Abstract
Immunohistochemistry on mouse tissue utilizing mouse monoclonal antibodies presents a challenge. Secondary antibodies directed against the mouse monoclonal primary antibody of interest will also detect endogenous mouse immunoglobulin in the tissue. This can lead to significant spurious staining. Therefore, a “mouse-on-mouse” staining strategy is needed to yield credible data. This paper presents a method that is easy to use and highly flexible to accommodate both an avidin-biotin detection system as well as a biotin-free polymer detection system. The mouse primary antibody is first combined with an Fab fragment of an anti-mouse antibody in a tube and allowed sufficient time to form an antibody complex. Any non-complexed secondary antibody is bound up with mouse serum. The mixture is then applied to the tissue. The flexibility of this method is confirmed with the use of different anti-mouse antibodies followed by a variety of detection reagents. These techniques can be used for immunohistochemistry (IHC), immunofluorescence (IF), as well as staining with multiple primary antibodies. This method has also been adapted to other models, such as using human antibodies on human tissue and using multiple rabbit antibodies in dual immunofluorescence.
Keywords: mouse-on-mouse, endogenous immunoglobulin, immunohistochemistry, monoclonal antibodies, dual immunohistochemistry, dual immunofluorescence, Fab fragment, multiple immunostaining
Introduction
Mouse models are important tools for studying human disease and immunohistochemistry is often required to analyze these samples. However, many of the antibodies that can detect mouse proteins are also created in the mouse. Secondary antibodies directed against mouse monoclonal antibodies of interest will also detect endogenous mouse immunoglobulin in the tissue, leading to significant spurious staining. Therefore, “mouse-on-mouse” staining strategies are needed to yield credible data.
Our goal was to develop a flexible working system for the use of mouse antibodies on mouse tissue, while also creating a system for using multiple antibodies from the same species for dual staining procedures. The method presented in this paper uses a procedure to create a complex of the secondary reagent with the primary antibody prior to applying to tissue using commercially available components. Normal mouse serum is then included to bind up any non-complexed secondary antibody. A similar strategy has been used in the past (Brown et al. 2004; Negoescu et al. 1994). This method is more flexible than commercial kits, because it can be used with standard avidin/biotin reagents as well as biotin-free polymers. In addition, this staining approach was easily adapted from histochemical detection systems to fluorescence staining, and further adapted for its use in multi-colored immunofluorescence staining.
Materials & Methods
Primary Mouse Antibodies
Anti-smooth muscle actin (SMA clone 1A4, #M0851, Dako; Glostrup, Denmark) was used at a dilution of 1:25 (2.8 µg/ml) after heat-induced epitope retrieval (HIER) with Tris-EDTA buffer, pH 9.0 (final concentration 10 mM Tris Base, 1 mM EDTA), or Trilogy (#920P-10, Cell Marque; Rockland, CA). Anti-muscle actin antibody (clone HHF35 #M0635, Dako) was used at a dilution of 1:50 (2.12 µg/ml) with no antigen retrieval. Anti-β-catenin (clone 14, #610153 BD Biosciences; San Jose, CA) was used at 1:50 (5 µg/ml) after HIER with citrate buffer, pH 6.0 (#S1699, Dako). Anti-estrogen receptor (clone 1D5, #M7047, Dako) was used at 1:75 dilution (5.2 µg/ml) after HIER with Trilogy. Anti-desmin (clone D33, # M0760, Dako) was used at 1:50 (4.7 µg/ml) after HIER with Trilogy. Anti-aspergillus (clone WF-AF-1, # MCA2576; AbD Serotec, Raleigh, NC) was used at a dilution of 1:25 (approximately 4 µg/ml) after Proteinase K (S3020, Dako) digestion for 5 min. Anti-SV-40 (clone PAb 101, #554149, BD Pharmingen, San Diego, CA) was used at 1:100 (5 µg/ml) after HIER with Trilogy. Anti-hepatocyte (clone OCH1E5 #M7158, Dako) was used at 1:75 (1.07 µg/ml) after HIER with Trilogy. Anti-cytokeratin (clone AE1/AE3, #M3515, Dako) was used at 1:25 (5 µg/ml) after HIER with citrate buffer, pH 6.0. We used mouse IgG (# I-2000, Vector Laboratories, Burlingame, CA) as our isotype control antibody.
Basic Staining Protocol
Normal mouse tissues were fixed in formalin for a minimum of three days, and then processed and embedded in paraffin. Four-micron sections were cut, baked at 60C for at least 30 min and cooled. Sections were deparaffinized in three changes of xylene for 5 min each and then rehydrated to distilled water using graded alcohols. If required, HIER was used at this time by steaming the slides for 20 min, cooling them for 20 min, and then transferring the slides into wash buffer for 5 min. The antigen retrieval buffers used were citrate buffer at pH 6.0, Tris-EDTA buffer at pH 9.0, or Trilogy. The slides were transferred to TBS-Tween (TBS-T) wash buffer (final concentration 0.05 M Tris, 0.15 M NaCl, 0.05% Tween-20, pH 7.6 ± 0.1) for 5 min, and then loaded onto the Dako Autostainer Plus. Our antibodies were diluted in TBS containing 1% BSA at pH 7.5 ± 0.1. Endogenous peroxidase was blocked with a 3% hydrogen peroxide solution for 8 min (#33502-644, VWR International; Radnor, PA) (see Table 1). If needed, endogenous biotin was blocked with Avidin/Biotin System (#AB972M, Biocare Medical; Concord, CA) for 10 min each. When we used the biotinylated goat anti-mouse secondary antibody, we used 15% normal goat serum (Jackson ImmunoResearch Catalog # 005-000-121) in TBS with 1% BSA for 10 min as the protein block. When the secondary antibody was not biotinylated (either derived from goat or rabbit), we used TCT buffer (0.05 M Tris, 0.15 M NaCl, 0.25% Casein and 0.1% Tween-20) for 10 min as the protein block. To develop the signal, we used two sequential applications of 3, 3’-diaminobenzidine using the DAB Plus (#K3468, Dako) for 4 min each, followed by a 2 min incubation of hematoxylin as the counterstain (Automation Hematoxylin #S3301, Dako). After staining, the slides were dehydrated in graded alcohols, cleared in xylene, and coverslipped.
Table 1.
Protocol Steps in Sequential Order Depending on the Secondary Antibody.
| Biotinylated Goat Anti-Mouse | Unconjugated Goat Anti-Mouse | Unconjugated Rabbit Anti-Mouse | |
|---|---|---|---|
| Endogenous peroxidase block | Yes | Yes | Yes |
| Endogenous biotin block | Yes | No | No |
| Protein block | 15% goat serum | TCT buffer | TCT buffer |
| Antibody complex | 1°+2°-B | 1°+2° | 1°+2° |
| Tertiary | SA-HRP | anti-goat polymer-HRP | anti-rabbit polymer-HRP |
| Substrate | DAB | DAB | DAB |
1°, primary antibody; 2°, secondary antibody; B, biotin. SA-HRP, streptavidin horseradish peroxidase; TCT, buffer containing 0.05 M Tris, 0.15 M NaCl, 0.25% Casein and 0.1% Tween-20; DAB, 3, 3’-diaminobenzidine.
Staining Method Using a Biotinylated Goat Secondary
To create an antibody complex, we combined a biotinylated goat anti-mouse IgG Fab fragment (#115-067-003; Jackson ImmunoResearch Laboratories; West Grove, PA), the desired mouse primary antibody and antibody diluent in a tube at room temperature for 20 min. The ratio used was 2 µg of the secondary antibody for each 1 µg primary antibody (Brown et al. 2004). To ensure that non-complexed secondary antibody cannot bind to endogenous immunoglobulin in the mouse tissue, 10 µl of normal mouse serum (#015-000-120; Jackson ImmunoResearch) was added per 1 µg of secondary antibody and the tube incubated for an additional 10 min (Tuson et al. 1990). This complex was then added to the slide and followed with streptavidin-HRP (# 016-030-084; Jackson ImmunoResearch) at 1:2000 for 30 min. Slides were visualized with DAB (see Table 1).
Staining Method Using an Unconjugated Goat or Rabbit Secondary
To create an antibody complex, we combined the unconjugated secondary, either a rabbit anti-mouse IgG Fab fragment (#315-007-003, Jackson ImmunoResearch) or a goat anti-mouse IgG Fab fragment with the desired mouse primary antibody and antibody diluent in a tube as described above. To ensure that non-complexed secondary antibody cannot bind to endogenous immunoglobulin in the mouse tissue, normal mouse serum was added. A biotin-free polymer, either Mach2 rabbit HRP-Polymer (#RHRP520L, Biocare Medical) or goat HRP-Polymer Kit (# GHRP516H, Biocare Medical) was added to the tissue for 30 min and the staining visualized with DAB (see Table 1).
Staining Method for Triple Immunofluorescence
The anti-SMA antibody complex was created utilizing a biotinylated Fab fragment of a goat anti-mouse antibody. This was followed with an Alexa Fluor 647-labeled Streptavidin 1:200 (#S21374, Invitrogen; Carlsbad, CA). The anti-β−catenin antibody complex was created utilizing an Fab fragment of a rabbit anti-mouse antibody. This was detected with a goat anti-rabbit Alexa Fluor 568-labeled antibody diluted 1:50 (#A11011, Invitrogen). The anti-muscle actin antibody complex was created using an HRP-labeled goat anti-mouse secondary antibody (#810-1302, Rockland Immunochemicals Inc.; Gilbertsville, PA). This was then amplified using the CSAII amplification reagent (#K1497, Dako). The sections were then counterstained with DAPI and coverslipped using ProLong Gold mounting media (Invitrogen).
Imaging
Brightfield images were captured using a Nikon DS-Fi1 camera (Melville, NY). Immunofluorescence images were captured using the Aperio FL (Vista CA), which utilizes a black and white monochrome camera. Each fluorochrome is represented as a pseudo-color in images.
Results
A variety of antigen retrieval buffers were first tested to see if they contributed differently to the amount of endogenous antibody staining observed with anti-mouse secondary reagents. Tissue was pretreated with Trilogy or the pH 6.0 and 9.0 buffers and then incubated with biotinylated goat anti-mouse secondary followed by streptavidin-labeled HRP and developed with DAB. Tissue sections pretreated with Trilogy produced the most endogenous antibody staining (data not shown). The pH 6.0 and the pH 9.0 buffers produced less endogenous antibody staining (data not shown). We selected Trilogy for the two subsequent experiments to monitor how much endogenous antibody staining could be eliminated with the following technique.
Previous studies showed that combining reagents in a small volume increased the signal strength (Brown et al. 2004). To test this theory, antibody complexes were created by initially restricting the diluent volume to only 20% of the total diluent volume. After 20 min, mouse serum was added and allowed to bind any remaining non-complexed secondary antibodies. After 10 min, this mixture was brought to the full working dilution by adding the remaining 80% of the diluent. This method did not show improved staining in our hands (data not shown).
To demonstrate the strength and pattern of staining when the secondary antibody binds to the endogenous mouse antibodies, kidney, spleen, and intestine tissue samples were stained with secondary antibody only (Fig. 1). The slides showed heavy endogenous antibody staining in all three tissues. In the kidney, the renal cortex had ubiquitous staining of the corpuscle, tubules and, especially, the peritubular capillaries (A). In the spleen, darkly stained cells were seen in the red pulp and just beneath the splenic capsule (D). In the intestine, there was slight background on the epithelium and dark staining inside the blood vessels and the lymphoid cells in the lamina propria (G). The isotype control slides, which included the mouse serum in order to bind any non-complexed secondary antibodies, showed little to no endogenous antibody staining. If present, there was some staining on the immune cells in the spleen (E) and intestinal villi (H). To test the effectiveness of our method on mouse tissues, these tissues were stained with anti-smooth muscle actin (SMA). The anti-SMA antibody was complexed with a biotinylated goat anti-mouse Fab fragment in a tube and then the non-complexed secondary was bound up with normal mouse serum. This was visualized with streptavidin labeled-HRP and developed with DAB. The anti-SMA staining was strongly positive on all three tissues. Positive staining was also observed in smooth muscle cells of the blood vessels in the kidney (C), smooth muscle cells of the blood vessels, splenic capsule, and, most notably, around the periarteriola lymphocyte sheath (PALS) in the spleen (F), the muscularis mucosae and in the lamina propria of the intestine (I).
Figure 1.
Mouse-on-mouse staining method on mouse kidney (A, B, C), spleen (D, E, F), and intestine (G, H, I) tissue samples. Panels (A), (D), and (G) illustrate the amount of staining from endogenous mouse immunoglobulin if only anti-mouse secondary antibody is added to tissue. Panels (C), (F), and (I) are stained with the anti- smooth muscle actin (SMA) antibody complexed with the biotinylated secondary antibody. Panels (B), (E), and (H) are stained with an isotype control antibody complexed with the secondary antibody at the same concentration as the anti-SMA antibody. Images were captured at 10×. Scale bar is 100 µm.
All biologically active tissues contain endogenous biotin. Some tissues, including brain, kidney, liver andadipose, express very high levels. This is true for formalin-fixed paraffin-embedded (FFPE) tissues and is especially noticeable in frozen tissues (Wood and Warnke 1981). Streptavidin-labeled detection reagents can interact with endogenous biotin causing significant background. Avidin/biotin blocking reagents can be effective in blocking this background; however, biotin-free detection methods can be favorable. Therefore, a biotin-free mouse-on-mouse system can be very useful. To create a biotin-free detection system that can be utilized with mouse antibodies on mouse tissue, we combined a rabbit anti-mouse Fab fragment with mouse antibodies and then followed this complex with an anti-rabbit polymer (Fig. 2). We also used a goat anti-mouse Fab fragment followed by an anti-goat polymer. Both methods yielded strong SMA staining on mouse kidney with very little background.
Figure 2.
A biotin-free mouse-on-mouse method on mouse kidney tissue. The top row shows positive anti-SMA staining using an unconjugated secondary antibody made in goat (A) or rabbit (C) and developed with biotin-free polymers. The bottom row is the concentration-matched isotype control and shows minimal background with these biotin-free reagents (B and D). Images were captured at 10×. Scale bar is 100 µm.
We continued to test this method using a variety of mouse primary antibodies in a range of mouse tissues (Fig. 3). Primary antibodies were complexed with a Fab fragment of a rabbit anti-mouse antibody in a tube. After allowing ample time to form this complex, mouse serum was added to bind up any non-complexed secondary antibody. This was applied to the tissue and detected with an anti-rabbit-HRP polymer, then visualized with DAB. The anti-HHF35 complex for actin positively stained the muscle fibers in mouse uterus (A). The anti-β−catenin complex stained the epithelial cell junctions in the mouse intestine (B). The anti-estrogen receptor alpha complex stained nuclei in mouse uterus (C). The anti-desmin complex stained mouse skeletal muscle fibers (D). Anti-aspergillus complex specifically stained the wall and septae of the Aspergillus, along with minor hyphae cytoplasmic staining in Aspergillus-infected mouse lung (E). The anti-SV-40 complex stained the nuclei of genetically modified mouse prostate tumor cells (F). The anti-SMA complex stained the smooth muscle cells of blood vessels and muscle of the mouse uterus (G). The anti-hepatocyte complex stained hepatocytes in the mouse liver (H). The anti-cytokeratin AE1/AE3 complex stained the epithelial layer and glands of the mouse skin (I).
Figure 3.
Multiple mouse antibodies demonstrate positive staining with a biotin-free mouse-on-mouse method. The primary antibody was combined with the Fab fragment of a rabbit anti-mouse antibody and detected using an anti-rabbit polymer. The images show staining for anti-muscle actin (HHF35) (A), anti-β−catenin (B), anti-estrogen receptor (C), anti-desmin (D), anti-aspergillus (E), anti-SV-40 (F), anti-smooth muscle actin (SMA) (G), anti-hepatocyte (H), anti-cytokeratin (AE1/AE3) (I). Images were captured at 40x. Scale bar is 50 µm.
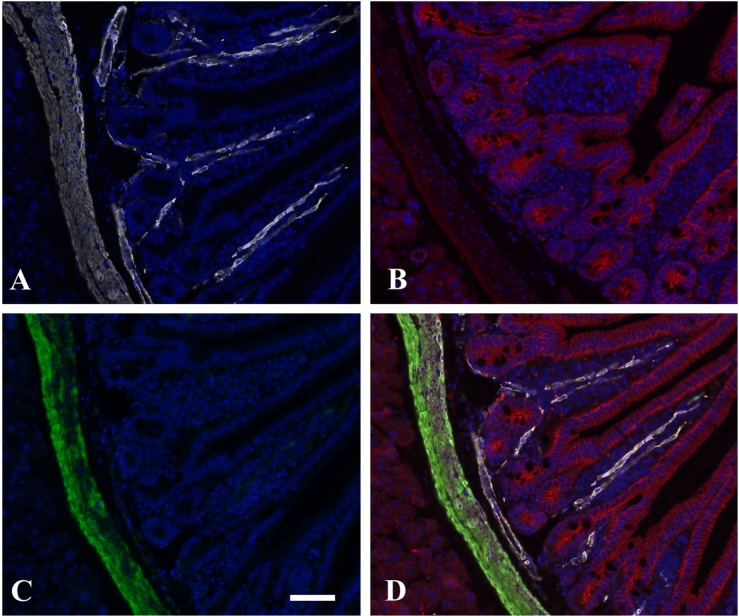
To test the flexibility of this method, we wanted to see how this approach would work in multi-colored immunofluorescence (IF). It is a challenge to perform staining when two or more primary antibodies are made in the same species. The secondary reagent used to detect the second primary antibody can also attach to the first primary antibody, yielding false-positive staining. The staining procedure is further complicated if the primary antibodies are made in the same species as the tissue being stained. Here, we incorporated an avidin/biotin detection system with a biotin-free detection system. In addition, it can be difficult to stain FFPE tissue with fluorochromes in the green range, such as FITC or Alexa 488, because there is often intense autofluorescence in the green range. In our experience, we have often needed to use a great deal of amplification to separate the antibody staining signal from this autofluorescence. Therefore, we utilized DAKO CSAII reagents, which contain a FITC-labeled tyramide, to amplify our third antibody signal. We used HIER with EDTA buffer at pH 9.0 on mouse intestinal tissue samples, as described earlier. We then used anti-SMA complexed with a Fab fragment of a biotinylated goat anti-mouse followed by streptavidin-labeled with Alexa Flour 647 (Fig. 4A). We then used anti-β−catenin antibody complexed with the Fab fragment of a rabbit anti-mouse antibody followed by a goat anti-rabbit antibody conjugated to Alexa Fluor 568 (Fig. 4B). For the third mouse primary antibody, we coupled HHF35 (actin) with a peroxidase-conjugated goat anti-mouse and amplified this signal with the CSA II amplification reagent (Fig. 4C). Figure 4 shows the anti-SMA (pseudo-colored white) staining of the lamina propria and submucosa, and the anti-β−catenin (pseudo-colored red) staining of the cellular junctions of the columnar epithelium and crypts of Lieberkuhn, as expected. The anti-muscle actin (HHF35) (pseudo-colored green) stains mostly the submucosa. When the images were combined, the merge shows the specificity of each antibody staining, with no cross-over between secondary reagents (Fig. 4D).
Figure 4.
Triple immunofluorescence staining using three mouse antibodies on mouse intestinal tissue. Anti-smooth muscle actin (SMA) labeled with Alexa Fluor 647 and pseudo-colored white (A), anti-β−catenin labeled with Alexa Fluor 568 and pseudo-colored red (B), anti-muscle actin (HHF35) labeled with FITC and pseudo-colored green (C), and the merged image showing all three antibodies (D). The tissue section was counterstained with DAPI (pseudo-colored blue). Images were captured at 10×. Scale bar is 100 µm.
Discussion
Our goal was to create a mouse-on-mouse staining method that was reliable, cost-effective and could easily be modified for various uses. The cornerstone of this method is to first combine a mouse primary antibody with an anti-mouse secondary antibody in a tube in order to form a complex. Normal mouse serum is then added to bind up the non-complexed secondary antibodies. The resulting antibody complex can then be added to the tissue.
Using this technique we eliminated all or most of the endogenous antibody staining in the kidney, spleen, and intestinal tissues (Fig. 1). In mouse spleen, however, some of the cells in the white pulp were still lightly stained showing that not all free secondary antibody could be efficiently blocked from binding endogenous immunoglobulin in all tissues when using aggressive antigen retrieval methods. We are currently investigating new techniques to solve this issue. We recommend using a pH 6.0 citrate antigen retrieval buffer where possible in order to avoid this type of unwanted staining. This undesirable staining in the spleen also shows the importance of including isotype controls to accurately interpret data using mouse monoclonal antibodies on mouse tissue. As in the spleen, a subset of cells that stained in intestinal tissue could not be completely eradicated with our technique when using stronger antigen retrieval.
SMA staining of these tissues demonstrated robust and specific staining in smooth muscle cells surrounding the vasculature in all of the tissues, as well as the capsule and the marginal zone of the spleen and the muscularis propria and plicae circulares of the intestine. Furthermore, the staining pattern and elimination of endogenous mouse immunoglobulin staining was consistent whether using biotinylated reagents or biotin-free detection (Fig. 1 and Fig. 2).
Once optimized, we tested this technique with a number of mouse monoclonal antibodies to verify that the staining patterns were correct for a number of antigens (Fig. 3). These slides showed the expected staining patterns for nuclear, cytoplasmic, and cell surface markers. In addition, this technique detected viral antigen SV40 expressed by a genetically engineered mouse model (Yan et al. 1997) and fungal antigens in a mouse model of Aspergillus infection (Jhingran et al. 2012). This technique also worked well with various antibody isotypes, including IgG1, IgG2a and IgM.
This method also proved to be highly flexible. This technique could be used with other enzymes, including streptavidin-labeled alkaline phosphatase, and other chromagens, such as Ferangi Blue, Permanent Red or Substrate Kit III. Staining was easily converted from visible substrates to fluorescence by simply changing the labeling of the streptavidin from HRP to a fluorochrome. In addition, a biotin-free system was created by using a non-biotinylated secondary antibody followed by a biotin-free polymer. In our case, we used both a goat anti-mouse Fab fragment secondary antibody followed by a goat polymer and a rabbit anti-mouse Fab fragment secondary antibody followed by a rabbit polymer. This biotin-free system is desirable when working with biotin-rich tissues, such as kidney, and eliminates the need for costly and time-consuming avidin/biotin blocking steps. This method also increases the usability of a mouse-on-mouse system when performing dual IHC, where a biotin-free amplification could be used alongside a biotin-dependent amplification system. These methods were also used to convert tyramide amplification reagents into mouse-on-mouse systems. Anti-muscle actin was complexed to an anti-mouse secondary conjugated with HRP in a tube and then developed with the CSAII kit (Fig. 4).
We have used this technique on mouse tissue with mouse antibodies and also to stain human tissue with human antibodies; therefore, it is likely that this technique could be used to stain rat tissue with a rat primary antibody as well. For example, anti-rat secondary antibody Fab fragments would be required in order to form the complex with the rat antibody. Rat serum would then be used to block unbound secondary antibodies. The technique could also be used with a rabbit primary on rabbit tissue or goat antibody on goat tissue (with the appropriate secondary antibody and serum).
Finally, this method offers a way to stain any tissue with two or more primary antibodies made in the same species. We used three mouse primary antibodies to stain mouse tissue (Fig. 4). It is important to demonstrate that the reagents used to detect the second or third primary antibodies do not cross-react with the first primary. In order to do this, we included a control that stained for the first antigen (anti-SMA) and then followed this with only the detection for the second primary antibody. It is critical to run this control for each antibody set to verify the specificity of staining. If the second or third detection system binds to the first or second primary antibody (respectively) the staining will not be useful. The power of this method is that it can be used in different scenarios. For example, we used this method to stain human tissue with two rabbit antibodies and others have used commercial kits utilizing the same strategy to stain human tissue with two rat primary antibodies (van der Loos and Gobel 2000).
We believe that this mouse-on-mouse method offers laboratories an affordable and flexible alternative to commercial kits for the use of mouse antibodies on mouse tissue. Furthermore, this method can be used to expand our immunohistochemistry tool box when it comes to using two or more antibodies of the same species in immunohistochemistry and immunofluorescence.
Acknowledgments
A special thanks to Kimberly Melton and Sunni Farley for their valuable feedback while reviewing this paper for submission.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was support by NCI 5 P30 CA015704-39.
References
- Brown JK, Pemberton AD, Wright SH, Miller HR. (2004). Primary antibody-Fab fragment complexes: a flexible alternative to traditional direct and indirect immunolabeling techniques.J Histochem Cytochem 52:1219-1230 [DOI] [PubMed] [Google Scholar]
- Jhingran A, Mar KB, Kumasaka DK, Knoblauch SE, Ngo LY, Segal BH, Iwakura Y, Lowell CA, Hamerman JA, Lin X, Hohl TM. (2012). Tracing conidial fate and measuring host cell antifungal activity using a reporter of microbial viability in the lung. Cell Rep 2:1762-1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negoescu A, Labat-Moleur F, Lorimier P, Lamarcq L, Guillermet C, Chambaz E, Brambilla E. (1994). F(ab) secondary antibodies: a general method for double immunolabeling with primary antisera from the same species. Efficiency control by chemiluminescence. J Histochem Cytochem 42:433-437 [DOI] [PubMed] [Google Scholar]
- Tuson JR, Pascoe EW, Jacob DA. (1990). A novel immunohistochemical technique for demonstration of specific binding of human monoclonal antibodies to human cryostat tissue sections. J Histochem Cytochem 38:923-926 [DOI] [PubMed] [Google Scholar]
- van der Loos CM, Gobel H. (2000). The animal research kit (ARK) can be used in a multistep double staining method for human tissue specimens. J Histochem Cytochem 48:1431-1438 [DOI] [PubMed] [Google Scholar]
- Wood GS, Warnke R. (1981). Suppression of endogenous avidin-binding activity in tissues and its relevance to biotin-avidin detection systems. J Histochem Cytochem 29:1196-1204 [DOI] [PubMed] [Google Scholar]
- Yan Y, Sheppard PC, Kasper S, Lin L, Hoare S, Kapoor A, Dodd JG, Duckworth ML, Matusik RJ. (1997). Large fragment of the probasin promoter targets high levels of transgene expression to the prostate of transgenic mice. Prostate 32: 129-139 [DOI] [PubMed] [Google Scholar]