Abstract
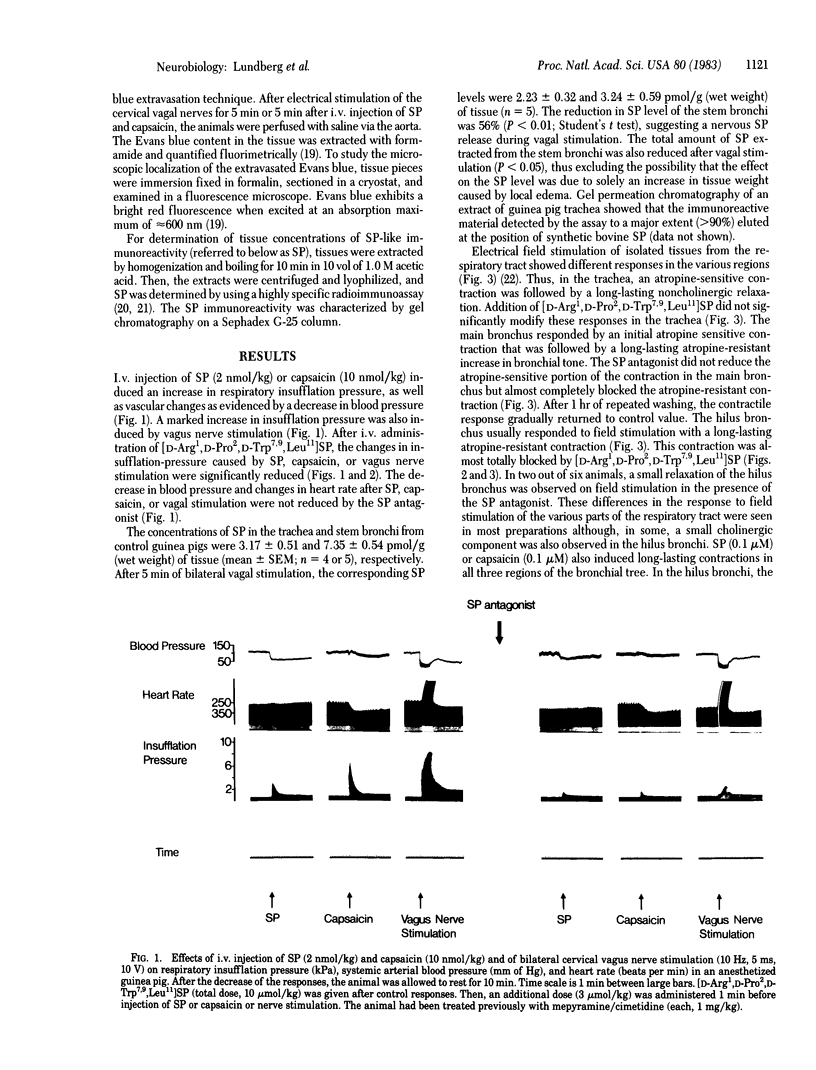
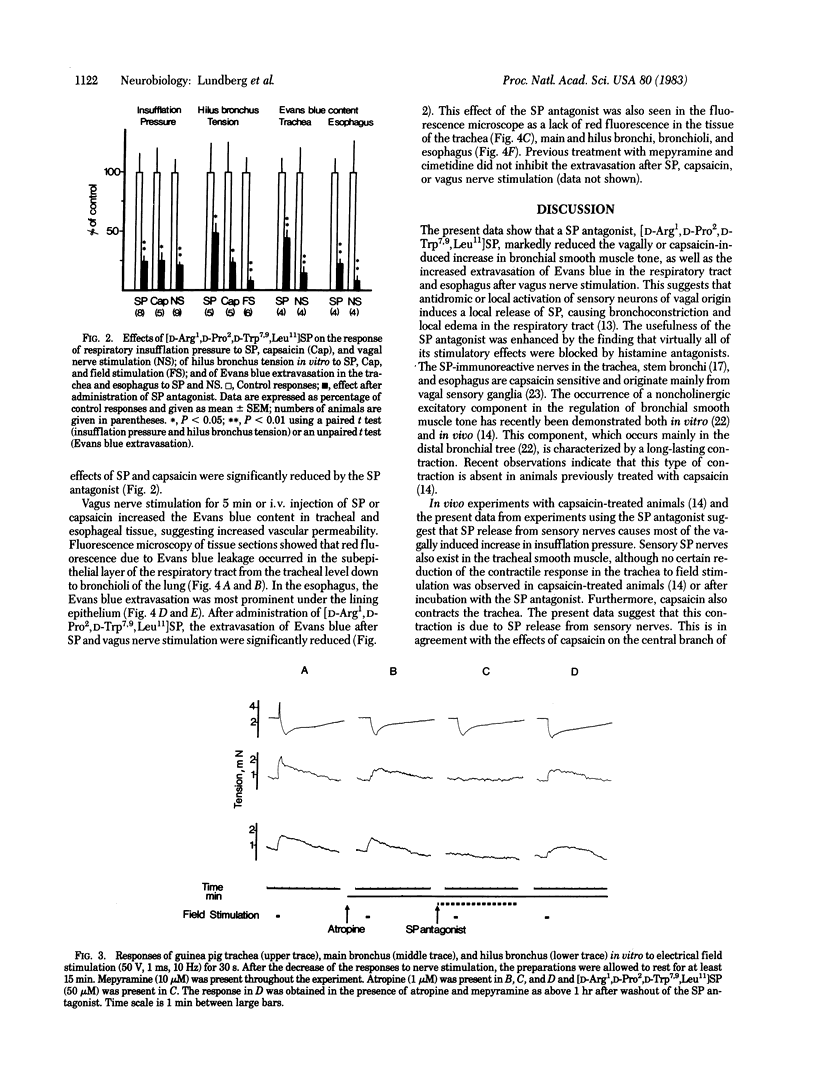
Electrical stimulation of the cervical vagus nerve in anesthetized guinea pigs induced a rapid increase in respiratory insufflation pressure, suggesting increased airway resistance. After intravenous administration of a substance P (SP) antagonist, [D-Arg1,D-Pro2,D-Trp7,9,Leu11]SP, the insufflation pressure response to vagal stimulation was reduced by 78% while the cardiovascular effects were unchanged. Histamine receptor-blocking agents were used to inhibit the effects of histamine release induced by the SP-antagonist. [D-Arg1,D-Pro2,D-Trp7,9,Leu11]SP also reduced the increase in insufflation pressure caused by intravenous SP or capsaicin. The long-lasting noncholinergic contraction of the main and hilus bronchi induced by field stimulation in vitro, as well as the contractile effects of SP and capsaicin, were also blocked by the SP antagonist. The cholinergic contractions and the noncholinergic tracheal relaxation on field stimulation in vitro were, however, not blocked by the antagonist. Vagal stimulation in vivo also increased vascular permeability in the respiratory tract and esophagus, causing a subepithelial edema as indicated by Evans blue extravasation. Previous treatment with [D-Arg1,D-Pro2,D-Trp7,9,Leu11]SP inhibited the permeability increase induced by both vagus nerve stimulation and exogenous SP. SP release from vagal sensory nerves was indirectly shown by reduction in the bronchial levels of SP after nerve stimulation in vivo. The data suggest that a major portion of the vagally or capsaicin-induced increase in smooth muscle tone is caused by SP release from sensory neurons. In addition, activation of vagal SP-containing sensory nerves induces local edema. Tracheobronchial afferent SP-containing C fibers may thus exert local control of smooth muscle tone and vascular permeability in normal and pathophysiological conditions.
Keywords: vascular permeability, respiratory tract, bronchoconstriction
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayliss W. M. On the origin from the spinal cord of the vaso-dilator fibres of the hind-limb, and on the nature of these fibres. J Physiol. 1901 Feb 28;26(3-4):173–209. doi: 10.1113/jphysiol.1901.sp000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bill A., Stjernschantz J., Mandahl A., Brodin E., Nilsson G. Substance P: release on trigeminal nerve stimulation, effects in the eye. Acta Physiol Scand. 1979 Jul;106(3):371–373. doi: 10.1111/j.1748-1716.1979.tb06412.x. [DOI] [PubMed] [Google Scholar]
- Brodin E., Gazelius B., Olgart L., Nilsson G. Tissue concentration and release of substance P-like immunoreactivity in the dental pulp. Acta Physiol Scand. 1981 Feb;111(2):141–149. doi: 10.1111/j.1748-1716.1981.tb06717.x. [DOI] [PubMed] [Google Scholar]
- Brodin E., Nilsson G., Folkers K. Characterization of two substance P antisera. Acta Physiol Scand. 1982 Jan;114(1):53–57. doi: 10.1111/j.1748-1716.1982.tb06951.x. [DOI] [PubMed] [Google Scholar]
- Ennis M. Histamine release from human pulmonary mast cells. Agents Actions. 1982 Apr;12(1-2):60–63. doi: 10.1007/BF01965108. [DOI] [PubMed] [Google Scholar]
- Gamse R., Molnar A., Lembeck F. Substance P release from spinal cord slices by capsaicin. Life Sci. 1979 Aug 13;25(7):629–636. doi: 10.1016/0024-3205(79)90558-7. [DOI] [PubMed] [Google Scholar]
- Grundström N., Andersson R. G., Wikberg J. E. Pharmacological characterization of the autonomous innervation of the guinea pig tracheobronchial smooth muscle. Acta Pharmacol Toxicol (Copenh) 1981 Aug;49(2):150–157. doi: 10.1111/j.1600-0773.1981.tb00884.x. [DOI] [PubMed] [Google Scholar]
- Holmdahl G., Håkanson R., Leander S., Rosell S., Folkers K., Sundler F. A substance P antagonist, [D-Pro2, D-Trp7,9]SP, inhibits inflammatory responses in the rabbit eye. Science. 1981 Nov 27;214(4524):1029–1031. doi: 10.1126/science.6171036. [DOI] [PubMed] [Google Scholar]
- Jancsó G., Kiraly E., Jancsó-Gábor A. Pharmacologically induced selective degeneration of chemosensitive primary sensory neurones. Nature. 1977 Dec 22;270(5639):741–743. doi: 10.1038/270741a0. [DOI] [PubMed] [Google Scholar]
- Jancsó N., Jancsó-Gábor A., Szolcsányi J. Direct evidence for neurogenic inflammation and its prevention by denervation and by pretreatment with capsaicin. Br J Pharmacol Chemother. 1967 Sep;31(1):138–151. doi: 10.1111/j.1476-5381.1967.tb01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell T. M., Iversen L. L., Cuello A. C. Capsaicin-induced depletion of substance P from primary sensory neurones. Brain Res. 1978 Aug 18;152(1):183–188. doi: 10.1016/0006-8993(78)90146-4. [DOI] [PubMed] [Google Scholar]
- LOEFSTROEM B., PERNOW B., WAHREN J. VASODILATING ACTION OF SUBSTANCE P IN THE HUMAN FOREARM. Acta Physiol Scand. 1965 Mar;63:311–324. doi: 10.1111/j.1748-1716.1965.tb04070.x. [DOI] [PubMed] [Google Scholar]
- Lembeck F., Holzer P. Substance P as neurogenic mediator of antidromic vasodilation and neurogenic plasma extravasation. Naunyn Schmiedebergs Arch Pharmacol. 1979 Dec;310(2):175–183. doi: 10.1007/BF00500282. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Saria A. Bronchial smooth muscle contraction induced by stimulation of capsaicin-sensitive sensory neurons. Acta Physiol Scand. 1982 Dec;116(4):473–476. doi: 10.1111/j.1748-1716.1982.tb07170.x. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Saria A. Capsaicin-sensitive vagal neurons involved in control of vascular permeability in rat trachea. Acta Physiol Scand. 1982 Aug;115(4):521–523. doi: 10.1111/j.1748-1716.1982.tb07116.x. [DOI] [PubMed] [Google Scholar]
- Nilsson G., Pernow B., Fischer G. H., Folkers K. Presence of substance P-like immunoreactivity in plasma from man and dog. Acta Physiol Scand. 1975 Aug;94(4):542–544. doi: 10.1111/j.1748-1716.1975.tb05915.x. [DOI] [PubMed] [Google Scholar]
- Olgart L., Gazelius B., Brodin E., Nilsson G. Release of substance P-like immunoreactivity from the dental pulp. Acta Physiol Scand. 1977 Dec;101(4):510–512. doi: 10.1111/j.1748-1716.1977.tb06040.x. [DOI] [PubMed] [Google Scholar]
- Rosell S., Olgart L., Gazelius B., Panopoulos P., Folkers K., Hörig J. Inhibition of antidromic and substance P-induced vasodilatation by a substance P antagonist. Acta Physiol Scand. 1981 Mar;111(3):381–382. doi: 10.1111/j.1748-1716.1981.tb06752.x. [DOI] [PubMed] [Google Scholar]