Abstract
Modeling integrated human physiology in vitro is a formidable task not yet achieved with any of the existing cell/tissue systems. However, tissue engineering is becoming increasingly successful at authentic representation of the actual environmental milieu of tissue development, regeneration and disease progression, and in providing real-time insights into morphogenic events. Functional human tissue units engineered to combine biological fidelity with the high-throughput screening and real-time measurement of physiological responses are poised to transform drug screening and predictive modeling of disease. In this review, we focus on the in vitro engineering of functional human myocardium that mimics heart tissue for analysis of myocardial function, in the context of physiological studies, drug screening for therapeutics, and safety pharmacology.
During the last decade, new sources of human cardiomyocytes and biomaterial scaffolds that mimic native heart tissue have been identified. These are being used to develop in vitro models for studying human heart disease.
For decades, tissue engineering has been driven by biologists, engineers, and clinicians to meet the growing need to repair or replace tissues lost by disease, injury, or degeneration in an aging population. After some spectacular successes (Atala et al. 2006; Macchiarini et al. 2008), some failures, and the development of some promising new approaches ( Zimmermann et al. 2006; Caspi et al. 2007; Dahl et al. 2011), it is becoming clear that engineering biological substitutes of tissues, or perhaps even organs, is increasingly plausible but is still facing major hurdles. The large size of clinically useful tissues often means that immediate perfusion with blood is required. There is also a need for robust, renewable cell sources with which to make authentic constructs. Combined with complex regulatory procedures, these factors have slowed down progress toward clinical implementation although in vitro applications are now meeting success.
Engineered human tissues that are most widely used at present time are for repairing bone and other tissues of the skeleton. A range of new in vitro applications, such as safety pharmacology and creating disease models suitable for drug discovery, are also being considered, with the goal of translating the laboratory research to treatments for patients. The engineered tissue is, in this case, not a medicinal product, but the vehicle for testing treatments. In this more immediate application of tissue engineering, the tissues are small enough to avoid diffusional constraints of oxygen supply and the regulatory requirements are only minimal. Although it is unreasonable to expect these three-dimensional (3D) tissue models to respond exactly as a native tissue, such models can recapitulate certain physiological functions and be used in early stage research to investigate the efficacy, safety, and mode of action of therapeutic agents. Instead of attempting to mimic the complexity of the whole organ, a reasonable goal would be to replicate the tissue-specific architecture so that it recapitulates a subset of most relevant physiological functions for the tissue of interest. The focus here is on tissue-engineered structures for human myocardium to model normal and disease physiology of the heart.
Potential cell sources for cells of the heart are stem cells because human cardiomyocytes differ significantly from those of rodents, most obviously in their electrophysiological properties. In addition, the mouse heart typically beats at approximately 500 times per minute (bpm) whereas the beating rate of the human heart is usually ∼60–90 bpm, human stem cells are a preferred source. Currently, there are three main sources of stem cells that can be used as a source of human cardiomyocytes: (1) human embryonic stem cells obtained from donated human blastocysts (Thomson et al. 1998), (2) human-induced pluripotent stem cells (hiPSC) obtained by reprogramming adult somatic cells by overexpressing key transcription factors (Takahashi et al. 2007), and (3) human cardiac progenitor cells derived from fetal or possibly adult heart (Goumans et al. 2007; Smits et al. 2009). All of these cell types can form cardiomyocytes in vitro either by differentiating in the presence of specific growth factors (methods reviewed in Mummery et al. 2012), coculture with a mouse endodermal cell line (Mummery et al. 2003), or treatment with 5-azacytidine and TGF-β (Goumans et al. 2007). Stem cell-derived cardiomyocytes usually show spontaneous electrical activity with cyclic contraction and measurable action potentials that are sensitive to chronotropic drugs (which change the heart rate), features typical of immature or fetal cardiomyocytes (Wobus and Boheler 2005; Davis et al. 2011). Furthermore, the progenitor cells from pluripotent cells or the heart appear to differentiate into cardiac fibroblasts and endothelial cells, also essential components of the heart and comprising up to 60% of its cellular content.
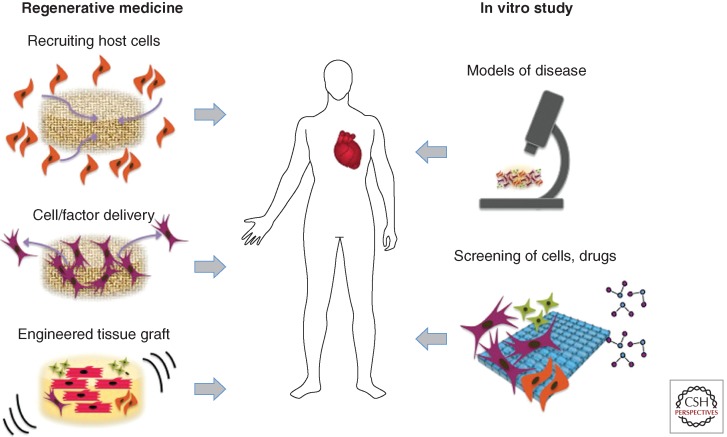
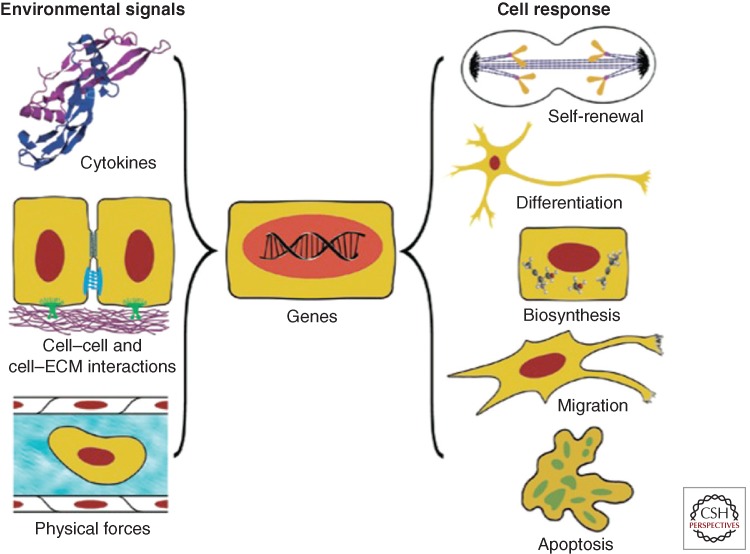
The term “myocardial tissue engineering” refers, in a broad sense, to the application of principles and methods of engineering and life sciences toward fundamental understanding of structure–function relationships in normal and pathological heart tissues and the development of biological substitutes to restore, maintain, or improve tissue function (Fig 1). Key aspects include the 3D nature of the scaffolding material, and the environmental control and provision of spatial and temporal sequences of biophysical factors via bioreactors (Fig. 2).
Figure 1.
Myocardial tissue engineering. The classical paradigm of myocardial tissue engineering involves cultivation of cells on scaffolds, with the application of molecular and physical factors (via bioreactors), for implantation. Alternatively, host cells can be recruited to the repair site by implanted scaffolds, with or without cells. More recently, myocardial tissue engineering is being increasingly used for modeling disease and in high-throughput platforms for screening of drugs and therapeutic targets.
Figure 2.
Environmental regulation of cell function. Both in vivo and in vitro, cells interact with the entire context of their environment: cytokines, surrounding cells, extracellular matrix, and physical forces (hydrodynamic, mechanical, electrical). In response to the combined effects of these factors, the cells may take a number of different fates—from differentiation to apoptosis and death. At the same time, the cells modulate their environment by secreting cytokines, remodeling the extracellular matrix, affecting the surrounding cells, and generating forces. Cardiomyocytes are an example of the cells that actively interact with their environment throughout development, adult life, as well as under pathological conditions. The designs of advanced systems for myocardial tissue engineering are “biomimetic” in nature as they recapitulate key regulatory factors, molecular and physical, acting in vivo. (From Vunjak-Novakovic and Scadden 2011; reproduced, with permission, from the author.)
A tissue-engineering paradigm for using cardiac cells involves coordinated use of biomaterial scaffolds providing a structural template for cell attachment and tissue formation, biochemical and physical regulatory signals (Vunjak-Novakovic and Scadden 2011). To use the full biological potential of the cells, the in vitro system needs to recapitulate some aspects of the native tissue milieu. This requirement for “biomimetics” is not easy to meet as cells respond to the entire context of their microenvironment, not simply to a few dominant signals (reviewed in Vunjak-Novakovic et al. 2010). For engineering cardiac muscle, scaffold requirements include an appropriate porosity (to foster cell migration and attachment, and the exchange of nutrients, most critically oxygen, and metabolites), and hierarchical structure (orientation, anisotropy, channels for vascular conduits). Ideally, a scaffold is designed to mimic the properties of native heart matrix and thereby mediate tissue formation. A scaffold needs to actively interact with the cells at multiple scales: “molecular” (incorporated ligands and growth factors), “cellular” (migration, cell–cell contacts, stiffness), and “tissue” (interfaces, structural and mechanical anisotropy) (Langer and Tirrell 2004; Lutolf and Hubbell 2005; Tibbitt and Anseth 2009).
With the recent advances in both stem cell and tissue engineering technologies, the time is now ripe to apply functional 3D myocardium from human pluripotent stem cells (hPSC) to preclinical drug development, basic research, and patient-specific disease modeling. Increasingly automated and miniaturized assay formats provide higher content than standard two-dimensional (2D) cultures, are more stable, and drive cardiomyocyte maturation toward mature heart tissue ( Hirt et al. 2014).
CELLS OF THE HEART
The adult heart is composed of at least three major cells types. Atrial and ventricular myocytes make up 30% of the heart and cardiac fibroblasts (±60%). The remaining 10% are pacemaker cells, endothelial cells/endocardium, epicardium, and pericytes, which, although only a relative minority, are essential for normal cardiac function and a major cause of disease when functionally impaired or defective. The fetal heart has proportionally more cardiomyocytes and fewer cardiac fibroblasts. Within these broad categories of cell types, there are functionally distinct subtypes in both the adult and embryonic heart. For example, cardiomyocytes can have atrial, ventricular, or pacemaker identities with distinct electrophysiological features and gene expression profiles, although it is still a matter of debate when or how these identities are specified in normal development.
The heart forms soon after gastrulation from anterior mesodermal cells that migrate between the ectoderm and endoderm cell layers in the primitive streak. Heart-forming cells (or cardiac progenitors) are primarily localized in the middle of the primitive streak. Signals from adjacent cells, in the endoderm in particular, are thought to promote the induction of cardiac mesoderm and its subsequent patterning. Three families of protein growth factors or their inhibitors identified by disruption of normal development (reviewed by Olson and Schneider 2003) are among the endoderm-derived instructive signals: bone morphogenetic proteins (BMPs), the Wnt proteins, and the fibroblast growth factors (FGFs). BMP signaling generally promotes cardiogenesis, whereas the Wnt proteins and FGFs are involved in providing positional cues for cell specification.
Overall, the timing and relative expression of different combinations of growth factors induce and pattern the cardiogenic mesoderm (reviewed in Davis et al. 2011). During anterior migration, the mesoderm receives signals that switch on a highly conserved heart-specific combination of transcription factors establishing the cardiac identity. The mesodermal precursor cells in the primitive streak express transcription factors such as the T-box factor Brachyury (T) and the homeodomain protein, Mixl1. Subsequently, these cells transiently activate the basic helix–loop–helix transcription factor mesoderm posterior 1 to enter a “precardiac” mesoderm stage of development.
A subset of these cells begins to express the homeodomain transcription factor Nkx2-5, the T-box protein Tbx5, and Isl1, all early markers of the cardiac lineage that are activated during the formation of the primary and secondary heart fields (Mjaatvedt et al. 2001; Waldo et al. 2001; Cai et al. 2003; Meilhac et al. 2004; Buckingham et al. 2005). Nkx2-5 and Tbx5 associate with members of the GATA family of transcription factors (GATA4/5/6) and serum response factor to activate cardiac structural genes such as actin, myosin light chain, myosin heavy chain, troponins, and desmin. Myocyte enhancer factor 2 family members regulate cardiac muscle structural genes.
Thus, multiple complex interactions between these highly conserved gene regulatory networks control the initial differentiation, proliferation, and maturation of cardiomyocytes although the expression of single molecular markers cannot be linked unambiguously to one cardiac progenitor cell lineage. Because of their functional roles, many of these cardiac genes can be used as markers of emerging cardiomyocytes in differentiating cultures of hPSC.
Methods for inducing stem cell differentiation are being driven by our increased understanding of the underlying developmental biology. Genetic marking and identification of the different cell subtypes and application of developmental signals in an appropriate time and concentration-dependent manner enabled derivation of many cardiac and vascular subtypes from stem cells. Today, we have various methods for their specification, selection, and sometimes expansion in culture available (reviewed in Mummery et al. 2012). These methods have moved the field significantly beyond the initial studies in which only <1% of cells in the spontaneously beating regions of cell aggregates or “embryoid bodies” were actually cardiomyocytes, to the present state of the art in which up to 80% of the cells may now be cardiomyocytes. Crucial to improving efficiency and reproducibility of differentiation methods have been (1) the use of standard aggregate size and format by using identical cell numbers in each and aggregating by centrifugation in 96-well plates of use of Aggrewells, (2) cross-titration of the principle mesoderm inducing growth factors BMP4 and activin to find optimal batch and cell line concentrations, (3) timed addition and removal of the growth factors, (4) the use of serum-free (commercial) medium formulations, and (5) small molecules to activate the Wnt signaling pathway. In addition, some methods do not require aggregation and instead derive cardiomyocytes in monolayer cultures. They use much the same combination of growth factors, but often include additional small molecules like XAV. Recent advances in efficiency are thus largely attributable to the use of defined culture media, specific growth factors, and small molecular inhibitors (Elliott et al. 2011; Kattman et al. 2011; reviewed in Mummery et al. 2012).
Whereas it is presently possible to generate atrial, ventricular, and pacemaker-like cells from hPSCs, cell differentiation into these specific phenotypes is still random and cannot be controlled in a directed fashion and under defined and reproducible conditions. Moreover, methods for generating endocardium, epicardium, and cardiac fibroblasts have not been described to date, yet these cells are crucial components of cardiac repair after damage. Exceptionally, derivation of specific subtypes of smooth muscle cells has now been described (reviewed in Majesky and Mummery 2012). Much research is presently ongoing in these areas toward deriving all cells of the heart specifically and efficiently. The success of this work is critical for enabling reconstruction of synthetic human myocardium “in a dish” for tissue engineering purposes.
One important problem with developing physiological and disease models based on cardiomyocytes derived from hPSCs is that these differentiated derivatives are very immature, for the heart perhaps no more than an equivalent of early gestation, as evidenced by the low upstroke velocities of their action potentials, irregular shape, poor sarcomere organization, and gene expression (Mummery et al. 2003). Although the cardiomyocytes derived from hPSC show many typical features of their in vivo equivalents, overt symptoms of many diseases at the organ level develop only in adults late in life (although at the cellular level, some defects may be evident earlier). Signals that induce cell maturation during development are likely to vary depending on the individual cell type. However, these signals are generally derived from the local tissue “niche,” through its spatial organization or molecular, electrical, and mechanical signals. The heart, for example, undergoes cyclic work and this is thought to contribute to cardiomyocyte maturation (Sheehy et al. 2012).
Not only do the functional phenotypes of cardiovascular cells change during maturation (e.g., gene expression changes, spontaneous beating stops), but the chromatin structure and epigenetic status also change. Polycomb repressive complex 2 (PRC2), which trimethylates histone H3 at lysine 27, for example, establishes H3K27Me3 repressive epigenetic marks that promote tissue-specific differentiation by silencing ectopic gene programs. In heart development, the PRC2 subunit EZH2 is controlled by the cardiac transcription factor Nkx2.5 and EZH2, in turn, represses a multiplicity of cardiac transcription factors; if the epigenetic landscape established by EZH2 is disrupted in early development, this can lead to sustained disruptive effects in later development and cardiac maturation (He et al. 2012). These parameters provide opportunities to develop readouts for the state of cell maturation, and at the same time present potential targets through which maturation might be induced. Tissue engineering in 3D settings is an effective approach used for this purpose, in particular, in conjunction with the use of reporter lines derived by homologous recombination that enable assessment of functional readouts in real time and optimization of each differentiation event in a stepwise fashion.
BIOMATERIALS FOR ENGINEERING MYOCARDIUM
The native heart matrix is a highly ordered anisotropic structure that supports densely packed cardiomyocytes and supporting cells, the function of which is governed by the propagation of electrical signals inducing synchronous mechanical contractions that pump blood. Collagen is the primary load-bearing protein in the heart that transduces the forces generated by the myocytes in systole and provides passive stiffness during diastole (Fomovsky et al. 2010), acting in concert with titin, a major intracellular contributor to passive elasticity (Chung and Granzier 2011) and modulating the cell phenotype (Engler et al. 2006). The extracellular matrix of the adult heart also includes fibronectin, laminin, vitronectin, and elastin, all of which contribute to cell adhesion and the load-bearing capacity of the heart (Godier-Fournemont and Vunjak-Novakovic 2012).
Biomaterial scaffolds should provide a 3D environment for cells to attach, interact with each other, transmit load, and conduct electrical signals. An effective scaffold for cardiac tissue engineering has the capacity to induce alignment, provide appropriate stiffness for the cells to generate physiological force, and enzymatically degrade over time to be replaced by cell-secreted extracellular matrix proteins. Moreover, highly ordered anisotropic layers within native myocardium are critical for alignment of cardiomyocytes and the transduction of mechanical and electrical signals. Conceivably, for normal function, cardiac cells need a matrix with cardiac-like molecular composition, structure, and mechanical properties. Two broad classes of cardiac tissue engineering scaffolds are natural and synthetic materials.
Frequently used natural materials include collagen (Eschenhagen et al. 1997; Radisic et al. 2004a; Zimmermann et al. 2006), fibrin (Christman et al. 2004; (Hansen et al. 2010), hyaluronic acid (HA) (Khademhosseini et al. 2007), Matrigel ( Zimmermann et al. 2002; Radisic et al. 2004b; Laflamme et al. 2007), and preparations of native heart matrix (Duan et al. 2011; Godier-Furnemont et al. 2011; Singelyn et al. 2012). Natural materials readily provide signals to cells by surface receptor interactions, uptake, and degradation of the matrix molecules, but are difficult to process without disrupting potentially important hierarchical structures. Hydrogels formed from natural materials are particularly suitable for tissue engineering applications because their mechanical properties can be adjusted to values inherent to native heart matrix (about 5 kPa) (Marsano et al. 2010).
Frequently used synthetic materials include polyesters such as poly(lactic acid) and poly (glycolic acid) (Bursac et al. 1999; Carrier et al. 1999), polylactones (Ishii et al. 2005), polyurethanes (McDevitt et al. 2005), and polysebasic acid (Radisic et al. 2006; Engelmayr et al. 2008; You et al. 2010; Marsano et al. 2013). Synthetic biomaterials can be readily customized, but may be limited in functional cellular interactions, and therefore are often modified to incorporate adhesion peptides or release biological molecules.
In a series of studies by Eschenhagen and Zimmermann, cardiac constructs were made using a mixture of liquid collagen I, Matrigel, and growth supplements to encapsulate heart cells (Zimmermann et al. 2000, 2002, 2004, 2006). The cell-hydrogel solution was reconstituted in circular molds to form ring-shaped constructs 15 mm in diameter and 1–4-mm thick, and subjected to mechanical loading (10% stretch, 2 Hz). After a short culture period, these constructs developed contractile and electrophysiological properties of working myocardium. Alternatively, cellular tension was induced via excitation–contraction coupling by applying cardiac-like electrical stimulation to cultured cells on scaffolds using bioreactors fitted with conducting electrodes (Radisic et al. 2004a). The electrically stimulated cells conducted electrical pacing signals, and contracted synchronously at the frequency of stimulation.
Another natural biomaterial that has been successfully applied to cardiac tissue engineering is HA. Although HA macromolecules maintain the biomechanical properties of cardiac tissue, their fragments are highly bioactive and involved in many regulatory events including cellular function and development, tumor progression, angiogenesis, inflammation, wound healing, and regeneration. Although HA has not received as much attention as other biomaterials for cardiac tissue engineering, it has been shown to support a differentiated cell phenotype in adult cardiomyocytes (Khademhosseini et al. 2007).
An excellent synthetic scaffold for cardiac tissue engineering is the elastomer poly(glycerol sebacate) (PGS) (Radisic et al. 2006). PGS has been extensively used in cardiac tissue applications because of its high and controllable elasticity and stiffness, as well as slow degradation via hydrolysis. The pores, stiffness, and channel geometry in this scaffold can be designed to enable engineering of vascularized cardiac tissue (Marsano et al. 2010). This material was used to fabricate scaffolds in the form of an accordion-like honeycomb with anisotropic structure and biomechanical properties of native cardiac muscle (Engelmayr et al. 2008). When cultured with neonatal heart myocytes, this scaffold induced cell alignment and coupling, and a direction-dependent contractile behavior.
Several groups have sought to use biological tissues as scaffolds for cardiac tissue engineering. A 2008 study reported by Taylor (Ott et al. 2008) revealed that decellularized rat hearts could be seeded with cardiomyocytes and endothelial cells, resulting in regions of contractile activity after four days in culture. Decellularization of tissues, pioneered by the Badylak laboratory (Robinson et al. 2005), removes all cellular elements from a tissue, leaving an intact, functional extracellular matrix. Thin sheets of fully decellularized human heart matrix were an excellent substrate for in vitro cultivation and in vivo delivery of cells for cardiac regeneration (Godier-Furnemont et al. 2011). Such scaffolds can provide a combination of local control (through preserved protein content and native mechanical properties) and long-range signaling (through well-preserved anisotropic structure and topology of the extracellular matrix).
We also investigated cardiogenesis and maturation of human cardiomyocytes derived by staged molecular induction of embryonic stem cells (Yang et al. 2008) in hydrogels derived by blending native cardiac extracellular matrix (ECM) with type I collagen without any additional growth factors (Duan et al. 2011). Maturation of cardiac function was documented in terms of synchronous contractile behavior, expression of mRNA for cardiac-specific markers, and striation patterns. Hydrogel with high ECM content (75% ECM, 25% collagen, no supplemental factors) increased the fraction of cells expressing cardiac marker troponin T when compared with either hydrogel with low ECM content (25% ECM, 75% collagen, no supplemental factors) or collagen hydrogel (100% collagen, with supplemental factors) (Duan et al. 2011).
The scaffold is a key component of almost all tissue-engineered systems as it determines interactions between the cells and their environment. Specialized scaffolds are now available that guide cell alignment, improve mass transport by incorporation of perfusion channels, and provide the mechanical properties and degradation necessary for the formation of functional tissue constructs. Although much has been achieved in the development of “designer” scaffolds customized for use with cardiac cells, further advances in tissue engineering may require a new generation of scaffolds, perhaps those with ability to change their properties in response to the conditions of perfusion and loading. However, the cardiac constructs used for in vitro drug screening and modeling of disease can be simpler than those engineered for implantation. Consequently, the scaffold materials can also be simpler and, in many cases, just a hydrogel under tension can form a basis for minimally functional small tissue organoids.
FUNCTIONAL MYOCARDIUM IN VITRO
The main advantage of engineered functional myocardium is that it is a more physiological surrogate of native heart than cardiac myocytes cultured on plastic dishes or a slice of native myocardium. As such, engineered myocardium extends the promises of all cell culture work: (1) direct experimental access to the cell/tissue without interference with systemic, compensatory mechanisms such as sympathetic nervous activation, (2) relative stability over days or weeks, a clear advantage over isolated muscle work, (3) relative ease of genetic manipulation, for example, by adenoviral or adeno-associated virus (AAV)-mediated gene transfer or small interfering RNA-mediated gene knockdown, (4) replacement of ethically critical animal experiments, and (5) unique potential to study biomedical questions in human heart muscle in vitro. It is, particularly, the latter argument that drives the field forward.
At present, only some of this potential has been fulfilled. It is interesting that pharmaceutical companies still rely on animal experiments and did not switch to cell culture work at a larger scale. A major limitation for adopting the in vitro models remains the immaturity of hPSC-derived cardiac myocytes as discussed above (which also applies to all other derivatives of PSC), as well as the imperfection of cardiac tissue quality in 3D models and the difficulties to control culture conditions at a level required by “good laboratory practice” standards (see Table 1 for comparison of animal experiments, isolated heart tissues, and engineered cardiac constructs). Nevertheless, functional myocardium, particularly that derived from hPSC, will play an increasing role in biomedical and pharmaceutical research because it allows studying questions that could not be answered otherwise, either in animals or in classic 2D cell monolayers, for the following reasons.
Table 1.
Relative advantages and disadvantages of drug testing using animal experimentation, isolated cardiac tissues, and engineered cardiac tissues
| Parameter | Animal experiments | Isolated cardiac tissues | Engineered cardiac tissues |
|---|---|---|---|
| Advantages | Integrate complexity of whole organism Allow long-term studies Identify effects of metabolites Validity (effects in animals are “real”) Full repertoire of genetic manipulation Long-term experiences Regulatory demand |
Direct access to cardiac muscle or vessel function Intact mature myocardium/vessel Full repertoire of methods to determine function Long-term experiences |
Human Direct access Simple interventions Stability for days/weeks Simple genetic manipulation Simple multiplexed functional readout |
| Disadvantages | Species differences Strain differences Lack of good disease models Ethics Integrate complexity of whole organism complicates interpretation Costs |
Only short-term experiments High degree of experimental artefact Species and strain differences Difficult genetic manipulation |
Unclear validity for human biology High degree of experimental artefact Limited maturity Limited experiences Methods to determine function undefined |
(1) Cardiotoxicity of drugs is an increasing problem because many of the new “targeted” anticancer drugs inhibit signaling pathways that are not only important for tumor growth, but also for the defense of cardiac myocytes under stress ( Eschenhagen et al. 2011; Force 2012). Despite some undisputable examples such as trastuzumab (Piccart-Gebhart et al. 2011), the scope of the problem remains controversial with oncologists generally not very interested in this aspect. The exact mechanisms of toxicity are unknown, and standard toxicity assays based on cell death, lactate dehydrogenase (LDH), or troponin-release are often not valid, and simple cell culture experiments tend to overestimate the toxicity of anticancer drugs (Force and Kolaja 2011). Finally, drugs such as trastuzumab are human specific and therefore cannot be studied in animals. For all these reasons, more sophisticated experiments with functional human myocardium are needed. Readouts of greatest interest are contractile force, kinetics and reversibility of contractions, and cell ultrastructure, under normal and stressed conditions. These measurements, conducted in a 3D setting of engineered cardiac muscle would help understand the degree and mechanisms of cardiotoxicity for numerous existing and candidate drugs before they enter clinical studies and the market.
(2) Similar reasons argue for using functional myocardium for studying proarrhythmic actions of drugs. The scope of the problem is enormous with an estimated 10% of all new chemical entities (NCE) having relevant repolarization-inhibitory effects (Fenichel et al. 2004) that can provoke torsade-de-pointe arrhythmias, ventricular fibrillation, and sudden death. Regulatory agencies such as the Food and Drug Administration (FDA) and European Medicines Agency (EMA) therefore require all NCE to undergo extensive tests, typically including patch clamp experiments on cells expressing a major human potassium channel (hERG), measurements of action potentials in isolated heart muscle preparations, and systemic application in telemetrically surveyed dogs (Darpo et al. 2006). Yet, the predictive value of these batteries of tests remains unsatisfactory. Experiments in human functional myocardium could offer significant advantages by providing an integrated readout of the effects of the drug on the entire set of proteins involved in excitation–contraction coupling. In contrast to hERG-assays or isolated muscle work, drug effects can be studied in engineered cardiac muscle over prolonged periods of time to assess the possible role of delayed pharmacokinetics.
(3) Finally, functional myocardium will be instrumental for hiPSC-mediated modeling of human cardiac disease. Whereas the potential value of this approach has already been shown (Davis et al. 2012), its practical application will require robust, standardized and quantitative, high-content readouts. It seems unlikely that the subtle phenotypes typically seen in inherited cardiac diseases, even more relevant in multigenic variations, will be detectable with the necessary precision in isolated cells randomly intermingled among an undefined mix of other cells. The use of synthetic functional myocardium would have multiple advantages: physiological longitudinally oriented, well-organized structure of myocytes and nonmyocytes, defined conditions of pre- and afterload, use of integrated functional readouts from thousands of cells, and minimal impact of random variations in individual cell function normally seen in classic cell cultures. Tissue engineering was shown to maintain one of the critical ultrastructural features of neonatal rodent cardiomyocytes—T-tubuli (Zimmermann et al. 2002), a feature yet to be shown in ES- and iPS-derived cardiomyocytes (Lieu et al. 2009).
To have utility for drug testing, functional myocardium needs to show more physiological responses than 2D cardiac cell culture systems and overcome some of their limitations. Specifically, the engineered myocardium should drive cardiac myocyte maturation toward an adult state, provide 3D organization of myocytes and nonmyocytes reminiscent of heart tissue, and develop force (Shimizu et al. 2002; Zimmermann et al. 2002; Radisic et al. 2004a; Tiburcy et al. 2011). The experimental system should be simple, robust, easily accessible to experimental manipulation, automatable, and cost effective. Obviously, some of these requirements are contradictory so a compromise would need to be tailored to specific requirements.
Among the three major tissue engineering techniques (cell-hydrogels, cellularization of prefabricated matrices, cell-sheet layering), only the hydrogel technique has been developed with the goal of creating functional myocardium as a paradigm for preclinical testing. The other two approaches also provide well-developed 3D functional heart muscle constructs (Shimizu et al. 2002; Radisic et al. 2004a,b), but are not well suited for functional tests because the constructs need to be mechanically fixed to allow transducers to measure function. The hydrogel technique, in contrast, can be designed in a way that heart muscles develop on devices (Eschenhagen et al. 1997) or in a geometrical form (Zimmermann et al. 2002) that allows easy attachment to force transducers. Variations of this technique include strip-like muscles mounted on a FlexerCell system (de Lange et al. 2011), needles (Birla et al. 2005), or silicone posts (Hansen et al. 2010; Boudou et al. 2012). In most systems, the muscles are fixed to isometric force transducers in organ baths and subjected to the entire spectrum of analyses classically performed with papillary muscles or isolated muscle strips. However, this approach cannot be scaled to larger numbers and requires tedious manual work. In contrast, the silicone post technique involves only minimal handling and allows miniaturization and automated readout of contractile function. Moreover, it provides the developing heart tissue with an autoadapted preload against which the tissue can perform contractile work, a perfect stimulus for longitudinal alignment and muscle development ( Eschenhagen et al. 2012). We propose that this technique is preferred for preclinical research.
In vitro assays based on the use of heart muscle engineered using hiPSC have potential to address numerous biomedical questions, some of which are described below.
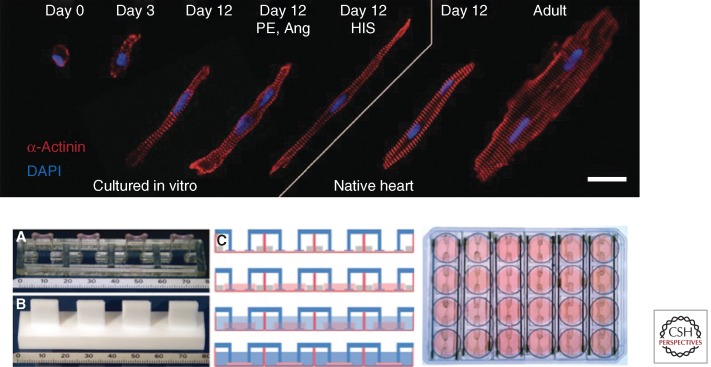
(1) Specification and maturity of engineered cardiac tissue. Most engineering methods available to date result in the formation of immature, fetal-quality cardiac tissues, whereas a certain level of maturity is necessary for pharmacological studies predictive of human situation (Fig. 3). Moreover, cardiac muscle contains a variety of muscle and nonmuscle cells that form distinct structures: the atrial and ventricular muscle, valves, pacemaker and conduction system, aortic and pulmonary outflow tract, coronary arterial system, and the endocardium (Vunjak-Novakovic et al. 2011). Although much progress has been made in recent years, further studies are needed to engineer different types of cardiac muscle and mature tissue organization and cell function.
Figure 3.
In vitro testing platforms. (Top) Engineered cardiac tissues can reach the level of advanced morphologic differentiation and maturation typical of their age-matched in vivo counterparts. Hypertrophic cardiomyocyte growth in hydrogel with the application of mechanical stretch is compared to native heart. Cardiomyocytes from engineered tissues at culture days 0, 3, and 12 from three experimental groups: (1) untreated, (2) treated with phenylephrine (PE, 20 µmol/L), angiotensin II (Ang, 100 nmol/L), and (3) treated with hypertrophy-inducing serum (HIS) are compared to cardiomyocytes from rat myocardium. Red, α-actinin; blue, DAPI-labeled nuclei. Scale bar, 20 µm. The image is an assembly of individual immunostains of representative cells from each group. (From Tiburcy et al. 2011; reproduced, with permission, from the author.) (Bottom) Experimental setup for casting and cultivation of cardiac tissue. (A) Silicone post rack with four tissues, turned upside down, scale in mm. (B) Teflon spacer to generate agarose molds, turned upside down, scale in mm. (C) Generation of cardiac tissues. First lane, Casting molds are generated in 24-well plates using agarose and Teflon spacers. Silicone racks are placed on the dish, pairs of posts reach into each mold. Second lane, Cell suspension in Matrigel, fibrinogen, and thrombin is pipetted into the molds. Third lane, Two hours later, the hydrogel is polymerized, with silicone posts embedded in hydrogel at both ends. The constructs are removed from the molds and transferred to 24-well plates. Fourth lane, The constructs are maintained in culture for 15 to 30 days. (From Hansen et al. 2010; reproduced, with permission, from the author.)
(2) Mechanisms of cardiac actions of drugs. Engineered heart tissue (EHT) was instrumental in detecting an antiadrenergic effect of the cholesterol-lowering drugs atorvastatin on the heart. Pretreatment with atorvastatin desensitized EHTs to the positive inotropic effect of isoprenaline because of reduced isoprenylation of the signal-transducing G protein subunit γ3, which then lead to a drop out of the stimulatory G protein Gαs from the membrane (Mühlhäuser et al. 2006).
(3) Proarrhythmic effects of drugs. Drugs known to cause torsade-de-pointes arrhythmias in patients (e.g., quinidine, erythromycin, dofetilide, thioridazine) generally cause action potential prolongation by inhibiting a specific K+ channel (hERG). Preclinical toxicology experiments in EHTs suggest that prolongation of contraction twitches, particularly the relaxation phase, is a valid surrogate of the proarrhythmic effect. In human EHTs, the effect was closely related to the inhibition of hERG (Schaaf et al. 2011). In contrast, in rat EHTs, which do not contain relevant hERG-like activity, other effects are involved that may point to additional, currently unknown mechanisms of potentially lethal side effects of drugs (Hansen et al. 2010).
(4) The role of specific proteins in heart muscle function. A powerful approach to study protein function in the heart is to generate cardiac-specific knockout mouse lines. Future applications will likely use hiPSC from patients with natural mutations in the same genes. EHTs can serve an important purpose in target validation studies. We have knocked down PKC-α in EHTs using adenovirus-encoding-specific short hairpin RNA and observed contractile force to negatively correlate with the concentration of the remaining PKC-α (El-Armouche et al. 2007), suggesting that PKC-α exerts a constitutive negative influence on contractile force in the heart, making it a potential target for drug development.
(5) Functional genomics. In a similar manner, EHTs can serve as a test bed for analyzing the consequences of disease-associated mutations in cardiac proteins. By comparing the effects of AAV-mediated overexpression of wild-type and mutants of the FHL1, a protein suggested to be involved in stress sensing in the sarcomere, we have shown that mutant FHL1 exerted pathological effects on EHT contractile function (Friedrich et al. 2012). These data support the notion that the mutants indeed cause the development of inherited cardiomyopathies.
(6) Hypertrophy and heart failure mechanisms. Cardiac hypertrophy is a physiological mechanism of heart adaptation to increased hemodynamic demand (growth, pregnancy, exercise). It is also a major risk factor, however, for the development of heart failure. The key question that is still unanswered is whether the pathways involved in these two phenomena are identical and to what extent pathways can be identified that cause a pathological, maladaptive form of hypertrophy. Answering this question would require in-depth molecular analysis and accurate assessment of contractile function. In this respect, we have recently developed a new EHT-based hypertrophy model in which EHTs are subjected to increased afterload by stiffening the silicone posts for a few days. This manipulation induced the full spectrum of pathological hypertrophy, including decreased contractile function and increased metabolic demand (Hirt et al. 2012).
CONCLUDING REMARKS
New sources of healthy and diseased human cardiomyocytes over the last decade based on both hiPSC and targeted mutations in control hPSC have developed in parallel with major advances in designing biomaterial scaffolds that mimic the composition, structure, and biomechanics of native human heart tissue. These two research areas are now poised to exploit the technology to the full and develop the much needed in vitro models for studying human heart disease and screening drugs under conditions representative of whole-body physiology.
A major remaining challenge is to refine methods for directed differentiation to atrial, ventricular, and pacemaker-like cells from hPSC is possible, as well as other crucial cellular components of the heart, endocardium, epicardium, and cardiac fibroblasts. Developmental biology is here leading the way toward this goal. Combining these “organs on a chip” formats that not only contain the tissue of interest, like the myocardium and adjacent epicardium and endocardium, but functional blood vessels that can transport culture medium, synthetic or real blood, and drugs, means that new perspectives are on the horizon that can address complex disease paradigms and multidrug treatments.
However, modeling integrated human physiology in vitro is a formidable goal that has not been reached yet with any of the existing cell/tissue systems. Tissue engineering is now becoming increasingly successful in more authentically representing the actual environmental milieu of development, regeneration, and disease progression, and providing real-time insights into cellular and morphogenic events. Instead of attempting to recapitulate the entire complexity of the organ, a more reasonable goal is to replicate the tissue-specific architecture (as a basis for function) and a subset of most relevant functions in a way predictable of human physiology. It is thus of interest to identify the simplest functional tissue unit allowing high-throughput, high-content in vitro studies. Such minimally functional human tissue units, engineered to combine biological fidelity with the use in high-throughput platforms and online analytics, are expected to replace animal experimentation and transform drug screening and modeling of disease.
Footnotes
Editors: Margaret Buckingham, Christine L. Mummery, and Kenneth R. Chien
Additional Perspectives on The Biology of Heart Disease available at www.perspectivesinmedicine.org
REFERENCES
- Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB 2006. Tissue-engineered autologous bladders for patients needing cystoplasty. The Lancet 367: 1241–1246 [DOI] [PubMed] [Google Scholar]
- Birla RK, Borschel GH, Dennis RG, Brown DL 2005. Myocardial engineering in vivo: Formation and characterization of contractile, vascularized three-dimensional cardiac tissue. Tissue Eng 11: 803–813 [DOI] [PubMed] [Google Scholar]
- Boudou T, Legant WR, Mu A, Borochin MA, Thavandiran N, Radisic M, Zandstra PW, Epstein JA, Margulies KB, Chen CS 2012. A microfabricated platform to measure and manipulate the mechanics of engineered cardiac microtissues. Tissue Eng Part A 18: 910–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham M, Meilhac S, Zaffran S 2005. Building the mammalian heart from two sources of myocardial cells. Nat Rev Genet 6: 826–835 [DOI] [PubMed] [Google Scholar]
- Bursac N, Papadaki M, Cohen RJ, Schoen FJ, Eisenberg SR, Carrier R, Vunjak-Novakovic G, Freed LE 1999. Cardiac muscle tissue engineering: Towards an in vitro model for electrophysiological studies. Am J Physiol 277: H433–H444 [DOI] [PubMed] [Google Scholar]
- Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S 2003. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell 5: 877–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier R, Papadaki M, Rupnick M, Schoen FJ, Bursac N, Langer R, Freed LE, Vunjak-Novakovic G 1999. Cardiac tissue engineering: Cell seeding, cultivation parameters and tissue construct characterization. Biotechnol Bioeng 64: 580–589 [DOI] [PubMed] [Google Scholar]
- Caspi O, Lesman A, Basevitch Y, Gepstein A, Arbel G, Habib IH, Gepstein L, Levenberg S 2007. Tissue engineering of vascularized cardiac muscle from human embryonic stem cells. Circ Res 100: 263–272 [DOI] [PubMed] [Google Scholar]
- Christman KL, Vardanian AJ, Fang Q, Sievers RE, Fok HH, Lee RJ 2004. Injectable fibrin scaffold improves cell transplant survival, reduces infarct expansion, and induces neovasculature formation in ischemic myocardium. J Am Coll Cardiol 44: 654–660 [DOI] [PubMed] [Google Scholar]
- Chung CS, Granzier HL 2011. Contribution of titin and extracellular matrix to passive pressure and measurement of sarcomere length in the mouse left ventricle. J Mol Cell Cardiol 50: 731–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl SLM, Kypson AP, Lawson JH, Blum JL, Strader JT, Li Y, Manson RJ, Tente WE, DiBernardo L, Hensley MT, et al. 2011. Readily available tissue-engineered vascular grafts. Sci Transl Med 3: 68ra9. [DOI] [PubMed] [Google Scholar]
- Darpo B, Nebout T, Sager PT 2006. Clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs. J Clin Pharmacol 46: 498–507 [DOI] [PubMed] [Google Scholar]
- Davis RP, van den Berg CW, Casini S, Braam SR, Mummery CL 2011. Pluripotent stem cell models of cardiac disease and their implication for drug discovery and development. Trends Mol Med 17: 475–484 [DOI] [PubMed] [Google Scholar]
- Davis RP, Casini S, van den Berg CW, Hoekstra M, Remme CA, Dambrot C, Salvatori D, Oostwaard DW, Wilde AA, Bezzina CR, et al. 2012. Cardiomyocytes derived from pluripotent stem cells recapitulate electrophysiological characteristics of an overlap syndrome of cardiac sodium channel disease. Circulation 125: 3079–3091 [DOI] [PubMed] [Google Scholar]
- de Lange WJ, Hegge LF, Grimes AC, Tong CW, Brost TM, Moss RL, Ralphe JC 2011. Neonatal mouse-derived engineered cardiac tissue: A novel model system for studying genetic heart disease. Circ Res 109: 8–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Y, Liu Z, O’Neill J, Wan L, Freytes DO, Vunjak-Novakovic G 2011. Hydrogel derived from native heart matrix induces cardiac differentiation of human embryonic stem cells without supplemental growth factors. J Cardiovasc Transl Research 4: 605–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Armouche A, Singh J, Naito H, Wittköpper K, Didié M, Laatsch A, Zimmermann WH, Eschenhagen T 2007. Adenovirus-delivered short hairpin RNA targeting PKC-α improves contractile function in reconstituted heart tissue. J Mol Cell Cardiol 43: 371–376 [DOI] [PubMed] [Google Scholar]
- Elliott DA, Braam SR, Koutsis K, Ng ES, Jenny R, Lagerqvist EL, Biben C, Hatzistavrou T, Hirst CE, Yu QC, et al. 2011. NKX2-5(eGFP/w) hESCs for isolation of human cardiac progenitors and cardiomyocytes. Nat Methods 8: 1037–1040 [DOI] [PubMed] [Google Scholar]
- Engelmayr GC Jr, Cheng M, Bettinger CJ, Borenstein JT, Langer R, Freed LE 2008. Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nat Mater 7: 1003–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE 2006. Matrix elasticity directs stem cell lineage specification. Cell 126: 677–689 [DOI] [PubMed] [Google Scholar]
- Eschenhagen T, Fink C, Remmers U, Scholz H, Wattchow J, Weil J, Zimmermann W, Dohmen HH, Schäfer H, Bishopric N, et al. 1997. Three-dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: A new heart muscle model system. FASEB J 11: 683–694 [DOI] [PubMed] [Google Scholar]
- Eschenhagen T, Didié M, Heubach J, Ravens U, Zimmermann WH 2002. Cardiac tissue engineering. Transpl Immunol 9: 315–321 [DOI] [PubMed] [Google Scholar]
- Eschenhagen T, Force T, Ewer MS, de Keulenaar GW, Suter TM, Anker SD, Avkiran M, de Azambuja W, Balligand JL, Brutsaert DL, et al. , 2011. Cardiovascular side effects of cancer therapies: A position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 13: 1–10 [DOI] [PubMed] [Google Scholar]
- Eschenhagen T, Eder A, Vollert I, Hansen A 2012. Physiological aspects of cardiac tissue engineering. Am J Physiol Heart Circ Physiol 303: H133–H143 [DOI] [PubMed] [Google Scholar]
- Fenichel RR, Malik M, Antzelevitch C, Sanguinetti M, Roden DM, Priori SG, Ruskin JN, Lipicky RJ, Cantilena LR 2004. Drug-induced torsades de pointes and implications for drug development. J Cardiovasc Electrophysiol 15: 475–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fomovsky GM, Thomopoulos S, Holmes JW 2010. Contribution of extracellular matrix to the mechanical properties of the heart. J Mol Cell Cardiol 48: 490–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Force T 2012. Double-edged sword of the new cancer therapeutics. Circulation 125: 2057–2058 [DOI] [PubMed] [Google Scholar]
- Force T, Kolaja KL 2011. Cardiotoxicity of kinase inhibitors: The prediction and translation of preclinical models to clinical outcomes. Nat Rev Drug Discov 10: 111–126 [DOI] [PubMed] [Google Scholar]
- Friedrich FW, Wilding BR, Reischmann S, Crocini C, Lang P, Charron P, Müller OJ, McGrath MJ, Vollert I, Hansen A, et al. 2012. Evidence for FHL1 as a novel disease gene for isolated hypertrophic cardiomyopathy. Hum Mol Genet 21: 3237–3254 [DOI] [PubMed] [Google Scholar]
- Godier-Furnémont A, Martens T, Koeckert M, Wan LQ, Parks J, Zhang G, Hudson J, Vunjak-Novakovic G 2011. Composite scaffold provides a cell delivery platform for cardiovascular repair. Proc Natl Acad Sci 108: 7974–7979 [Research highlights. 2011. Bioengineering Bio: Patching up the heart Nature 472: 393]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godier-Furnémont AFG, Vunjak-Novakovic G 2012. Cardiac muscle tissue engineering. In Biomaterials science: An introduction to materials in medicine, 3rd ed. (ed. Ratner B, Hofman A, Lemons JE, Schoen FJ). Academic, San Diego [Google Scholar]
- Goumans MJ, de Boer TP, Smits AM, van Laake LW, van Vliet P, Metz CH, Korfage TH, Kats KP, Hochstenbach R, Pasterkamp G, et al. 2007. TGF-β1 induces efficient differentiation of human cardiomyocyte progenitor cells into functional cardiomyocytes in vitro. Stem Cell Res 1: 138–149 [DOI] [PubMed] [Google Scholar]
- Hansen A, Eder A, Bönstrup M, Flato M, Mewe M, Schaaf S, Aksehirlioglu B, Schwoerer AP, Uebeler J, Eschenhagen T 2010. Development of a drug screening platform based on engineered heart tissue. Circ Res 107: 35–44 [DOI] [PubMed] [Google Scholar]
- He A, Ma Q, Cao J, von Gise A, Zhou P, Xie H, Zhang B, Hsing M, Christodoulou D, Cahan P, et al. 2012. Polycomb repressive complex 2 regulates normal development of the mouse heart. Circ Res 110: 406–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt MN, Sörensen NA, Bartholdt LM, Boeddinghaus J, Schaaf S, Eder A, Vollert I, Stöhr A, Schulze T, Witten A, et al. 2012. Increased afterload induces pathological cardiac hypertrophy: A new in vitro model. Basic Res Cardiol 107: 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt M, Hansen A, Eschenhagen T 2014. Cardiac tissue engineering—State of the art. Circ Res 114: 354–367 [DOI] [PubMed] [Google Scholar]
- Ishii O, Shin M, Sueda T, Vacanti JP 2005. In vitro tissue engineering of a cardiac graft using a degradable scaffold with an extracellular matrix-like topography. J Thorac Cardiovasc Surg 130: 1358–1363 [DOI] [PubMed] [Google Scholar]
- Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A, Ellis J, Keller G 2011. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell 8: 228–240 [DOI] [PubMed] [Google Scholar]
- Khademhosseini A, Eng G, Yeh J, Kucharczyk P, Langer R, Vunjak-Novakovic G, Radisic M 2007. Microfluidic patterning for fabrication of contractile cardiac organoids. Biomed Microdevices 9: 149–157 [DOI] [PubMed] [Google Scholar]
- Laflamme M, Chen K, Naumova A, Muskheli V, Fugate J, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, et al. 2007. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol 25: 1015–1024 [DOI] [PubMed] [Google Scholar]
- Langer R, Tirrell DA 2004. Designing materials for biology and medicine. Nature 428: 487–492 [DOI] [PubMed] [Google Scholar]
- Lieu DK, Liu J, Siu CW, McNerney GP, Tse HF, Abu-Khalil A, Huser T, Li RA 2009. Absence of transverse tubules contributes to non-uniform Ca2+ wavefronts in mouse and human embryonic stem cell-derived cardiomyocytes. Stem Cells Dev 18: 1493–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutolf MP, Hubbell JA 2005. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol 23: 47–55 [DOI] [PubMed] [Google Scholar]
- Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, Dodson A, Martorell J, Bellini S, Parnigotto PP, et al. 2008. Clinical transplantation of a tissue-engineered airway. Lancet 372: 2023–2030 [DOI] [PubMed] [Google Scholar]
- Majesky MW, Mummery CL 2012. Smooth muscle diversity from human pluripotent cells. Nat Biotechnol 30: 152–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsano A, Maidhof R, Wan LQ, Wang Y, Gao J, Tandon N, Vunjak-Novakovic G 2010. Scaffold stiffness affects the contractile function of engineered cardiac constructs. Biotechnol Prog 26: 1382–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsano A, Maidhof R, Luo J, Fujikara K, Konofagou E, Banfi A, Vunjak-Novakovic G 2013. Controlled expression of VEGF by transduced cells in a cardiac patch improves vascularization and cardiac function in a mouse model of myocardial infarction. Biomaterials 34: 393–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt TC, Laflamme MA, Murry CE 2005. Proliferation of cardiomyocytes derived from human embryonic stem cells is mediated via the IGF/PI3-kinase/Akt signaling pathway. J Mol Cell Cardiol 39: 865–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meilhac SM, Esner M, Kelly RG, Nicolas JF, Buckingham ME 2004. The clonal origin of myocardial cells in different regions of the embryonic mouse heart. Dev Cell 6: 685–698 [DOI] [PubMed] [Google Scholar]
- Mjaatvedt CH, Nakaoka T, Moreno-Rodriguez R, Norris RA, Kern MJ, Eisenberg CA, Turner D, Markwald RR 2001. The outflow tract of the heart is recruited from a novel heart-forming field. Dev Biol 238: 97–109 [DOI] [PubMed] [Google Scholar]
- Mühlhäuser U, Zolk O, Rau T, Münzel F, Wieland T, Eschenhagen T 2006. Atorvastatin desensitizes β-adrenergic signaling in cardiac myocytes via reduced isoprenylation of G-protein γ-subunits. FASEB J 20: 785–787 [DOI] [PubMed] [Google Scholar]
- Mummery C, Ward-van Oostwaard D, Doevendans P, Spijker R, van den Brink S, Hassink R, van der Heyden M, Opthof T, Pera M, de la Riviere AB, et al. 2003. Differentiation of human embryonic stem cells to cardiomyocytes: Role of coculture with visceral endoderm-like cells. Circulation 107: 2733–2740 [DOI] [PubMed] [Google Scholar]
- Mummery CL, Zhang J, Ng ES, Elliott DA, Elefanty AG, Kamp TJ 2012. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: A methods overview. Circ Res 111: 344–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EN, Schneider MD 2003. Sizing up the heart: Development redux in disease. Genes Dev 17: 1937–1956 [DOI] [PubMed] [Google Scholar]
- Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA 2008. Perfusion-decellularized matrix: Using nature’s platform to engineer a bioartificial heart. Nat Med 14: 213–221 [DOI] [PubMed] [Google Scholar]
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, et al. 2011. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353: 1659–1672 [DOI] [PubMed] [Google Scholar]
- Radisic M, Park H, Shing H, Consi T, Schoen F, Langer R, Freed LE, Vunjak-Novakovic G 2004a. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc Natl Acad Sci 101: 18129–18134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisic M, Yang L, Boublik J, Cohen RJ, Langer R, Freed LE, Vunjak-Novakovic G 2004b. Medium perfusion enables engineering of compact and contractile cardiac tissue. Am J Physiol 286: H507–H516 [DOI] [PubMed] [Google Scholar]
- Radisic M, Park H, Chen F, Wang Y, Dennis R, Langer R, Freed LE, Vunjak-Novakovic G 2006. Biomimetic approach to cardiac tissue engineering: Oxygen carriers and channeled scaffolds. Tissue Eng 12: 2077–2091 [DOI] [PubMed] [Google Scholar]
- Robinson KA, Li J, Mathison M, Redkar A, Cui J, Chronos NA, Matheny RG, Badylak SF 2005. Extracellular matrix scaffold for cardiac repair. Circulation 112: I135– I143 [DOI] [PubMed] [Google Scholar]
- Schaaf S, Shibamiya A, Mewe M, Eder A, Stöhr A, Hirt MN, Rau T, Zimmermann WH, Conradi L, Eschenhagen T, et al. 2011. Human engineered heart tissue as a versatile tool in basic research and preclinical toxicology. PLoS ONE 6: e26397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehy SP, Grosberg A, Paker KK 2012. The contribution of cellular mechanostransduction to cardiomyocyte form and function. Biomech Model Mechanobiol 11: 1223–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Yamato M, Isoi Y, Akutsu T, Setomaru T, Abe K, Kikuchi A, Umezu M, Okano T 2002. Fabrication of pulsatile cardiac tissue grafts using a novel 3-dimensional cell sheet manipulation technique and temperature-responsive cell culture surfaces. Circ Res 90: e40. [DOI] [PubMed] [Google Scholar]
- Singelyn JM, Sundaramurthy S, Johnson TD, Schup-Magoffin PJ, Hu DP, Faulk DM, Wang J, Mayle KM, Bartels K, Salvatore M, et al. 2012. Catheter-deliverable hydrogel derived from decellularized ventricular extracellular matrix increases endogenous cardiomyocytes and preserves cardiac function post-myocardial infarction. JACC 59: 751–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits AM, van Vliet P, Metz CH, Korfage T, Sluijter JP, Doevendans PA, Goumans MJ 2009. Human cardiomyocyte progenitor cells differentiate into functional mature cardiomyocytes: An in vitro model for studying human cardiac physiology and pathophysiology. Nat Protoc 4: 232–243 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S 2007. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131: 861–872 [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM 1998. Embryonic stem cell lines derived from human blastocysts. Science 282: 1145–1147 [DOI] [PubMed] [Google Scholar]
- Tibbitt MW, Anseth KS 2009. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng 103: 655–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiburcy M, Didie M, Boy O, Christalla P, Doker S, Naito H, Karikkineth BC, El-Armouche A, Grimm M, Nose M, et al. 2011. Terminal differentiation, advanced organotypic maturation, and modeling of hypertrophic growth in engineered heart tissue. Circ Res 109: 1105–1114 [DOI] [PubMed] [Google Scholar]
- Vunjak-Novakovic G, Scadden DT 2011. Biomimetic platforms for human stem cell research. Cell Stem Cell 8: 252–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vunjak-Novakovic G, Tandon N, Godier A, Martens T, Maidhof R, Marsano A, Radisic M 2010. Challenges in cardiac tissue engineering. Tissue Eng Part B Rev 16: 169–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vunjak-Novakovic G, Lui KO, Tandon N, Chien K 2011. Bioengineering heart muscle: A paradigm for regenerative medicine. Annual Rev Biomed Eng 13: 245–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldo KL, Kumiski DH, Wallis KT, Stadt HA, Hutson MR, Platt DH, Kirby ML 2001. Conotruncal myocardium arises from a secondary heart field. Development 128: 3179–3188 [DOI] [PubMed] [Google Scholar]
- Wobus AM, Boheler KR 2005. Embryonic stem cells: Prospects for developmental biology and cell therapy. Physiol Rev 85: 635–678 [DOI] [PubMed] [Google Scholar]
- Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, et al. 2008. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature 453: 524–528 [DOI] [PubMed] [Google Scholar]
- You Z, Cao H, Gao J, Shin PH, Day BW, Wang Y 2010. A functionalizable polyester with free hydroxyl groups and tunable physiochemical and biological properties. Biomaterials 31: 3129–3138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann WH, Fink C, Kralisch D, Remmers U, Weil J, Eschenhagen T 2000. Three-dimensional engineered heart tissue from neonatal rat cardiac myocytes. Biotechnol Bioeng 68: 106–114 [PubMed] [Google Scholar]
- Zimmermann WH, Schneiderbanger K, Schubert P, Didié M, Münzel F, Heubach JF, Kostin S, Neuhuber WL, Eschenhagen T 2002. Tissue engineering of a differentiated cardiac muscle construct. Circ Res 90: 223–230 [DOI] [PubMed] [Google Scholar]
- Zimmermann WH, Melnychenko I, Eschenhagen T 2004. Engineered heart tissue for regeneration of diseased hearts. Biomaterials 25: 1639–1647 [DOI] [PubMed] [Google Scholar]
- Zimmermann W, Melnychenko I, Wasmeier G, Didie M, Naito H, Nixdorff U, Hess A, Budinsky L, Brune K, Michaelis B, et al. 2006. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med 12: 452–458 [DOI] [PubMed] [Google Scholar]