Abstract
Purpose: Single-incision videoendoscopic surgery has recently become popular as a result of the ongoing search for less invasive procedures. The aim of this study was to evaluate the safety and efficacy of endoscopic single-port nipple-sparing mastectomy, axillary lymphadenectomy, and immediate reconstruction in patients with breast cancer.
Patients and Methods: From May 14, 2012 through January 23, 2013, 10 patients underwent videoendoscopic single-port nipple-sparing mastectomy and axillary dissection via a single, limited incision and immediate prosthetic reconstruction. Patient charts were reviewed, and demographic data, operative time, complications and pathology results were analyzed.
Results: In all patients, videoendoscopic surgery was performed successfully. Of 10 patients, 7 were diagnosed as having invasive ductal carcinoma, 2 had a ductal carcinoma in situ, and 1 underwent bilateral prophylactic mastectomy. The weight of the resected gland was 300–650 g, with a mean of 420 g. There were no operative complications, and the mean operative time was 250 minutes (range, 160–330 minutes). One-stage reconstruction with implants was performed on 4 patients, whereas expanders were placed in the remaining 6. Surgical margins of all cases were pathologically negative, and there were no recurrences observed during the early follow-up period.
Conclusions: Videoendoscopic single-port nipple-sparing mastectomy is technically feasible even in larger breasts, enabling immediate reconstruction with good cosmetic outcomes. However, further studies with larger clinical series and long-term follow-up are required to compare the safety and efficacy of the technique with those of the standard nipple-sparing mastectomy.
Introduction
Breast cancer is the most common cancer of the female population.1 Today, lumpectomy is considered to be the standard operation for early-stage breast cancer to maintain quality of life without major alteration in the body image.2,3 However, for those cases beyond the indications for breast-conserving surgery, including patients with multiple tumors, generalized microcalcifications, and/or multicentricity, mastectomy is the preferred choice of treatment.4 In surgical practice, subcutaneous mastectomy has proven to be oncologically safe for early breast cancer.5,6 The removal of the nipple as a part of the mastectomy has routinely been performed owing to the presumed risk of occult nipple involvement.7–9 However, many patients are not satisfied with the cosmesis of the reconstructed nipple regarding shape, size, position, and color match.10 For those patients without skin and nipple involvement, nipple-sparing mastectomy (NSM) offers the opportunity to preserve the breast envelope and the nipple–areola complex by excising only breast tissue and avoiding multiple surgical procedures for reconstruction.
Surgery for breast cancer has evolved from radical resections to breast-conserving techniques and less invasive procedures throughout history.11–13 Minimally invasive techniques have been used in many fields of surgery, enabling improved body cavity access, enhanced visualization via magnification, and minimalized tissue trauma.14–18 Since the first use of the endoscope in breast surgery, several reports on the application of the technique to lump excision, breast augmentation, subcutaneous mastectomy, and axillary dissection have been published.19–22 However, there are scant data in the literature regarding endoscopic NSM,23 with certain limitations such as its use exclusively on patients with small breast size,24 use of multiple access sites, thus creating multiple scars,25,26 and compromised cosmesis due to lack of immediate reconstruction.26
The aim of this study was to share our experience of videoendoscopic single-port NSM and show that it can be safely and effectively performed in a wider spectrum of patients including those with larger breasts, with enhanced cosmesis due to facilitation of a single access site and immediate reconstruction.
Patients and Methods
Between May 14, 2012 and January 23, 2013, in total, 355 consecutive patients with primary operable breast cancers underwent surgery at our institution. Among these patients, 263 (74%) underwent breast-conserving surgery, and the remaining 92 (26%) received mastectomies. For 10 of these these patients, we performed 11 videoendoscopic single-port NSM procedures. The indication of the operations was assessed at our clinic's surgical-oncology meetings prior to each procedure. Indications for endoscope-assisted NSM included multicentric breast carcinoma, extensive intraductal neoplasia, and large unicentric carcinomas that would not be suitable for breast-conserving surgery. The exclusion criteria for the procedure were skin involvement and a centrally located tumor that was suspicious for nipple and areola involvement as assessed by physical examination or radiological imaging studies. Informed consent forms were obtained from all the patients before procedures.
The patients who underwent videoendoscopic single-port NSM and axillary dissection via a single 3–5 cm axillary incision were evaluated retrospectively from our prospectively maintained archives; such evaluation does not require institutional review board approval. The patients' demographic data, operative time, tumor size, tumor location, complications, surgical margins, and final pathology results were analyzed. Prosthetic reconstruction was performed through the same incision to complement the procedure in all cases.
Surgical technique
The patient, under general anesthesia with endotracheal intubation, was positioned supine on the operating table with the arm abducted maximally. Before the videoendoscopic mastectomy procedure was performed, sentinel lymph node biopsy was carried out routinely under gamma probe guidance.
At first, a 3-cm-long axillary incision was made along the extension of the axillary fold to the lateral breast border. Then the dissection was carried out, and the sentinel lymph node was found with the help of gamma probe guidance through the axillary fascia. After the specimen was sent to frozen section, the videoendoscopic procedure was then continued from the same incision.
A 4–6-cm incision was made along the lateral border of the pectoralis major muscle. At first, the subcutaneous flap was dissected under direct vision. Then we obtained a working space for the introduction of the endoscopic port in order to overcome the blind spots, which can be practically very difficult for the dissection by endoscopic instruments around the port entry site as Kitamura et al.23 defined previously.
A SILS™ port (12 mm; Covidien, Norwalk, CT) was inserted into the incision by using a Kelly clamp. Two 5-mm trocars and one 10-mm trocar were inserted through the SILS port (Fig. 1). The SILS port was connected to an insufflator (Karl Storz, Tuttlingen, Germany) to keep the pressure at 8–10 mm Hg. The endoscopic view was observed through a 30° 10-mm-diameter straight-angled rigid endoscope (Karl Storz). Dissection was performed with an Endo Grasp™ (Autosuture™, Covidien), an endoscissors (Endo Mini-Shears™ 5-mm instrument with unipolar cautery; Covidien), LigaSure™ V (Valleylab Inc., Boulder, CO), and Harmonic ACE® curved shears (Ethicon Endo-Surgery, Cincinnati, OH). The optic system, all the trocars, and the endoscopic instruments were taken from the laparoscopy instrumentation box. No specific adaptations were performed. The video-assisted NSM required a superficial dissection of the gland, moving from the axillary toward the nipple; it then continued over the nipple up to the breast fold along the lateral margin (Fig. 2). Once the skin flaps were developed, the operation proceeded with the deep layer dissection. In the first 2 cases, we made the dissection posterior to anterior as Sakamoto et al.26 previously mentioned in the literature. In this technique, the dissection plane starts along from the posterior pectoral fascia in which the breast tissue is pulled up to create a sufficient working space until the breast tissue along with the pectoral fascia was dissected off the major pectoral muscle; however, we found that after the breast tissue is completely separated from the posterior fascia, the manipulation of the breast and dissection of the anterior parts beneath the skin became more difficult. Thus, we preferred skin dissection first by starting from anterior in the remaining cases and did not encounter any technical difficulties. The dissection of the last attachment from the inferior breast fold was completed, thus fully mobilizing the gland for extraction. The specimen was then removed through the axillary skin incision. The nodes were dissected at levels 1 and 2. The dissected axillary content was removed through the incision. Following copious irrigation of the mastectomy pocket, the lateral border of the pectoralis major muscle was elevated, and the submuscular pocket was dissected medially to the sternal border, taking care not to injure the intercostal perforators. Inferiorly, the dissection was continued to exceed the inframammarian fold, below which the muscle was released to carry the dissection to the subcutaneous plane, thus allowing for adequate expansion. In the lateral border, the superficial fascia of the serratus anterior muscle was dissected posteriorly in a limited fashion, just enough to accommodate the lateral border of the expander. Subsequent to the formation and antibiotic irrigation of the total submuscular pocket, an expander or implant (Mentor Worldwide LLC, Santa Barbara, CA) was inserted, drains were placed in both submuscular and subcutaneous planes, and the free muscle edges were sutured laterally. Before the subcutaneous layer was closed, the expander was inflated with an adequate volume of saline, avoiding tension in the muscle suture line and pressure on the skin flaps. The skin was closed using 3/0 absorbable sutures.
FIG. 1.

Photograph showing the bimanual dissection of the nipple to effectively core out the ductal extensions.
FIG. 2.
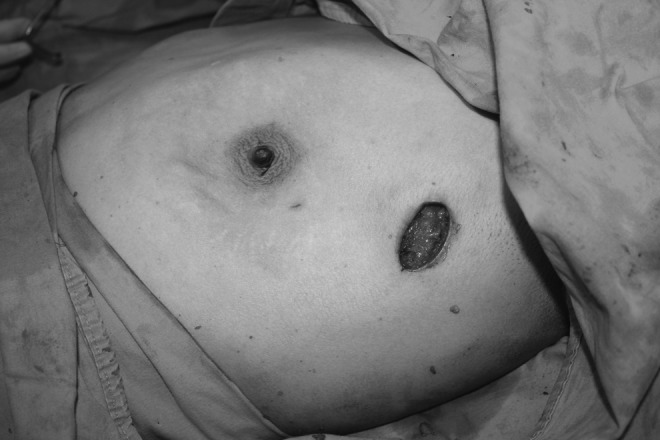
Operative photograph taken after the completion of gland resection.
During the frozen section assessment, if the sentinel lymph node biopsy was found to be positive, an axillary node dissection involving levels 1 and 2 was performed. A tissue biopsy specimen from the posterior margin of the nipple was also examined routinely by frozen section, according to which the decision to excise the nipple was made in case of tumor involvement.
Statistical analysis
Data are expressed as mean±standard deviation values. All statistical analyses were performed with SPSS version 15.0 statistics software (SPSS Inc., Chicago, IL).
Results
Ten patients received 11 videoendoscopic single-port NSM procedures. Mean patient age was 41±7 years (range, 30–51 years). Seven patients were premenopausal, and 3 patients were menopausal. Four patients came to the hospital because of palpable breast masses, 4 patients were detected during screening examinations, 1 patient underwent prophylactic surgery because of positive family history and BRCA 1 positivity, and 1 patient consulted us after having an axillary node biopsy in another institution with a diagnosis of carcinoma metastasis. In the last patient with axillary biopsy in a different institute, we performed a single-incision endoscopic NSM, axilllary dissection, and tissue expander from the same previous biopsy incision (Fig. 3).
FIG. 3.
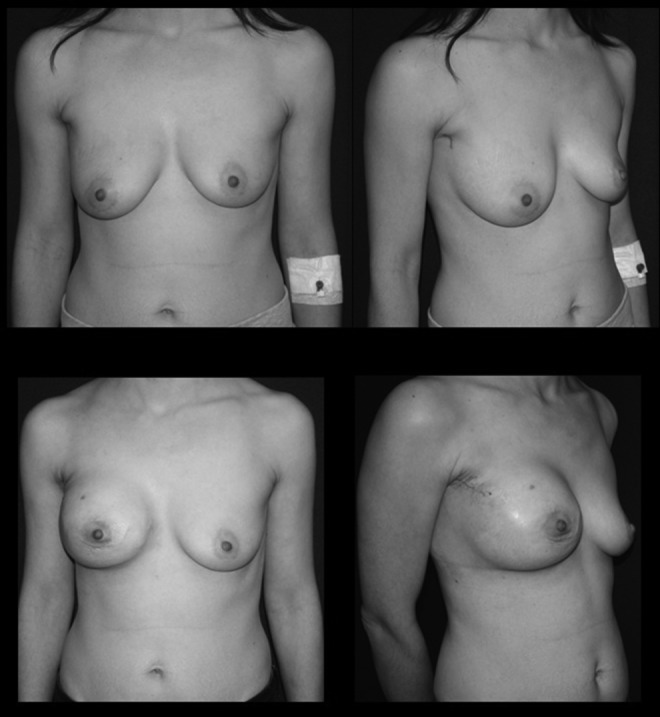
(Upper panels) Photographs showing a patient with invasive ductal carcinoma of the right breast. (Lower panels) Three weeks after videoendoscopic nipple-sparing mastectomy of the right breast and immediate insertion of a 450-mL expander.
The mean distance between the areola and the sternum was 26 cm (range, 21–29 cm). The weight of the resected specimen was 300–650 g, with a mean of 420 g. The mean operative time was 250 minutes (range, 140–330 minutes), including axillary lymphadenectomy. When the second half of the patient series was compared with the first half, it was seen that the operative time was shorter for the second half (186.6±24.8 minutes versus 290.4±39.1 minutes, respectively). There were no perioperative complications; however, there were 3 cases of nipple sloughing, which healed uneventfully without any complete or partial nipple loss (Fig. 4). One of the patients developed a hematoma at postoperative week 1, in whom the implant was saved after drainage of the hematoma, and another patient developed infection within the expander region at postoperative week 6, while receiving chemotherapy. This patient was also treated conservatively and with antibiotic adminsitration. Sentinel lymph node biopsy was positive in 4 procedures, which required axillary lympadenectomy. The average volume of implant was 370 (range, 320–420) mL, and the expander volume was 550 mL (range, 450–650) mL. The average intraoperative fill volume for expanders was 250 (range, 150–350) mL.
FIG. 4.
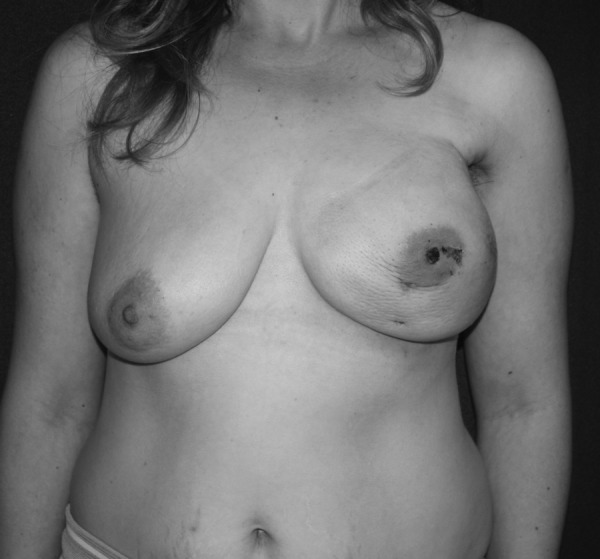
Invasive ductal carcinoma of the right breast: nipple sloughing at 1 week following videoendoscopic nipple-sparing mastectomy and immediate insertion of a 650-mL expander.
Final pathology surgical margins of all cases were pathologically negative. In another patient, the frozen section of the retro nipple area came back positive and led to nipple resection. The final pathology of the specimens was invasive ductal carcinoma in 6 patients and ductal carcinoma in situ in the remaining 4. There were 6 patients with stage 2 disease and 2 with stage 1 disease. Mean tumor size was 14.1±6.5 mm (range, 8–29 mm). Except for 1 case with nipple resection, the remaining cases' permanent pathological reports showed that the primary tumors were >2 cm away from the nipple–areola complex. No major complications were observed, and mean hospital stay was 3 days. All patients were satisfied with the cosmetic outcome at the 3-month follow-up after surgery.
Discussion
Endoscopic breast surgery was first developed in the field of plastic surgery by Kompatscher27 in 1992. Although several studies have reported the feasibility and safety of endoscopic mastectomy,24–26,28 it has not become popular because a substantial benefit over the conventional approach has not been shown in the literature.
The surgical technique for NSM is under constant evolution. We have indeed come a long way in terms of gentle tissue handling with minimal retractor use as well as finding the right dissection plane and appreciating variations in flap thickness. Dilute infiltration and the use of long facelift scissors are the two weapons of the modern NSM techniques with minimal incisions.29 However, the disadvantages are twofold: first of all, the dilute infiltration used to minimize bleeding increases the thickness of the subcutaneous layer and may lead to erroneous preparation of a rather thin flap with compromised circulation. Second, the blind use of long scissors may inadvertently damage the intercostal perforators, especially the second and third, which contribute significantly to the overall circulation of both the nipple–areola complex and the mastectomy flaps and become all the more important when coupled with implant insertion. The use of the endoscope overcomes both of these drawbacks by eliminating the need for infiltration and enabling direct visualization, which lead to a significantly more refined and elegant technique.
The tranaxillary technique for breast lesions via ipsilateral axillary was first introduced by Agarwal et al.30 in 2008. In another report, Tamaki et al.31 shared their experience with transaxillary endoscopic partial mastectomy for early-stage breast cancer and mentioned that, from the oncological aspect, they decided to use retractors to maintain the work space instead of CO2 insufflation to avoid subcutaneous emphysema and possible wide dissemination of cancer cells into the soft tissues. In contrast to that study, we prefer to maintain the work space with CO2 insufflation. In our opinion, the inflation positively affects the dissection by retracting Cooper's ligaments, and because we did not perform any endoscopic breast-conserving procedures, during the mastectomy operation, we did not encounter any dissemination of cancer cells.
The individual use of a single incision has previously been advocated by different authors in both endoscopy-assisted mastectomy23 and axillary dissection.32 Our technique enables the performance of both NSM and axillary dissection as well as prosthetic reconstruction through the same, limited incision.
In a recent study, Sakamoto et al.26 reported on 89 patients who underwent endoscopic NSM procedures; they noted total nipple necrosis and partial nipple necrosis rates of 24% and 18%, respectively. In our study we did not encounter any nipple necrosis, probably because of avoidance of the additional periareolar incision defined in the aforementioned technique26; however, there were 3 cases of nipple sloughing, which healed uneventfully without any complete or partial nipple loss.
One technical point that we believe is essential to facilitate the procedure would be the order of dissection; in the previous reports, the initial dissection plane was determined as the inferior surface of the gland off the pectoralis fascia, followed by the development of skin flaps. We found that once the gland is mobilized off the chest wall, it becomes quite difficult to dissect it from the skin, which is always the most tricky part of the procedure in an NSM. It would not be wrong to speculate that changing the order of dissection would facilitate the gland dissection, thus avoiding the extra incisions mentioned in the previous reports.26 Also, we note that, because we perform endoscopic mastectomy via a single incision and with CO2 insufflation, it is feasible for our technique to dissect the posterior pectoral part after dissecting it anterior from the skin with the help of insufflation, which makes Cooper's ligaments tighten, thus making it easy for dissection.
Immediate reconstruction is the sine qua non of NSM for optimum cosmesis. One major challenge in immediate prosthetic reconstruction is creating an optimal pocket to safely host the implant and avoid complications including those related to the adjuvant therapy span.33 Total submuscular techniques as well as the use of acellular dermal matrices are advocated to accomplish the above-mentioned goal. In our series, we managed to easily create a sufficient total submuscular pocket in most of our patients with the help of direct visualization provided by the endoscopic technique and also were able to correctly fix a piece of acellular dermal matrix to cover the lower pole of a high-volume implant with extra projection in one particular case.
However, our study has several limitations. Because the number of patients in the current series was limited and the follow-up period was relatively short, we did not perform a head-to-head comparison between our endoscopic group and those patients who underwent conventional open surgery. However, in the literature, several other investigators have shown significant advantages of endoscopic breast surgery in terms of cosmetics and postoperative pain levels.23,31 In our dataset, pain management was no different than for those who underwent open surgery because we believe it is actually the submuscular dissection with immediate prosthetic reconstruction that causes actual pain and pressure rather than the site or the size of the access incision.
Endoscopic breast surgery appears to be a well-tolerated and relatively safe technique. Complications observed following conventional open breast surgery, such as seroma, hematoma, and infection, are less frequently seen following endoscopic surgery. However, to gain acceptance in clinical practice, the procedure must prove itself to be at least as efficacious as open surgery in terms of oncological outcomes. In a recent review, Leff et al.34 reported that the inital results of endoscopic breast surgery are encouraging and suggested that obtaining equivalent oncological results to open surgery should be achievable.
Although there are several advantages of endoscopic surgery compared with conventional open surgery, including smaller incision, enchanced visualization via magnification, and minimalized tissue trauma resulting in increased flap viability and earlier recovery, there are also endoscopic limitations that related to small working space, rigid instrumentation, and instrument collision.24–26 Concurrent with these data, the operative time in our study was also significantly longer than for our open technique; however, we still believe that operative time in endoscopic surgery depends on the surgeon's experience, the learning curve of the technique, and the extent of pathology. Adaptation of the robotic techniques with angulated instrumentation and a three-dimensional view might help improve the operative times in endoscopic breast surgery in the near future.15
In conclusion, we believe that videoendoscopic single-port NSM can be safely and effectively performed in a wider spectrum of patients, including those with larger breasts, with enhanced cosmesis due to facilitation of a single access site and immediate reconstruction. Although the learning curve might be steep with prolonged operative times initially, it is easily improved in the hands of experienced breast surgeons working in high-turnover breast centers.
Disclosure Statement
No competing financial interests exist.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277–300 [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Anderson S, Redmond CK, Wolmark N, Wickerham DL, Cronin WM. Reanalysis and results after 12 years of follow-up in a randomized clinical trial comparing total mastectomy with lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med 1995;333:1456–1461 [DOI] [PubMed] [Google Scholar]
- 3.Veronesi U, Salvadori B, Luini A, Greco M, Saccozzi R, del Vecchio M, et al. Breast conservation is a safe method in patients with small cancer of the breast. Long-term results of three randomised trials on 1,973 patients. Eur J Cancer 1995;31A:1574–1579 [DOI] [PubMed] [Google Scholar]
- 4.Niemeyer M, Paepke S, Schmid R, Plattner B, Müller D, Kiechle M. Extended indications for nipple-sparing mastectomy. Breast J 2011;17:296–299 [DOI] [PubMed] [Google Scholar]
- 5.Hinton CP, Doyle PJ, Blamey RW, Davies CJ, Holliday HW, Elston CW. Subcutaneous mastectomy for primary operable breast cancer. Br J Surg 1984;71:469–472 [DOI] [PubMed] [Google Scholar]
- 6.Kroll SS, Ames F, Singletary SE, Schusterman MA. The oncologic risks of skin preservation at mastectomy when combined with immediate reconstruction of the breast. Surg Gynecol Obstet 1991;172:17–20 [PubMed] [Google Scholar]
- 7.Laronga C, Kemp B, Johnston D, Robb GL, Singletary SE. The incidence of occult nipple-areola complex involvement in breast cancer patients receiving a skin-sparing mastectomy. Ann Surg Oncol 1999;6:609–613 [DOI] [PubMed] [Google Scholar]
- 8.Cense HA, Rutgers EJ, Lopes Cardozo M, Van Lanschot JJ. Nipple-sparing mastectomy in breast cancer: A viable option? Eur J Surg Oncol 2001;27:521–526 [DOI] [PubMed] [Google Scholar]
- 9.Chung AP, Sacchini V. Nipple-sparing mastectomy: Where are we now? Surg Oncol 2008;17:261–266 [DOI] [PubMed] [Google Scholar]
- 10.Jabor MA, Shayani P, Collins DR, Jr, Karas T, Cohen BE. Nipple-areola reconstruction: Satisfaction and clinical determinants. Plast Reconstr Surg 2002;110:457–463; discussion 464–465. [DOI] [PubMed] [Google Scholar]
- 11.Hermann RE, Esselstyn CB, Jr, Crile G, Jr, Cooperman AM, Antunez AR, Hoerr SO. Long-term results of modified radical and conservative operations of breast cancer. Chirurg 1984;55:193–199 [PubMed] [Google Scholar]
- 12.Winchester DP, Trabanino L, Lopez MJ. The evolution of surgery for breast cancer. Surg Oncol Clin N Am 2005;14:479–498 [DOI] [PubMed] [Google Scholar]
- 13.Chung AP, Sacchini V. Nipple-sparing mastectomy: Where are we now? Surg Oncol 2008;17:261–266 [DOI] [PubMed] [Google Scholar]
- 14.Barbaros U, Demirel T, Gozkun O, Serin K, Bilge O, Kalayci M, et al. A new era in minimally invasive liver resection (MILR) single-incision laparoscopic liver resection (SIL-LR): The first two cases. Surg Technol Int 2011;XXI:81–84 [PubMed] [Google Scholar]
- 15.Agcaoglu O, Aliyev S, Siperstein A, Berber E. Robotic transaxillary central neck dissection: Video description of the technique. Surg Laparosc Endosc Percutan Tech 2012;22:e197–e198 [DOI] [PubMed] [Google Scholar]
- 16.Paranjape C, Ojo OJ, Carne D, Guyton D. Single-incision laparoscopic total colectomy. JSLS 2012;16:27–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holloway RW, Patel SD, Ahmad S. Robotic surgery in gynecology. Scand J Surg 2009;98:96–109 [DOI] [PubMed] [Google Scholar]
- 18.Simforoosh N, Aminsharifi A. Laparoscopic management in stone disease. Curr Opin Urol 2013;23:169–174 [DOI] [PubMed] [Google Scholar]
- 19.Avrahami R, Nudelman I, Watenberg S, Lando O, Hiss Y, Lelchuk S. Minimally invasive surgery for axillary dissection. Cadaveric feasibility study. Surg Endosc 1998;12:466–468 [DOI] [PubMed] [Google Scholar]
- 20.Tagaya N, Kubota K. Experience with endoscopic axillary lymphadenectomy using needlescopic instruments in patients with breast cancer: A preliminary report. Surg Endosc 2002;16:307–309 [DOI] [PubMed] [Google Scholar]
- 21.Eaves FF, 3rd, Bostwick J, 3rd, Nahai F, Murray DR, Styblo TM, Carlson GW. Endoscopic techniques in aesthetic breast surgery. Augmentation, mastectomy, biopsy, capsulotomy, capsulorrhaphy, reduction, mastopexy, and reconstructive techniques. Clin Plast Surg 1995;22:683–695 [PubMed] [Google Scholar]
- 22.Tsangaris TN, Trad K, Brody FJ, Jacobs LK, Tsangaris NT, Sackier JM. Endoscopic axillary exploration and sentinel lymphadenectomy. Surg Endosc 1999;13:43–47 [DOI] [PubMed] [Google Scholar]
- 23.Kitamura K, Ishida M, Inoue H, Kinoshita J, Hashizume M, Sugimachi K. Early results of an endoscope-assisted subcutaneous mastectomy and reconstruction for breast cancer. Surgery 2002;131(1 Suppl):S324–S329 [DOI] [PubMed] [Google Scholar]
- 24.Ho WS, Ying SY, Chan AC. Endoscopic-assisted subcutaneous mastectomy and axillary dissection with immediate mammary prosthesis reconstruction for early breast cancer. Surg Endosc 2002;16:302–306 [DOI] [PubMed] [Google Scholar]
- 25.Ito K, Kanai T, Gomi K, Watanabe T, Ito T, Komatsu A, et al. Endoscopic-assisted skin-sparing mastectomy combined with sentinel node biopsy. ANZ J Surg 2008;78:894–898 [DOI] [PubMed] [Google Scholar]
- 26.Sakamoto N, Fukuma E, Higa K, Ozaki S, Sakamoto M, Abe S, et al. Early results of an endoscopic nipple-sparing mastectomy for breast cancer. Ann Surg Oncol 2009;16:3406–3413 [DOI] [PubMed] [Google Scholar]
- 27.Kompatscher P. Endoscopic capsulotomy of capsular contracture after breast augmentation: A very challenging therapeutic approach. Plast Reconstr Surg 1992;90:1125–1126 [DOI] [PubMed] [Google Scholar]
- 28.Fan LJ, Jiang J, Yang XH, Zhang Y, Li XG, Chen XC, et al. A prospective study comparing endoscopic subcutaneous mastectomy plus immediate reconstruction with implants and breast conserving surgery for breast cancer. Chin Med J (Engl) 2009;122:2945–2950 [PubMed] [Google Scholar]
- 29.Davies K, Allan L, Roblin P, Ross D, Farhadi J. Factors affecting post-operative complications following skin sparing mastectomy with immediate breast reconstruction. Breast 2011;20:21–25 [DOI] [PubMed] [Google Scholar]
- 30.Agarwal BB, Agarwal S, Gupta M, Mahajan K. Transaxillary endoscopic excision of benign breast lumps: A new technique. Surg Endosc 2008;22:407–410. Erratum in: Surg Endosc 2010;24:1514. [DOI] [PubMed] [Google Scholar]
- 31.Tamaki Y, Nakano Y, Sekimoto M, Sakita I, Tomita N, Ohue M, et al. Transaxillary endoscopic partial mastectomy for comparatively early-stage breast cancer. An early experience. Surg Laparosc Endosc 1998;8:308–312 [PubMed] [Google Scholar]
- 32.Uras C, Aytac E, Aydogan F. Videoendoscopic single-port axillary dissection. J Minim Access Surg 2011;7:246–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Celet Ozden B, Guven E, Aslay I, Kemikler G, Olgac V, Soluk Tekkesin M, et al. Does partial expander deflation exacerbate the adverse effects of radiotherapy in two-stage breast reconstruction? World J Surg Oncol 2012;10:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leff DR, Vashisht R, Yongue G, Keshtgar M, Yang GZ, Darzi A. Endoscopic breast surgery: Where are we now and what might the future hold for video-assisted breast surgery? Breast Cancer Res Treat 2011;125:607–625 [DOI] [PubMed] [Google Scholar]


