estimates of associations between exposure to PM2.5 and HRV by adding a dummy variable for the use of statin (Yes/No) before the visit to refit the previously described model.[46]
Results
Table 1 describes the demographic and clinical characteristics, HRV measurements of the study population, and environmental variable measurements. The study subjects were all male and their average age was 73.8 (95% confidence interval (CI): 60.8, 86.7). The sample was predominantly white (97.1%). The mean of PM2.5 was 11.8 µg/m3 (SD 6.2 µg/m3). The correlation between apparent temperature and PM2.5 was modest (r = 0.26). The mean of apparent temperature was 10.4 °C (SD 9.7 °C). Although the apparent temperature considerably varied, the room temperature was relatively steady (mean: 24.0 °C, SD: 1.7 °C).
Table 1.
Descriptive statistics of demographic, health and environmental factors among visits of the Normative Aging Study participants with heart rate variation measures between November 2000 and December 2007 in Boston, Massachusetts, USA
| Variable | First visit (n=583) ¶ | Total visits (n=839) ¶¶ |
|---|---|---|
| Age, years * | 72.98 (6.60) | 73.77 (6.61) |
| Body mass index, kg/m2 | 28.16 (4.01) | 28.07 (4.06) |
| Mean arterial pressure, mmHg | 93.16 (10.74) | 91.45 (10.84) |
| Fasting blood glucose, mg/dl | 107.51 (27.25) | 107.40 (25.16) |
| Season, n (%) | ||
| Spring | 153 (26.24) | 204 (24.31) |
| Summer | 139 (23.84) | 205 (24.43) |
| Fall | 166 (28.47) | 254 (30.27) |
| Winter | 125 (21.44) | 176 (20.98) |
| Smoking status, n (%) | ||
| Never smoker | 173 (29.67) | 259 (30.87) |
| Current smoker | 25 (4.29) | 27 (3.22) |
| Former smoker | 385 (66.04) | 553 (65.91) |
| Alcohol intake (≥ 2 drinks/day), n (%) | 107 (18.35) | 154 (18.36) |
| Use of β-blocker, n (%) | 209 (35.85) | 320 (38.14) |
| Use of Ca-Channel blocker, n (%) | 79 (13.55) | 121 (14.42) |
| Use of angiotensin-converting enzyme inhibitor, n (%) | 128 (21.96) | 200 (23.84) |
| Log10 standard devision of normal-to-normal intervals, msec | 1.53 (0.28) | 1.52 (0.29) |
| Log10 high frequency, msec2 | 2.02 (0.59) | 1.94 (0.75) |
| Log10 low frequency, msec2 | 1.95 (0.73) | 2.00 (0.60) |
| PM2.5, µg/m3 ** | 11.32 (6.53) | 11.80 (6.18) |
| apparent temperature, °C ** | 10.68 (9.75) | 10.38 (9.71) |
| Room temperature, °C | 24.24 (1.80) | 23.96 (1.69) |
| Cholesterol, mg/dL | 270.62 (145.06) | 259.65 (139.59) |
for the first time visits of participants;
for the total visits of participants;
Values are mean± SD when appropriate.
Two day moving averages.
Table 2 shows the genetic distributions of APOE, LPL and VEGF. Among 583 subjects, the wild types for APOE-T530C, APOE-T392C, APOE-A491T, APOE-T427C and each of LPL polymorphisms were dominant, but the situation was different for APOE-G219C, APOE-G113C, and VEGF-G634C, of which heterozygous type shared large proportions (48.1%, 44.7% and 44.7%, respectively). Heterozygous types for APOE-T392C, APOE-A491T consisted of 21.2% and 32.6%, respectively. Among APOE haplotypes, E3/E3 was the most frequent combination (63.8%). E3/E2 and E3/E4 were found in 12.8% and 19.4% of the study participants, respectively. Other haplotype combinations (E2/E2, E4/E4 and E2/E4) were rare (3.9%).
Table 2.
Genotype distributions of Apoprotein E, lipoprotein lipase and vascular endothelial growth of the Normative Aging participants between November 2000 and December 2007 in Boston, Massachusetts, USA
| Polymorphism | N (%) | |
|---|---|---|
| APOE-T530C (rs7412) | Wild | 491 (85.1) |
| Heterozygous | 83 (14.4) | |
| Homozygous | 3 (0.5) | |
| APOE-G219T (rs405509) | Wild | 162 (28.7) |
| Heterozygous | 272 (48.1) | |
| Homozygous | 131 (23.2) | |
| APOE-T392C (rs429358) | Wild | 435 (76.9) |
| Heterozygous | 120 (21.2) | |
| Homozygous | 11 (1.9) | |
| APOE-G113C (rs440446) | Wild | 220 (39.8) |
| Heterozygous | 247 (44.7) | |
| Homozygous | 86 (15.6) | |
| APOE-A491T (rs449647) | Wild | 353 (63.2) |
| Heterozygous | 182 (32.6) | |
| Homozygous | 24 (4.3) | |
| APOE-T427C (rs769446) | Wild | 486 (85.1) |
| Heterozygous | 75 (13.1) | |
| Homozygous | 10 (1.8) | |
| LPL-N291S (rs268) | Wild Type | 555 (97.2) |
| Heterozygous | 15 (2.6) | |
| Homozygous | 1 (0.2) | |
| LPL-S447X (rs328) | Wild Type | 460 (83.3) |
| Heterozygous | 86 (15.6) | |
| Homozygous | 6 (1.1) | |
| LPL-D9N (rs1801177) | Wild Type | 537 (97.8) |
| Heterozygous | 12 (2.3) | |
| Homozygous | 0 (0.0) | |
| VEGF-G634C (rs2010963) | Wild Type | 233 (42.5) |
| Heterozygous | 245 (44.7) | |
| Homozygous | 70 (12.8) | |
| APOE haplotype | E3/E3 | 358 (63.8) |
| E3/E2 | 72 (12.8) | |
| E3/E4 | 109 (19.4) | |
| Other | 22 (3.9) |
The sum of the subjects in each genotype may not add up to the total number of subjects due to missing genotyping data. Missing genotyping is due to a variable number of samples for each locus for which genotyping was not successful.
Results show that a 10-µg/m3 increase of PM2.5 was significantly associated with 3.8% (95% CI: 0.2%, 7.4%), 7.8% (95% CI: 0.4%, 15.3%) and 10.6% (95% CI: 1.8%, 19.4%) decreases for SDNN, LF and HF, respectively. We also examined whether genotype variations are related to HRV and results did not show any evidence that genetic polymorphisms were associated with HRV in this study. As a sensitive analysis, we adjusted for an additional covariate, current statin use (yes/no). In this sensitive analysis, a 10-µg/m3 increase of PM2.5 was significantly associated with 3.9% (95% CI: 0.3%, 7.5%), 7.9 % (95% CI: 0.5%, 15.3%) and 10.6% (95% CI: 1.8%, 19.4%) decreases for SDNN, LF and HF, respectively. As these results suggest that the use of statin did not confound the associations between exposure to particulate pollution and HRV, we did not adjust for it in the following analyses.
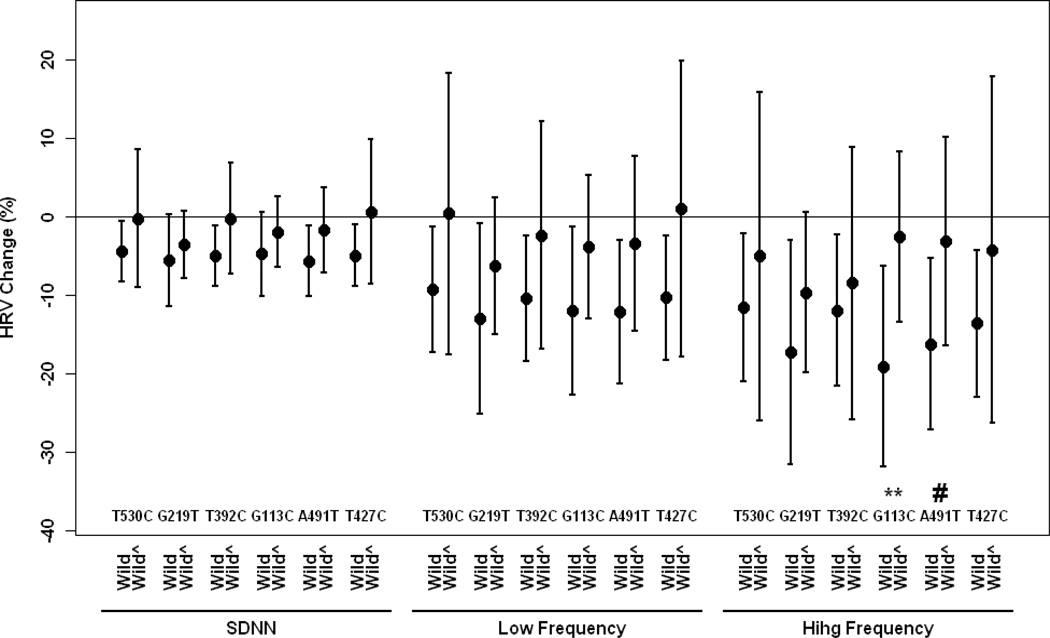
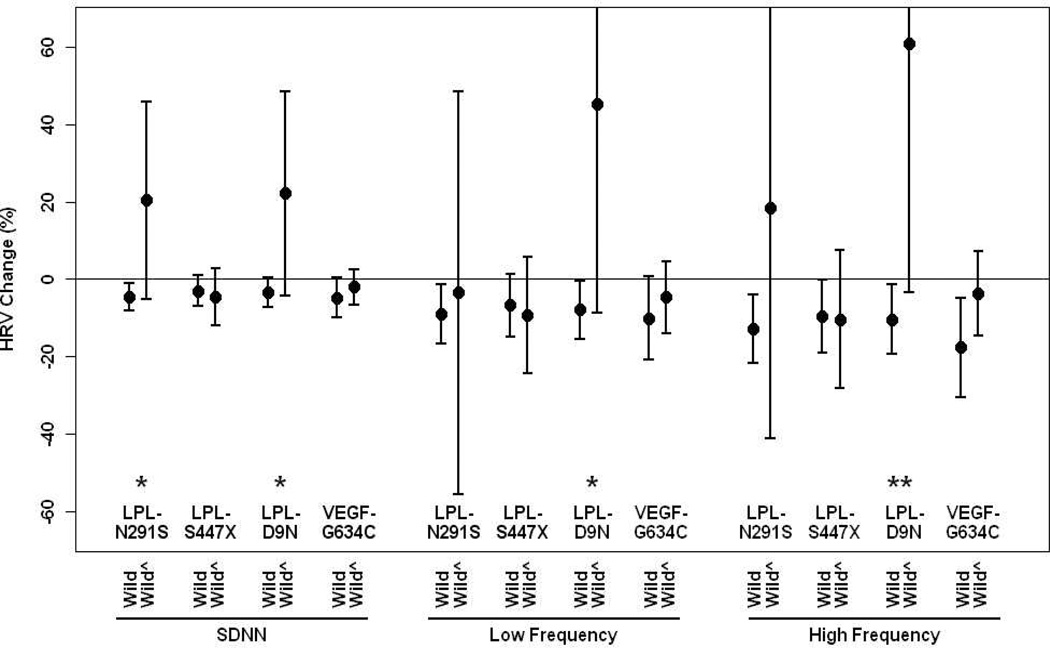
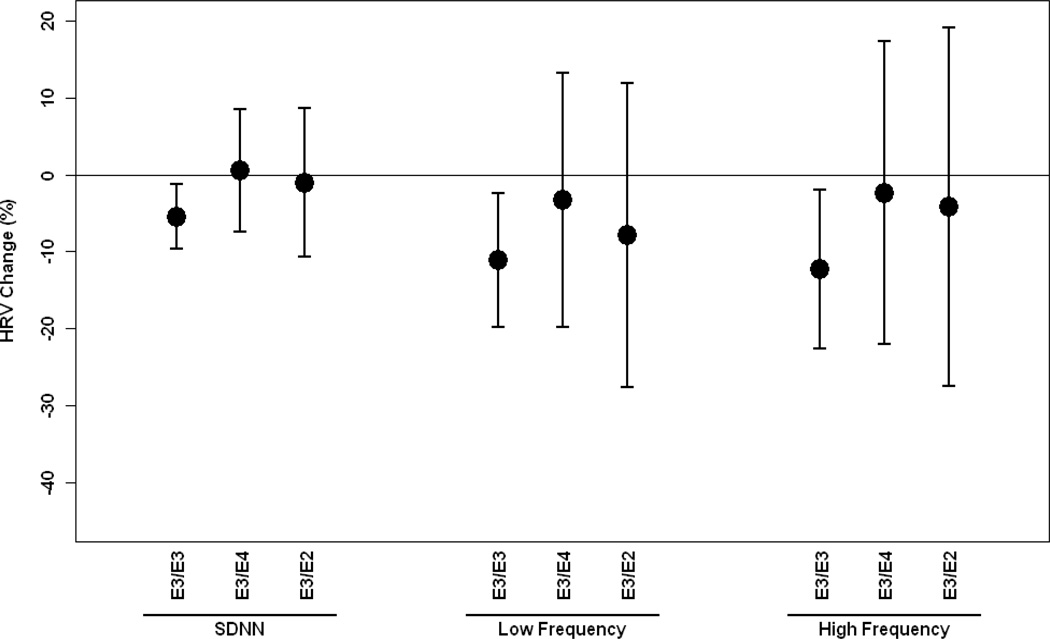
We then estimated associations between PM2.5 and HRV by genetic polymorphisms after adjustment for potential confounders. Figure 1–2 show associations between PM2.5 and HRV by APOE, LPL and VEGF gene polymorphisms. In general, individuals with the wild types of APOE, LPL and VEGF showed stronger effects of PM2.5 on the decrease of HRV compared to those with non-wild genotypes, but not for LPL-S447X showing the slightly stronger effect of PM2.5 on the decrease of HRV among those carrying non-wild genotypes. Significant associations were only observed for wild-type gene carriers, but not for non-wild carriers for any examined gene polymorphisms. Significant interaction effects were found for APOE-G113C, APOE-A491T, LPL-N291S, LPL-D9N, especially for LPL-D9N at 0.10 or 0.05 level. We also examined whether associations between PM2.5 and HVR were modified by APOE haplotypes. In general, there were stronger associations between PM2.5 and HRV for E3/E3 compared to E4/E2 and E3/E4, but no significant interactive effects were observed (Figure 3). We categorized concentrations of cholesterol and triglyceride by low and high quartiles and examined the effect of modification by cholesterol and triglyceride. We found the same trends as those of genes. In general, subjects with higher levels of cholesterol or triglyceride experienced lower risk of HRV by PM2.5. The full set of estimates are presented in the supplement.
Figure 1.
Percent change (95% CI) in Standard deviation of normal-to-normtal interval, low frequency and high frequency associated with a 10-µg/m3 increase in PM2.5 by ApoE-T530C, ApoE-G291T, ApoE-T392C, ApoE-G113C, ApoE-A491T and ApoE-T427C gene polymorphisms, wild type vs non-wild type (wild^) adjusting for age, body mass index, mean arterial blood pressure, fasting blood glucose, cigarette smoking, alcohol consumption, season, room temperature and outdoor apparent temperature and uses of β-blockers, angiotension-converting enzyme inhibitors and calcium channel blockers.
#: p<0.20, ** p<0.05 for interaction terms between PM2.5 and gene polymorphism (wild vs non-wild); SDNN: standard deviation of normal-to-normal intervals.
Figure 2.
Percent change (95% CI) in Standard deviation of normal-to-normtal interval, low frequency and high frequency associated with a 10-µg/m3 increase in PM2.5 by LPL-N291S, LPL-S447S, LPL-D9N and VEGF-G634C gene polymorphisms, wild type vs non-wild types (wild^) adjusting for age, body mass index, mean arterial blood pressure, fasting blood glucose, cigarette smoking, alcohol consumption, season, room temperature and outdoor apparent temperature and uses of β-blockers, angiotension-converting enzyme inhibitors and calcium channel blockers.
*p<0.10, ** p<0.05 for interaction terms between PM2.5 and gene polymorphism (wild vs non-wild); SDNN: standard deviation of normal-to-normal intervals.
Figure 3.
Percent change (95% CI) in Standard deviation of normal-to-normtal interval, low frequency and high frequency associated with a 10-µg/m3 increase in PM2.5 by ApoE haplotypes E3/E3, E3/E2, E3/E4 gene polymorphisms adjusting for age, body mass index, mean arterial blood pressure, fasting blood glucose, cigarette smoking, alcohol consumption, season, room temperature and apparent temperature and uses of β-blockers, angiotension-converting enzyme inhibitors and calcium channel blockers.
SDNN: standard deviation of normal-to-normal intervals.
In order to explore the relationship of concentrations of cholesterol and triglyceride with variants of APOE variants, we fit linear regression model using log of concentrations of cholesterol or triglyceride as dependent variable, all APOE genes (wild vs non-wild) as predictors adjusting for age, body mass index, mean arterial pressure, fasting blood glucose, cigarette smoking (never/former/current), alcohol consumption (≥2 drinks per day, yes/no), use of β-blockers, calcium channel blockers, angiotensin-converting enzyme inhibitors, room temperature, season, and 48-hour moving average of outdoor apparent temperature[18]. Results show that there were no significant relationships between concentrations of cholesterol/triglyceride and each of APOE variants except for APOE-T530C associated with triglyceride (subjects with non-wild APOE-T530C variants had higher triglyceride concentration).
Discussion
This study shows that exposure to PM2.5 exerted effects on the decrease of HRV while its short-term health significance effect has not well established. Genetic variations related to lipid metabolisms and VEGF modified associations between exposure to ambient particulate matter and heart rate variability. In general, the associations between PM2.5 exposure and HRV were weaker for individuals carrying variants of LPL, APOE and VEGF genes than those carrying wild types. Significant associations between PM2.5 and HRV often appeared among individuals with wild types of the genes and E3/E3 APOE variants, but not for those with hetero- and homozygous types for any APOE, LPL and VEGF genotypes or other APOE haplotypes. LPL-N291S, LPL-D9N and ApoE-G113C significantly modified the effect of PM2.5 on HRV at the 0.10 or 0.05 level (Figure 1–3).
There are six major classes of apolipoproteins (A, B, C, D, E, H). APOE is an apolipoprotein found in the chylomicron and intermediate-density lipoproteins that binds to a specific receptor on liver cells and peripheral cells. It is essential for the normal catabolism of triglyceride-rich lipoprotein constituents APOE transports lipoproteins, fat-soluble vitamins, and cholesterol into the lymph system and then into the blood. APOE consists of 299 amino acids. APOE gene (ApoE) is mapped to chromosome 19, consisting of four exons and three introns, totaling 3597 base pairs. Structures and functions of the three common alletic isoforms, ApoE2, ApoE3 and ApoE4, have been extensively studied. The isoforms differ at at positions 112 and 158, corresponding to APOE-T530C and APOE-T392C. ApoE3, the most common isoform, contains cysteine and arginine, respectively, whereas ApoE2 has two cysteines and apoE4 has two arginies at the two positions.[47] ApoE3 and ApoE4 bind to LDL receptors with similarly high affinity, but the binding of ApoE2 is 50- to 100-times weker.[48]
APOE is a multifunctional protein that plays a crucial role in the metabolism of triglycerides and cholesterol by binding to receptors on the liver to help mediate clearance of chylomicrons and very low-density lipoproteins from the bloodstream.[49–51] Variants in the APOE gene are related to different disease occurrences, including cardiovascular diseases, Alzheimer’s disease and biliary tract diseases.[35, 49, 52] Many studies have shown that APOE polymorphisms are associated with cardiovascular diseases.[35, 49, 53] For example, a large meta-analysis conducted by Bennet et al.[35] shows that E2 carriers have a 20% lower risk of coronary heart disease and E4 carriers have a slightly higher risk compared with E3 carriers. Some studies have shown that APOE polymorphisms are also related to plaque formation with the E4 variants being associated with more plaque formation.[54–55]
Enzyme lipoprotein lipase has a central role in lipoprotein metabolisms by catalyzing hydrolysis of triglycerides in chylomicrons and very low-density lipoprotein particles. LPL also functions as a mediator facilitating binding and/or incorporation of series of lipoproteins through either lipoprotein receptors or heparin sulfate proteoglycans into several lines of cells.[56–57] Therefore, the gene for LPL is an obvious candidate for contributing to inherited predisposition for dyslipidaemia and risk of atherosclerosis. Since the gene was cloned in 1989, more than 100 LPL gene mutations have been identified, the majority of which cause only mild detrimental enzymatic function.[58] Three variants LPL-D9N, LPL-N291S and LPL-S447X, causing the replace of an aspartic acid to an asparagine residue, an asparagine to a serine residue and a serine to another at position 9, 291 and 447, have been extensively studied. Variants of LPL-D9N, LPL-N291S occur at high frequencies and the general population (up to 5%) and result in the decreased lipolytic activity compared to non-variant.[59, 60] LPL-S447X mutation will introduce change a premature stop codon and result in a mature protein that lacks the C-terminal serine and glycine. In contrast to all other variants, this mutation is associated with beneficial effects on lipid homeostasis and atheroprotection.[58]
Variations of LPL genes have been shown to be associated with different diseases, including cardiovascular diseases and severe retinopathy.[61–64] Fisher et al.[61] reported that mutation of LPL-S447X was a favorable factor for lipid profile but mutations for two others are risk factors (LPL-N291S, LPL-D9N). A meta-analysis conducted by Hu et al.[62] shows that the N291S variant in the LPL gene is a risk factor for dyslipidemia and is associated with coronary heart disease and the risk of type 2 diabetes mellitus. Together with our study, we found weaker effects of PM2.5 on HRV in those with LPL variants for lipid metabolisms (Figure 2).
Vascular endothelial growth factor, a mitogen that specifically acts on endothelial cells, has different effects, including mediating increased vascular permeability, inducing angiogenesis, cell growth, migration, and inhibition of apoptosis.[65–68] Studies have shown that polymorphisms of VEGF genes are associated with severity of atherosclerosis and severe diabetic retinopathy.[39–41] Howell et al.[41] examined whether VEGF polymorphisms were associated with atherosclerosis and found that the genotypes had significantly different distributions with and without myocardial infarction. Al-Kateb et al.[39] found that multiple VEGF variants were associated with the development of severe retinopathy in type 1 diabetes patients. An experimental study shows that VEGF significantly alters the rate of atherosclerotic plaque development.[69]
Effect modification by the genetic factors on associations between air pollution and cardiovascular events has not been widely examined. Our recent studies examined the effect modifications and found that the mutations of genes modified the associations between HRV and PM2.5, including glutathione S-transferase M1, dietary intakes of methyl nutrients, hemochromatosis and oxidative stress genes.[18, 46, 70, 71] However, so far, few studies have examined effect modifications of the mutations of genes related to lipid metabolism on the associations between air pollution and cardiovascular events.
While this study found the evidence that APOE, LPL and VEGF gene polymorphisms modified association between PM2.5 effects and HRV, this finding was inconsistent with the one expected, i.e., the exposure to PM2.5 elevates lipid concentrations and consequently promotes the plaque formation. And this finding should be carefully interpreted and remains to be identified by other studies. The current study shows that PM2.5 consistently produced lower effects on HRV reductions among the subjects with unfavorable mutations of genes to lipid metabolism compared to those without the variations. However, it remains unclear how lipid metabolism might render subjects with variants of the genes more likely to experience a decrease in HRV with a short-term exposure to fine particles. A few studies found that the gene variation of LPL-D9N modified smoking risk on cardiovascular diseases.[38, 64] They used stably transfected cell lines in vitro to examine the role of variants of LPL-D9N gene in lipoprotein metabolisms and found that compared to the wild-type structure, LPL-N9 cells bind and internalize twofold to fourfold more LDL and ox-LDL. They only speculated on the mechanism via its bringing function in the enzymes. Studies show that ApoE2 has 50- to 100-times weaker affinity to LDL receptor.[48] Nevertheless, a large meta-analysis shows that E2 carriers have a 20% lower risk of coronary heart disease compared with E3 or E4 carriers.[35] Therefore, mechanisms of gene variation on health effect are complex and need to be further clarified. At this stage, we can only speculate that it might be caused via interaction of fine particles with lipid receptors or that high cholesterol or triglyceride may probably influence the absorption of metal ion.
We note that genes and their protein products are pleiotropic, and the effects of these genetic variants on HRV and the autonomic nervous system may be independent from their effects on placque formation and myocardial infarction. In this respect, while on the surface these results may seem counter-intuitive, without research which directly addresses this question one cannot be sure. For example, APOE receptors are mediators of synapse formation in neuronal tissue, a function which may be related to their effects on brain development, but this function is independent of effects on arterial cholesterol plaques. Indeed, several groups have reported that APOE4 variants are associated with improved cognitive function in children, a finding which is opposite to effects seen in adults.[72–74] Thus, unexpected findings for these variants have been reported before for other health effects. We believe these data demonstrate that the function of these receptors on HRV deserves further study.
There are some limitations in this study. First, we used a single ambient monitoring site as a surrogate for recent exposure to PM2.5, which might result in exposure misclassification. The extent of errors depends on the spatial homogeneity of the exposure because we only contrasted exposures with temporality. A recent study comparing ambient concentrations in this area with personal exposures in Boston has shown high longitudinal correlations between two measurements.[75] They reported that PM2.5 was homogeneous over the Boston area. This suggests that the use of ambient monitoring measurements is reasonable and the resulting exposure error is likely to be nondifferential. Second, the sample size was not large enough, especially for small frequencies of the gene mutations, such as LPL-N291S, LPL-D9N (Table 2), and therefore, attention should be paid to the interpretations of the present studies. Finally, this study was conducted in an aged population that consists of older men, of which white are dominant. Therefore, results are difficult to be generalized to other populations.
In conclusion, this study found evidence that variations of lipid-related genes modified associations between PM2.5 and HRV. In general, Exposure to PM2.5 appeared to have stronger risks on SDNN, LF and HF among subjects carrying wild types of APOE, LPL and VEGF comparing to those carrying unfavorable variants of genes related to lipid metabolism. Significant effect modifications were found in LPL-N291S, LPL-D9N, APOE-G113C and APOE-A491T. The mechanism of such modification remains to be clarified.
Supplementary Material
What this paper adds?
What is already known on this subject?
Many studies have shown that particulate air pollution is associated with cardiovascular diseases but the mechanism remains to be clarified. One of suggested mechanistic pathways is related to the formation of atheromatous plaques in which metabolisms of lipid and cholesterol play a vital role and are regulated many enzymes and protein. Lipid mechanism-related gene variations may influence the functions of related enzymes but few study have examined whether the genetic polymorphisms modify the effects of the exposure to particulate matter on heart disease.
What does this study add?
We examined whether variations of a set of lipid-related genes modified the associations between heart rate variability (HRV) and exposure to PM2.5. Results show that carriers of wild types of apolipoprotein E, lipoprotein lipase and vascular endothelial growth factor genes had stronger effects of particles on HRV compared to those with hetero- or homozygous types. It implies that lipid-related gene variations may be involved into the mechanistic pathways between particle exposure and heart disease. The further mechanisms need to be clarified.
Acknowledgement
This study was supported by the U.S. Environmental Protection Agency grants EPA R827353 and R832416; National Institute of Environmental Health Sciences (NIEHS) grants RO1-ES015172, ES00002, PO1_ES008925, ES05257, P42-ES05947, ES-014663 and ES10798. The VA Normative Aging Study is supported by the Cooperative Studies Program/Epidemiology Research and Information Center of the U.S. Department of Veterans Affairs and is a component of the Massachusetts Veterans Epidemiology Research and Information Center, Boston, Massachusetts.
Footnotes
License Statement
"The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive license (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in JECH editions and any other BMJPGL products to exploit all subsidiary rights, as set out in our license (http://jech.bmj.com/ifora/licence.pdf)".
Contributor Information
Cizao Ren, Exposure, Epidemiology, and Risk Program, Harvard School of Public Health, Boston, MA, USA.
Andrea Baccarelli, Center of Molecular and Genetic Epidemiology, Department of Environmental and Occupational Health, Maggiore Hospital Policlinico, Mangiagalli and Regina Elena IRCCS Foundation, University of Milan, Italy.
Elissa Wilker, Environmental and Occupational Medicine and Epidemiology Program, Harvard School of Public Health, Boston, MA, USA.
Helen Suh, Exposure, Epidemiology, and Risk Program, Harvard School of Public Health, Boston, MA, USA.
David Sparrow, VA Normative Aging Study, Veterans Affairs Boston Healthcare System and the Department of Medicine, Boston University School of Medicine, Boston, MA, USA.
Pantel Vokonas, VA Normative Aging Study, Veterans Affairs Boston Healthcare System and the Department of Medicine, Boston University School of Medicine, Boston, MA, USA.
Robert Wright, Department of Pediatrics, Children’s Hospital, Boston and Department of Environmental Health, Harvard School of Public Health, Boston, MA, USA.
Joel Schwartz, Exposure, Epidemiology, and Risk Program, Harvard School of Public Health, Boston, MA, USA.
References
- 1.Schwartz J, Morris R. Air pollution and hospital admissions for cardiovascular disease in Detroit, Michigan. Am J Epidemiol. 1995;142:23–35. doi: 10.1093/oxfordjournals.aje.a117541. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz J. Air pollution and hospital admissions for heart disease in eight U.S. counties. Epidemiology. 1999;10:17–22. [PubMed] [Google Scholar]
- 3.Samet JM, Dominici F, Curriero FC, Coursac I, Zeger SL. Fine particulate air pollution and mortality in 20 U.S. cities, 1987–1994. N Engl J Med. 2000;343:1742–1749. doi: 10.1056/NEJM200012143432401. [DOI] [PubMed] [Google Scholar]
- 4.Pope CA, III, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, Thurston GD. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287:1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ren C, Tong S. Temperature modifies the health effects of particulate matter in Brisbane, Australia. Int J Biometeorol. 2006;51:87–96. doi: 10.1007/s00484-006-0054-7. [DOI] [PubMed] [Google Scholar]
- 6.Laden F, Schwartz J, Speizer FE, Dockery DW. Reduction in fine particulate air pollution and mortality -- extended follow-up of the Harvard six cities study. Am J Repir Crit Care Med. 2006;173:667–672. doi: 10.1164/rccm.200503-443OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz J. What are people dying of on high air pollution days? Environ Res. 1994;64:26–35. doi: 10.1006/enrs.1994.1004. [DOI] [PubMed] [Google Scholar]
- 8.Peters A, Dockery D, Muller JE, Mittleman MA. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001;103:2810–2815. doi: 10.1161/01.cir.103.23.2810. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan J, Sheppard L, Schreuder A, Ishikawa N, Siscovick D, Kaufman J. Relation between short-term fine-particulate matter and onset of myocardial infarction. Epidemiology. 2005;16:41–48. doi: 10.1097/01.ede.0000147116.34813.56. [DOI] [PubMed] [Google Scholar]
- 10.Forastiere F, Stafoggia M, Picciotto S, Bellander T, D'Ippoliti D, Lanki T, Klot SV, Nyberg F, Paatero P, Peters A, Pekkanen J, Sunyer J, Perucci CA. A Case-Crossover Analysis of Out-of-Hospital Coronary Deaths and Air Pollution in Rome, Italy. Am J Respir Crit Care Med. 2005;172:1549–1555. doi: 10.1164/rccm.200412-1726OC. [DOI] [PubMed] [Google Scholar]
- 11.Zanobetti A, Schwartz J. The effect of particulate air pollution on emergency admissions for myocardial infarction: a multi-city case-crossover analysis. Environ Health Perspect. 2005;113:978–982. doi: 10.1289/ehp.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rich DQ, Schwartz J, Mittleman MA, Link M, Luttmann-Gibson H, Catalano PJ, Speizer FE, Dockery DW. Association of short-term ambient air pollution concentrations and ventricular arrhythmias. Am J Epidemiol. 2005;161:1123–1132. doi: 10.1093/aje/kwi143. [DOI] [PubMed] [Google Scholar]
- 13.Rich DQ, Kim MH, Turner JR, Mittleman MA, Schwartz J, Catalano PJ, Dockery DW. Association of ventricular arrhythmias detected by implantable cardioverter defibrillator and ambient air pollutants in the St Louis, Missouri metropolitan area. Occup Environ Med. 2006;63(9):591–596. doi: 10.1136/oem.2005.023457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsui H, Larson MG, Venditti FJ, Jr, Manders ES, Evans JC, Feldman CL, Levy D. Impact of reduced heart rate variability on risk for cardiac events: the Framingham Heart Study. Circulation. 1996;94:2850–2855. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- 15.Bigger JT, Jr, Fleiss JL, Steiman RC, Rolnitzky LM, Kleiger RE, Rottman JN. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation. 1992;85:164–171. doi: 10.1161/01.cir.85.1.164. [DOI] [PubMed] [Google Scholar]
- 16.Rovere DTL, Bigger JT, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet. 1998;351:478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 17.Zuanetti G, Neilson JM, Latini R, Santoro E, Maggioni A, Ewing DJ. Prognostic Significance of Heart Rate Variability in Post–Myocardial Infarction Patients in the Fibrinolytic Era: The GISSI-2 Results. Circulation. 1996;94:432–436. doi: 10.1161/01.cir.94.3.432. [DOI] [PubMed] [Google Scholar]
- 18.Baccarelli A, Gassano PA, Litonjua A, Park SK, Suh H, Sparrow D, Vokonas P, Schwartz J. Cardiac autonomic dysfunction: effects from particulate air pollution and protection by dietary methyl nutrients and metabolic polymorphisms. Circulation. 2008;117:1802–1809. doi: 10.1161/CIRCULATIONAHA.107.726067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devlin RB, Ghio AJ, Kehrl H, Sanders G, Cascio W. Elderly humans exposed to concentrated air pollution particles have decreased heart rate variability. Eur Respir J (Suppl) 2003;40:76s–80s. doi: 10.1183/09031936.03.00402403. [DOI] [PubMed] [Google Scholar]
- 20.Gold DR, Litonjua A, Schwartz J, Lovett E, Larson A, Nearing B, Allen G, Verrier M, Cherry R, Verrier R. Ambient pollution and heart rate variability. Circulation. 2000;101:1267–1273. doi: 10.1161/01.cir.101.11.1267. [DOI] [PubMed] [Google Scholar]
- 21.Pope CA, III, Hansen ML, Long RW, Nielsen KR, Eatough NL, Wilson WE, Eatough DJ. Ambient particulate air pollution, heart rate variability, and blood markers of inflammation in a panel of elderly subjects. Environ Health Perspect. 2004;112:339–345. doi: 10.1289/ehp.6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz J, Litonjua A, Suh H, Verrier M, Zanobetti A, Syring M, Nearing B, Verrier R, Stone P, MacCallum G, Speizer FE, Gold DR. Traffic related pollution and heart rate variability in a panel of elderly subjects. Thorax. 2005;60:455–461. doi: 10.1136/thx.2004.024836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC, Jr, Tager I. Air pollution and cardiovascular disease: a statement for healthcare professionals from the expert panel on population and prevention science of the American Heart Association. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- 24.Donaldson K, Stone V, Seaton A, MacNee W. Ambient particle inhalation and the cardiovascular system: potential mechanisms. Environ Health Perspect. 2001;109(suppl 4):523–527. doi: 10.1289/ehp.01109s4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Utell MJ, Frampton MW. Cardiovascular effects associated with air pollution: potential mechanisms and methods of testing. Inhal Toxicol. 2002;14:1231–1247. doi: 10.1080/08958370290084881. [DOI] [PubMed] [Google Scholar]
- 26.Eller NH, Malmberg B, Bruhn P. Heart rate variability and intima media thickness. Int J Behav Med. 2006;13:201–213. doi: 10.1207/s15327558ijbm1303_3. [DOI] [PubMed] [Google Scholar]
- 27.Gottsäter A, Ahlgren AR, Taimour S, Sundkvist G. Decreased heart rate variability may predict the progression of carotid atherosclerosis in type 2 diabetes. Clin Auton Res. 2006;16:228–234. doi: 10.1007/s10286-006-0345-4. [DOI] [PubMed] [Google Scholar]
- 28.Huikuri HV, Jokinen V, Syvänne M, Nieminen MS, Airaksinen KEJ, Ikäheimo MJ, Koistinen JM, Kauma H, Kesäniemi AY, Majahalme S, Niemelä KO, Frick MH. Heart rate variability and progression of coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 1999;19:1979–1985. doi: 10.1161/01.atv.19.8.1979. [DOI] [PubMed] [Google Scholar]
- 29.Nagata K, Sasaki E, Goda K, Yamamoto N, Sugino M, Yamamoto K, Narabayashi I, Hanafusa T. Differences in heart rate variability in non-hypertensive diabetic patients correlate with the presence of underlying cerebrovascular disease. Clin Physiol Funct Imaging. 2006;26:92–98. doi: 10.1111/j.1475-097X.2006.00654.x. [DOI] [PubMed] [Google Scholar]
- 30.Sajadieh A, Nielsen OW, Rasmussen V, Hein HO, Abedini S, Hansen JF. Increased heart rate and reduced heart-rate variability are associated with subclinical inflammation in middle-aged and elderly subjects with no apparent heart disease. Eur Heart J. 2004;25:363–370. doi: 10.1016/j.ehj.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Sloan RP, McCreath H, Tracey KJ, Sidney S, Liu K, Seeman T. RR interval variability is inversely related to inflammatory markers: the CARDIA study. Mol Med. 2007;13:178–184. doi: 10.2119/2006-00112.Sloan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suwa T, Hogg JC, Quinlan KB, Ohgami A, Vincent R, van Eeden SF. Particulate air pollution induces progression of atherosclerosis. J Am Coll Cardiol. 2002;39(6):935–942. doi: 10.1016/s0735-1097(02)01715-1. [DOI] [PubMed] [Google Scholar]
- 33.Malcom GT, McMahan CA, McGill HC, Herderick EE, Tracy RE, Troxclair DA, Strong JP. Associations of arterial tissue lipids with coronary heart disease risk factors in young people. Atherosclerosis. 2008;203:515–521. doi: 10.1016/j.atherosclerosis.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 34.Steinberg D. The pathogenesis of atherosclerosis. An interpretive history of the cholesterol controversy, part IV: the 1984 coronary primary prevention trial ends it-almost. J Lipid Res. 2006;47:1–14. doi: 10.1194/jlr.R500014-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Bennet AM, Angelantonio ED, Ye Z, Wensley F, Dahlin A, Ahlbom A, Keavney B, Collins R, Wiman B, Faire U, Danesh J. Association of apolipoprotein E genotypes with lipid levels and coronary risk. JAMA. 2007;298:1300–1311. doi: 10.1001/jama.298.11.1300. [DOI] [PubMed] [Google Scholar]
- 36.Bersano A, Ballabio E, Bresolin N, Candelise L. Genetic polymorphisms for the study of multifactorial stroke. Hum Mutat. 2008;29:776–795. doi: 10.1002/humu.20666. [DOI] [PubMed] [Google Scholar]
- 37.Noh H, Yamashita H, Goldberg IJ. Cardiac metabolism and mechanics are altered by genetic loss of lipoprotein triglyceride lipolysis. Cardiovasc Drugs Ther. 2006;20:441–444. doi: 10.1007/s10557-006-0633-1. [DOI] [PubMed] [Google Scholar]
- 38.Talmud PJ, Stephens JW. Lipoprotein lipase gene variants and the effect of environmental factors on cardiovascular disease risk. Diabetes Obes Metab. 2004;6:1–7. doi: 10.1111/j.1463-1326.2004.00304.x. [DOI] [PubMed] [Google Scholar]
- 39.Al-Kateb H, Mirea L, Xie X, Sun L, Liu M, Chen H, Bull SB, Boright AP, Paterson AD. Multiple variants in vascular endothelia growth factor (VEGFA) are risk factors for time to severe retinopathy in type 1 diabetes: the DCCT/EDIC genetics study. Diabetes. 2007;56:2161–2168. doi: 10.2337/db07-0376. [DOI] [PubMed] [Google Scholar]
- 40.Churchill AJ, Carter JG, Ramsden C, Turner SJ, Yeung A, Brenchley PE, Ray DW. VEGF polymorphisms are associated with severity of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2008;49:3611–3616. doi: 10.1167/iovs.07-1383. [DOI] [PubMed] [Google Scholar]
- 41.Howell WM, Ali S, Rose-Zerilli MJ, Ye S. VEGF polymorphisms and severity of atherosclerosis. J Med Genet. 2005;42:485–490. doi: 10.1136/jmg.2004.025734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bell B, Rose C, Damon A. The normative aging study: an interdisciplinary and longitudinal study of health and aging. Aging Human Develop. 1972;3:4–17. [Google Scholar]
- 43.Park SK, O’Neill MS, Vokonas PS, Sparrow D, Schwartz J. Effects of air pollution on heart rate variability: the VA Normative Aging Study. Environ Health Perspect. 2005;113:304–309. doi: 10.1289/ehp.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun X, Ding H, Hung K, Guo B, Journal O. A new MALDI-TOF based mini-sequencing assay for genotyping of SNPS. Nucleic Acids Research. 2000;28:e68. doi: 10.1093/nar/28.12.e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee LG, Connell CR, Bloch W. Allelic discrimination by nick-translation PCR with luorogenic probes. Nucleic Acids Res. 1993;21:3761–3766. doi: 10.1093/nar/21.16.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwartz J, Park SK, O’Neill MS, Vokonas PS, Sparrow D, Weiss S, Kelsey Glutathione-S-transferase M1, obesity, statins, and autonomic effects of particles. Am J Respir Crit Care Med. 2005;172:1529–1533. doi: 10.1164/rccm.200412-1698OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hatters DM, Peters-Libeu CA, Weisgraber KH. Apolipoprotein E structure: insights into function. Trends Biochem Sci. 2006;31:445–454. doi: 10.1016/j.tibs.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 48.Weisgraber KH, Innerarity TL, Mahley RW. Abnormal lipoprotein receptor-biding activity of the human E apoprotein due to cysteine-arginine interchange at a single site. J Biol Chem. 1982;257:2518–2521. [PubMed] [Google Scholar]
- 49.Eichner JE, Dunn ST, Perveen G, Thompson DM, Stewart KE, Stroehla BC. Apolipoprotein E polymorphism and cardiovascular disease: a HuGE review. Am J Epidemiol. 2002;155:487–495. doi: 10.1093/aje/155.6.487. [DOI] [PubMed] [Google Scholar]
- 50.Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 51.Utermann G, Langenbeck U, Beisiegel U, Weber W. Genetics of the apolipoprotein E system in man. Am J Hum Genet. 1980;32(3):339–347. [PMC free article] [PubMed] [Google Scholar]
- 52.Andreotti G, Chen J, Gao Y, Rashid A, Chen BE, Rosenberg P, Sakoda LC, Deng J, Shen M, Wang B, Han T, Zhang B, Yeager M, Welch R, Chanock S, Fraumeni JF, Jr, Hsing AW. Polymorphisms of genes in the lipid metabolism pathway and risk of biliary tract cancers and stones: a population-based case-control study in Shanghai, China. Cancer Epidemiol Biomarkers Prev. 2008;17(3):525–534. doi: 10.1158/1055-9965.EPI-07-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paternoster L, González NAM, Lewis S, Sudlow C. Association between apolipoprotein E genotype and carotid intima-media thickness may suggest a specific effect on large artery atherothrombotic stroke. Stroke. 2008;39:48–54. doi: 10.1161/STROKEAHA.107.488866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stoll G, Bendszus M. Inflammation and atherosclerosis: novel insight into plaque formation and destabilization. Stroke. 2006;37:1923–1932. doi: 10.1161/01.STR.0000226901.34927.10. [DOI] [PubMed] [Google Scholar]
- 55.Tikellis C, Jandeleit-Dahm KA, Sheehy K, Murphy A, Chin-Dusting J, Kling D, Sebokova E, Cooper ME, Mizrahi J, Woollard KJ. Reduced plaque formation induced by rosglitazone in an STZ-diabetes mouse model of atherosclerosis is associated with downregulation of adhesion molecules. Atherosclerosis. 2008;199:55–64. doi: 10.1016/j.atherosclerosis.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 56.Chappell DA, Inoue I, Fry GL, Pladet MW, Bowen SL, Iverius PH, Lalouel J, Strickland DK. Cellular catabolism of normal very low density lipoproteins via the low density lipoprotein receptor-related protein/α2-macroglobulin receptor is induced by the C-terminal domain of lipoprotein lipase. J Biol Chem. 1994;269:18001–18006. [PubMed] [Google Scholar]
- 57.Zheng C, Murdoch SJ, Brunzell JD, Sacks FM. Lipoprotein Lipase Bound to Apolipoprotein B Lipoproteins Accelerates Clearance of Postprandial Lipoproteins in Humans. Arterioscler Thromb Vasc Biol. 2006;26:891–896. doi: 10.1161/01.ATV.0000203512.01007.3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wittrup HH, Tybjaerg-Hansen A, Nordestgaard BG. Lipoprotein lipase mutations, plasma lipids and lipoproteins, and risk of ischemic heart disease: a meta-analysis. Circulation. 1999;99:2901–2907. doi: 10.1161/01.cir.99.22.2901. [DOI] [PubMed] [Google Scholar]
- 59.Mailly F, Tugrul Y, Reymer PW, Bruin T, Seed M, Groenemeyer BF, Asplund-Carlson A, Vallance D, Winder AF, Miller GJ, Kastelein J, Hamsten A, Olivecrona G, Humphries SE, Talmud PJ. A common variant in the gene for lipoprotein lipase (Asp9->Asn): Functional implications and prevalence in normal and hyperlipidemic subjects. Arterioscler Thromb Vasc Biol. 1995;15:468–478. doi: 10.1161/01.atv.15.4.468. [DOI] [PubMed] [Google Scholar]
- 60.Reymer PW, Gagne E, Groenemeyer BE, Zhang H, Forsyth I, Jansen H, Seidell JC, Krombout D, Lie KE, Kastelein J, Hayden MR. A lipoprotein lipase mutation (Asn291Ser) is associated with reduced HDL cholesterol levels in premature atherosclerosis. Nat Genet. 1995;10:28–34. doi: 10.1038/ng0595-28. [DOI] [PubMed] [Google Scholar]
- 61.Fisher RM, Humphries SE, Talmud PJ. Common variation in the lipoprotein lipase gene: effects on plasma lipids and risk of atherosclerosis. Atherosclerosis. 1997;135:145–159. doi: 10.1016/s0021-9150(97)00199-8. [DOI] [PubMed] [Google Scholar]
- 62.Hu Y, Liu W, Huang R, Zhang X. A systematic review and meta-analysis of the relationship between lipoprotein lipase Asn291Ser variant and diseases. J Lipid Res. 2006;47:1908–1914. doi: 10.1194/jlr.M600108-JLR200. [DOI] [PubMed] [Google Scholar]
- 63.Rip J, Nierman MC, Ross CJ, Jukema JW, Hayden MR, Kastelein JJ, Stroes ES, Kuivenhoven JA. Lipoprotein lipase S447X: a naturally occurring gain-of-function mutation. Arterioscler Thromb Vasc Biol. 2006;26:1236–1245. doi: 10.1161/01.ATV.0000219283.10832.43. [DOI] [PubMed] [Google Scholar]
- 64.Talmud PJ, Bujac SR, Hall S, Miller GJ, Humphries SE. Substitution of asparagines for aspartic acid at residue 9 (D9N) of lipoprotein lipase markedly augments risk of ischaemic heart disease in male smokers. Atherosclerosis. 2000;149:75–81. doi: 10.1016/s0021-9150(99)00309-3. [DOI] [PubMed] [Google Scholar]
- 65.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 66.Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 67.Leung DW, Cachianes G, Kuang W, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 68.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 69.Celletti FL, Waugh JM, Amabile PG, Brendolan A, Hilfiker PR, Dake MD. Vascular endothelial growth factor enhances atherosclerotic plaque progression. Nat Med. 2001;7:425–429. doi: 10.1038/86490. [DOI] [PubMed] [Google Scholar]
- 70.Chahine T, Baccarelli A, Litonjua A, Wright RO, Suh H, Gold DR, Sparrow D, Vokonas, Schwartz J. Particulate air pollution, Oxidative stress genes, and heart rate variability in an elderly cohort. Environ Health Perspect. 2007;115:1617–1622. doi: 10.1289/ehp.10318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Park SK, O’Neill MS, Wright RO, Hu H, Vokonas PS, Sparrow D, Suh H, Schwartz J. HFE genotype, particulate air pollution, and heart rate variability: a gene-environment interaction. Circulation. 2006;114:2798–2805. doi: 10.1161/CIRCULATIONAHA.106.643197. [DOI] [PubMed] [Google Scholar]
- 72.Wright RO, Hu H, Silverman EK, Tsaih SW, Schwartz J, Bellinger D, Palazuelos E, Weiss ST, Hernandex-Avila M. Apolipoprotein E genetype predicts 24-month Bayley scales infant development score. Pediatr Res. 2003;54:819–825. doi: 10.1203/01.PDR.0000090927.53818.DE. [DOI] [PubMed] [Google Scholar]
- 73.Puttonen S, Elovainio M, Kivimäki M, Lehtimäki T, Keltikangas-Jarvinen L. The combined effects of apolipoprotein E polymorphism and low-density lipoprotein cholesterol on cognitive performance in young adults. Neuropsychobiology. 2003;48:35–40. doi: 10.1159/000071827. [DOI] [PubMed] [Google Scholar]
- 74.Oriá RB, Patrick PD, Zhang H, Lorntz B, de Castro Costa CM, Brito GA, Barrett LJ, Lima AA, Guerrant RL. APOE 4 protects the cognitive development in children with heavy diarrhea burdens in northeast Brazil. Pediatr Res. 2005;57:310–316. doi: 10.1203/01.PDR.0000148719.82468.CA. [DOI] [PubMed] [Google Scholar]
- 75.Sarnat JA, Brown KW, Schwartz J, Coull BA, Koutrakis P. Ambient Gas Concentrations and Personal Particulate Matter Exposures: Implications for Studying the Health Effects of Particles. Epidemiology. 2005;16:385–395. doi: 10.1097/01.ede.0000155505.04775.33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.