Abstract
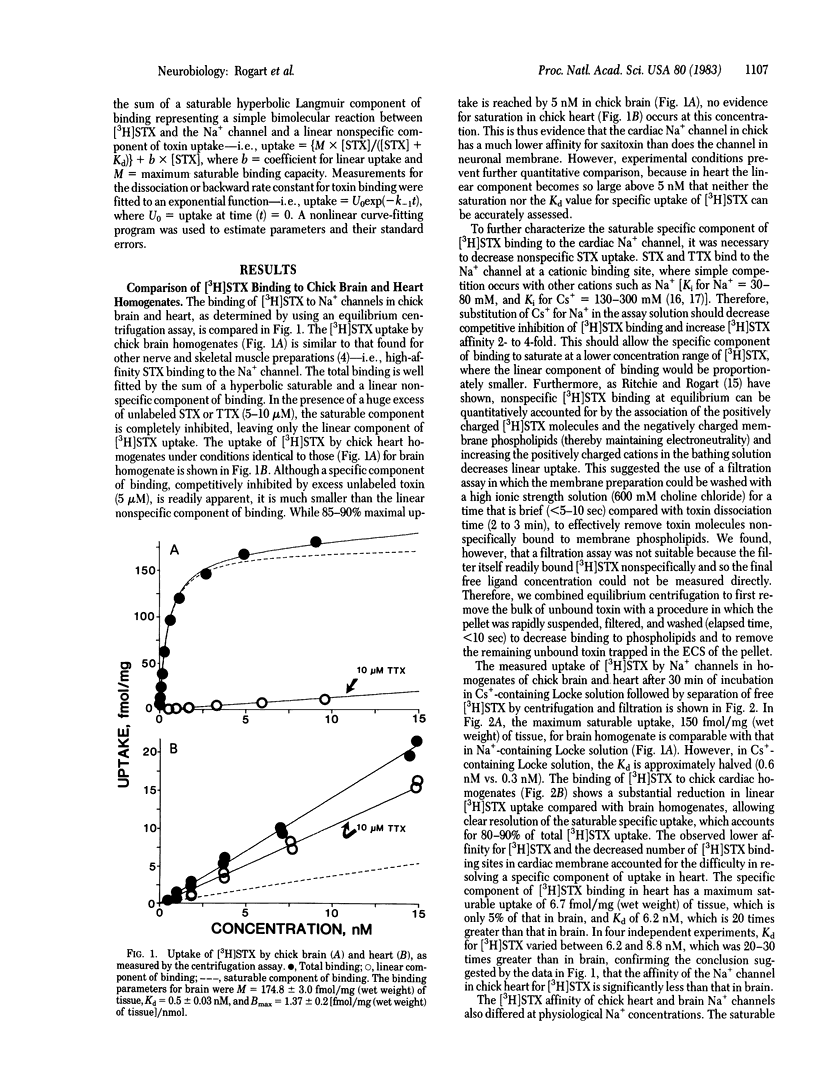
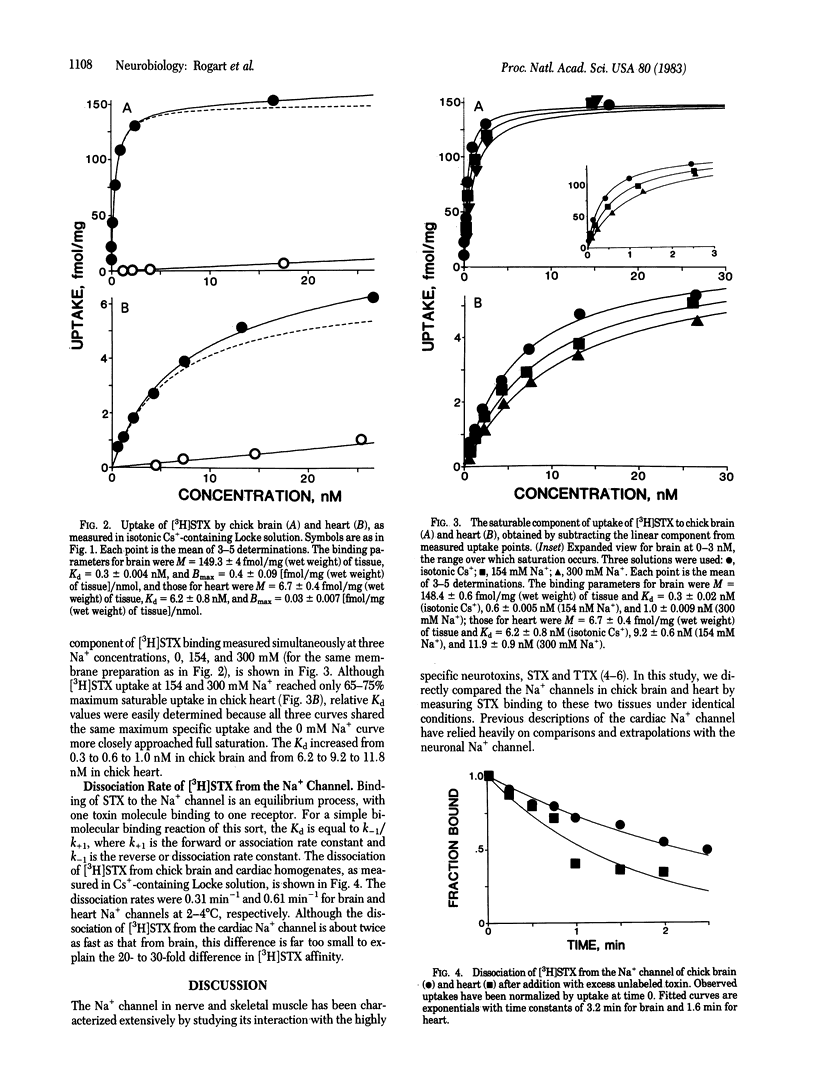
Na+ channels in chick brain and heart have been directly compared by measuring binding of tritium-labeled saxitoxin ([3H]STX) to the two tissues under identical conditions. Maximum saturable uptake and toxin affinity were considerably less in chick heart than in chick brain, requiring the development of an assay method to resolve specific [3H]STX uptake in heart. With this method, binding to both preparations consisted of a specific saturable component and a linear nonspecific component. The equilibrium dissociation constant for [3H]STX measured in chick heart (6.2-8.8 nM) was 20-30 times higher than that measured in chick brain (0.3 nM). The dissociation rate for [3H]STX was only about twice as fast in heart as it was in brain, indicating that the decrease in toxin affinity in heart results predominantly from a slowed toxin association rate. The decreased affinity for [3H]STX found at the chick heart Na+ channel is compared with toxin-resistant Na+ channels in other preparations. The existence of two Na+ channel subtypes is proposed, with high affinity and low affinity for saxitoxin and tetrodotoxin; the significance of this classification is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antman E. M., Stone P. H., Muller J. E., Braunwald E. Calcium channel blocking agents in the treatment of cardiovascular disorders. Part I: Basic and clinical electrophysiologic effects. Ann Intern Med. 1980 Dec;93(6):875–885. doi: 10.7326/0003-4819-93-6-875. [DOI] [PubMed] [Google Scholar]
- Baer M., Best P. M., Reuter H. Voltage-dependent action of tetrodotoxin in mammalian cardiac muscle. Nature. 1976 Sep 23;263(5575):344–345. doi: 10.1038/263344a0. [DOI] [PubMed] [Google Scholar]
- Barchi R. L., Weigele J. B. Characteristics of saxitoxin binding to the sodium channel of sarcolemma isolated from rat skeletal muscle. J Physiol. 1979 Oct;295:383–396. doi: 10.1113/jphysiol.1979.sp012975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A. Activation and inhibition of the action potential Na+ ionophore of cultured rat muscle cells by neurotoxins. Biochem Biophys Res Commun. 1976 Jan 12;68(1):136–142. doi: 10.1016/0006-291x(76)90020-6. [DOI] [PubMed] [Google Scholar]
- Catterall W. A. Inhibition of voltage-sensitive sodium channels in neuroblastoma cells by antiarrhythmic drugs. Mol Pharmacol. 1981 Sep;20(2):356–362. [PubMed] [Google Scholar]
- Catterall W. A. Neurotoxins that act on voltage-sensitive sodium channels in excitable membranes. Annu Rev Pharmacol Toxicol. 1980;20:15–43. doi: 10.1146/annurev.pa.20.040180.000311. [DOI] [PubMed] [Google Scholar]
- Cohen C. J., Bean B. P., Colatsky T. J., Tsien R. W. Tetrodotoxin block of sodium channels in rabbit Purkinje fibers. Interactions between toxin binding and channel gating. J Gen Physiol. 1981 Oct;78(4):383–411. doi: 10.1085/jgp.78.4.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couraud F., Rochat H., Lissitzky S. Binding of scorpion neurotoxins to chick embryonic heart cells in culture and relationship to calcium uptake and membrane potential. Biochemistry. 1980 Feb 5;19(3):457–462. doi: 10.1021/bi00544a009. [DOI] [PubMed] [Google Scholar]
- Fosset M., De Barry J., Lenoir M. C., Lazdunski M. Analysis of molecular aspects of Na+ and Ca2+ uptakes by embryonic cardiac cells in culture. J Biol Chem. 1977 Sep 10;252(17):6112–6117. [PubMed] [Google Scholar]
- Harris J. B., Thesleff S. Studies on tetrodotoxin resistant action potentials in denervated skeletal muscle. Acta Physiol Scand. 1971 Nov;83(3):382–388. doi: 10.1111/j.1748-1716.1971.tb05091.x. [DOI] [PubMed] [Google Scholar]
- Hondeghem L. M., Katzung B. G. Time- and voltage-dependent interactions of antiarrhythmic drugs with cardiac sodium channels. Biochim Biophys Acta. 1977 Nov 14;472(3-4):373–398. doi: 10.1016/0304-4157(77)90003-x. [DOI] [PubMed] [Google Scholar]
- Hondeghem L. M. Validity of Vmax as a measure of the sodium current in cardiac and nervous tissues. Biophys J. 1978 Jul;23(1):147–152. doi: 10.1016/S0006-3495(78)85439-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima T., Pappano A. J. Ontogenetic increase of the maximal rate of rise of the chick embryonic heart action potential. Relationship to voltage, time, and tetrodotoxin. Circ Res. 1979 Mar;44(3):358–367. doi: 10.1161/01.res.44.3.358. [DOI] [PubMed] [Google Scholar]
- Jover E., Martin-Moutot N., Couraud F., Rochat H. Binding of scorpion toxins to rat brain synaptosomal fraction. Effects of membrane potential, ions, and other neurotoxins. Biochemistry. 1980 Feb 5;19(3):463–467. doi: 10.1021/bi00544a010. [DOI] [PubMed] [Google Scholar]
- Lombet A., Renaud J. F., Chicheportiche R., Lazdunski M. A cardiac tetrodotoxin binding component: biochemical identification, characterization, and properties. Biochemistry. 1981 Mar 3;20(5):1279–1285. doi: 10.1021/bi00508a036. [DOI] [PubMed] [Google Scholar]
- Marcus N. C., Fozzard H. Tetrodotoxin sensitivity in the developing and adult chick heart. J Mol Cell Cardiol. 1981 Mar;13(3):335–340. doi: 10.1016/0022-2828(81)90322-9. [DOI] [PubMed] [Google Scholar]
- McCammon J. A., Northrup S. H. Gated binding of ligands to proteins. Nature. 1981 Sep 24;293(5830):316–317. doi: 10.1038/293316a0. [DOI] [PubMed] [Google Scholar]
- Minneman K. P., Molinoff P. B. Classification and quantitation of beta-adrenergic receptor subtypes. Biochem Pharmacol. 1980 May 15;29(10):1317–1323. doi: 10.1016/0006-2952(80)90424-4. [DOI] [PubMed] [Google Scholar]
- Minneman K. P., Pittman R. N., Molinoff P. B. Beta-adrenergic receptor subtypes: properties, distribution, and regulation. Annu Rev Neurosci. 1981;4:419–461. doi: 10.1146/annurev.ne.04.030181.002223. [DOI] [PubMed] [Google Scholar]
- Pappone P. A. Voltage-clamp experiments in normal and denervated mammalian skeletal muscle fibres. J Physiol. 1980 Sep;306:377–410. doi: 10.1113/jphysiol.1980.sp013403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J. K., Raftery M. A. Properties of the tetrodotoxin binding component in plasma membranes isolated from Electrophorus electricus. Biochemistry. 1976 Mar 9;15(5):944–953. doi: 10.1021/bi00650a002. [DOI] [PubMed] [Google Scholar]
- Ritchie J. M., Rogart R. B. Characterization of exchange-labeled saxitoxin and the origin of linear uptake by excitable tissue. Mol Pharmacol. 1977 Nov;13(6):1136–1146. [PubMed] [Google Scholar]
- Ritchie J. M., Rogart R. B., Strichartz G. R. A new method for labelling saxitoxin and its binding to non-myelinated fibres of the rabbit vagus, lobster walking leg, and garfish olfactory nerves. J Physiol. 1976 Oct;261(2):477–494. doi: 10.1113/jphysiol.1976.sp011569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie J. M., Rogart R. B. The binding of labelled saxitoxin to the sodium channels in normal and denervated mammalian muscle, and in amphibian muscle. J Physiol. 1977 Jul;269(2):341–354. doi: 10.1113/jphysiol.1977.sp011905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie J. M., Rogart R. B. The binding of saxitoxin and tetrodotoxin to excitable tissue. Rev Physiol Biochem Pharmacol. 1977;79:1–50. doi: 10.1007/BFb0037088. [DOI] [PubMed] [Google Scholar]
- Rogart R. Sodium channels in nerve and muscle membrane. Annu Rev Physiol. 1981;43:711–725. doi: 10.1146/annurev.ph.43.030181.003431. [DOI] [PubMed] [Google Scholar]
- Singh B. N., Collett J. T., Chew C. Y. New perspectives in the pharmacologic therapy of cardiac arrhythmias. Prog Cardiovasc Dis. 1980 Jan-Feb;22(4):243–301. doi: 10.1016/0033-0620(80)90011-0. [DOI] [PubMed] [Google Scholar]
- Strichartz G., Cohen I. Vmax as a measure of GNa in nerve and cardiac membranes. Biophys J. 1978 Jul;23(1):153–156. doi: 10.1016/S0006-3495(78)85440-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton M., Fozzard H. A. The relation of Vmax to INa, GNa, and h infinity in a model of the cardiac Purkinje fiber. Biophys J. 1979 Mar;25(3):407–420. doi: 10.1016/S0006-3495(79)85312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]



