Abstract
Background. Human immunodeficiency virus type 1 (HIV-1)–infected women have lower viral loads than men but similar rates of disease progression. We hypothesized that sex-based differences in CCR5 expression mediate viral load differences.
Methods. CCR5 was analyzed by flow cytometry in disaggregated lymph node cells from untreated HIV-1–infected women (n = 28) and men (n = 27). The frequencies of HIV-1 RNA–producing cells in the lymph node were determined by in situ hybridization. Linear and generalized linear regression models were used.
Results. The percentage of CCR5+CD3+CD4+ cells was lower in women (mean, 12%) than men (mean, 16%; P = .034). Neither the percentage of CCR5+CD3+CD4+ cells nor the CCR5 density predicted viral load or HIV-1 RNA–producing lymph node cells (P ≥ .24), after adjusting for CD4+ T-cell count, race, and age. Women had marginally fewer HIV-1 RNA–producing cells (mean, 0.21 cells/mm2) than men (mean, 0.44 cells/mm2; P = .046). After adjusting for the frequency of HIV-1 RNA–producing cells and potential confounders, the viral load in women were 0.46 log10 copies/mL lower than that in men (P = .018).
Conclusions. Reduced lymph node CCR5 expression in women did not account for the viral load difference between sexes. CCR5 expression did not predict viral load or frequencies of HIV-1 RNA–producing cells, indicating that physiologic levels of CCR5 do not limit HIV-1 replication in lymph node. Less plasma virus was associated with each HIV-1 RNA–producing cell in women as compared to men, suggesting that women may either produce fewer virions per productively infected cell or more effectively clear extracellular virus.
Keywords: Lymph node, CCR5, Immune activation, Sex differences, HIV-1
Plasma viral load is used to monitor responses to antiretroviral therapy (ART) and to assess risk of disease progression in human immunodeficiency virus type 1 (HIV-1)–infected individuals. Lower viral load in HIV-1–infected women, compared with HIV-1–infected men, has been observed throughout the world [1–3]. Viral load differences between the sexes are most pronounced in early disease and wane in more-advanced stages of infection [1, 2]. Nevertheless, although viral load is a predictor of disease progression [4], most studies before the availability of ART did not demonstrate sex-based differences in rates of disease progression or death [5, 6]. Reasons for sex-based differences in viral load and why a lower viral load is not associated with a slower rate of disease progression in women, compared with men, are not understood. A better understanding of the mechanisms underlying sex-based differences in viral load could provide important insight into fundamental principles that govern the relationship between virus replication and disease progression.
Expression of the HIV-1 chemokine coreceptor CCR5 is related to the susceptibility to HIV-1 infection. Individuals who are homozygous for a 32-base-pair deletion (Δ32) in the CCR5 receptor that abrogates cell surface CCR5 expression are resistant to R5 HIV-1 infection [5]. CCR5-Δ32 heterozygotes have lower cell surface CCR5 expression, lower viral load, and delayed disease progression [7]. In vitro, the amount of CCR5 on the CD4+ T-cell surface corresponds to susceptibility to infection [7–10]. One study demonstrated less CCR5 expression on peripheral blood CD4+ T cells in seronegative women, compared with seronegative men [11]. Whether there are sex-based differences in CCR5 expression in HIV-1–seropositive individuals and whether these differences account for sex-based differences in viral load is unknown.
The present study investigated whether differences in CCR5 expression mediate sex-based differences in viral load. Because most HIV-1 replication occurs in secondary lymphoid tissues [12–14], we reasoned that there might be sex-based differences in production of HIV-1 within those tissues. We obtained lymph nodes from HIV-1–infected women and men not receiving ART, determined levels of CCR5 expression on lymph node CD4+ T cells, and evaluated their relationship vis-a-vis sex and viral load. We also examined CCR5 expression on activated (HLA-DR+CD38+ [hereafter, “DR+38+”]) lymph node CD4+ T cells, as we previously demonstrated that these cells harbor the majority of HIV-1 in lymph nodes in vivo [15]. Finally, we determined frequencies of HIV-1 RNA–producing cells within lymph node tissue sections, anticipating that sex-based differences in CCR5 expression would result in significantly fewer HIV-1 RNA–producing cells in women, compared with men.
METHODS
Study Subjects and Clinical Specimens
Inguinal lymph nodes were obtained by excisional biopsy as described previously [16] from individuals with documented HIV-1 infection for at least 4 months, CD4+ T-cell counts of >300 cells/mm3, and no history of AIDS. Subjects who had previously used ART were included if they had discontinued therapy at least 6 months before lymph node donation and were at their nadir CD4+ T-cell count. Women underwent lymph node excision without attention to menstrual cycle phase. Recruitment proceeded on the basis of subject eligibility and interest until close to the end of the study, when it was noted that there was a relative deficiency of HIV-1–seropositive men with a high viral load who had CD4+ T-cell counts of <500 cells/mm3. To adjust for this skewing, the last 5 male subjects were recruited specifically because they had CD4+ T-cell counts of <500 cells/mm3 and HIV-1 RNA concentrations of >50,000 copies/mL. Informed consent was obtained from all study participants, and the study was approved by the Colorado Multiple Institutional Review Board.
Portions of lymph nodes were disaggregated, and the remainder was snap frozen in OCT as described previously [16]. Peripheral blood obtained on the same day as lymph node excision was used to determine CD4+ T-cell counts, plasma HIV-1 RNA concentration (Roche Amplicor HIV-1 Monitor [1997–2002], Roche COBAS Amplicor HIV-1 test [2002–2008], and Roche COBAS Ampliprep/Taqman 96 HIV-1 test [2008–2011], Indianapolis, IN), and HIV-1 coreceptor tropism (Trofile test, Monogram Biosciences, South San Francisco, CA) [17]. Plasma estradiol and progesterone levels were measured by mass spectrometry (Esoterix, Calabasas Hills, CA).
Analyses of CCR5 Expression
Disaggregated lymph node cells (5 × 106) were stained with antibodies to CD3-PEcy5 (BD Biosciences, San Jose, CA), CD4-APC-H7, CD38-FITC (Invitrogen Life Science), HLA-DR-APC (BD Biosciences), and CCR5-PE (manufactured by BD Biosciences with a known 1:1 ratio of PE to antibody), evaluated by flow cytometry (LSR II, BD Immunocytometry Systems), and analyzed using FlowJo (Tree Star, Ashland, OR). Percentages of CCR5+ cells and the mean number of CCR5 molecules (QuantiBRITE beads [BD Biosciences, San Diego, CA]) were determined as described elsewhere [15, 18].
In Situ Hybridization for HIV-1 RNA
In situ hybridization for HIV-1 RNA was performed on 6-µm lymph node sections as described previously [16]. Control sections hybridized with sense probes uniformly had demonstrated no staining. Numbers of HIV-1 RNA–producing cells from each tissue section were summed and divided by the total area of the tissue sections, as quantified by a computerized image analysis system (Leica Q5001W Image Analysis; Leica, Cambridge, United Kingdom), to determine the frequency of RNA-producing cells per square millimeter of tissue.
CCR5-Δ32 Genotyping
DNA was extracted from peripheral blood mononuclear cells (Qiagen Blood and Tissue Kit; Germantown, MD), and CCR5-Δ32 genotype determined by polymerase chain reaction analysis as previously described [18].
Statistical Analysis
Analyses assumed a 2-sided test of hypothesis with a significance level of 0.05. The Fisher exact tests and t tests were used for analysis of demographic data. Normalizing log transforms were used for right-skewed data. Means or geometric means and Pearson correlations are reported. Ordinary least squares regression was used to model continuous outcomes while controlling for potential confounders or mediators. An interaction term was considered to test whether linear relationships differed by sex or race. For our primary explanatory variables, the percentage of an effect mediated by an additional covariate was based on the ratio of adjusted to crude parameter estimates, with corresponding variance estimated using the delta method. Count data were modeled using a negative binomial generalized linear model with a log link to account for overdispersion. An offset was included to control for between-subject differences in total lymph node area measured.
RESULTS
Clinical Characteristics of Study Subjects
Clinical characteristics of the 28 women and 27 men who donated lymph nodes are shown in Table 1. There were no significant differences between men and women in age or race. Sexual contact with men was the most common HIV-1 risk factor for women (79%) and men (86%). The majority (86%) of women were premenopausal, and 4 were postmenopausal, based on menstrual histories. None were receiving exogenous hormone therapy.
Table 1.
Demographic and Clinical Characteristics of Study Subjects
| Women (n = 28) | Men (n = 27) | P | |
|---|---|---|---|
| Age, y | 38 (34–41) | 35 (32–38) | .30 |
| Race/ethnicity | .32 | ||
| White | 10 (36) | 16 (59) | |
| Black | 13 (46) | 7 (26) | |
| Hispanic | 4 (14) | 3 (11) | |
| Native American | 1 (4) | 1 (4) | |
| HIV-1 risk factor | |||
| Sex between men | … | 24 (89) | |
| Sex between men and women | 22 (79) | 1 (4) | <.001 |
| Injection drug use | 3 (11) | 2 (7) | 1.0 |
| Othera | 3 (11) | 0 (0) | |
| CD4+ T-cell count, cells/mm2 | 525 (440–610) | 619 (531–708) | .11 |
| Plasma HIV-1 RNA load, log10 copies/mL | 3.88 (3.56–4.2) | 4.49 (4.17–4.8) | .01 |
| Previous antiretroviral therapyb | 8 (28) | 4 (15) | .3 |
| R5-tropic HIV-1c | 20 (100) | 20 (100) | 1.0 |
| Heterozygous for CCR5-Δ32 mutation | 6 (21) | 7 (26) | .76 |
Data are mean (95% confidence interval) or no. (%) of subjects.
Abbreviation: HIV-1, human immunodeficiency virus type 1.
a Needlestick (n = 2) and blood transfusion (n = 1).
b All subjects discontinued antiretroviral therapy >6 months before entering the study.
c One woman and 4 men did not have sufficient plasma available, and Trofile test results were indeterminate for 7 women and 3 men. Subjects with indeterminate results of tropism assays had significantly lower viral loads than subjects with definitive results (mean, 3.3 vs 4.4 log10 copies/mL; P < .001).
The mean CD4+ T-cell count was 571 cells/mm3 (95% confidence interval [CI], 511–632) and did not differ significantly between men and women. As expected, women had lower viral loads than men, with a mean difference of 0.61 log10 copies/mL (P = .01). Among all study subjects, 22% had a prior history of ART receipt, and this did not differ by sex. There was no sex-based difference in time since ART discontinuation between women and men (geometric mean, 2.4 and 2.1 years, respectively; P = .9). All 40 subjects with available tropism results harbored only R5-tropic virus. Overall, 24% of subjects were heterozygous for the CCR5-Δ32 mutation, and there were no sex-based differences in the frequency of CCR5 heterozygosity. Viral load tended to be lower in CCR5-Δ32 heterozygotes, compared with wild-type homozygotes (Supplementary Table 1).
Women Had Significantly Lower Percentages of Lymph Node CCR5+CD4+ T Cells Than Men
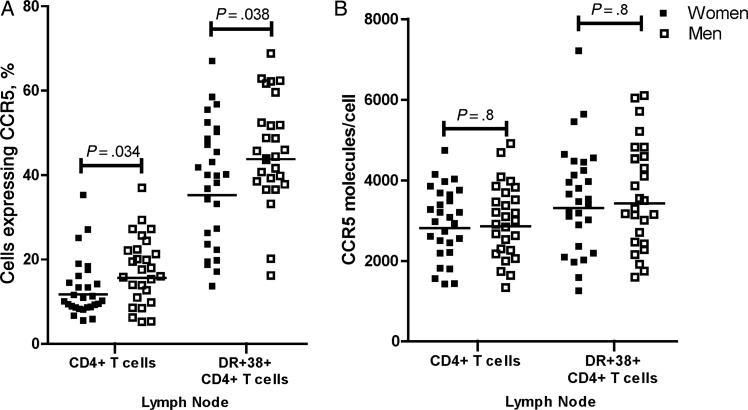
Geometric mean percentages of lymph node CCR5+CD4+ T cells were lower in HIV-1–infected women (12%), compared with HIV-1–infected men (16%; Figure 1A). When CCR5 expression was evaluated on activated CD4+ T cells, geometric mean percentages of CCR5+ cells were lower in women (35%), compared with men (44%), as well (Figure 1A). There were no sex-based differences in the density of CCR5 molecules on CD4+ T cells or activated CD4+ T cells (Figure 1B). The percentage of cells expressing CCR5 and the CCR5 density were lower among CCR5-Δ32 heterozygotes, compared with wild-type homozygotes (Supplementary Table 1). Neither the percentage nor the density of CCR5+CD4+ T cells was significantly related to plasma progesterone or estradiol levels (data not shown).
Figure 1.
Percentages (A) and concentrations (B) of CCR5 on CD4+ T cells and HLA-DR+CD38+CD4+ T cells in lymph nodes from human immunodeficiency virus type 1–seropositive women (n = 28) and men (n = 27). Each symbol represents the value for an individual subject. Horizontal lines indicate geometric means.
Sex-Based Differences in CCR5 Expression on Lymph Node CD4+ T Cells Did Not Account for Sex-Based Differences in Viral Load
Neither the percentage of CCR5+ cells nor the density of CCR5 on CD4+ T cells or the activated subset was predictive of viral load (P ≥ .4), after adjusting for CD4+ T-cell count, race, and age. In addition, CCR5 expression did not predict viral load after adjusting for CD4+ T-cell count, Δ32 heterozygosity, and age (P ≥ .5). When analyses were limited to subjects known to harbor R5-tropic virus, outcomes did not change (P ≥ .3).
Percentages of Activated CD4+ T Cells in Lymph Nodes Predicted Viral Load, But Did Not Account for Sex-Based Differences in Viral Load
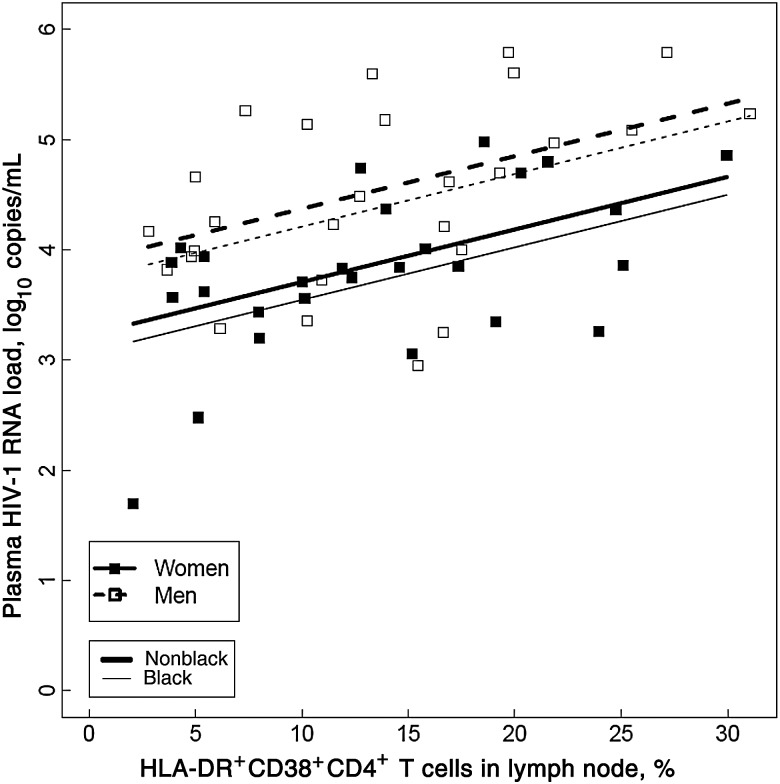
The geometric mean percentage of lymph node DR+38+CD4+ T cells was 11% (95% CI, 10–14) and did not differ significantly between women (12%; 95% CI, 9–15) and men (11%; 95% CI, 9–15; P = .85). In a model that adjusted for multiple clinical factors, including sex, race, age, and CD4+ T-cell count, an increase of 5% in DR+38+CD4+ T cells predicted an increase of 0.24 log10 copies/mL (95% CI, .06–.42) in viral load (P = .012). In the same model, after controlling for the percentage of DR+38+CD4+ T cells, race, age, and CD4+ T-cell count, women still had a viral load that was 0.66 log10 copies/mL (95% CI, .62–.69) lower than that in men (P = .002; Figure 2).
Figure 2.
After adjusting for race, age, sex, and CD4+ T-cell count, the percentage of HLA-DR+CD38+CD4+ T cells in lymph nodes predicted log10 number of human immunodeficiency virus type 1 (HIV-1) RNA copies/mL (P = .012). The model also showed persistent sex-based differences in plasma HIV-1 RNA load (P = .002), after controlling for HLA-DR+CD38+CD4+ T cells, race, age, and CD4+ T-cell count. The slopes of the lines were not significantly different by sex (P = .9) or race (P = .2).
Women Had Lower Frequencies of HIV-1 RNA–Producing Lymph Node Cells Than Men
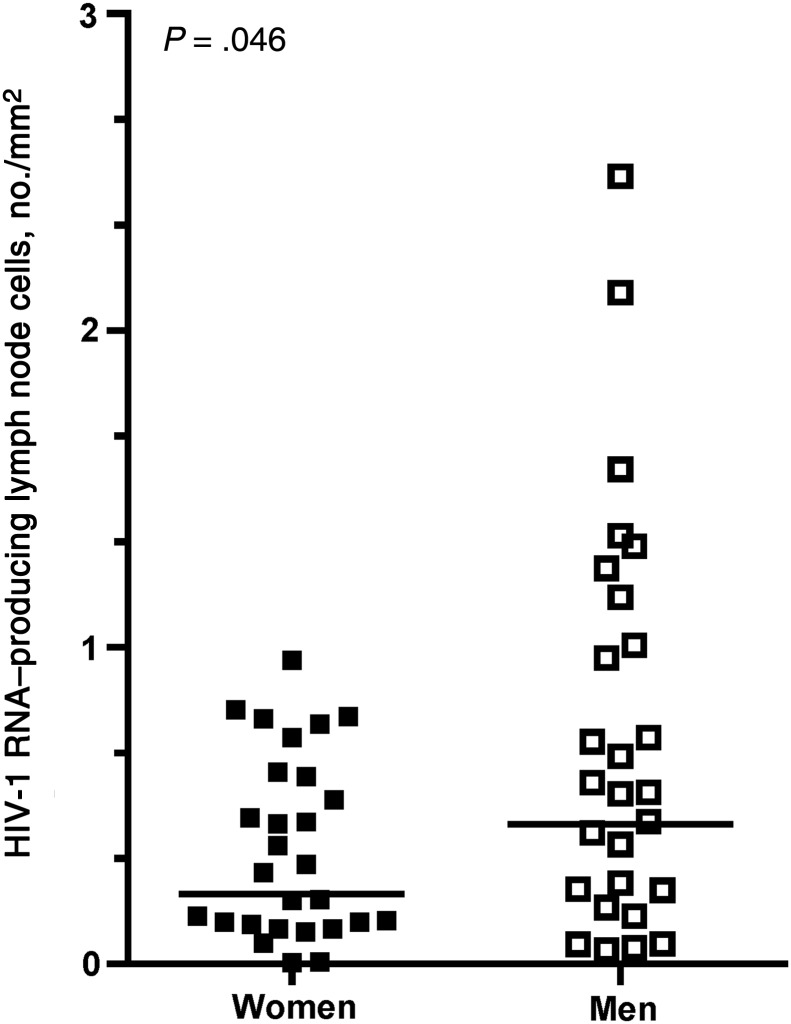
The geometric mean lymph node weight was 888 mg and did not differ between women (818 mg; 95% CI, 561–1193; n = 28) and men (973 mg; 95% CI, 722–1312; n = 25; P = .4). The mean area of a single lymph node tissue section was 33 mm2 (95% CI, 27,–38), and this also did not differ between women (mean, 33 mm2; 95% CI, 24–41) and men (mean, 32 mm2; 95% CI, 25–40; P = .9). The geometric mean frequency of HIV-1 RNA–producing cells was 0.30 cells/mm2 (95% CI, .21–.43). Women had lower frequencies of HIV-1 RNA–producing cells than men (Figure 3; geometric mean, 0.21 vs 0.44 cells/mm2; P = .046). Sex-based differences in HIV-1 RNA–producing cells mediated 45% (95% CI, 4–86) of the sex-based differences in viral load (P = .031).
Figure 3.
Frequencies of human immunodeficiency virus type 1 (HIV-1) RNA–producing lymph node cells per square millimeter, as determined by in situ hybridization for HIV-1 RNA, were significantly higher in men, compared with women. Each symbol represents the sum of the HIV-1 RNA–producing cells from all lymph node sections, divided by the total area examined for an individual subject. A geometric mean of 6 (95% confidence interval [CI], 5–8) sections and 168 mm2 (95% CI, 128–222 mm2) of tissue were analyzed per subject. Horizontal lines indicate geometric means.
Neither CCR5 Expression Nor Activated CD4+ T Cells Accounted for Sex-Based Differences in HIV-1 RNA–Producing Lymph Node Cells
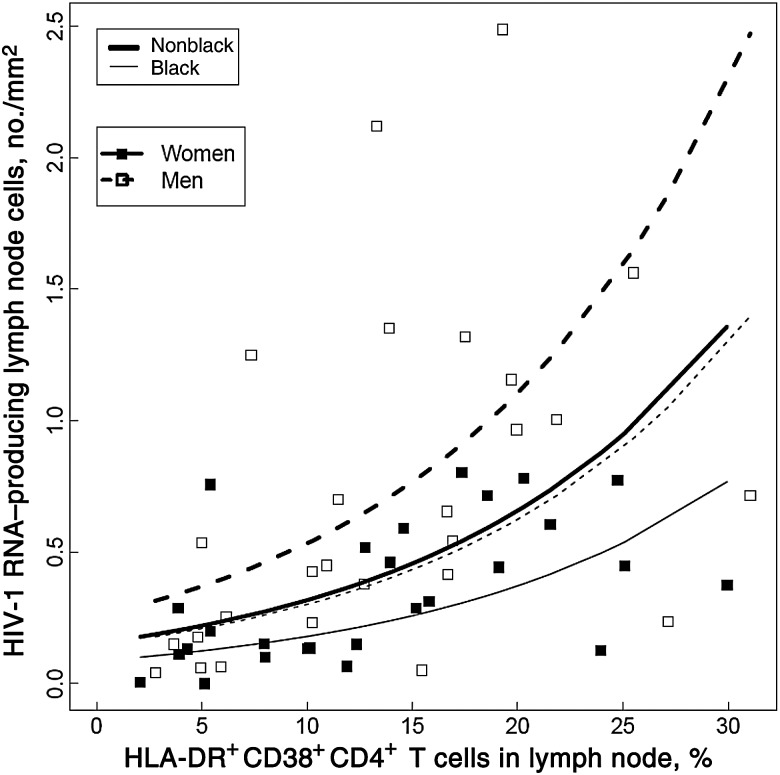
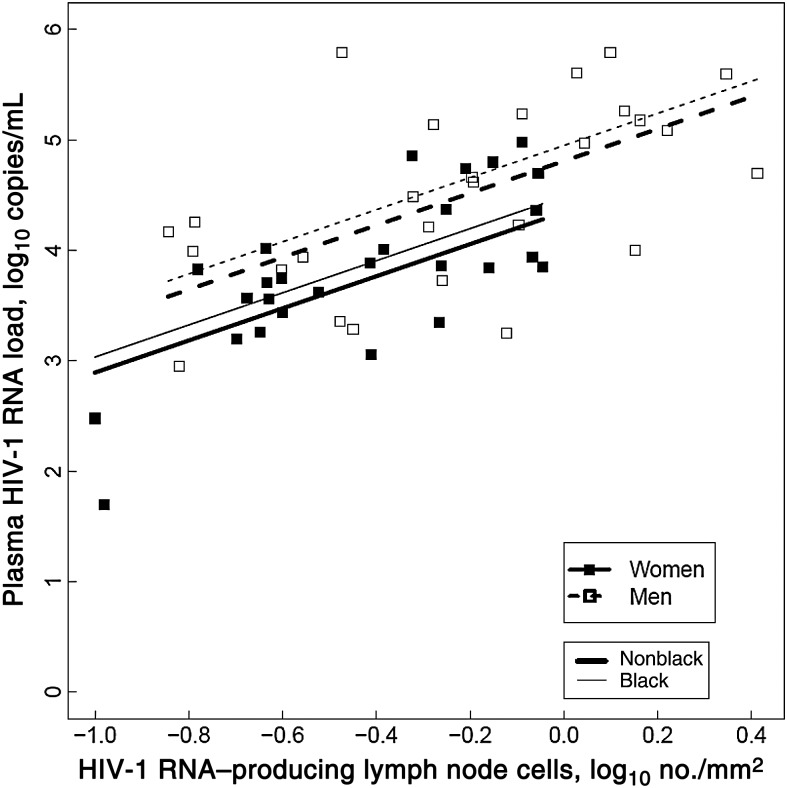
To evaluate whether CCR5 expression or the percentage of activated CD4+ T cells was a determinant of the frequency of HIV-1 RNA–producing cells a negative binomial generalized linear model was used. Neither CCR5 density nor percentage of CD4+ or DR+38+CD4+ T cells expressing CCR5 was predictive of HIV-1 RNA–producing cells (P ≥ .24), after adjusting for CD4+ T-cell count, race, and age. CCR5 expression also did not predict the number of HIV-1 RNA–producing cells per millimeter, after adjusting for CD4+ T-cell count, Δ32 heterozygosity, and age (P ≥ .4). An increase of 5% in DR+38+CD4+ T cells predicted a 44% (95% CI, 17–77) increase in HIV-1 RNA–producing cells in univariate analysis (P < .001). After adjusting for race, percentage of DR+38+CD4+ T cells, age, and CD4+ T-cell count, women still had 40% (95% CI, 6–62) fewer HIV-1 RNA–producing cells than men (P = .023; Figure 4).
Figure 4.
Percentages of HLA-DR+CD38+CD4+ T cells predicted increases in the number of human immunodeficiency virus type 1 (HIV-1) RNA–producing cells per square millimeter in a model that controlled for sex, race, and CD4+ T-cell count (P < .001). After adjustment for the percentage of T cells in the lymph node that were HLA-DR+CD38+CD4+, race, and CD4+ T-cell count, women still had lower proportions of HIV-1 RNA–producing cells, compared with men (P = .023). Black women (thin solid line) had 66% (95% confidence interval, 40–81) lower HIV-1 RNA–producing cells than nonblack men (thick dashed line; P < .001), but there was no significant difference between black men (thin dashed line) and nonblack women (thick solid line; P = .9). The slopes of the lines were not significantly different by sex (P = .9) or race (P = .7).
HIV-1 RNA–Producing Lymph Node Cells Were Associated With a Lower Viral Load in Women, Compared With Men
Frequencies of log10 HIV-1 RNA–producing cells correlated significantly with log10 viral load (Pearson r = 0.68; 95% CI, .50–.80; P < .001). To determine whether there were sex-based differences in the relationship between HIV-1 RNA–producing cells and viral load, we used a linear regression model that adjusted for multiple clinical parameters (Figure 5). An increase of 0.2 log10 HIV-1 producing cells/mm2 predicted an increase in viral load of 0.29 log10 copies/mL (95% CI, .17–.41; P < .001). Furthermore, in this same model, women had a significantly lower log10 viral load (0.46; 95% CI, .085–.84; P = .018) than men, after adjustment for HIV-1 RNA–producing cells, CD4+ T-cell count, race, and age. Similar results were obtained after controlling for HIV-1 RNA–producing cells, CD4+ T-cell count, CCR5-Δ32 heterozygosity, and age (log10 viral load difference, 0.46; P = .017)
Figure 5.
Human immunodeficiency virus type 1 (HIV-1) RNA–producing lymph node cells predicted plasma viral load, after adjusting for sex, race, age, and peripheral blood CD4+ T-cell count (P < .001). After adjusting for race, age, CD4+ T-cell count, and HIV-1 RNA–producing cells, women still had a significantly lower viral load than men (P = .018). Viral load did not significantly differ between blacks and all other races (P = .46). The slope did not differ by sex (P = .16).
DISCUSSION
This is the first study to investigate sex-based differences in viral load in secondary lymphoid tissues, the primary site of HIV-1 replication [12–14]. Women had significantly lower percentages of lymph node CD4+ T cells and activated CD4+ T cells that expressed CCR5. Surprisingly, neither of these parameters predicted viral load or HIV-1 RNA–producing cells, and reduced lymph node CCR5 expression in women did not account for sex-based differences in viral load. Percentages of activated CD4+ T cells in the lymph node predicted the plasma viral load, similar to what has been previously reported for peripheral blood mononuclear cells [19, 20] and lymph nodes [21]. Proportions of activated CD4+ T cells in lymph nodes, however, did not differ between women and men and failed to explain sex-based differences in viral load. Women had significantly lower frequencies of HIV-1 RNA–producing cells in lymph nodes than men, but the difference accounted for less than half of the sex-based differences in viral load in our cohort. Intriguingly, after adjusting for the number of HIV-1 RNA–producing cells, women had 0.46 log10 lower viral load, compared with men. These data indicate that less plasma virus is associated with each HIV-1 RNA–producing cell in women, compared with men.
Before this study, sex-based differences in CCR5 expression had only been described in peripheral blood of HIV-1–seronegative individuals [11]. In that study, the density but not the percentage of CCR5+CD4+ T cells was lower in women than in men. In contrast, percentages of CCR5+CD3+CD4+ cells but not the density of CCR5 differed between the sexes in our study. Reasons for these differences are unclear but could be related to HIV-1 serostatus or differences between peripheral blood and lymph node CD4+ T cells. Whether CCR5 expression is modulated by sex hormones is controversial [22–24]. Although there was no relationship between hormone levels and CCR5 in the present study, this does not exclude a relationship between cycle phase or peak hormone levels and CCR5 expression, because women in our study underwent lymph node excision randomly, without regard to menstrual cycle phase. Irrespective of the mechanism that underlies sex-based differences in CCR5, the finding is important because it may translate into sex-based differences related to the primary function of CCR5, which is chemotaxis of immune cells to sites of pathology, and impact a broad range of diseases [25].
The findings that CCR5 expression was not a predictor of viral load or HIV-1 RNA–producing cells were surprising and contrary to our initial hypothesis, as well as to peripheral blood studies from one group [26–28]. One possible explanation is that HIV-1 RNA–producing lymph node cells are not major contributors to viral load. Several lines of evidence weigh against this explanation. It is well accepted that the majority of HIV-1 replication occurs in secondary lymphoid tissues [12, 13] and that they produce the majority of the viral load [29]. Lymph nodes are the largest secondary lymphoid organ, collectively harboring approximately two-thirds of CD4+ T cells in secondary lymphoid tissues [30]. Frequencies of HIV-1 RNA–producing cells in our study correlated with viral load, suggesting that lymph node cells are representative of cells that produce the viral load. Finally, phylogenetic analyses of virus in plasma and secondary lymphoid tissues of humans and macaques suggest that lymph nodes are a major source of plasma viremia, whereas gut-associated lymphoid tissue (GALT) is a minor contributor [31, 32]. Our study cannot exclude the possibility that a correlation exists between CCR5 expression and virus replication in the spleen or GALT. It is unlikely, however, that the studies reported here would be repeated in the spleen, owing to the risks associated with splenic biopsies. Furthermore, both the enzymatic digestion necessary to disaggregate GALT and the prolonged period between obtaining fresh tissue and measurement of CCR5 would render GALT measurements highly suspect because of the lability of the CCR5 molecule ([33] and unpublished data). Regardless, spleen and GALT each harbor only approximately one-sixth of the CD4+ T cells in secondary lymphoid tissues [30] and are less important than lymph nodes in terms of their contribution to viral load.
The failure of lymph node CCR5 expression to correlate with viral load or HIV-1 RNA–producing cells is difficult to reconcile with observations that CCR5-Δ32 heterozygotes have a lower viral load than wild-type homozygotes [34]. It has been widely assumed that this difference is mediated by CCR5 expression. Nevertheless, another potential explanation is that individuals who are heterozygous for the Δ32 allele have more-vigorous cell-mediated immunity than others [35, 36]. Independent support for our findings of a lack of correlation between CCR5 expression and viral load or HIV-1 RNA–producing cells is provided by a study that demonstrated that CCR5 expression restricted R5-tropic HIV-1 replication in vitro only at levels of <2000 molecules/cell [9], which is close to the lower limit of CCR5 expression observed in our study (range, 1261–7214 molecules/cell). Thus, although CCR5 expression is clearly necessary for infection with R5 HIV-1, our study suggests that physiologic levels of CCR5 are not limiting and consequently are not a major determinant of R5 HIV-1 production in lymph nodes. These findings have important implications for strategies currently under development to treat HIV-1 infection through interruption of CCR5 expression [37], because they suggest that profound reductions in CCR5 expression may be necessary to successfully reduce virus replication in lymph nodes.
Women had fewer HIV-1 RNA–producing cells than men, although this difference was marginally significant (P = .046) and explained less than half of the sex-based difference in viral load. This finding could be related to recruitment of more men with a high viral load than would have occurred naturally in an untreated population. As described in Methods, near the end of study recruitment, a deficiency of men with a CD4+ T-cell count of <500 cells/mm3 and a high viral load was noted, likely because of earlier initiation of ART by men in the United States [38–42]. Our efforts to correct for this skewing may have resulted in overrepresentation of men with a high viral load. Indeed, the sex-based difference in viral load observed in our study (−0.64 log10 copies/mL) was higher than that reported in other studies (range, −0.14 to −0.53 log10 copies/mL) [1–3], supporting the notion that men with a high viral load were overrepresented. HIV-1 RNA–producing cells are likely related to disease progression, because frequencies of HIV-1 RNA–producing cells predict rates of disease progression in simian immunodeficiency virus (SIV)–infected macaques [43]. If HIV-1–infected women do not have substantially different frequencies of HIV-1 RNA–producing cells than men, this could explain why they progress at similar rates as men to AIDS and death despite different viral loads.
The sex-based difference in viral load that remained after controlling for HIV-1 RNA–producing cells was −0.46 log10 copies/mL, which is consistent with the sex-based difference seen in many epidemiologic studies [1, 3]. Although differences in amounts of lymphoid tissue could account for this, there were no sex-based differences in size or weight of lymph nodes. Another possible explanation would be production of fewer virions per HIV-1 RNA–producing cell in women, compared with men. Future studies that measure intracellular levels of HIV-1 RNA in lymph node cells could be useful in evaluating this. Finally, more-efficient clearance of extracellular HIV-1 in women as compared to men could account for the sex-based discordance between HIV-1 RNA–producing cells and viral load. Although a previous study concluded that there were no sex-based differences in plasma HIV-1 RNA clearance, based on analysis of decay rates after initiation of ART [44], the median CD4+ T-cell count was 261 cells/mm3, and there were no sex-based differences in viral load, suggesting the study had insufficient statistical power to detect sex-based differences. Thus, it remains possible that sex-based differences in clearance of extracellular virus exist, particularly in individuals with relatively preserved CD4+ T-cell counts, such as those in our study.
This analysis has several limitations. Recruitment relied primarily on identification of chronically HIV-1–infected subjects who were not receiving therapy. Because of differences in rates of initiation of ART among men and women and among individuals of different races in the United States [38–42], this could have introduced bias. Furthermore, we sought to correct for skewing in the study population by recruiting additional men with low CD4+ T-cell counts and high viral loads, which may have introduced other biases. There may also have been some bias toward recruitment of CCR5-Δ32 heterozygotes; they constituted 24% of all study subjects, whereas the CCR5-Δ32 allele is found in only 20% of healthy white individuals in Denver and, likely, in fewer individuals with a race or ethnicity other than white [45]. Importantly, all multivariate analyses controlled for CD4+ T-cell count, an indirect measure of length of infection, as well as age and race, and additional analyses controlling for CCR5-Δ32 heterozygosity did not affect the results of the study. Nevertheless, it is possible that there were biases that were not controlled for by these measures.
In the past, when viral load was one of the criteria used in the United States to determine when to initiate ART, it was suggested that women might benefit from initiation of ART at a lower viral load than that for men [46]. The present study provides a biologic basis for that recommendation, as it suggests there are not large sex-based differences in numbers of lymph node HIV-1 RNA–producing cells but that HIV-1 RNA–producing cells are associated with a lower viral load in women than in men. Because viral load is no longer used to determine when to initiate therapy and because US guidelines recommend early initiation of therapy for everyone [47], sex-based differences in viral load are less clinically relevant. Nevertheless, care providers should be cognizant that a woman with the same viral load as a man might have a larger burden of productively infected lymph node cells and consequently may experience more-rapid disease progression in the absence of ART. The findings of this study also shed light on the observation that viral load fails to account for all of the variation in rates of disease progression among HIV-1–infected individuals even in analyses that control for sex [48, 49]. Results of this study suggest that differences in the amount of virions produced per productively infected cell and/or clearance of extracellular virus may explain this. This study has demonstrated that examination of sex-based differences in viral load can provide important insights into HIV-1 immunopathogenesis. Further investigation of whether sex-based differences exist in production or clearance of extracellular virions could provide critical insights into fundamental principles that govern virus production and disease progression, which are essential to the development of novel therapeutic strategies to treat and cure HIV-1 infection.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Kevin Ryan, the NIH program officer for the grant, without whose support this study would not have been possible; Karen Whalen and Kimberly Poland, for nursing assistance during lymph node excisions; Steven Johnson, Beverly Putnam, Cathi Basler, M. Graham Ray, and John Koeppe, University of Colorado Anschutz Medical Campus, and William Burman and Patricia Caraway, Denver Heath ID Clinic, for assistance in study subject recruitment; and the subjects who participated in this study.
Financial support. This work was supported by the National Institute of Health (grants R21HD051450 and R21HD051450-02S1) and the University of Colorado Center for AIDS Research (grant AI054907).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Gandhi M, Bacchetti P, Miotti P, Quinn TC, Veronese F, Greenblatt RM. Does patient sex affect human immunodeficiency virus levels? Clin Infect Dis. 2002;35:313–22. doi: 10.1086/341249. [DOI] [PubMed] [Google Scholar]
- 2.Grinsztejn B, Smeaton L, Barnett R, et al. Sex-associated differences in pre-antiretroviral therapy plasma HIV-1 RNA in diverse areas of the world vary by CD4(+) T-cell count. Antivir Ther. 2011;16:1057–62. doi: 10.3851/IMP1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Napravnik S, Poole C, Thomas JC, Eron JJ., Jr Gender difference in HIV RNA levels: a meta-analysis of published studies. J Acquir Immune Defic Syndr. 2002;31:11–9. doi: 10.1097/00126334-200209010-00002. [DOI] [PubMed] [Google Scholar]
- 4.Mellors JW, Rinaldo CR, Jr, Gupta P, White RM, Todd JA, Kingsley LA. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–70. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 5.Prins M, Meyer L, Hessol NA. Sex and the course of HIV infection in the pre- and highly active antiretroviral therapy eras. AIDS. 2005;19:357–70. doi: 10.1097/01.aids.0000161765.75663.27. [DOI] [PubMed] [Google Scholar]
- 6.Sterling TR, Vlahov D, Astemborski J, Hoover DR, Margolick JB, Quinn TC. Initial plasma HIV-1 RNA levels and progression to AIDS in women and men. N Engl J Med. 2001;344:720–5. doi: 10.1056/NEJM200103083441003. [DOI] [PubMed] [Google Scholar]
- 7.Hogan CM, Hammer SM. Host determinants in HIV infection and disease. Part 2: genetic factors and implications for antiretroviral therapeutics. Ann Intern Med. 2001;134:978–96. doi: 10.7326/0003-4819-134-10-200105150-00012. [DOI] [PubMed] [Google Scholar]
- 8.Lee B, Sharron M, Montaner LJ, Weissman D, Doms RW. Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proc Natl Acad Sci U S A. 1999;96:5215–20. doi: 10.1073/pnas.96.9.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Platt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72:2855–64. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reeves JD, Gallo SA, Ahmad N, et al. Sensitivity of HIV-1 to entry inhibitors correlates with envelope/coreceptor affinity, receptor density, and fusion kinetics. Proc Natl Acad Sci U S A. 2002;99:16249–54. doi: 10.1073/pnas.252469399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Portales P, Clot J, Corbeau P. Sex differences in HIV-1 viral load due to sex difference in CCR5 expression. Ann Intern Med. 2001;134:81–2. doi: 10.7326/0003-4819-134-1-200101020-00023. [DOI] [PubMed] [Google Scholar]
- 12.Embretson J, Zupancic M, Ribas JL, et al. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993;362:359–62. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- 13.Pantaleo G, Graziosi C, Demarest JF, et al. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993;362:355–8. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- 14.Tenner-Racz K, Racz P, Bofill M, et al. HTLV-III/LAV viral antigens in lymph nodes of homosexual men with persistent generalized lymphadenopathy and AIDS. Am J Pathol. 1986;123:9–15. [PMC free article] [PubMed] [Google Scholar]
- 15.Meditz AL, Haas MK, Folkvord JM, et al. HLA-DR+CD38+CD4+ T lymphocytes have elevated CCR5 expression and produce the majority of R5-tropic HIV-1 RNA in vivo. J Virol. 2011;85:10189–200. doi: 10.1128/JVI.02529-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Folkvord JM, Armon C, Connick E. Lymphoid follicles are sites of heightened human immunodeficiency virus type 1 (HIV-1) replication and reduced antiretroviral effector mechanisms. AIDS Res Hum Retroviruses. 2005;21:363–70. doi: 10.1089/aid.2005.21.363. [DOI] [PubMed] [Google Scholar]
- 17.Whitcomb JM, Huang W, Fransen S, et al. Development and characterization of a novel single-cycle recombinant-virus assay to determine human immunodeficiency virus type 1 coreceptor tropism. Antimicrob Agents Chemother. 2007;51:566–75. doi: 10.1128/AAC.00853-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meditz AL, Moreau KL, MaWhinney S, et al. CCR5 expression is elevated on endocervical CD4+ T cells in healthy postmenopausal women. J Acquir Immune Defic Syndr. 2012;59:221–8. doi: 10.1097/QAI.0b013e31823fd215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deeks SG, Kitchen CM, Liu L, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104:942–7. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 20.Hazenberg MD, Otto SA, van Benthem BH, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS. 2003;17:1881–8. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- 21.Yang OO, Ferbas JJ, Hausner MA, et al. Effects of HIV-1 infection on lymphocyte phenotypes in blood versus lymph nodes. J Acquir Immune Defic Syndr. 2005;39:507–18. [PubMed] [Google Scholar]
- 22.Mo R, Chen J, Grolleau-Julius A, Murphy HS, Richardson BC, Yung RL. Estrogen regulates CCR gene expression and function in T lymphocytes. J Immunol. 2005;174:6023–9. doi: 10.4049/jimmunol.174.10.6023. [DOI] [PubMed] [Google Scholar]
- 23.Vassiliadou N, Tucker L, Anderson DJ. Progesterone-induced inhibition of chemokine receptor expression on peripheral blood mononuclear cells correlates with reduced HIV-1 infectability in vitro. J Immunol. 1999;162:7510–8. [PubMed] [Google Scholar]
- 24.Asin SN, Heimberg AM, Eszterhas SK, Rollenhagen C, Howell AL. Estradiol and progesterone regulate HIV type 1 replication in peripheral blood cells. AIDS Res Hum Retroviruses. 2008;24:701–16. doi: 10.1089/aid.2007.0108. [DOI] [PubMed] [Google Scholar]
- 25.Guergnon J, Combadière C. Role of chemokines polymorphisms in diseases. Immunol Lett. 2012;145:15–22. doi: 10.1016/j.imlet.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 26.Reynes J, Baillat V, Portales P, Clot J, Corbeau P. Relationship between CCR5 density and viral load after discontinuation of antiretroviral therapy. JAMA. 2004;291:46. doi: 10.1001/jama.291.1.46. [DOI] [PubMed] [Google Scholar]
- 27.Reynes J, Portales P, Segondy M, et al. CD4 T cell surface CCR5 density as a host factor in HIV-1 disease progression. AIDS. 2001;15:1627–34. doi: 10.1097/00002030-200109070-00004. [DOI] [PubMed] [Google Scholar]
- 28.Reynes J, Portales P, Segondy M, et al. CD4+ T cell surface CCR5 density as a determining factor of virus load in persons infected with human immunodeficiency virus type 1. J Infect Dis. 2000;181:927–32. doi: 10.1086/315315. [DOI] [PubMed] [Google Scholar]
- 29.De Boer RJ, Ribeiro RM, Perelson AS. Current estimates for HIV-1 production imply rapid viral clearance in lymphoid tissues. PLoS Comput Biol. 2010;6:e1000906. doi: 10.1371/journal.pcbi.1000906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ganusov VV, De Boer RJ. Do most lymphocytes in humans really reside in the gut? Trends Immunol. 2007;28:514–8. doi: 10.1016/j.it.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Vanderford TH, Bleckwehl C, Engram JC, et al. Viral CTL escape mutants are generated in lymph nodes and subsequently become fixed in plasma and rectal mucosa during acute SIV infection of macaques. PLoS Pathog. 2011;7:e1002048. doi: 10.1371/journal.ppat.1002048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lerner P, Guadalupe M, Donovan R, et al. The gut mucosal viral reservoir in HIV-infected patients is not the major source of rebound plasma viremia following interruption of highly active antiretroviral therapy. J Virol. 2011;85:4772–82. doi: 10.1128/JVI.02409-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berhanu D, Mortari F, De Rosa SC, Roederer M. Optimized lymphocyte isolation methods for analysis of chemokine receptor expression. J Immunol Methods. 2003;279:199–207. doi: 10.1016/s0022-1759(03)00186-8. [DOI] [PubMed] [Google Scholar]
- 34.de Roda Husman AM, Koot M, Cornelissen M, et al. Association between CCR5 genotype and the clinical course of HIV-1 infection. Ann Intern Med. 1997;127:882–90. doi: 10.7326/0003-4819-127-10-199711150-00004. [DOI] [PubMed] [Google Scholar]
- 35.Catano G, Chykarenko ZA, Mangano A, et al. Concordance of CCR5 genotypes that influence cell-mediated immunity and HIV-1 disease progression rates. J Infect Dis. 2011;203:263–72. doi: 10.1093/infdis/jiq023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Connick E, Schlichtemeier RL, Purner MB, et al. Relationship between human immunodeficiency virus type 1 (HIV-1)-specific memory cytotoxic T lymphocytes and virus load after recent HIV-1 seroconversion. J Infect Dis. 2001;184:1465–9. doi: 10.1086/324488. [DOI] [PubMed] [Google Scholar]
- 37.Cannon P, June C. Chemokine receptor 5 knockout strategies. Curr Opin HIV and AIDS. 2011;6:74–9. doi: 10.1097/COH.0b013e32834122d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gebo KA, Fleishman JA, Conviser R, et al. Racial and gender disparities in receipt of highly active antiretroviral therapy persist in a multistate sample of HIV patients in 2001. J Acquir Immune Defic Syndr. 2005;38:96–103. doi: 10.1097/00126334-200501010-00017. [DOI] [PubMed] [Google Scholar]
- 39.Giordano TP, White AC, Jr, Sajja P, et al. Factors associated with the use of highly active antiretroviral therapy in patients newly entering care in an urban clinic. J Acquir Immune Defic Syndr. 2003;32:399–405. doi: 10.1097/00126334-200304010-00009. [DOI] [PubMed] [Google Scholar]
- 40.Lemly DC, Shepherd BE, Hulgan T, et al. Race and sex differences in antiretroviral therapy use and mortality among HIV-infected persons in care. J Infect Dis. 2009;199:991–8. doi: 10.1086/597124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McNaghten AD, Hanson DL, Dworkin MS, Jones JL Adult/Adolescent Spectrum of HIVDG. Differences in prescription of antiretroviral therapy in a large cohort of HIV-infected patients. J Acquir Immune Defic Syndr. 2003;32:499–505. doi: 10.1097/00126334-200304150-00006. [DOI] [PubMed] [Google Scholar]
- 42.Meditz AL, MaWhinney S, Allshouse A, et al. Sex, race, and geographic region influence clinical outcomes following primary HIV-1 infection. J Infect Dis. 2011;203:442–51. doi: 10.1093/infdis/jiq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chakrabarti L, Cumont MC, Montagnier L, Hurtrel B. Variable course of primary simian immunodeficiency virus infection in lymph nodes: relation to disease progression. J Virol. 1994;68:6634–43. doi: 10.1128/jvi.68.10.6634-6643.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuritzkes DR, Ribaudo HJ, Squires KE, et al. Plasma HIV-1 RNA dynamics in antiretroviral-naive subjects receiving either triple-nucleoside or efavirenz-containing regimens: ACTG A5166s. J Infect Dis. 2007;195:1169–76. doi: 10.1086/512619. [DOI] [PubMed] [Google Scholar]
- 45.Sato H, Silveira L, Spagnolo P, et al. CC chemokine receptor 5 gene polymorphisms in beryllium disease. Eur Respir J. 2010;36:331–8. doi: 10.1183/09031936.00107809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sterling TR, Lyles CM, Vlahov D, Astemborski J, Margolick JB, Quinn TC. Sex differences in longitudinal human immunodeficiency virus type 1 RNA levels among seroconverters. J Infect Dis. 1999;180:666–72. doi: 10.1086/314967. [DOI] [PubMed] [Google Scholar]
- 47.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf.E1 ; November 13, 2013; p.47.
- 48.Mellors JW, Margolick JB, Phair JP, et al. Prognostic value of HIV-1 RNA, CD4 cell count, and CD4 Cell count slope for progression to AIDS and death in untreated HIV-1 infection. JAMA. 2007;297:2349–50. doi: 10.1001/jama.297.21.2349. [DOI] [PubMed] [Google Scholar]
- 49.Rodriguez B, Sethi AK, Cheruvu VK, et al. Predictive value of plasma HIV RNA level on rate of CD4 T-cell decline in untreated HIV infection. JAMA. 2006;296:1498–506. doi: 10.1001/jama.296.12.1498. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.