Abstract
Background
Brassica vegetable consumption may confer a protective effect against cancer, possibly attributable to their glucosinolates. Glucobrassicin is a predominant glucosinolate and is the precursor of indole-3-carbinol (I3C), a compound with anti-cancer effects. However, objective assessments of I3C uptake from Brassica vegetables have not been successful.
Methods
We conducted a randomized, crossover trial to test whether 3,3′-diindolylmethane (DIM, a metabolite of I3C) excreted in the urine after consumption of raw Brassica vegetables with divergent glucobrassicin concentrations is a marker of I3C uptake from such foods. Twenty-five subjects were fed 50 g of either raw ‘Jade Cross’ Brussels sprouts (high glucobrassicin concentration) or ‘Blue Dynasty’ cabbage (low glucobrassicin concentration) once daily for three days. All urine was collected for 24 h after vegetable consumption each day. After a washout period, subjects crossed over to the alternate vegetable. Urinary DIM was measured using a novel liquid chromatography-electrospray ionization-tandem mass spectrometry-selected reaction monitoring (LC-ESI-MS/MS-SRM) method with [2H2]DIM as internal standard.
Results
Urinary DIM was consistently and significantly higher after Brussels sprouts feeding than after cabbage feeding, as evidenced by an average difference of 8.73 pmol/mg creatinine (95% CI 5.36-12.10, p=0.00002).
Conclusion
We have successfully quantified urinary DIM after uptake of I3C from food, and demonstrated that differences in glucobrassicin exposure are reflected in urinary DIM levels.
Impact
Our LC-ESI-MS/MS-SRM method and the results of our study indicate urinary DIM is a measure of I3C uptake from Brassica vegetables, a finding that can be utilized in prospective epidemiological and chemoprevention studies.
Keywords: 3,3′-diindolylmethane; indole-3-carbinol; Brassica; chemoprevention; glucobrassicin
Introduction
Brassica vegetables are abundant in glucosinolates, widely thought to mediate their anti-cancer effects.(1) Glucobrassicin, a predominant glucosinolate,(2) is converted upon mastication of vegetables into indole-3-carbinol (I3C), which then undergoes acid condensation in the stomach, predominantly to 3,3′-diindolylmethane (DIM).(3) I3C and DIM possess remarkable pleiotropic anti-cancer properties.(3, 4) Epidemiological evidence links a high intake of Brassica vegetables to reduced cancer risk;(5) however, the association is inconsistent,(6) highlighting the necessity of developing biomarkers to quantify phytonutrient uptake. No published study has successfully correlated vegetable consumption with I3C metabolites in humans. We conducted a randomized, crossover trial to test the hypothesis that higher glucobrassicin intake from food results in higher DIM levels excreted in the urine. To do so, we developed a liquid chromatography-electrospray ionization-tandem mass spectrometry-selected reaction monitoring (LC-ESI-MS/MS-SRM) method, and compared urinary DIM levels after consumption of ‘Jade Cross’ Brussels sprouts (high glucobrassicin) or ‘Blue Dynasty’ cabbage (low glucobrassicin).
Materials and Methods
Study Design
Twenty-five healthy, non-vegetarian, non-smoking adults were recruited for this randomized, two-period crossover trial. All subjects completed study procedures concurrently. Subjects abstained from glucosinolates starting 7 days prior to consumption of the first study vegetable. Compliance was ascertained from self-reported food diaries and DIM level in spot urine samples collected immediately prior to consumption of study vegetables on the first day of each period. Subjects were randomized to consume 50 g of raw ‘Jade Cross’ Brussels sprouts or ‘Blue Dynasty’ cabbage in a single sitting at the study center once every 24 h for three days. Urine was collected for 24 h after each vegetable feeding. After a 5-day washout period, subjects consumed the alternate vegetable and collected urine in the same manner. Urine was processed and stored at -20°C. Vegetables were prepared fresh daily. Fasting was not required. The protocol and consent form were approved by the Institutional Review Board at the University of Minnesota. All subjects provided informed consent.
‘Jade Cross’ Brussels sprouts and ‘Blue Dynasty’ cabbage (Jordan Seeds, Woodbury, MN) were selected for their divergent glucobrassicin concentrations (unpublished data) and were grown for the study. Samples were taken at three time points for glucobrassicin analysis.
Glucobrassicin Concentration
Samples were prepared on the day of collection by boiling in water, blending and homogenizing (2 min, 12,000 RPM; Polytron PT 1300D; Brinkmann Instruments, Westbury, NY), centrifuging, extracting desulpho-glucobrassicin using strong anion-exchange (SAX) solid-phase extraction (SPE), and filtering the extract through a 0.2-μm PTFE syringe filter pre-wetted with methanol before sample storage at -30°C. Samples were stored at -10°C after blending until homogenization. Further details of the methods are described elsewhere.(7) HPLC analysis was carried out as previously described(7) with minor modifications, using an Agilent 1200 series HPLC system (Agilent Technologies, Santa Clara, CA) equipped with a solvent degasser and diode array detector. The injection volume was 50 μL. The solvent gradient was (A = H2O, B = acetonitrile): 0 to 2 min, 5 to 15% B; 2 to 20 min, 15 to 47% B; 20 to 23 min, 47 to 100% B; 23 to 30 min, 100% B; 30 to 33 min, 100 to 5% B. Peaks were verified using known retention times and absorbance spectra, and integrated using Chemstation software (Agilent Technologies). Desulpho-GB was quantified using a desulphosinigrin standard curve and a response factor of 0.29.(8)
Analysis of Urinary DIM
Chemicals
All chemicals were HPLC, LC-MS, or Optima grade. DIM was from LKT Labs (Saint Paul, MN). Indole, ammonium acetate, t-butyl methyl ether, and β-glucuronidase (E.coli G8295) were from Sigma-Aldrich (St. Louis, MO). [2H2]Formaldehyde was from Cambridge Isotope Laboratories, Inc. (Andover, MA).
[2H2]DIM Synthesis
[2H2]DIM synthesis was carried out essentially as previously described.(9) [2H2]Formaldehyde (20% w/w aqueous solution; 1.5 mL) was added to indole (1.17 mg, 0.01 mol) suspended in a mixture of glacial acetic acid (0.6 g, 0.01 mol) in 2.5 mL of D2O. The mixture was vigorously stirred under nitrogen at 90°C for 5 h, cooled, and purified using the HPLC system below. The retention time of [2H2]DIM was 41 min (0.6 g, 50%, isotopic purity >99%). 1H NMR ([2H6]DMSO) showed absence of methylene protons, isotopic purity >99%). 1H NMR ([2H6]DMSO) showed absence of methylene protons, δ 10.7 (2H, 1, 1′NH), 7.5 (d, J= 15.6 Hz, 2H, 4, 4′H), 7.25 (d, J= 16.2 Hz, 2H, 7, 7′H), 7.18 (d, J= 3.6 Hz, 2H, 2, 2′H), 7.05 (dd, J= 1.8, 14.4 Hz, 2H, 5, 5′H), 6.95 (dd, J= 1.2, 14.4 Hz, 2H, 6, 6′H). The MS (positive ion ESI): m/z (relative intensity) 249 [M+1] (25); 131[M-indole-C2H2] (95). The HPLC system consisted of a Waters model 680 gradient controller, two Waters 510 pumps, Waters 440 absorbance detector (254 nm) (Waters, Milford, MA), Hewlett Packard 1100 series autosampler (Palo Alto, CA), and Luna C18 reverse-phase column 250 × 4.6 mm, 5μm (Phenomenex, Torrance, CA) with a linear gradient from 10% CH3OH in H2O to 100% CH3OH in 40 min and held for an additional 10 min at a flow rate of 0.7 mL/min.
Urine Sample Preparation
Urine samples were prepared using a published technique(10) with modifications. Two thousand units of E. coli β-glucuronidase in 0.1 mL of phosphate buffered saline (0.26%, w/v) and 10 pmol [2H2]DIM internal standard were added to 1 mL of urine. Following a 20 h incubation at 37°C, each sample was extracted two times with an equal volume of t-butyl methyl ether. The extracts were evaporated to dryness using a Savant SpeedVac evaporator (Thermo Fisher Scientific, Waltham, MA) and reconstituted to 20 μL with acetonitrile/10 mM ammonium acetate (30/70, v/v). Appropriate controls were included.
LC-ESI-MS/MS-SRM
Analyses were done on a TSQ Quantum Discovery Max instrument (Thermo Fisher Scientific) in the positive ion mode with N2 as the nebulizing and drying gas. MS parameters: spray voltage 3.2 kV; sheath gas 25; capillary temperature 250°C; collision energy 17 V; scan width 0.05 amu; Q2 gas pressure 1.0 mTorr; source CID 9 V; tube lens offset 104 V; Q1 0.2 amu and Q3 0.7 FWHM. MS data were acquired and processed by Xcalibur software version 1.4 (Thermo Fisher Scientific). Eight μL of the sample were injected from an autosampler using an Agilent 1100 capillary LC system (Agilent Technologies) equipped with a 5μm, 150 × 0.5 mm Zorbax SB-C18 column (Agilent Technologies). The flow rate was 15 μL/min for the first 3 min then 10 μL/min with a gradient from 60% methanol in 15 mM NH4OAc to 100% methanol in 8 min and held for an additional 29 min. The mass transitions monitored were m/z 247.12→130.07 for DIM (typical retention time 16.6 min) and m/z 249.12→132.07 for [2H2]DIM (typical retention time 16.5 min). Quantitation was done by comparing the MS peak area ratio of DIM to that of [2H2]DIM using a calibration curve prepared by plotting the MS peak area ratio of DIM to [2H2]DIM against their concentration ratios using standard mixes containing constant levels of [2H2]DIM and varying levels of DIM. The assay limit of quantitation (LOQ) was 132 fmol. Results below the assay LOQ were computed as 50% LOQ (0.07 pmol/mL).
Accuracy and Precision
Accuracy and precision were determined using two 24 h urine collections from an individual who abstained from Brassica vegetables for 7 days (negative control) and then consumed 200 g of commercial Brussels sprouts for 7 days (positive control). Five replicates were analyzed on two different days. To validate precision over a range of DIM concentrations, 1.25, 2.55, 5.05, 10.1, 20.25, or 30.35 pmol of DIM was added to negative control urine and analyzed in duplicate.
Urine Creatinine
Urine creatinine was measured at the University of Minnesota Medical Center using Vitros Crea slides (Ortho-Clinical Diagnostics, Rochester, NY)
Statistical Analysis
We subtracted measurements from the same subject (Brussels sprouts – cabbage) in both treatment sequences to eliminate possible individual effects. We assumed standard additive normal models for these two sets of differences which included “treatment effect” and “order effect” parameters. Data was analyzed using Proc GLIM of SAS (version 9.2; Cary, NC). The effect of body mass index (BMI), sex, age, and race/ethnicity on urinary DIM was analyzed using linear regression. A 5% significance level was used.
Results
Subjects
Ten males and 15 females ages 22 to 63 years (mean 35.8 ± 11.8 years) completed the study. Twelve subjects were randomized to cabbage first, 13 to Brussels sprouts. None were taking proton pump inhibitors, H2-antagonists, antacids, or antibiotics. Six of the 150 twenty four h urine collections (4%) from 5 different subjects were missing one partial urine void.
Glucobrassicin Concentration
The glucobrassicin concentration in the vegetables is shown in Table 1 and was consistently ∼4-fold lower in cabbage as compared to Brussels sprouts.
Table 1. Glucobrassicin concentration (± SEM) of harvested vegetables and salads.
Halved cabbages (n=4, ∼125 g each) were sampled at harvest. Brussels sprouts (n=3, ∼200 g each) were sampled on the day they were separated from stalks, 2 days after harvest. Brussels sprouts and cabbage salads were sampled on the first day of each vegetable-feeding period (n=4, ∼125 g each and n=3, ∼100 g each, respectively). The mean glucobrassicin concentration was significantly higher in Brussels sprouts than in cabbage
| μmol glucobrassicin/100 gfw | ||||
|---|---|---|---|---|
| Harvest | Period 1 | Period 2 | Mean | |
| Cabbage | 47.4 ± 13.3 | 44.2 ± 1.4 | 45.3 ± 2.0 | 45.8 ± 5.0* |
| Brussels sprouts | 165.3 ± 2.2 | 194.8 ± 6.4 | 191.6 ± 5.5 | 183.9 ± 6.4 |
p<0.001
Abbreviations: gfw = gram food weight
Accuracy and Precision of the Urinary DIM Assay
Accuracy averaged 93% (86-112%), intra-day precision averaged 8.5%, and the inter-day precision averaged 16% (n=5 positive control samples). Precision over a range of DIM concentrations averaged 3% with excellent correlation between added and measured DIM (R2 =0.999).
Urinary DIM Levels
Typical LC-ESI-MS/MS-SRM chromatograms are illustrated in Figure 1A-C. Urinary DIM levels are summarized in Table 2. DIM was undetectable in 38 of the 50 baseline samples; nine subjects produced a peak at the retention time of DIM. Mean 24 h DIM level was higher after consumption of either cabbage or Brussels sprouts compared to baseline, except in 4 subjects who had no detectable DIM after cabbage and one subject with no change after cabbage. Brussels sprouts consumption consistently led to higher mean DIM compared to cabbage consumption. The average difference was 8.73 pmol/mg creatinine (95% CI 5.36, 12.10, p=0.00002). The within subject variation was small compared to the variation across subjects, particularly after Brussels sprouts (ICC=0.85). Mean DIM averaged 0.03 ± 0.01% (Brussels sprouts) and 0.01 ± 0.00% (cabbage) of the glucobrassicin consumed, ranging from 1.40 to 44.00 pmol/mg creatinine (Brussels sprouts) and 0 to 1.47 pmol/mg creatinine (cabbage). Mean urinary DIM did not correlate with age, sex, race/ethnicity, and BMI (p=0.23 for overall model).
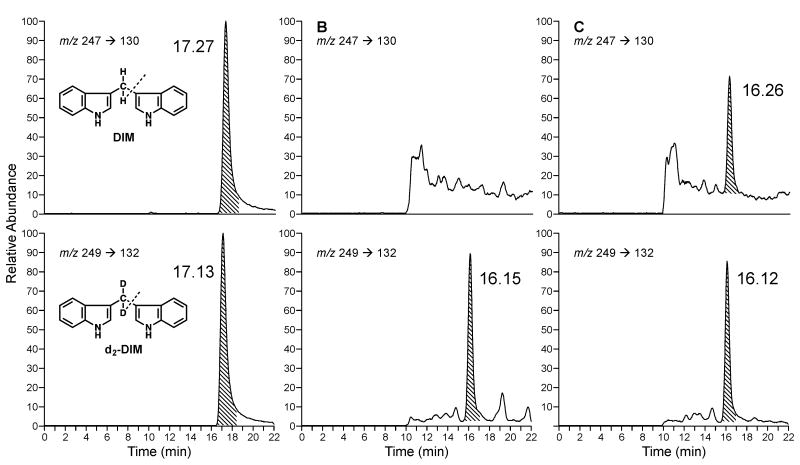
Figure 1.
Representative LC-ESI-MS/MS-SRM chromatograms obtained upon analysis of A, standard DIM (top) and internal standard [2H2]DIM (bottom); B, a urine sample from a subject before eating Brussels sprouts (top) and internal standard [2H2]DIM (bottom), and C, a urine sample from a subject after eating Brussels sprouts (top) and internal standard [2H2]DIM (bottom).
Table 2.
Mean 24 h urinary DIM among each of the 25 subjects after consumption of Brussels sprouts or cabbage once daily for 3 consecutive days. Each mean value represents the mean of three 24 h urine collections. Baseline urinary DIM is also shown. Feeding order refers to which vegetable – Brussels sprouts or cabbage – the subject was randomized to eat during the first vegetable-eating period of the study. Overall mean urinary DIM after Brussels sprouts was significantly higher than after cabbage, with an average difference of 8.73 (95% CI 5.36, 12.10, p=0.00002), taking into account order effect. Mean urinary DIM was slightly higher in the second vegetable-feeding period than in the first, but the difference was not significant (p=0.21). Abbreviations: C, cabbage; B, Brussels sprouts; ND, not detected; Cr, urine creatinine
| Subject # | Feeding order | Baseline urinary DIM pmol/mg Cr | Mean 24h Urinary DIM pmol/24 h ± SD | Mean 24h Urinary DIM pmol/mg Cr ± SD | Difference pmol/mg Cr | |||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| B | C | B | C | B | C | |||
| 1 | C | ND | ND | 13,466 ± 4,272 | 973 ± 504 | 9.18 ± 3.17 | 0.60 ± 0.37 | 8.58 |
| 2 | C | 0.95 | ND | 60,673 ± 40,675 | 2,233 ± 528 | 44.00 ± 31.04 | 1.47 ± 0.33 | 42.53 |
| 3 | C | ND | 0.02 | 32,128 ± 10,987 | 1,135 ± 674 | 21.84 ± 8.48 | 0.85 ± 0.51 | 20.99 |
| 4 | C | ND | ND | 15,067 ± 7,509 | 144 ± 143 | 4.88 ± 2.45 | 0.05 ± 0.05 | 4.83 |
| 5 | C | ND | ND | 19,069 ± 4,959 | 320 ± 116 | 7.02 ± 2.03 | 0.12 ± 0.04 | 6.90 |
| 6 | C | 0.05 | 0.02 | 10,937 ± 3,839 | 350 ± 304 | 5.17 ± 1.85 | 0.17 ± 0.15 | 5.00 |
| 7 | C | ND | ND | 7,028 ± 673 | 0 | 5.22 ± 0.42 | ND | 5.22 |
| 8 | C | ND | ND | 17,539 ± 18,348 | 0 | 8.89 ± 8.33 | ND | 8.89 |
| 9 | C | ND | ND | 7,842 ± 6,249 | 167 ± 190 | 7.58 ± 2.63 | 0.12 ± 0.13 | 7.46 |
| 10 | C | ND | 0.67 | 16,151 ± 7,533 | 762 ± 421 | 10.18 ± 4.96 | 0.56 ± 0.31 | 9.62 |
| 11 | C | 0.07 | 0.05 | 4,812 ± 2,956 | 154 ± 74 | 4.03 ± 2.22 | 0.14 ± 0.07 | 3.89 |
| 12 | C | ND | ND | 10,092 ± 3,678 | ND | 5.76 ± 2.28 | ND | 5.76 |
| 13 | B | ND | ND | 11,438 ± 6,449 | 864 ± 1,048 | 5.84 ± 3.33 | 0.42 ± 0.51 | 5.42 |
| 14 | B | 0.42 | 0.43 | 14,100 ± 2,304 | 985 ± 348 | 13.08 ± 3.06 | 0.56 ± 0.40 | 12.52 |
| 15 | B | ND | ND | 20,787 ± 5,675 | 891 ± 1543 | 11.74 ± 4.04 | 0.62 ± 1.08 | 11.08 |
| 16 | B | ND | ND | 4,913 ± 3,934 | 285 ± 243 | 5.17 ± 3.81 | 0.28 ± 0.24 | 4.89 |
| 17 | B | ND | ND | 13,175 ± 4,488 | 598 ± 497 | 9.83 ± 3.34 | 0.44 ± 0.37 | 9.39 |
| 18 | B | ND | ND | 26,597 ± 9,642 | 815 ± 410 | 15.60 ± 5.50 | 0.41 ± 0.20 | 15.19 |
| 19 | B | 0.06 | 0.04 | 8,118 ± 2,764 | 565 ± 499 | 3.08 ± 0.75 | 0.20 ± 0.17 | 2.88 |
| 20 | B | ND | ND | 6,340 ± 4,728 | 1,447 ± 2,506 | 5.33 ± 3.49 | 1.03 ± 1.78 | 4.30 |
| 21 | B | ND | ND | 7,832 ± 8,628 | 725 ± 727 | 5.22 ± 5.66 | 0.51 ± 0.47 | 4.71 |
| 22 | B | ND | ND | 3,984 ± 3,436 | 0 | 1.40 ± 1.25 | ND | 1.40 |
| 23 | B | 0.48 | ND | 7,383 ± 3,609 | 284 ± 279 | 5.40 ± 1.78 | 0.24 ± 0.22 | 5.16 |
| 24 | B | ND | ND | 15,954 ± 789 | 1,201 ± 823 | 6.12 ± 0.49 | 0.48 ± 0.27 | 5.64 |
| 25 | B | ND | 0.46 | 10,999 ± 11,324 | 2,129 ± 1,372 | 4.66 ± 1.68 | 0.92 ± 0.74 | 3.74 |
Discussion
This study is the first to successfully quantify urinary DIM, a biomarker of I3C uptake, after feeding Brassica vegetables, and the first to show that higher glucobrassicin exposure from food consistently results in higher urinary DIM levels. Characterizing I3C uptake using urinary DIM is advantageous, as the glucosinolate concentration in vegetables can vary widely based on factors such as vegetable type, growth conditions and preparation technique.(2) Measurement of I3C itself is problematic since it becomes rapidly undetectable in vivo.(11, 12)
Our LC-ESI-MS/MS-SRM method represents a notable improvement to previous assays.(10, 12-14) The use of [2H2]DIM as internal standard results in accurate identification and quantitation of DIM. Use of recombinant E. coli derived β-glucuronidase ameliorated the confounding from H. pomatia-derived β-glucuronidase observed during assay development(15) and improved detection. Additionally, our technique makes it possible to quantify the small amount of DIM that results from food consumption, particularly after cooking, which denatures myrosinase, decreasing glucobrassicin conversion to I3C. In contrast, taking I3C supplements results in urinary DIM levels ∼5000 times greater than in our subjects who ate Brussels sprouts.(13)
The proportion of glucobrassicin excreted in the urine as DIM was low, most likely from a low rate of conversion of I3C to DIM in the stomach due to the complex heterogeneity of gastric contents. Additionally, the major route of I3C or DIM excretion in humans is unknown. Prior studies have examined I3C distribution and metabolism after administration of radiolabeled I3C in animals and overall suggest that I3C and DIM may not be readily absorbed by the gut or are excreted in the bile.(11, 16, 17) The inter-individual differences in urinary DIM observed were expected and are consistent with other biomarker studies after administration of Brassica vegetables or their phytonutrients.(12, 18-20)
Other questions remain before urinary DIM can be used as a biomarker, focusing on the pharmacokinetics of I3C and DIM after Brassica vegetable consumption, longitudinal assessments, effect of cooking, and identification of biologically relevant doses in humans. Further investigation is underway.
In summary, urinary DIM is an objective measure of assessing I3C uptake from Brassica vegetables containing divergent glucobrassicin concentrations. Our highly sensitive LC-ESI-MS/MS-SRM method and the results of our study provide a strong foundation to further validate urinary DIM for wider application as a biomarker, particularly in studies of Brassica vegetables in cancer chemoprevention.
Acknowledgments
Grant support: N. Fujioka was supported by NHLBI training grant 5T32HL007062-34 and by Minnesota Masonic Charities. C. Ainslee-Waldman was supported by NCI training grant T32CA321670. This work was supported in part by NIH P30 CA77598 utilizing the Masonic Cancer Center, University of Minnesota, Analytical Biochemistry shared resource. The study was supported by institutional funds from the University of Minnesota to SSH.
Footnotes
Conflict of Interest Disclosure: The authors have no conflicts of interest to disclose.
References
- 1.Herr I, Büchler MW. Dietary constituents of broccoli and other cruciferous vegetables: Implications for prevention and therapy of cancer. Cancer Treat Rev. 2010;36(5):377–83. doi: 10.1016/j.ctrv.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Ciska E, Martyniak-Przybyszewska B, Kozlowska H. Content of glucosinolates in cruciferous vegetables grown at the same site for two years under different climatic conditions. J Agric Food Chem. 2000;48(7):2862–7. doi: 10.1021/jf981373a. [DOI] [PubMed] [Google Scholar]
- 3.International Agency for Research on Cancer. IARC handbooks of cancer prevention. Vol. 9. Lyon: IARC Press; 2004. Cruciferous vegetables, isothiocyanates and indoles. [Google Scholar]
- 4.Aggarwal BB, Ichikawa H. Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle. 2005;4(9):1201–15. doi: 10.4161/cc.4.9.1993. [DOI] [PubMed] [Google Scholar]
- 5.Verhoeven DT, Goldbohm RA, van Poppel G, Verhagen H, van den Brandt PA. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5:733–48. [PubMed] [Google Scholar]
- 6.Kim MK, Park JH. Cruciferous vegetable intake and the risk of human cancer: epidemiological evidence. Proc Nutr Soc. 2009;68(01):103–10. doi: 10.1017/S0029665108008884. [DOI] [PubMed] [Google Scholar]
- 7.Fritz VA, Justen VL, Bode AM, Schuster T, Wang M. Glucosinolate enhancement in cabbage by jasmonic acid application. HortScience. 2010;45(8):1188–91. [Google Scholar]
- 8.European Economic Community. Oil seeds - determination of glucosinolates high performance liquid chromatography. Official Journal of the European Communities. 1990;L170:27–34. Regulation No. 1864/90, Enclosure VIII. [Google Scholar]
- 9.Jackson AH, Prasitpan N, Shannon PVR, Tinker AC. Electrophilic substitution in indoles. part 15. The reaction between methylenedi-indoles and p-nitrobenzenediazonium fluoroborate. J Chem Soc Perkin Trans 1. 1987:2543–51. [Google Scholar]
- 10.Hauder J, Winkler S, Bub A, Rüfer CE, Pignitter M, Somoza V. LC-MS/MS quantification of sulforaphane and indole-3-carbinol metabolites in human plasma and urine after dietary intake of selenium-fortified broccoli. J Agric Food Chem. 2011;59(15):8047–57. doi: 10.1021/jf201501x. [DOI] [PubMed] [Google Scholar]
- 11.Anderton MJ, Manson MM, Verschoyle RD, Gescher A, Lamb JH, Farmer PB, et al. Pharmacokinetics and tissue disposition of indole-3-carbinol and its acid condensation products after oral administration to mice. Clin Cancer Res. 2004;10(15):5233–41. doi: 10.1158/1078-0432.CCR-04-0163. [DOI] [PubMed] [Google Scholar]
- 12.Reed GA, Arneson DW, Putnam WC, Smith HJ, Gray JC, Sullivan DK, et al. Single-dose and multiple-dose administration of indole-3-carbinol to women: pharmacokinetics based on 3,3′-diindolylmethane. Cancer Epidemiol Biomarkers Prev. 2006;15(12):2477–81. doi: 10.1158/1055-9965.EPI-06-0396. [DOI] [PubMed] [Google Scholar]
- 13.Sepkovic DW, Bradlow HL, Bell M. Quantitative determination of 3,3′-diindolylmethane in urine of individuals receiving indole-3-carbinol. Nutr Cancer. 2001;41(1&2):57–63. doi: 10.1080/01635581.2001.9680612. [DOI] [PubMed] [Google Scholar]
- 14.Anderton MJ, Jukes R, Lamb JH, Manson MM, Gescher A, Steward WP, et al. Liquid chromatographic assay for the simultaneous determination of indole-3-carbinol and its acid condensation products in plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;787(2):281–91. doi: 10.1016/s1570-0232(02)00923-6. [DOI] [PubMed] [Google Scholar]
- 15.Ainslie-Waldman CE, Simpkins SW, Upadhyaya P, Carmella SG, Hecht SS, Trudo SP. Contamination of deconjugation enzymes derived from Helix pomatia with the plant bioactive compounds 3,3′-diindolylmethane, 5-methoxypsoralen, and 8-methoxypsoralen. Food Chem Toxicol. 2013;62(0):188–93. doi: 10.1016/j.fct.2013.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dashwood RH, Uyetake L, Fong AT, Hendricks JD, Bailey GS. In vivo disposition of the natural anti-carcinogen indole-3-carbinol after PO administration to rainbow trout. Food Chem Toxicol. 1989;27(6):385–92. doi: 10.1016/0278-6915(89)90144-0. [DOI] [PubMed] [Google Scholar]
- 17.Stresser DM, Williams DE, Griffin DA, Bailey GS. Mechanisms of tumor modulation by indole-3-carbinol. Disposition and excretion in male Fischer 344 rats. Drug Metab Dispos. 1995;23(9):11. [PubMed] [Google Scholar]
- 18.Fahey JW, Wehage SL, Holtzclaw WD, Kensler TW, Egner PA, Shapiro TA, et al. Protection of humans by plant glucosinolates: efficiency of conversion of glucosinolates to isothiocyanates by the gastrointestinal microflora. Cancer Prev Res. 2012;5(4):603–11. doi: 10.1158/1940-6207.CAPR-11-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egner PA, Chen JG, Wang JB, Wu Y, Sun Y, Lu JH, et al. Bioavailability of sulforaphane from two broccoli sprout beverages: results of a short-term, cross-over clinical trial in Qidong, China. Cancer Prev Res. 2011;4(3):384–95. doi: 10.1158/1940-6207.CAPR-10-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kensler TW, Chen JG, Egner PA, Fahey JW, Jacobson LP, Stephenson KK, et al. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthrene tetraols in a randomized clinical trial in He Zuo Township, Qidong, People's Republic of China. Cancer Epidemiol Biomarkers Prev. 2005;14(11):2605–13. doi: 10.1158/1055-9965.EPI-05-0368. [DOI] [PubMed] [Google Scholar]