Abstract
The expression of glucocorticoid receptors (GRs) was investigated immunohistochemically in two different lineages of oligodendrocytes, using carbonic anhydrase (CA) II and neuron glial antigen (NG) 2 as markers of mature oligodendrocytes and oligodendrocyte progenitors, respectively. We focused on the gray matter regions, including CA1, CA3 and the dentate gyrus of the hippocampus, the primary somatosensory cortex barrel field and the basolateral amygdala, and the white matter regions, including the corpus callosum, external capsule and fimbria of the hippocampus. More than 80% of CAII-immunoreactive (IR) cells and more than 95% of NG2-IR cells expressed GRs in various regions of the brain. In contrast, neither CAII-IR cells nor NG2-IR cells expressed mineralocorticoid receptors (MRs) in the same regions. The intensity of GR expression was drastically reduced in CA II-IR cells and NG2-IR cells in the same regions in adrenalectomized mice. Finally, steroid receptor co-activator (SRC)-1 and p300, both of which are cofactors for GR, were expressed in the gray and white matter regions in NG2-IR cells, but not in CAII-IR cells. These results suggest that the expression of GRs in oligodendrocytes and their progenitor cells mediates several functions in vivo, including differentiation and myelination, as a major target of glucocorticoids and their cofactors.
Keywords: Glucocorticoid receptor, oligodendrocyte, oligodendrocyte progenitor, corticosteroid (glucocorticoid), mineralocorticoid receptor, cofactor
Introduction
Glucocorticoids contribute to a wide range of actions in the nervous system, including mediating stress responses, energy metabolism, cell growth and differentiation, and immune and inflammatory responses (Sapolsky et al. 2000; de Kloet et al. 2005). These functions are exerted through two receptor targets, the glucocorticoid receptors (GRs) and mineralocorticoid receptors (MRs), which bind the same hormone (primarily cortisol in humans and corticosterone in rodents) and work as ligand-dependent transcription factors to exert classical glucocorticoid actions (Han et al. 2005). In contrast, rapid, non-genomic actions of glucocorticoids have also been reported (Cintra et al. 1994). The classical hormone actions are regulated by a subset of nuclear proteins called cofactors (coactivators and corepressors), such as steroid receptor coactivator 1 (SRC-1) and p300 (Leo et al. 2000; Wu et al. 2005). Corticosteroids are widely used in the treatment of various types of diseases, including neurodegenerative diseases that affect white matter, such as multiple sclerosis (MS) (Myhr and Mellgren 2009), whereas corticosteroids are ineffective in gray matter injuries, such as head injury and stroke (Sun et al. 2010). Furthermore, recent studies have demonstrated that corticosteroids are involved in various aspects of the regulation of oligodendrocytes, including the proliferation, differentiation, and protection of oligodendrocytes, independent of the treatment of MS. For example, it was recently demonstrated that plasma corticosterone activates SGK1 and induces morphological changes in oligodendrocytes (Miyata et al. 2011). Glucocorticoids also protect oligodendrocytes against excitotoxin (Sun et al. 2010). Thus, it would be interesting to elucidate whether the distribution patterns of GRs in oligodendrocytes in both white matter and gray matter are different.
Although many studies have reported GR expression in cultured oligodendrocytes (Bohn et al. 1991), very few investigations have reported GR expression in oligodendrocytes in vivo. The primary purpose of the present study was to investigate the expression of GRs and GR cofactors in oligodendrocytes in the brains of adult mice. To this end, we performed three major experiments, as follows. First, the expression of GRs in oligodendrocytes was investigated immunohistochemically in various regions of the brains of adult mice. Because GRs are involved in cell growth and differentiation in the central nervous system (CNS), the expression pattern of GRs in different lineages of oligodendrocytes would be helpful in understanding this role. We selected two markers for oligodendrocytes, carbonic anhydrase (CA) II and neuron glial antigen (NG) 2. CAII is one of seven CA isozymes that are expressed in the CNS and is considered as a marker of adult oligodendrocytes (Ghandour et al. 1980). In contrast, NG2 is an integral membrane chondroitin sulfate proteoglycan, which is expressed on oligodendrocyte progenitors (Nishiyama et al. 1999). We analyzed the gray matter regions, including CA1, CA3 and the dentate gyrus in the hippocampus, primary somatosensory cortex barrel field and basolateral amygdala, and on the white matter regions, including the corpus callosum, external capsule and fimbria of the hippocampus. The expression of another corticosteroid receptor, MR, was also investigated in oligodendrocytes in the same regions of the adult brain using the same oligodendrocyte markers. Next, the effect of reduction of corticosterone (CORT) levels on the expression of GRs in CAII-immunoreactive (IR) oligodendrocytes and NG2-IR oligodendrocyte progenitors was examined in adult mice that had undergone bilateral adrenalectomy to determine whether GRs expressed in oligodendrocytes are functional and responsive to its ligand, CORT. Finally, the expression of steroid receptor co-activator (SRC)-1 and p300 was investigated immunohistochemically in CAII-IR oligodendrocytes and NG2-IR oligodendrocyte progenitors, along with GR expression. The transcriptional activity of GRs is enhanced by the recruitment of one of the transcriptional co-activators of the p160 family (SRCs), with a docking platform for secondary co-activators, such as the cAMP-response element binding protein (CREB)-binding protein (CBP), or its close homologue p300. Although glucocorticoids have been reported to promote myelination through SRC-1 or p300 in Schwann cells, it is not known whether SRC-1 and p300 are localized in oligodendrocytes (Désarnaud et al. 2000). Therefore, clarification of SRC-1 and p300 expression in oligodendrocytes would help to determine whether GR function is regulated by these cofactors.
The results demonstrate for the first time that GRs are expressed in different lineages of oligodendrocytes, mature CAII-IR oligodendrocytes and NG2-IR oligodendrocyte progenitors in vivo, and suggest that GRs mediate several important functions, including the promotion of myelination, as a major target of glucocorticoids.
Materials & Methods
Animals
Adult male C57BL/6 mice (8-10 weeks old) were purchased from Japan SLC (Hamamatsu, Japan) and housed in groups (4 animals /case) in a room with a 12 hr light-dark cycle with the light turned on at 8:00 AM. Food pellets and drinking water were provided ad libitum. The protocols for this study were approved by the Animal Care Committee of Nara Medical University in accordance with the policies established by the NIH for the care and use of laboratory animals.
Preparation of the Brain Sections
Mice were deeply anesthetized in the morning (9:30-11:30 AM) by an intraperitoneal injection of pentobarbital sodium salt solution (Somnopencyl 0.1 ml/10 g bodyweight; Kyoritsu Seiyaku Corporation, Tokyo, Japan) and then perfused intracardially with ice-cold phosphate-buffered saline (PBS) followed by 4% paraformaldehyde (PFA), pH 7.4. After perfusion, the brains were removed and fixed in 4% PFA at 4C overnight. Coronal sections were cut (50-µm sections) with a linear slicer (Pro. 7, DOSAKA EM CO. (D.K.S.), Kyoto, Japan).
Adrenalectomy and Corticosterone (CORT) Assay
Six mice underwent bilateral adrenalectomy in the morning (9:30-11:30 AM) and another six mice underwent sham-operation for adrenalectomy. After the mice were anesthetized with isoflurane (Escain; Mylan Inc., Pittsburgh, PA), the skin of the right side of their flank was opened, the muscles dissected and the right adrenal gland removed. The left adrenal gland was removed through the same skin incision. After adrenalectomy, the mice were provided drinking water supplemented with 0.9% saline. Three days after adrenalectomy, the animals were sacrificed under pentobarbital anesthesia. Blood samples were collected by intracardiac puncture and stored in heparinized tubes. Plasma was obtained by centrifugation (2,000 ×g for 10 min at 4C) and stored at -80C until the day for subsequent assay. The plasma concentrations of CORT, the rodent glucocorticoid, were measured using an enzyme immunoassay (EIA0 kit (YK240, Yanaihara Institute, Inc., Shizuoka, Japan) to confirm resection of the adrenal glands. After the blood samples were obtained, the animals were perfused with ice-cold PBS followed by PFA. The brains were removed and fixed with PFA. Coronal sections (50-µm thick) were prepared as described above.
Immunohistochemistry
Free-floating coronal sections were washed with PBS, immersed in 25 mM glycine in PBS for 20 min, and permeabilized with 0.3% Triton-X 100 in PBS (PBS-T) for 15 min. After blocking in PBS-T supplemented with 5% normal goat serum (NGS) or 5% normal horse serum (NHS) at room temperature for 2 hr, rabbit polyclonal antibody against rat GR (1:1000; provided by Prof. M. Kawata [Morimoto et al. 1996]) and the mouse monoclonal antibody against rat MR (1:200; provided by Prof. C. E. Gomez-Sanchez [Gomez-Sanchez CE et al. 2006]) were used to detect GR- and MR-immunoreactivities, respectively. A sheep polyclonal antibody against human Carbonic Anhydrase II antibody (CAII, ab8953) (1:1000; Abcam, Cambridge, UK) was used to identify mature oligodendrocytes, and a polyclonal guinea pig antibody against rat NG2 (1:200; provided by Dr. W. B. Stallcup [Ozerdem et al. 2001; Stallcup 2002; Kucharova et al. 2011]) was used to identify oligodendrocyte progenitors. The antibodies used to examine the cofactors in the oligodendrocytes included a polyclonal rabbit antibody against human SRC-1 (M-341) (1:500; Santa Cruz Biotechnology, Dallas, TX), and a monoclonal mouse antibody against rat p300 (NM-11; 1:250; Calbiochem, Darmstadt, Germany). Sections were incubated with primary antibodies (diluted in PBS-T) with 1% NHS at 4C overnight. After washing, sections were incubated with either AlexaFluor-488 labeled donkey-anti-rabbit, AlexaFluor-488 labeled goat-anti-rabbit IgG, AlexaFluor-488 labeled donkey-anti-mouse IgG, AlexaFluor-488 labeled goat-anti-mouse IgG or AlexaFluor-546 labeled donkey-anti-sheep and AlexaFluor-546 labeled goat-anti-guinea pig (all secondary antibodies 1:1000, Molecular Probes, Carlsbad, CA) in PBS-T with 1% NHS for 2 hr at room temperature. After washing with PBS for 15 min, sections were mounted and shielded with Vectashield® mounting media (Vector Laboratories, Burlingame, CA) containing 4’, 6-diamino-2-phenylindole (DAPI), and stored in the dark until further analysis. Observations and image capture were carried out using a FluoView 1000 confocal microscope (Olympus, Tokyo, Japan).
Quantitative Analysis
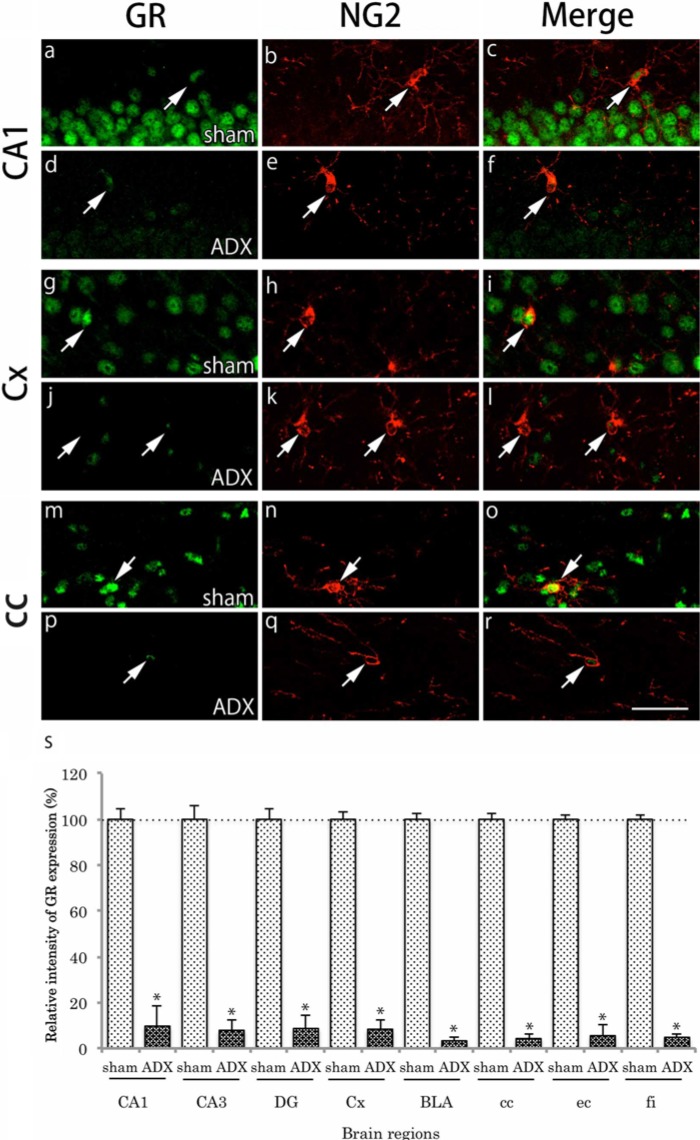
To quantify the percentages of GR- and MR-immunoreactivities in oligodendrocytes and to measure the fluorescence intensity of GR-immunoreactivity in oligodendrocytes from sham- and adrenalectomy-operated mice, brain sections were double-labeled with GR and CAII or GR and NG2, all of which were counterstained with DAPI. Sections were taken 1.4-1.7 mm posterior to the Bregma, according to the mouse brain atlas (Franklin and Paxinos 2008) were used for all analyses. Analyzed regions are illustrated in Figure 1. Fluorescence images were captured using a confocal laser microscope (Olympus Fluoview 1000) with the 40× objective lens. To count GR-IR cells in oligodendrocytes, four images per region were obtained per mouse and the ratio of GR-IR cells in CAII- or NG2-IR cells was calculated. The total number of CAII-IR- or NG2-IR-positive cells counted in each brain region ranged from 35 to 453 cells, with an average of 116 cells. The data is expressed as the mean value ± SD (n=4 mice). For signal intensity analyses, images of all samples were captured under the same microscopic conditions and four images per region were analyzed per mouse using the FV10-ASW software (Olympus). Briefly, cell bodies of the oligodendrocytes were outlined by means of CAII-or NG2-IR and the average fluorescence intensity of GR was measured within the outlined areas. The mean value of the sham-operated group (n=6) in each region is considered as 100% and the relative GR intensity in the adrenalectomy group (n=6) is shown.
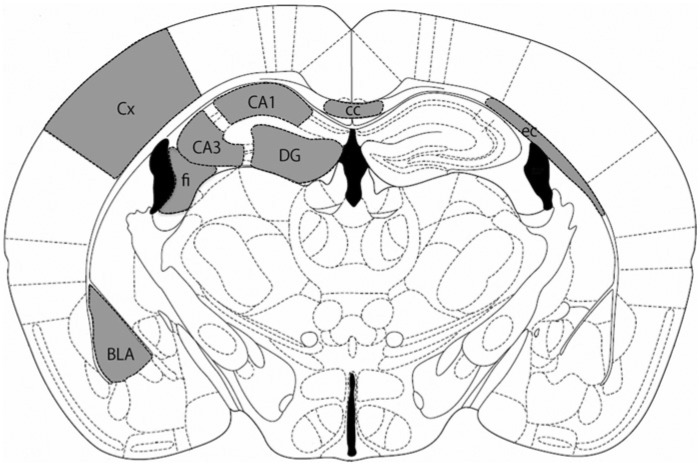
Figure 1.
Brain regions. The shaded regions were analyzed in all experiments. Sections were taken 1.4-1.7 mm posterior to the Bregma, according to the mouse brain atlas (Franklin and Paxinos 2008). For abbreviations, see Table 1.
Statistical Analysis
Statistical analyses were performed by t-test using JMP8 software (SAS Institute Japan, Tokyo, Japan) and P-values of less than 0.05 were considered statistically significant.
Results
GR Expression in CAII-IR Cells in the Gray and White Matter Regions
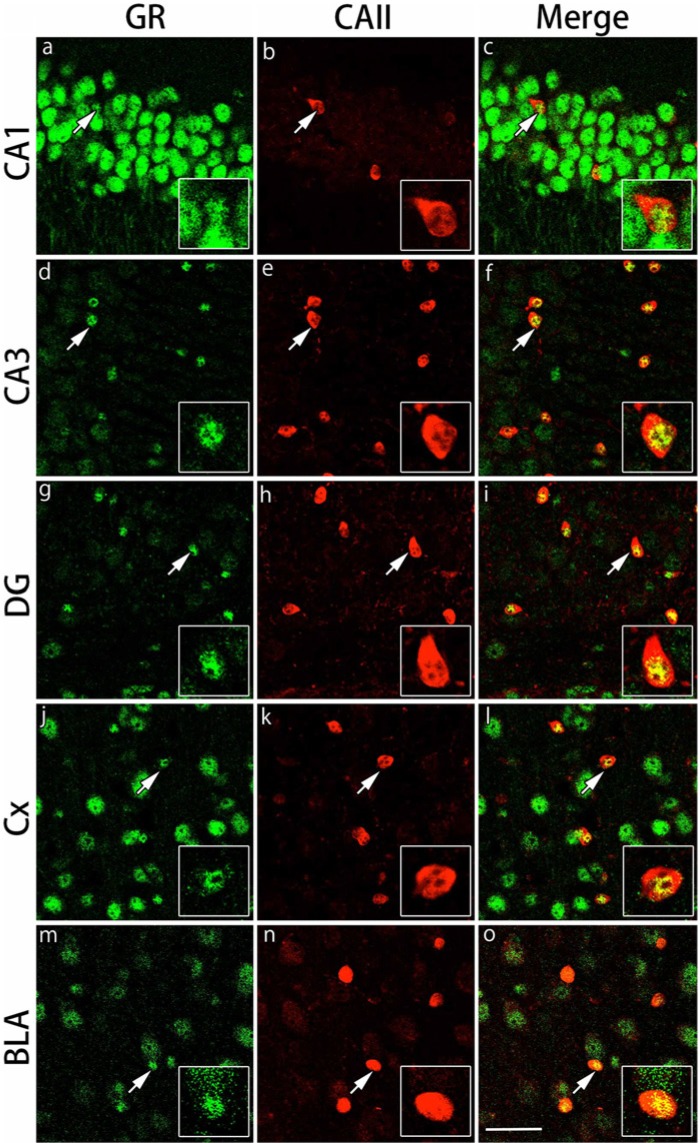
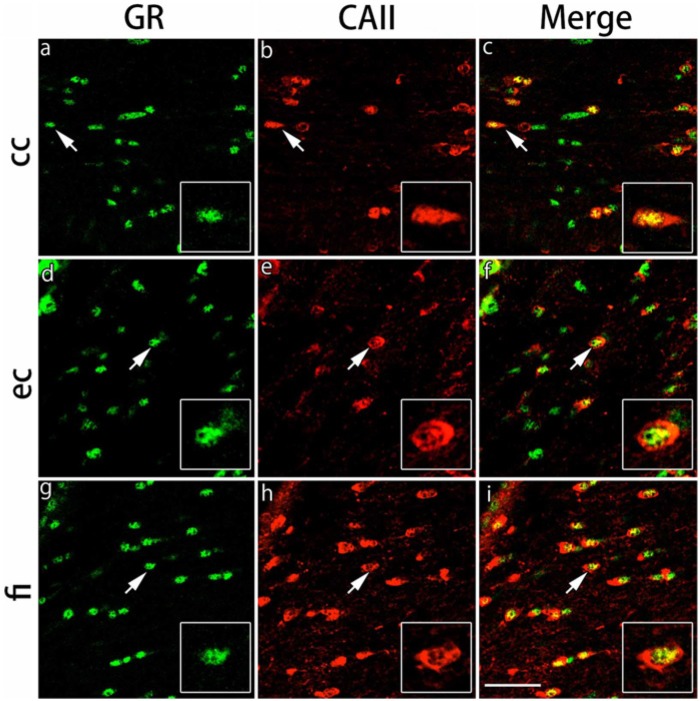
The expression of GRs in CAII-IR oligodendrocytes in the gray and white matter regions was examined by double immunofluorescence staining. We focused on CA1, CA3 and the dentate gyrus of the hippocampus, the primary somatosensory cortex barrel field and the basolateral amygdala in the gray matter regions and the corpus callosum, external capsule and fimbria of the hippocampus in the white matter regions. GRs were expressed broadly in various areas of the gray matter regions. The immunostaining pattern for GRs and CAII was somewhat different in each region. A recent paper demonstrated that different populations of oligodendrocytes are differentially distributed in various layers of the hippocampus (Vinet et al. 2010); thus, we analyzed the differential distribution patterns of GR in oligodendrocytes in the hippocampal subregions. In the CA1 region, GRs were expressed in many cells with strong intensity, but CAII-IR cells were sparse in this region (Fig. 2). In contrast, whereas the number of CAII-IR cells per field was slightly greater in the CA3 and dentate gyrus regions, the intensity of GR expression was weak in these regions (Fig. 2), which was similar to a previous neuronal study (Sarabdjitsingh et al. 2009). Most of the CAII-IR cells expressed GRs in the pyramidal cell layers of CA1 and CA3 and in the hilus of the dentate gyrus region. The expression of GRs in CAII-IR cells was observed in small nuclei with relatively strong intensity. In the primary somatosensory cortex barrel field and basolateral amygdala, most of the CAII-IR cells also expressed GRs (Fig. 2). In the basolateral amygdala, the intensity of GR immunoreactivity was weaker than that in other gray matter regions (Fig. 2). The ratio of double IR cells of GRs and CAII per total CAII-IR cells was calculated in each region. More than 80% of CAII-IR cells expressed GRs in the gray matter regions examined (Table 1). In the white matter regions, GRs were primarily expressed in small nuclei of the cells in the corpus callosum, external capsule and the fimbria of the hippocampus (Fig. 3). The number of CAII-IR cells per field was greater in the fimbria of the hippocampus compared with the corpus callosum and external capsule. More than 85% of CAII-IR cells expressed GRs in the white matter regions examined (Table 1).
Figure 2.
Double immunohistochemical labeling of GR and CAII in the gray matter regions. Brain slices were double immunofluorescently stained for glucocorticoid receptor (GR; green) and carbonic anhydrase II (CAII; red). The staining in hippocampal CA1 (a-c), hippocampal CA3 (d-f), dentate gyrus (DG) (g-i), primary somatosensory cortex barrel field (Cx) (j-l) and the basolateral amygdala (BLA) (m-o) are shown. The cells indicated by arrows show co-localization of CAII and GR (inset: magnified view). Scale bar: 50 µm.
Table 1.
Glucocorticoid Receptor (GR) Expression in Carbonic Anhydrase (CA)-II-immunoreactive (IR) or Neuron Glial Antigen (NG)-2-IR cells.
| Brain regions | GR- and CAII-double IR cells per total CAII-IR cells (%) |
GR- and NG2-double IR cells per total NG2-IR cells (%) |
|---|---|---|
| Gray matter regions | ||
| Hippocampus | ||
| CA1 | 83 ± 11 | 100 ± 0 |
| CA3 | 81 ± 5 | 99 ± 1 |
| Dentate gyrus (DG) | 91 ± 3 | 100 ± 0 |
| Primary somatosensory cortex barrel field (Cx) | 90 ± 2 | 99 ± 2 |
| Basolateral amygdala (BLA) | 87 ± 4 | 100 ± 0 |
| White matter regions | ||
| Corpus callosum (cc) | 91 ± 1 | 96 ± 3 |
| External capsule (ec) | 86 ± 2 | 100 ± 0 |
| Fimbria hippocampus (fi) | 91 ± 2 | 100 ± 0 |
CAII-IR or NG2-IR cells in each brain region were counted. Data are expressed as the mean ± SD (n=4 mice).
Figure 3.
Double immunohistochemical labeling of GR and CAII in the white matter regions. Brain slices were double immunofluorescently stained for glucocorticoid receptor (GR; green) and carbonic anhydrase II (CAII; red). The staining in the corpus callosum (cc) (a-c), external capsule (ec) (d-f) and fimbria of the hippocampus (fi) (g-i) are shown. The cells indicated by arrows show co-localization of CAII and GR (inset: magnified view). Scale bar: 50 µm.
GR Expression in NG2-IR Oligodendrocyte Progenitors in the Gray and White Matter Regions
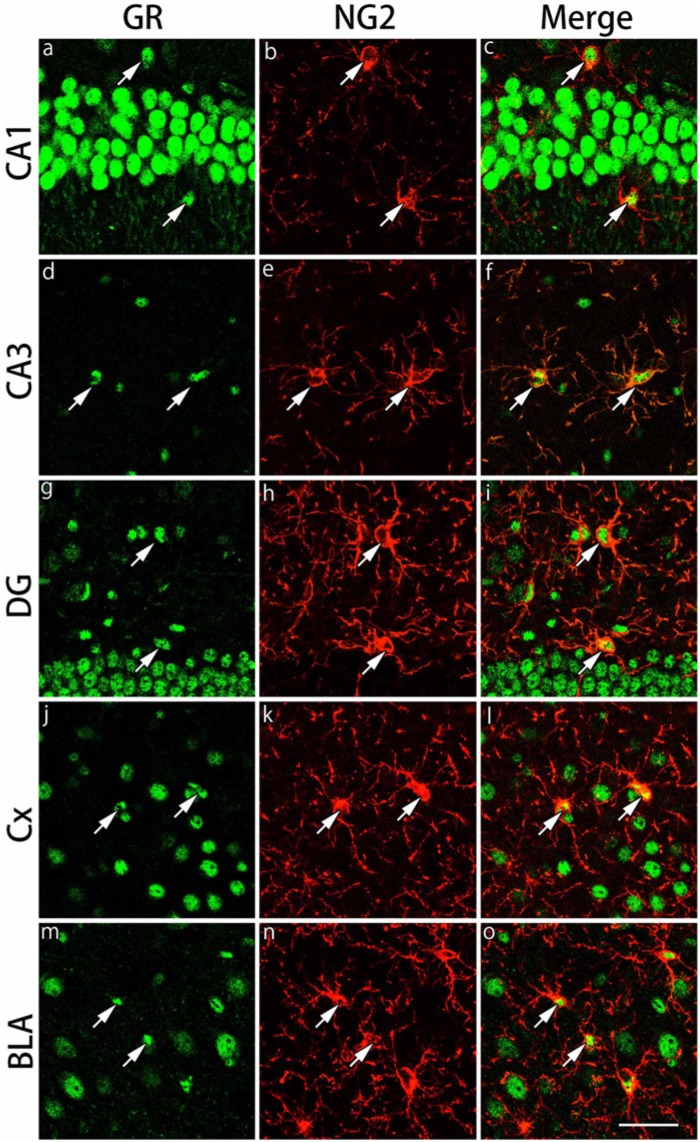
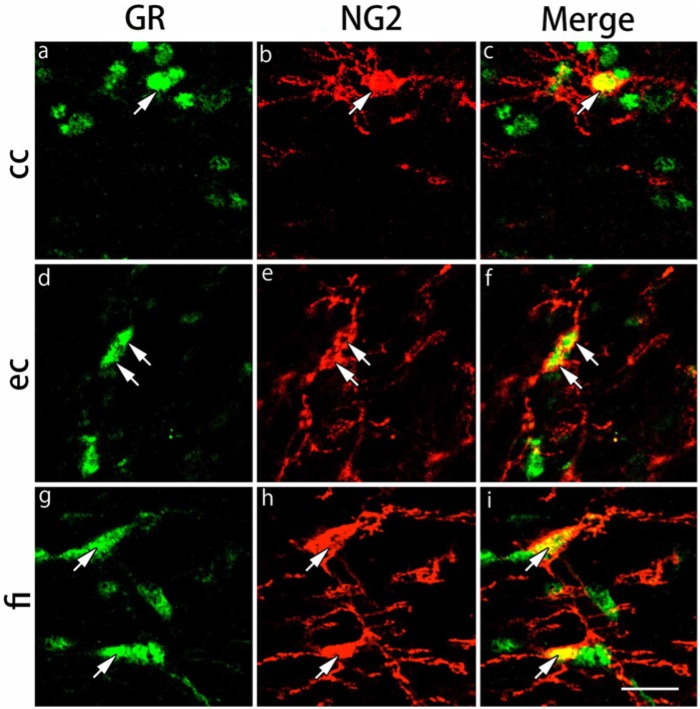
The expression of GRs in NG2-IR in oligodendrocyte progenitors of the gray and white matter regions was examined by double immunofluorescence staining. We examined the same brain regions as those of CAII-IR oligodendrocytes. NG2-IR cells were observed in the pyramidal cell layers of the hippocampus, CA1 and CA3 and hilus of dentate gyrus regions in the gray matter regions (Fig. 4). In the primary somatosensory cortex barrel field and basolateral amygdala, the majority of NG2-IR cells were large cells. Almost all NG2-IR cells expressed GRs in the gray matter regions (Table 1). In the white matter regions, NG2-IR cells exhibited small nuclei in the corpus callosum, external capsule and the fimbria of the hippocampus (Fig. 5). GRs were expressed in more than 95% of NG2-IR cells (Table 1).
Figure 4.
Double immunohistochemical labeling of GR and NG2 in the gray matter regions. Brain slices were double immunofluorescently stained for glucocorticoid receptor (GR; green) and neuron glial antigen-2 (NG2; red). The staining in hippocampal CA1 (a-c), hippocampal CA3 (d-f), dentate gyrus (DG) (g-i), primary somatosensory cortex barrel field (Cx) (j-l) and the basolateral amygdala (BLA) (m-o) are shown. The cells indicated by arrows show co-localization of GR and NG2. Scale bar: 50 µm.
Figure 5.
Double immunohistochemical labeling of GR and NG2 in the white matter regions. Brain slices were double immunofluorescently stained for glucocorticoid receptor (GR; green) and neuron glial antigen-2 (NG2; red). The staining in the corpus callosum (cc) (a-c), external capsule (ec) (d-f) and the fimbria of the hippocampus (fi) (g-i) are shown. The cells indicated by arrows show co-localization of GR and NG2. Scale bar: 25 µm.
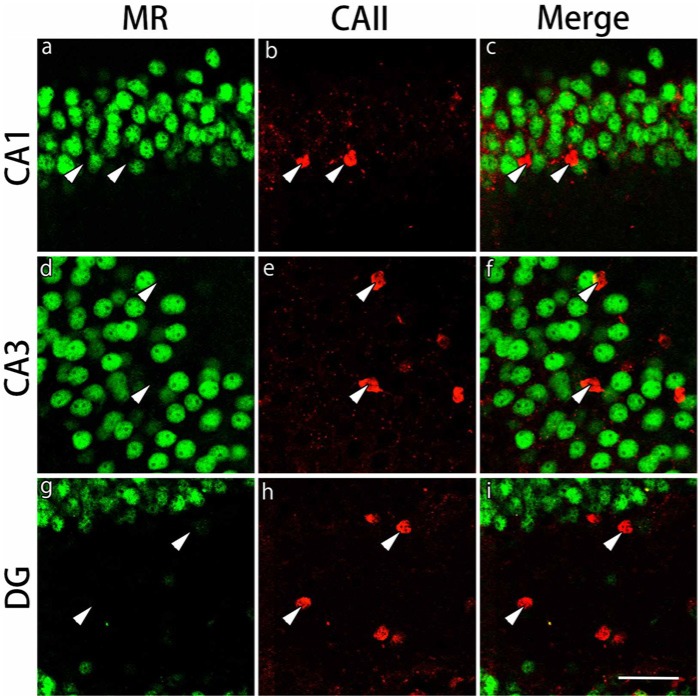
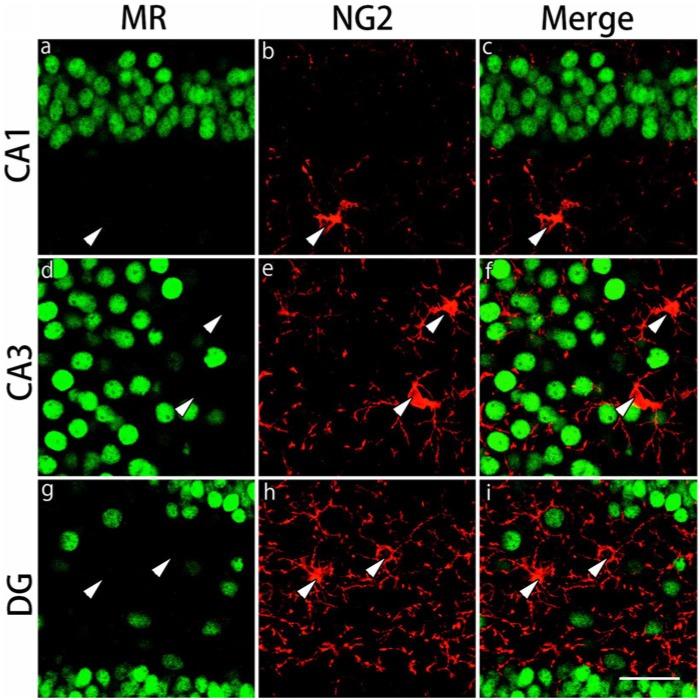
Absence of MR Expression in the CAII-IR or NG2-IR Cells in the Gray and White Matter Regions
The expression of MRs in CAII-IR or NG2-IR cells in the gray and white matter regions was examined by double staining. MRs were ubiquitously expressed in CA1, CA3 and dentate gyrus in the gray matter region (Figs. 6, 7). Most of the CAII-IR or NG2-IR cells were negative for MRs in these regions. MRs were also expressed in the corpus callosum, external capsule and the fimbria of the hippocampus in the white matter region (data not shown). Similarly, very few of the CAII-IR or NG2-IR cells expressed MRs in these regions (data not shown).
Figure 6.
Double immunohistochemical labeling of MR and CA II in the gray matter regions. Brain slices were double immunofluorescently stained for mineralocorticoid receptor (MR; green) and carbonic anhydrase-II (CAII; red). The staining in hippocampal CA1 (a-c), hippocampal CA3 (d-f) and the dentate gyrus (DG) (g-i) are shown. CAII-immunoreactive cells indicated by arrowheads in the merged images do not express MRs. Scale bar: 50 µm.
Figure 7.
Double immunohistochemical labeling of MR and NG2 in the gray matter regions. Brain slices were double immunofluorescently stained for mineralocorticoid receptor (MR; green) and neuron glial antigen 2 (NG2; red). The staining in hippocampal CA1 (a-c), hippocampal CA3 (d-f) and the dentate gyrus (DG) (g-i) are shown. NG2-immunoreactive cells indicated by arrowheads in the merged images do not express MRs. Scale bar: 50 µm.
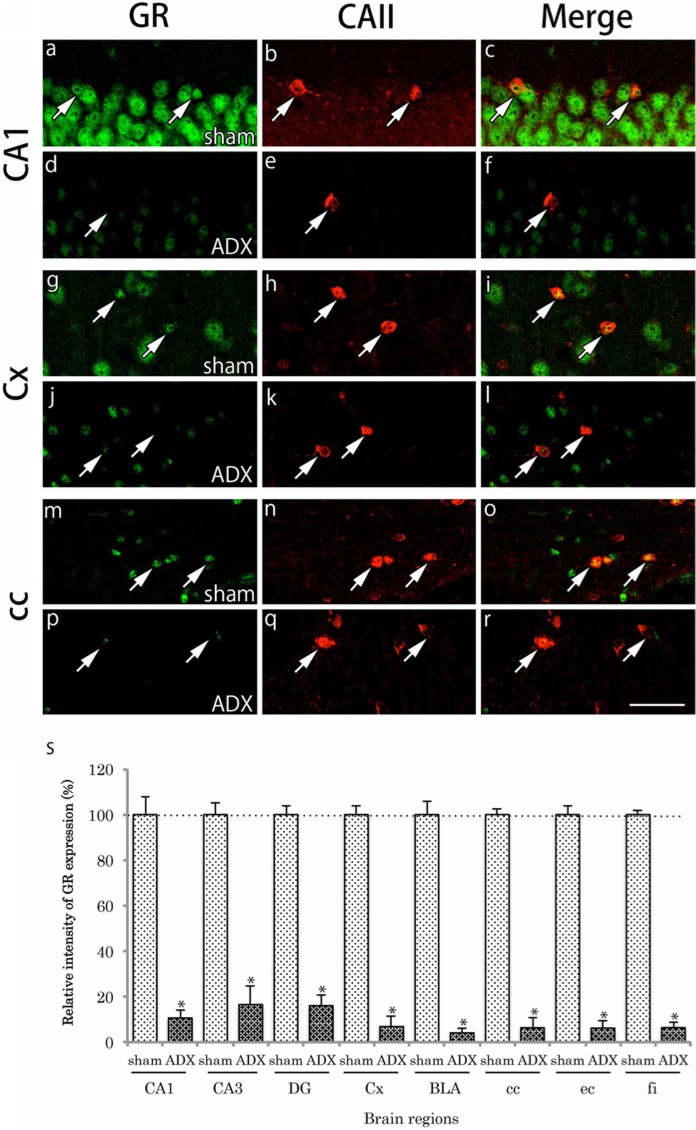
Effects of Serum CORT on GR Distribution in CAII-IR and NG2-IR Cells
In adrenalectomized mice, plasma levels of CORT decreased to about 20% of those in sham-operated mice (data not shown). This result indicates that plasma levels of CORT were successfully reduced by adrenalectomy. GR immunoreactivity was remarkably reduced in almost all brain regions in the adrenalectomy group. The reduction of GR immunoreactivity was also evident in both CAII-IR and NG2-IR cells in the regions examined (Figs. 8, 9). We show the representative regions, CA1, primary somatosensory cortex, and corpus callosum of the gray and white matter regions. The relative intensity of GR immunoreactivity in CAII-IR or NG2-IR cells was significantly reduced in adrenalectomized mice compared with sham-operated mice (Figs. 8, 9).
Figure 8.
Effects of adrenalectomy on GR immunohistochemical labeling in CAII-positive cells. Brain slices were double immunofluorescently stained for glucocorticoid receptor (GR; green) and carbonic anhydrase II (CAII; red). For comparison, the staining in the hippocampal CA1 region (a-c), primary somatosensory cortex (Cx) (g-i) and the corpus callosum (cc) (m-o) after sham operation are shown. The staining in CA1 (d-f), Cx (j-l), and cc (p-r) after adrenalectomy are also shown. GR immunoreactivity in the nucleus was very weak in the CAII-immunoreactive (IR) cells (arrows). Scale bar is 50 µm. The relative intensity of GR-immunoreactivity in CAII-IR cells in adrenalectomized mice (n=6) compared with that in sham-operated mice (n=6) is shown (s). The intensity of GR immunoreactivity in CAII-IR cells was notably reduced in adrenalectomized mice compared with that in sham-operated mice. The difference was statistically significant (*p<0.05). Data are expressed as the mean ± SD. Abbreviations: hippocampal CA3, CA3; basolateral amygdala, BLA; dentate gyrus, DG; external capsule, ec; and fimbria of the hippocampus, fi.
Figure 9.
Effects of adrenalectomy on GR immunohistochemical labeling in NG2-positive cells. Brain slices were double immunofluorescently stained for glucocorticoid receptor (GR; green) and neuron glial antigen 2 (NG2; red). For comparison, the staining in CA1 (a-c), primary somatosensory cortex (Cx) (g-i) and the corpus callosum (cc) (m-o) after sham operation are shown. The staining in the hippocampal CA1 (d-f), Cx (j-l), and cc (p-r) after adrenalectomy are also shown. GR immunoreactivity in the nucleus was very weak in NG2-immunoreactive (IR) cells (arrows). Scale bar: 50 µm. The relative intensity of GR immunoreactivity in CAII-IR cells in adrenalectomized mice (n=6) against that in sham-operated mice (n=6) is shown (s). The intensity of GR immunoreactivity in NG2-IR cells was remarkably reduced in adrenalectomized mice compared with that in sham-operated mice. The difference was statistically significant (*p<0.05). Data are expressed as the mean ± SD. Abbreviations: hippocampal CA3, CA3; basolateral amygdala, BLA; dentate gyrus, DG; external capsule, ec; and fimbria of the hippocampus, fi.
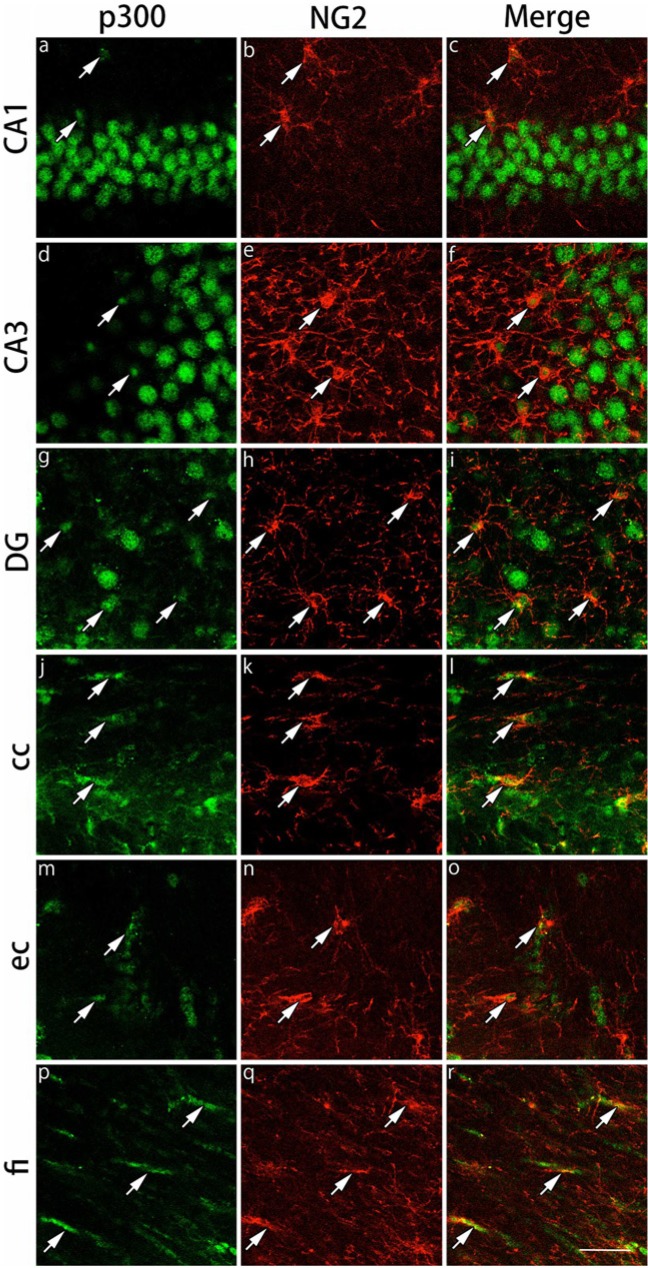
Expression of GR Cofactors in CAII-IR and NG2-IR Cells
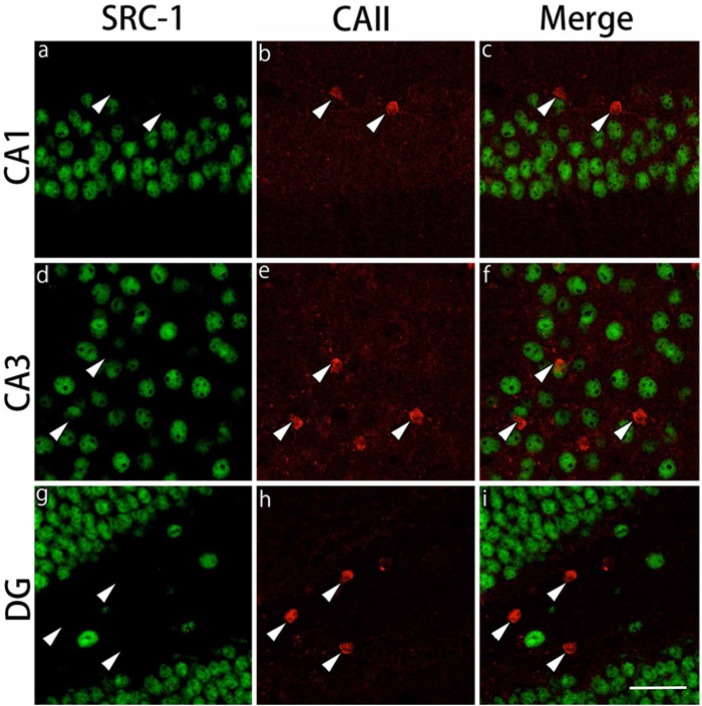
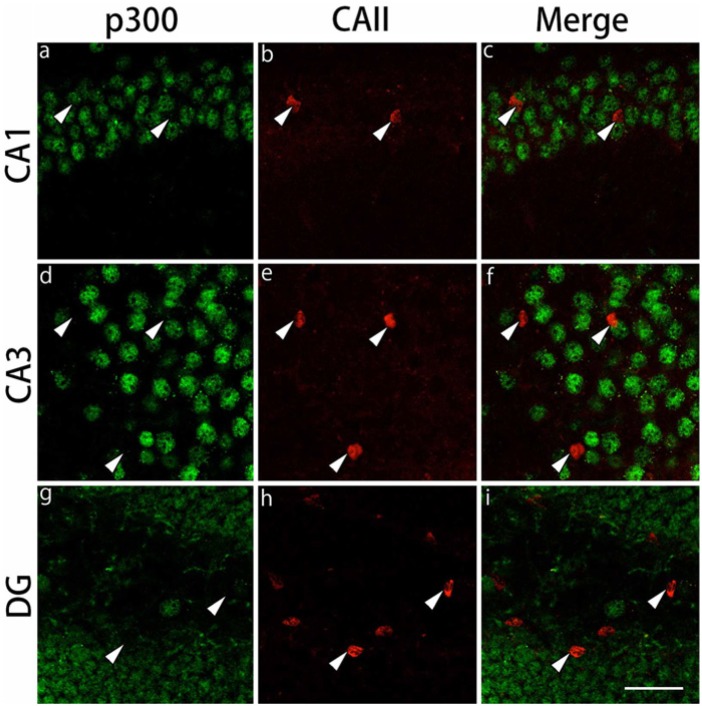
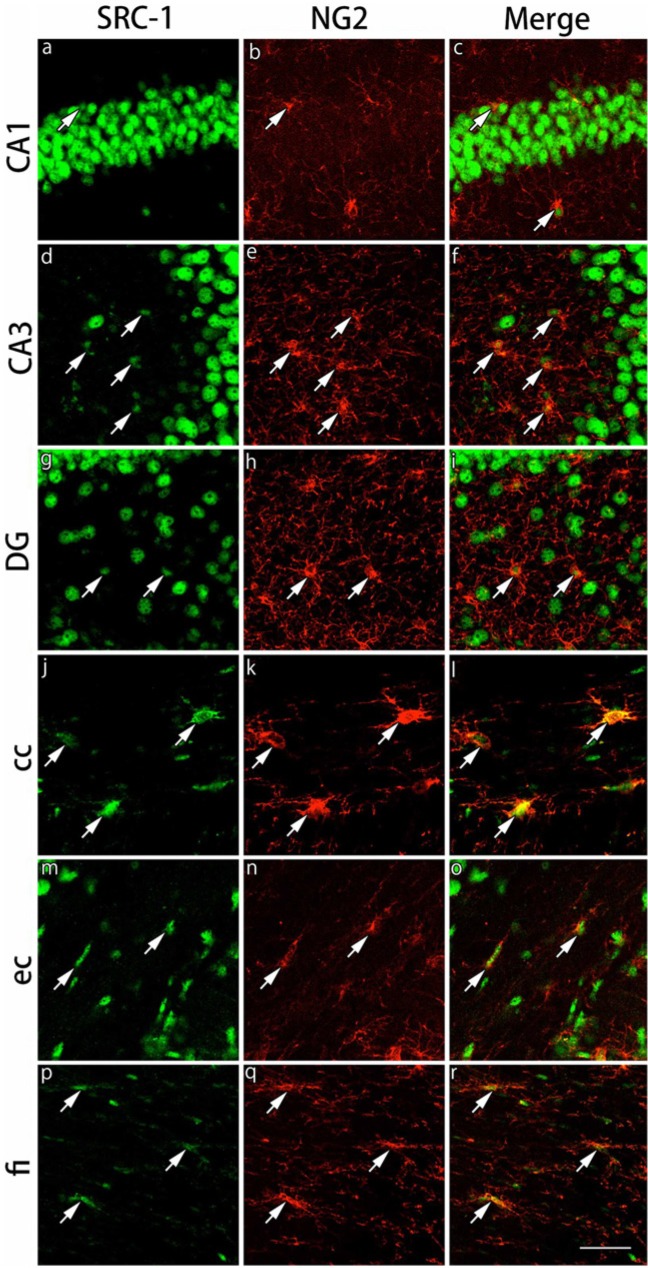
The expression of the GR cofactors, SRC-1 and p300, was examined in CAII-IR and NG2-IR cells. In the CAII-IR cells, neither SRC-1 nor p300 was expressed in the gray and white matter regions examined (Figs. 10, 11). On the other hand, both SRC-1 and p300 were expressed in NG2-IR cells in both the gray matter and white matter regions, showing more intensely and clearly in the corpus callosum and external capsule (Figs. 12, 13). Because NG2-IR cells express both SRC-1 and p300, we investigated colocalization of GRs with these cofactors in NG2-IR cells. We observed the co-expression of GR and p300 in NG2-IR oligodendrocyte progenitors by triple immunostaining (data not shown).
Figure 10.
Double immunohistochemical labeling of SRC-1 and CAII in the gray matter regions. Brain slices were double immunofluorescently stained for steroid receptor co-activator-1 (SRC-1; green) and carbonic anhydrase II (CAII; red). The staining in hippocampal CA1 (a-c), hippocampal CA3 (d-f) and the dentate gyrus (DG) (g-i) are shown. CAII-immunoreactive cells indicated by arrowheads do not express SRC-1 in these brain regions. Scale bar: 50 µm.
Figure 11.
Double immunohistochemical labeling of p300 and CAII in the gray matter regions. Brain slices were double immunofluorescently stained for p300 (green) and carbonic anhydrase II (CAII; red). The staining in hippocampal CA1 (a-c), hippocampal CA3 (d-f) and dentate gyrus (DG) (g-i) are shown. CAII-immunoreactive cells indicated by arrowheads do not express p300 in these brain regions. Scale bar: 50 µm.
Figure 12.
Double immunohistochemical labeling of SRC-1 and NG2 in the gray and white matter regions. Brain slices were double immunofluorescently stained for steroid receptor co-activator-1 (SRC-1; green) and neuron glial antigen 2 (NG2; red). The staining in hippocampal CA1 (a-c), hippocampal CA3 (d-f) and the dentate gyrus (DG) (g-i) in the gray matter region, and the corpus callosum (cc) (j-l), external capsule (ec) (m-o) and the fimbria of the hippocampus (fi) (p-r) in the white matter region are shown. The cells indicated by arrows show co-localization of SRC-1 and NG2. Expression of SRC-1 in NG2-immunoreactive cells in the white matter regions is stronger than that in the gray matter regions. Scale bar: 50 µm.
Figure 13.
Double immunohistochemical labeling of p300 and NG2 in the gray and white matter regions. Brain slices were double immunofluorescently stained for p300 (green) and neuron glial antigen 2 (NG2; red). The staining in hippocampal CA1 (a-c), hippocampal CA3 (d-f) and the dentate gyrus (DG) (g-i) in the gray matter region, and the corpus callosum (cc) (j-l), external capsule (ec) (m-o), and the fimbria of the hippocampus (fi) (p-r) in the white matter region are shown. The cells indicated by arrows show co-localization of p300 and NG2. Expression of p300 was stronger in the NG2-immunoreactive cells in the white matter region compared with the gray matter region. Scale bar: 50 µm.
Discussion
Previous studies have demonstrated that GRs are expressed in various regions of the adult brain (Cintra et al. 1994; Usuku et al. 2005). We confirmed that the distribution and staining pattern of GR immunoreactivity in the present study was consistent with those of previous studies using the same GR antibody (Morimoto et al. 1996). Many in vitro studies have demonstrated the expression of GRs in cultured oligodendrocytes and that GRs are involved in promoting myelination (Vielkind et al. 1990; Chan et al. 1998). Moreover, a recent in vivo study of the peripheral nervous system demonstrated that Schwann cells expressed GRs and that glucocorticoids enhanced the rate of myelin formation through GRs (Morisaki et al. 2010). In the present study, we investigated GR expression in two different lineages of oligodendrocytes using immunohistochemistry. Characterization of a number of specific biochemical markers has increased our knowledge regarding the stages of oligodendrocyte maturation, both in vitro and in vivo (Baumann and Pham-Dinh 2001). We found that GRs were expressed in mature CAII-IR oligodendrocytes in various brain regions of adult mice including both gray and white matter. These findings suggest that signal transduction through GRs in oligodendrocytes plays an important role in myelination of the adult brain in vivo. Furthermore, we observed that GRs were expressed in NG2-IR oligodendrocyte progenitors in various brain regions of adult mice, including both gray and white matter. Nishiyama and colleagues demonstrated that NG2-IR cells existed uniformly throughout the mature CNS, in both the gray and white matter, and that NG2-IR cells were antigenically oligodendrocyte progenitors that were differentiating into oligodendrocytes in vivo (Nishiyama et al. 1999). Careful examination of the relationship between NG2-IR cells and microglia showed no overlap between the two populations of cells. Thus, NG2 should be considered as one of the most reliable and widely used markers for oligodendrocyte progenitors in the CNS (Nishiyama et al. 1999; Polito and Reynolds 2005; Kucharova and Stallcup 2010). Our results, demonstrating GR expression in oligodendrocyte progenitors in the adult brain, suggest that GRs are involved in the differentiation processes of oligodendrocytes. These findings are consistent with the role of GRs in regulating cell growth and differentiation in the CNS (Chan et al. 1998; Alonso 2000; Schröter et al. 2009). It has been reported that NG2-IR oligodendrocyte progenitors are also present in MS lesions (Chang et al. 2002). The reasons for the absence of remyelination in MS lesions remain unclear and it remains controversial as to whether remyelinating cells are derived from pre-existing mature oligodendrocytes and/or from proliferating progenitor cells (Wood and Bunge 1991; Armstrong et al. 1992). Our findings of GR expression in more than 80% of mature CAII-IR oligodendrocytes and in more than 85% of NG2-IR oligodendrocyte progenitors demonstrate that GRs could be one of the possible targets for therapies intended to enhance remyelination in MS patients.
In contrast to the pattern of GR distribution in oligodendrocytes in the adult mouse brain, the other corticosteroid receptors, MRs, were not expressed in either CAII-IR cells or NG2-IR cells. In some regions of the brain, such as the hippocampal and cortex regions, GRs and MRs are co-expressed in the same neurons and exert various functions, including stress responses (Han et al. 2005). However, it remains to be determined whether oligodendrocytes co-express GRs and MRs. We did not observe any MR expression in either oligodendrocytes or oligodendrocyte progenitors in the adult mouse brain. These results indicate that MRs may not be involved in the myelination process and differentiation of oligodendrocytes. In contrast, it should be noted that MRs are expressed in other types of glial cells, astrocytes and microglia after ischemic insult (Hwang et al. 2006; Oyamada et al. 2008).
Next, we confirmed whether the distribution pattern of GRs in oligodendrocytes was reflective of the hormonal milieu. In the present study, adrenalectomy significantly reduced the plasma CORT level to about 20% of the basal level. Consequently, the intensity of GR expression in CAII-IR oligodendrocytes and NG2-IR oligodendrocyte progenitors was reduced significantly compared with sham-operated mice. Our finding is consistent with those of previous studies, in which there was a marked decrease of GR immunoreactivity in neurons (Ozawa et al. 1999; Usuku et al. 2005) and astrocytes (Gonzalez Deniselle et al. 1996), especially in the nuclear region, after adrenalectomy. These results suggest that GR expression is reflective of CORT levels in CAII-IR oligodendrocytes and NG2-IR oligodendrocyte progenitors. In the absence of CORT, GRs cannot translocate into the nucleus. However, the possibility also exists that the down-regulation of GR synthesis is induced by decreases in plasma CORT.
Finally, we investigated the expression of steroid hormone cofactors in mature oligodendrocytes and oligodendrocyte progenitors of the adult mouse brain. We focused on SRC-1 and p300, because the transcriptional activity of GRs is enhanced by the recruitment of one of the transcriptional co-activators of p160 family (SRCs), with docking platforms for secondary co-activators, such as CBP, or its close homologue p300 (Fonte et al. 2007). Previous in vitro studies have shown that SRC-1 is a co-activator for GR in Schwann cells and astrocytes (Grenier et al. 2004 and 2006), whereas CBP/p300 is a repressor of GR in Schwann cells and in astrocytes (Fonte et al. 2007). There has also been a report of the co-expression of SRC-1 and p300 in the adult rat brain (Ogawa et al. 2001). We found that there was no expression of SRC-1 or p300 in mature CAII-IR oligodendrocytes. In contrast, both cofactors were expressed in NG2-IR oligodendrocyte progenitors, with more intense expression in the white matter regions, such as the corpus callosum and external capsule. These results suggest that mature oligodendrocytes in the adult mouse brain do not use SRC-1 or p300, but use some other transcriptional cofactors. Furthermore, we confirmed that p300 was co-expressed with GRs in NG2-IR oligodendrocyte progenitors, whereas we have not yet successfully accomplished the triple immunostaining of GR, SRC-1 and NG2. Although further study is needed, these results may lead to the speculation that oligodendrocytes and their progenitor cells are regulated through GRs and their cofactors in region-specific manners. Future studies are necessary to elucidate which cofactors are required for GR action in CAII-IR mature oligodendrocytes.
In conclusion, the present study demonstrated for the first time that GRs are expressed in different cell lineages of oligodendrocytes; i.e., the CAII-IR cell, a typical adult-type, mature oligodendrocyte, and the NG2-IR cell, an oligodendrocyte progenitor, in various brain regions of adult mice, including both gray and white matter. Using adrenalectomy in mice, we confirmed that the GRs expressed in these oligodendrocytes and oligodendrocyte progenitors are reactive to CORT. Furthermore, cofactors for GR, SRC-1 and p300 are expressed in NG2-IR oligodendrocyte progenitors, more specifically, at high levels in the white matter regions, but are not expressed in mature CAII-IR oligodendrocytes.
Acknowledgments
We are grateful to Dr. Katsuhiko Ono for valuable advice.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a Grant-in-Aid for Scientific Research (B) from the Japan Society for the Promotion of Science to MN.
References
- Alonso G. (2000). Prolonged corticosterone treatment of adult rats inhibits the proliferation of oligodendrocyte progenitors present throughout white and gray matter regions of the brain. Glia 31:219-231 [DOI] [PubMed] [Google Scholar]
- Armstrong RC, Dorn HH, Kufta CV, Friedman E, Dubois-Dalcq ME. (1992). Pre-oligodendrocytes from adult human CNS. J Neurosci 12:1538-1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann N, Pham-Dinh D. (2001). Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev 81:871-927 [DOI] [PubMed] [Google Scholar]
- Bohn MC, Howard E, Vielkind U, Krozowski Z. (1991). Glial cells express both mineralocorticoid and glucocorticoid receptors. J Steroid Biochem Mol Biol 40:105-111 [DOI] [PubMed] [Google Scholar]
- Chan JR, Phillips LJ, Glaser M. (1998). Glucocorticoids and progestins signal the initiation and enhance the rate of myelin formation. Proc Natl Acad Sci U S A 95:10459-10464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A, Tourtellotte WW, Rudick R, Trapp BD. (2002). Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med 346:165-173 [DOI] [PubMed] [Google Scholar]
- Cintra A, Zoli M, Rosén L, Agnati LF, Okret S, Wikström AC, Gustaffsson JA, Fuxe K. (1994). Mapping and computer assisted morphometry and microdensitometry of glucocorticoid receptor immunoreactive neurons and glial cells in the rat central nervous system. Neuroscience 62:843-897 [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joëls M, Holsboer F. (2005). Stress and the brain: from adaptation to disease. Nat Rev Neurosci 6:463-475 [DOI] [PubMed] [Google Scholar]
- Désarnaud F, Bidichandani S, Patel PI, Baulieu EE, Schumacher M. (2000). Glucocorticosteroids stimulate the activity of the promoters of peripheral myelin protein-22 and protein zero genes in Schwann cells. Brain Res 865:12-16 [DOI] [PubMed] [Google Scholar]
- Fonte C, Trousson A, Grenier J, Schumacher M, Massaad C. (2007). Opposite effects of CBP and p300 in glucocorticoid signaling in astrocytes. J Steroid Biochem Mol Biol 104:220-227 [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. (2008). The mouse brain in stereotaxic coordinates, 3rd ed. ed. Netherlands, Amsterdam; Boston: Elsevier/Academic Press, 2008. [Google Scholar]
- Ghandour MS, Langley OK, Vincendon G, Gombos G, Filippi D, Limozin N, Dalmasso D, Laurent G. (1980). Immunochemical and immunohistochemical study of carbonic anhydrase II in adult rat cerebellum: a marker for oligodendrocytes.Neuroscience 5:559-571 [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez CE, de Rodriguez AF, Romero DG, Estess J, Warden MP, Gomez-Sanchez MT, Gomez-Sanchez EP. (2006). Development of a panel of monoclonal antibodies against the mineralocorticoid receptor. Endocrinology 147:1343-1348 [DOI] [PubMed] [Google Scholar]
- Gonzalez Deniselle MC, Gonzalez SL, Piroli GG, Lima AE, De Nicola AF. (1996). The 21-aminosteroid U-74389F increases the number of glial fibrillary acidic protein-expressing astrocytes in the spinal cord of control and Wobbler mice. Cell Mol Neurobiol 16:61-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier J, Trousson A, Chauchereau A, Amazit L, Lamirand A, Leclerc P, Guiochon-Mantel A, Schumacher M, Massaad C. (2004). Selective recruitment of p160 coactivators on glucocorticoid-regulated promoters in Schwann cells. Mol Endocrinol 18:2866-2879 [DOI] [PubMed] [Google Scholar]
- Grenier J, Trousson A, Chauchereau A, Cartaud J, Schumacher M, Massaad C. (2006). Differential recruitment of p160 coactivators by glucocorticoid receptor between Schwann cells and astrocytes. Mol Endocrinol 20:254-267 [DOI] [PubMed] [Google Scholar]
- Han F, Ozawa H, Matsuda K, Nishi M, Kawata M. (2005). Colocalization of mineralocorticoid receptor and glucocorticoid receptor in the hippocampus and hypothalamus. Neurosci Res 51:371-381 [DOI] [PubMed] [Google Scholar]
- Hwang IK, Yoo KY, Nam YS, Choi JH, Lee IS, Kwon YG, Kang TC, Kim YS, Won MH. (2006). Mineralocorticoid and glucocorticoid receptor expressions in astrocytes and microglia in the gerbil hippocampal CA1 region after ischemic insult. Neurosci Res 54:319-327 [DOI] [PubMed] [Google Scholar]
- Kucharova K, Chang Y, Boor A, Yong VW, Stallcup WB. (2011). Reduced inflammation accompanies diminished myelin damage and repair in the NG2 null mouse spinal cord. J Neuroinflammation 8:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharova K, Stallcup WB. (2010). The NG2 proteoglycan promotes oligodendrocyte progenitor proliferation and developmental myelination. Neuroscience 166:185-194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo C, Chen JD. (2000). The SRC family of nuclear receptor coactivators. Gene 245:1-11 [DOI] [PubMed] [Google Scholar]
- Miyata S, Koyama Y, Takemoto K, Yoshikawa K, Ishikawa T, Taniguchi M, Inoue K, Aoki M, Hori O, Katayama T, Tohyama M. (2011). Plasma corticosterone activates SGK1 and induces morphological changes in oligodendrocytes in corpus callosum. PLoS One 6:e19859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto M, Morita N, Ozawa H, Yokoyama K, Kawata M. (1996). Distribution of glucocorticoid receptor immunoreactivity and mRNA in the rat brain: an immunohistochemical and in situ hybridization study. Neurosci Res 26:235-269 [DOI] [PubMed] [Google Scholar]
- Morisaki S, Nishi M, Fujiwara H, Oda R, Kawata M, Kubo T. (2010). Endogenous glucocorticoids improve myelination via Schwann cells after peripheral nerve injury: An in vivo study using a crush injury model. Glia 58:954-963 [DOI] [PubMed] [Google Scholar]
- Myhr KM, Mellgren SI. (2009). Corticosteroids in the treatment of multiple sclerosis. Acta Neurol Scand Suppl:73-80 [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Chang A, Trapp BD. (1999). NG2+ glial cells: a novel glial cell population in the adult brain. J Neuropathol Exp Neurol 58:1113-1124 [DOI] [PubMed] [Google Scholar]
- Ogawa H, Nishi M, Kawata M. (2001). Localization of nuclear coactivators p300 and steroid receptor coactivator 1 in the rat hippocampus. Brain Res 890:197-202 [DOI] [PubMed] [Google Scholar]
- Oyamada N, Sone M, Miyashita K, Park K, Taura D, Inuzuka M, Sonoyama T, Tsujimoto H, Fukunaga Y, Tamura N, Itoh H, Nakao K. (2008). The role of mineralocorticoid receptor expression in brain remodeling after cerebral ischemia. Endocrinology 149:3764-3777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa H, Lion J, Xie CX, Nishi M, Steinbusch H, Kawata M. (1999). Down-regulation of ACTH and glucocorticoid receptor immunoreactivity in hypothalamic arcuate neurons after adrenalectomy in the rat. Neuroreport 10:1571-1575 [DOI] [PubMed] [Google Scholar]
- Ozerdem U, Grako KA, Dahlin-Huppe K, Monosov E, Stallcup WB. (2001). NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn 222:218-227 [DOI] [PubMed] [Google Scholar]
- Polito A, Reynolds R. (2005). NG2-expressing cells as oligodendrocyte progenitors in the normal and demyelinated adult central nervous system. J Anat 207:707-716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. (2000). How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21:55-89 [DOI] [PubMed] [Google Scholar]
- Sarabdjitsingh RA, Meijer OC, Schaaf MJ, de Kloet ER. (2009). Subregion-specific differences in translocation patterns of mineralocorticoid and glucocorticoid receptors in rat hippocampus. Brain Res 1249:43-53 [DOI] [PubMed] [Google Scholar]
- Schröter A, Lustenberger RM, Obermair FJ, Thallmair M. (2009). High-dose corticosteroids after spinal cord injury reduce neural progenitor cell proliferation. Neuroscience 161:753-763 [DOI] [PubMed] [Google Scholar]
- Stallcup WB. (2002). The NG2 proteoglycan: past insights and future prospects. J Neurocytol 31:423-435 [DOI] [PubMed] [Google Scholar]
- Sun YY, Wang CY, Hsu MF, Juan SH, Chang CY, Chou CM, Yang LY, Hung KS, Xu J, Lee YH, Hsu CY. (2010). Glucocorticoid protection of oligodendrocytes against excitotoxin involving hypoxia-inducible factor-1alpha in a cell-type-specific manner. J Neurosci 30:9621-9630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usuku T, Nishi M, Morimoto M, Brewer JA, Muglia LJ, Sugimoto T, Kawata M. (2005). [DOI] [PubMed] [Google Scholar]
- Visualization of glucocorticoid receptor in the brain of green fluorescent protein-glucocorticoid receptor knockin mice Neuroscience 135:1119-1128 [DOI] [PubMed] [Google Scholar]
- Vielkind U, Walencewicz A, Levine JM, Bohn MC. (1990). Type II glucocorticoid receptors are expressed in oligodendrocytes and astrocytes. J Neurosci Res 27:360-373 [DOI] [PubMed] [Google Scholar]
- Vinet J, Lemieux P, Tamburri A, Tiesinga P, Scafidi J, Gallo V, Sík A. (2010). Subclasses of oligodendrocytes populate the mouse hippocampus. Eur J Neurosci 31:425-438 [DOI] [PubMed] [Google Scholar]
- Wood PM, Bunge RP. (1991). The origin of remyelinating cells in the adult central nervous system: the role of the mature oligodendrocyte. Glia 4:225-232 [DOI] [PubMed] [Google Scholar]
- Wu RC, Smith CL, O’Malley BW. (2005). Transcriptional regulation by steroid receptor coactivator phosphorylation. Endocr Rev 26:393-399 [DOI] [PubMed] [Google Scholar]