Abstract
Background. Our goals were to describe azithromycin (AZI) pharmacokinetics in maternal plasma (MP), fetal plasma (FP), and amniotic fluid (AF) following intra-amniotic infection (IAI) with Ureaplasma in pregnant rhesus monkeys and to explore concentration-response relationships.
Methods. Following intra-amniotic inoculation of Ureaplasma parvum, rhesus monkeys received AZI (12.5 mg/kg every 12 hours intravenously for 10 days; n = 10). Intensive pharmacokinetic sampling of MP, FP, and AF was scheduled following the first (ie, single) dose and the last (ie, multiple) dose. Noncompartmental and pharmacokinetic modeling methods were used.
Results. The AF area under the concentration-time curve at 12 hours was 0.22 µg×h/mL following a single dose and 6.3 µg×h/mL at day 10. MP and AF accumulation indices were 8.4 and 19, respectively. AZI AF half-life following the single dose and multiple dose were 156 and 129 hours, respectively. The median MP:FP ratio in concomitantly drawn samples was 3.2 (range, 1.3–9.6; n = 9). Eradication of U. parvum occurred at 6.6 days, with a 95% effective concentration (EC95) of 39 ng/mL for the maximum AZI AF concentration.
Conclusions. Our study demonstrates that a maternal multiple-dose AZI regimen is effective in eradicating U. parvum IAI by virtue of intra-amniotic accumulation and suggests that antenatal therapy has the potential to mitigate complications associated with U. parvum infection in pregnancy, such as preterm labor and fetal sequelae.
Keywords: azithromycin, pharmacokinetics, pharmacodynamics, Ureaplasma, chorioamnionitis
Intrauterine infections are an important and potentially preventable cause of preterm delivery. Such infections cause the majority of very-low-birth-weight (VLBW) deliveries, which are characterized by a birth weight of <1500 g and delivery before 30 weeks of gestation. VLBW accounts for the highest rates of neonatal deaths, the most serious neonatal complications, and a disproportionate share of perinatal healthcare costs [1]. Intrauterine bacterial infections can cause inflammation of the fetal membranes, chorion, and amnion (chorioamnionitis). Ureaplasma urealyticum and Ureaplasma parvum are the microorganisms most frequently isolated from placenta with chorioamnionitis in women with preterm labor (47%) [2, 3]. Moreover, these bacteria are also the most common organisms isolated from the respiratory tracts of preterm infants and have been associated with neonatal pneumonia, severe respiratory failure, and bronchopulmonary dysplasia [4–7]. Among newborn infants, the frequency and severity of ureaplasma infection varies inversely with gestational age and weight, occurring most frequently among preterm infants with VLBW [8]. Indeed, detection of ureaplasmas in the chorioamnion is associated with delivery at <37 weeks gestational age [4, 9].
Intra-amniotic infection (IAI) often manifests without the mother's knowledge, making accurate diagnosis difficult and early intervention challenging to forestall upregulation of proinflammatory mediators that precede preterm birth and also play an important role in fetal and neonatal sequelae. Eradicating ureaplasma infection in pregnant women with chorioamnionitis could delay delivery and prevent complications in newborns. While Ureaplasma species lack a cell wall and are therefore resistant to β-lactam antibiotics, these bacteria are susceptible to macrolides and fluoroquinolones [4]. Tetracycline resistance occurs in up to 50% of ureaplasma clinical isolates because of the tetM transposon [4]. Isolated case reports demonstrate that it is possible to eradicate Ureaplasma species from the amniotic cavity by maternal administration of erythromycin [10]. However, in subsequent case reports, the rates of preterm delivery in treated versus untreated patients were similar, suggesting incomplete treatment or recolonization of the amniotic cavity [11]. While postnatal treatment with erythromycin is often ineffective at eliminating airway colonization with ureaplasmas in VLBW infants [12–14], macrolides remain the treatment of choice, especially in pregnant women and neonates [15].
Azithromycin (AZI) is a 15-membered semisynthetic macrolide antibiotic in the azalide subclass with some structural similarity to erythromycin, but with a prolonged duration of action, improved tissue penetration, a more favorable side-effect profile, and extended range of antimicrobial coverage [16]. Previous cross-sectional studies indicated that transplacental passage of AZI occurs at low rates and that the levels found in fetal plasma (FP) reach 2.6% of the levels in maternal circulation [14, 17, 18]. Several studies have demonstrated that AZI concentrations in tissues of the respiratory tract, including lung tissue, were several-fold higher than circulating AZI concentrations, providing promise that AZI will be useful against ureaplasmal infections in fetal lung tissue [19–22]. However, because of the limited placental permeability of AZI, there is controversy about whether antenatal antibiotic interventions prevents preterm labor, prolong gestation, or otherwise improves perinatal outcomes [23–25]. The use of prenatal antibiotics is debated with regard to the treatments' ability to prevent early labor, prolong gestation, and improve perinatal health. Conflicting results in the literature may be attributed to potential confounders and variations in study design [26–29]. There is now an urgent need to study specific antibiotic regimens for defined pathogens, to evaluate placental transfer, understand the pharmacokinetics and pharmacodynamics, and establish biological plausibility for the treatment of intra-uterine infections.
In the rhesus monkey (Macaca mulatta), hemochorial placentation, endocrine characteristics, anatomy, and singleton fetus pregnancies are analogues to those of human pregnancy and therefore permit longitudinal pharmacokinetic studies with serial sampling of both maternal and fetal compartments that is not possible with human subjects [30]. We provided proof of concept for the use of a specific macrolide antibiotic to eradicate U. parvum from the amniotic cavity and fetal tissues in the rhesus monkey [31, 32]. This maternal treatment targets amniotic fluid (AF) proinflammatory pathways and results in prolongation of gestation and reduced fetal lung injury [32]. Our goal was to extend this study to characterize the pharmacokinetics and pharmacodynamics of AZI in the maternal, fetal, and intra-amniotic compartments through modeling and to explore the relationships between U. parvum eradication and AZI exposure.
METHODS
Rhesus Macaque Model of U. parvum–Associated Chorioamnionitis
Our well-characterized nonhuman primate model of U. parvum IAI has provided preliminary data on the ability of AZI to eradicate intrauterine Ureaplasma infection, and we have used this model further to evaluate AZI pharmacokinetics and pharmacodynamics in this system [24, 31]. Protocols and procedures were approved by the Institutional Animal Care and Utilization Committee of the Oregon Health Sciences University, and guidelines for humane care were followed. Sixteen timed-mated pregnant rhesus monkeys (term is equal to day 168) were adapted to the vest and mobile catheter protection device at approximately 115 days of gestational age. Animals were catheterized (mean [±SD], 121.6 ± 1.1 days of gestational age; range, 115–127 days of gestational age) to implant maternal femoral artery and vein catheters, 2 open-tipped intra-amniotic catheters (for inoculation of bacteria, AF sampling, and uterine activity recording), and fetal electrocardiographic electrodes, as previously described [33]. Intravascular catheters were also placed in the fetal jugular vein and carotid artery for the analysis of AZI concentrations in the fetal circulation. Animals received tocolytics (ie, terbutaline sulfate and/or atosiban) for 1–5 days following surgery to control uterine irritability. Tocolytic medications were discontinued when uterine quiescence returned and at least 48 hours before experimental procedures.
Microorganism
On day 131 of gestation (range, 123–138 days), AF was inoculated with a low passage clinical isolate of U. parvum serovar 1 (107 colony-forming units [CFU]; n = 11) as a bolus at 1100 hours [33].
AZI Treatment and Sampling
Rhesus monkeys received maternal AZI alone (12.5 mg/kg every 12 hours intravenously for 10 days; n = 5); or in combination with the antiinflammatory drugs dexamethasone (4 mg/kg/day intravenously for 4 days) and indomethacin (100 mg/day orally for 5 days; n = 5) after 6–8 days of U. parvum IAI and with a concomitant increase in uterine contractions and/or cervical dilation over a 24-hour observation period, as determined by a modified Bishop score [32]. Both groups were combined for the purpose of these analyses. Following the first maternal AZI dose, paired samples were taken from MP and AF for measurement of AZI concentrations at time 0 (predose, prior to AZI treatment) 0.5, 1, 2, 3, 4, 6, 8, 12, and 24 hours, and then every 12 hours thereafter during the entire 10 day course of maternal AZI administration. Intensive sampling was repeated on day 10 following the final dose of AZI. Additional daily samples were collected after the last AZI dose until the onset of labor. To assess the placental-fetal transfer of AZI, intensive sampling of FP was scheduled following the first AZI dose, with limited serial samples collected throughout the treatment period. Fetal umbilical cord blood samples were paired with MP samples at delivery.
Culture and Detection of U. parvum
AF samples were collected, stored at −80°C, and transported on dry ice to the Diagnostic Mycoplasma Laboratory at the University of Alabama at Birmingham for analysis by culture and polymerase chain reaction (PCR). Samples for quantitative culture were thawed and processed using 10B broth and A8 agar. Colonies of U. parvum were identified on A8 agar by typical colonial morphology and urease production in the presence of a CaCl2 indicator, as previously described [34]. PCR was performed on batched specimens containing all samples from a single animal. The U. parvum primers used were directed toward the urease gene, using procedures previously described [35, 36]. Stringent precautions were used to prevent cross-contamination or PCR product carryover.
AZI Bioanalysis, Pharmacokinetics, and Pharmacodynamics
AZI pharmacokinetic studies were performed in the Division of Clinical Pharmacology at the University of Alabama at Birmingham. AZI was quantitated from 100 µL of plasma or AF, using a liquid-liquid extraction method coupled with high-performance liquid chromatography tandem mass spectrometry detection as previously described [31]. AZI concentration-time data were analyzed using noncompartmental methods and modeling. WinNonlin 5.3 (Pharsight, Mountain View, CA) was used for noncompartmental analyses. The area under the plasma concentration-time curve (AUC) was calculated using the linear-up/log-down trapezoidal rule. Maximum plasma concentration (Cmax) and time to maximum concentration (Tmax) were taken directly from the observed concentration-time data. Oral clearance (CL/F) was calculated as dose divided by AUCτ. Terminal apparent distribution volume (Vz/F) was calculated as dose divided by the product of the elimination rate constant (λz) and AUCτ. The elimination rate constant was determined by linear regression of the terminal elimination phase concentration-time points; elimination half-life (t1/2) was calculated as ln(2)/λz. Modeling was performed using ADAPT 5 [37], in which a triexponential model describing the AZI disposition in MP with the central plasma compartment linked to an amniotic compartment was used. For plasma data, maximum likelihood estimation maximization parameter estimation was applied using literature values following intravenous AZI administration. Using a sequential approach, fitted plasma parameters were then fixed for each animal to estimate the AF parameters. MP and amniotic pharmacokinetic parameter estimates were plotted against the time to U. parvum eradication to explore concentration-response relationships, and an inhibitory maximum effect (Emax) model was used to determine the concentration required to eradicate 50% of ureaplasma from AF (EC50).
RESULTS
A total of 10 animals received AZI treatment and had intensive plasma and amniotic AZI concentration-time data available following the first dose. Four animals had multiple dose (day 10) intensive plasma and amniotic concentration-time data available. Of the 6 animals not presented in the multiple dose data set, 2 received only 1 dose, 2 additional animals had only 3 or 4 days of dosing, and 2 animals did not undergo intensive sampling at day 10. Intensive sampling of FP was available for 2 animals. One had no samples collected, 1 had results that were all below the assay limit of detection, and the rest had only a single sample collected at various times after dosing, which were matched to MP samples. All animals received 12.5 mg/kg/dose in 2 divided doses each day, except for 1 animal, which received 10 mg/kg/dose because of a dilution error. These data were included since the resulting concentration-time profile and Cmax fell within the range of animals that received the higher dose. No AZI pharmacokinetic differences were observed between the animals that received AZI alone and those that received AZI plus dexamethasone/indomethacin.
Noncompartmental pharmacokinetic results are presented in Table 1. Only animals with at least 3 concentration-time points in the AF compartment collected ≥24 hours after the dose were included, to more accurately assess the elimination phase. Not all animals underwent extended sampling, because they went into labor at different times during or after AZI administration. For the first dose group, 2 animals (controls) had samples collected out to 125 and 170 hours, for an average t1/2 (±SD) of 156 ± 37 hours. In the multiple dose group, 3 animals had extended sampling (following the last dose on day 10) ranging from 72 to 289 hours, for an average t1/2 (±SD) of 129 ± 59 hours (Table 1).
Table 1.
Azithromycin Pharmacokinetic Parameters in Humans, Rhesus Monkeys, and Rhesus Monkey Amniotic Fluid
| Variable | First Dose |
Multiple Dose |
||||||
|---|---|---|---|---|---|---|---|---|
| Cmax, µg/mL | Tmax, h | AUC12, µg×h/mL | t1/2, h | Cmax, µg/mL | Tmax, h | AUC12, µg×h/mL | t1/2, h | |
| Humana | 3.63 ± 1.73 | 1.0 | 9.6 ± 4.8 | 68 | … | … | … | … |
| Rhesus MPb | 3.9 ± 1.3 | 0.7 ± 0.2 | 4.8 ± 1.3 | 34.9 ± 47.8 | 5.5 ± 1.8 | 0.5 | 7.4 ± 1.8 | 66.0 ± 45.1 |
| Rhesus AFc | 0.05 ± 0.05 | 8.2 ± 7.2 | 0.22 ± 0.03 | 156 ± 37 | 0.7 ± 0.5 | 4.9 ± 4.6 | 6.3 ± 4.5 | 129 ± 59 |
Data are mean ± standard deviation where available. Sample size is 10 for rhesus maternal plasma first dose and 4 for multiple dose.
Abbreviations: AF, amniotic fluid; AUC12, area under the concentration-time curve after 12 hours; Cmax, maximum concentration; MP, maternal plasma; Tmax, time to maximum concentration; t1/2, elimination half-life.
a Data are for a single 500-mg dose [38].
b Generated from noncompartmental analysis.
c The first dose and multiple dose t1/2 in amniotic fluid is based on data from 2 and 3 animals, respectively, who underwent extended sampling following that dose. First dose Cmax and Tmax are from 10 animals. For the remaining parameters in the amniotic fluid, the sample size is 4 animals each.
Following a single dose, the MP AZI Cmax and the time to Cmax (Tmax) are similar to that in humans receiving a single 500-mg intravenous infusion (approximately 7 mg/kg) [38], although the AUC and t1/2 were lower in rhesus monkeys (Table 1). Pharmacokinetic data are not available for steady-state intravenous doses in humans, but the MP t1/2 (66 hours) in rhesus monkeys appears consistent with prior pharmacokinetic studies in humans that used oral dosing [39]. The AF AUC12 was 0.22 µg×h/mL following the first dose and increased to 6.3 µg×h/mL at day 10, which was roughly equivalent to the corresponding MP AUC12 of 7.4 µg×h/mL. The AF accumulation index was 16 over the 10-day treatment period, using the formula 1/[1-e(−λz*t)]. By using the median ratio of steady-state (day 10; n = 3) AUC12 to first-dose AUC12 (n = 2), the accumulation index was 16.8. The AF Cmax increased from 0.05 to 0.7 mg/L over 10 days. FP was scheduled to be collected concurrently with MP at delivery, and data were available for 9 animals following multiple dosing. At a median time of 76.5 hours (range, 4.3–106 hours) after the dose, the MP:FP raw concentration ratio was 3.2 (range, 1.3–9.6). Two animals underwent 12-hour intensive pharmacokinetic sampling for MP and FP following the first dose. The MP:FP AUC12 ratios were 31 and 24.
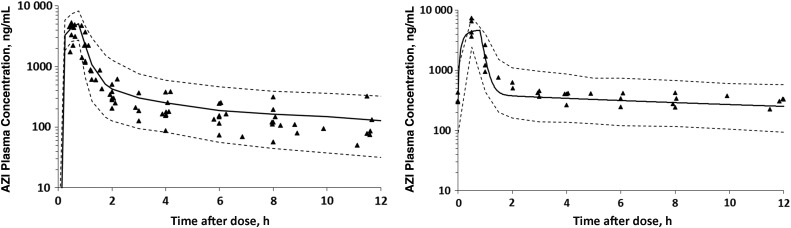
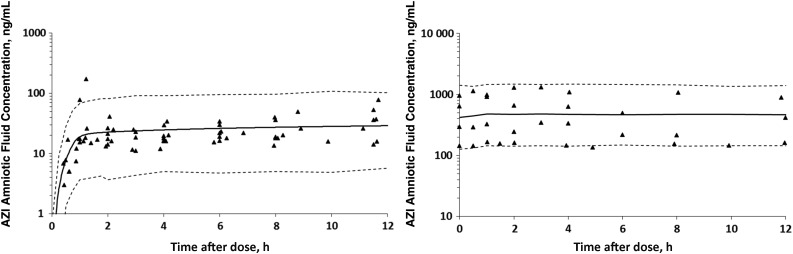
One-, 2-, and 3-compartment models were created in the attempt to fit the data. Ultimately, a 3-compartment MP model with a rate constant between the central and AF compartments was used because it best captured the long terminal phase in animals with extensive sampling. AZI plasma concentrations following the first dose and at day 10 and the simulated model fits are presented in Figure 1A and 1B. Figure 2A and 2B depict the AZI AF concentrations following the first dose and at day 10 with the simulated model fit. The modeled AF half-lives following the first dose and at day 10 were 153 hours and 113 hours, respectively. The modeled AF clearance (CL) was 0.37 L/hour following the first dose and 0.21 L/hour at day 10. Simulations suggest that the model fits were adequate, as evidenced by modest interindividual parameter variability and nearly all of the data points for MP and AF falling within the 10th–90th percentile of the simulated concentration-time curves (Figures 1 and 2).
Figure 1.
Azithromycin (AZI) concentrations in maternal plasma following the first dose (A; n = 10) and after 10 days of dosing (B; n = 4). Triangles represent the raw concentration-time data, the solid line is the simulated model fit, and the dashed lines represent the 10th–90th percentile confidence intervals.
Figure 2.
Azithromycin (AZI) concentrations in amniotic fluid following the first dose (A; n = 10) and at day 10 (B; n = 4). Triangles represent the raw concentration-time data, the solid line is the simulated model fit, and the dashed lines represent the 10th–90th percentile confidence intervals. Note the magnitude higher difference on the y-axis in panel B, compared with panel A.
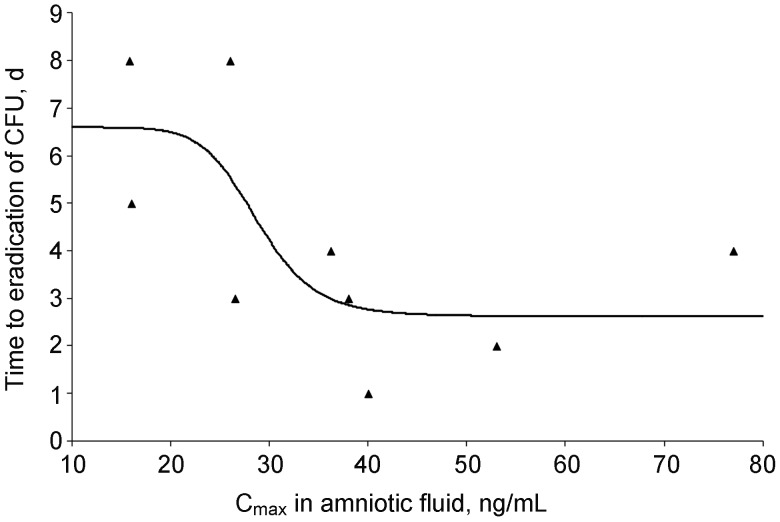
Concentration-response relationships between maternal and AF pharmacokinetic parameters and changes in the number of CFU per milliliter of U. parvum in AF were explored using a sigmoid inhibitory Emax model (Figure 3). No maternal pharmacokinetic parameters were related to changes in the number of CFU per milliliter over time (data not shown). The only clinically meaningful relationship was the AF Cmax versus the time to eradication of U. parvum in the AF, as quantitated by CFU. One animal was not included in the analysis as it went into early labor on day 3 of AZI treatment and U. parvum had not yet been eradicated. The AZI Cmax required to eradicate 50% of U. parvum (EC50) from the AF was 29 ng/mL, and the maximum drug induced effect (Emax – E0, where E0 is the baseline effect) occurred at 4 days. By using the Emax model parameters, the EC95 was calculated to be 39 ng/mL (0.039 µg/mL). The minimum inhibitory concentration (MIC) of the U. parvum isolate used in these studies (measured at pH 6.0, which is necessary to grow ureaplasmas in vitro) was 0.5 µg/mL, as measured by broth microdilution [34].
Figure 3.
Baseline inhibitory maximum effect model for the clearance of Ureaplasma from amniotic fluid (r = 0.72). The x-axis is the maximum concentration (Cmax) in amniotic fluid following a single dose. The maximum effect (Emax) minus baseline was 4 days with a concentration required to eliminate 50% of Ureaplasma species (EC50) of 29 ng/mL. The modeled EC95 was 39 ng/mL. Abbreviation: CFU, colony-forming units.
DISCUSSION
We used a chronically catheterized rhesus monkey model of U. parvum IAI to determine the transplacental pharmacokinetics of maternal AZI therapy. Our results indicate that maternal AZI administration (25 mg/kg/day) effectively reduce AF U. parvum colony counts to 5% of pretreatment levels within 24 hours, with maximal eradication of Ureaplasma within 6–7 days. Placental and fetal tissues were culture and PCR negative in 90% of cases [31]. Since AZI was administered by intravenous infusion, maternal peaks were concomitant with the administration of drug, whereas it took 5–8 hours for the AZI levels in AF to reach Cmax. This offset is likely a direct reflection of the time it took for the drug to distribute through multiple tissues, including the placenta and fetal tissues, before being excreted into the AF. The ability of AZI to traverse the placental barrier and provide therapeutic benefit in the intrauterine environment in humans with ureaplasmal infection has been a source of controversy. Human studies have relied on single doses of AZI administered to multiple subjects before delivery or placental perfusion in vitro [16, 17]. Our study is the first to evaluate multiple doses of AZI and multiple sampling times for both MP and AF in pregnant non-human primates infected with U. parvum. The MP concentrations measured were higher than those achieved in humans by using standard oral dosing, with first dose Cmax values ranging from 1.4 to 5.3 μg/mL in this study, compared with 0.5 ± 0.2 μg/mL following a single 500-mg oral dose in humans [40]. Following 1000-mg single oral doses in women scheduled for elective cesarean section, the peak concentrations in plasma (311 ng/mL) and AF concentrations at 12 hours (30 ng/mL) were consistent with the findings in this current study [16]. With repeated dosing, sustained levels of AZI (range, 75–300 ng/mL) were achieved for 10 days in the maternal circulation and AF. Pharmacokinetic modeling helped confirm that the rate of AZI clearance in the AF compartment was slow, resulting in significant accumulation over time (Figure 2A and 2B). A slow decay and prolonged t1/2 of AZI in the amniotic cavity is evidence for extended tissue penetration/accumulation and subsequent output in fetal urine.
Pharmacodynamic modeling was used to describe the relationship between the concentration of AZI achieved in AF following the first dose and the reduction of U. parvum (as described by the number of CFU per milliliter). The EC95 was 39 ng/mL, and the average Cmax in AF following the first dose was 50 ng/mL (Table 1), but by day 10 the average Cmax increased to 700 ng/mL. While the single AZI dose of 12.5 mg/kg every 12 hours achieves average maximum concentrations within the range needed to sterilize the intrauterine compartment from U. parvum in some animals, these data suggest that multiple doses allowing for drug accumulation in AF may be required in humans. As seen in Figure 2A, the best model fit for AF concentrations did not achieve the modeled EC95 following a single dose. But by day 10, all AZI concentrations exceeded this value (Figure 2B). Further studies will be needed to refine the optimum dose to be administered that achieves sterilization of the intrauterine compartment while using the shortest duration of therapy and that minimizes any potential adverse events. The MIC of U. parvum isolates in this study was 0.5 µg/mL (500 ng/mL). This MIC is clearly much higher than the in vivo modeled EC95 (39 ng/mL) required for eradication. The broth microdilution assay requires a pH of 6.0 in order for Ureaplasma MIC end points to be read [34]. The pH of AF is approximately 7.0 to 7.5 [40]. At this AF pH, it is likely that U. parvum susceptibility is considerably greater (lower MIC value) [41]. Importantly, AZI is bound to plasma protein in a concentration-dependent manner; from 51% at 20 ng/mL to 7% at 2000 ng/mL [39]. AF has considerably less protein components relative to plasma or serum [42, 43], so it is also likely that free AZI concentrations will be higher in this compartment, resulting in increased susceptibility (lower MIC). Additionally, the intra-amniotic pH is further elevated to ≥8.0 because of the production of ammonia by U. parvum infection itself (P. L. Grigsy, unpublished data). Taken together, the uniqueness of AF may create an environment where the susceptibility of U. parvum to AZI is considerably greater than suggested by the in vitro broth microdilution method.
This work provided the first model of transplacental transfer of AZI into the intrauterine environment. The net accumulation of AZI in AF over the dosing interval is explained by the rate of transfer into AF exceeding the rate of clearance from that compartment. It is likely that the sterilization of the intrauterine environment, including fetal tissues (lung), is aided by this accumulation. Based on the transplacental pharmacokinetics of AZI and the pharmacodynamic response of U. parvum to AZI measured in the AF, it is likely that sterilization of this compartment can be achieved if the mother is treated with an adequate dose, dosing interval, and duration of therapy. Alternative dosing strategies that achieve rapid and elevated AZI concentrations in the intrauterine environment should be explored.
Notes
Acknowledgments. We thank Dr Lois Colgin (senior veterinary pathologist, Oregon National Primate Research Center), for performing histopathologic examination of fetal lungs and placental membranes; Ms Kerri E. Sparks (research assistant, Oregon National Primate Research Center), for her histopathologic scoring of fetal lung tissue; Dr Li Xiao, Ms Donna Crabb, and Ms Amy Ratliff (University of Alabama at Birmingham), for their technical assistance and work in development and performance of quantitative Ureaplasma culture and PCR assays; Dr Michael D. Reed (Case Western Reserve University), for his contribution to our earlier AZI dose-finding studies; and Dr Michael G. Gravett (University of Washington), for his contributions and encouragement.
Financial support. This work was supported by the National Institute of Child Health and Human Development (grants R01 HD6159 and K99/R00 HD055059/HD055053), the National Institute of Allergy and Infectious Diseases (grant R01 A1072577), and the Division of Program Coordination, Planning, and Strategic Initiatives (grant 8P51 OD 011092–53 [formally, RR00163]).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Novy MJ, McGregor JA, Iams JD. New perspectives on the prevention of extreme prematurity. Clin Obstret Gynecol. 1995;38:790–808. doi: 10.1097/00003081-199538040-00013. [DOI] [PubMed] [Google Scholar]
- 2.Hillier SL, Martius J, Krohn M, Kiviat N, Holmes KK, Eschenbach DA. A case-control study of chorioamnionic infection and histological chorioamnionitis in prematurity. N Engl J Med. 1998;319:972–8. doi: 10.1056/NEJM198810133191503. [DOI] [PubMed] [Google Scholar]
- 3.Yoon BH, Romero R, Park JS, et al. Microbial invasion of the amniotic cavity with Ureaplasma urealyticum is associated with a robust host response in fetal, amniotic, and maternal compartments. Am J Obstet Gynecol. 1998;179:1254–60. doi: 10.1016/s0002-9378(98)70142-5. [DOI] [PubMed] [Google Scholar]
- 4.Waites KB, Katz B, Schelonka RL. Mycoplasmas and ureaplasmas as neonatal pathogens. Clin Microbiol Rev. 2005;18:757–89. doi: 10.1128/CMR.18.4.757-789.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldenberg RL, Thompson C. The infectious origins of stillbirth. Am J Obstet Gynecol. 2003;189:861–73. doi: 10.1067/s0002-9378(03)00470-8. [DOI] [PubMed] [Google Scholar]
- 6.Kirchner L, Helmer H, Heinze G, et al. Amnionitis with Ureaplasma urealyticum or other microbes leads to increased morbidity and prolonged hospitalization in very low birth weight infants. Eur J Obstet Gynecol Reprod Biol. 2007;134:44–50. doi: 10.1016/j.ejogrb.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Pinna GS, Skevaki CL, Kafetzis DA. The significance of Ureaplasma urealyticum as a pathogenic agent in the paediatric population. Curr Opin Infect Dis. 2006;19:283–9. doi: 10.1097/01.qco.0000224824.73223.e7. [DOI] [PubMed] [Google Scholar]
- 8.Kafetzis DA, Skevaki CL, Skouteri V, et al. Maternal genital colonization with Ureaplasma urealyticum promotes preterm delivery: association of the respiratory colonization of premature infants with chronic lung disease and increased mortality. Clin Infect Dis. 2004;39:1113–22. doi: 10.1086/424505. [DOI] [PubMed] [Google Scholar]
- 9.Schelonka RL, Waites KB. Ureaplasma infection and neonatal lung disease. Semin Perinatol. 2007;31:2–9. doi: 10.1053/j.semperi.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Mazor M, Chaim W, Horowitz S, Leiberman JR, Glezerman M. Successful treatment of preterm labour by eradication of Ureaplasma urealyticum with erythromycin. Arch Gynecol Obstet. 1993;253:215–8. doi: 10.1007/BF02766648. [DOI] [PubMed] [Google Scholar]
- 11.Berg TG, Philpot KL, Welsh MS, Sanger WG, Smith CV. Ureaplasma/Mycoplasma-infected amniotic fluid: pregnancy outcome in treated and nontreated patients. J Perinatol. 1999;19:275–7. doi: 10.1038/sj.jp.7200185. [DOI] [PubMed] [Google Scholar]
- 12.Baier RJ, Loggins J, Kruger TE. Failure of erythromycin to eliminate airway colonization with ureaplasma urealyticum in very low birth weight infants. BMC Pediatr. 2003;3:10. doi: 10.1186/1471-2431-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mabanta CG, Pryhuber GS, Weinberg GA, Phelps D. Erythromycin for the prevention of chronic lung disease in intubated preterm infants at risk for, or colonized or infected with Ureaplasma urealyticum. Cochrane Database Syst Rev. 2003:CD003744. doi: 10.1002/14651858.CD003744. (4) [DOI] [PubMed] [Google Scholar]
- 14.Watterberg K, Laine K, Neuvonen PJ, Ekblad U. Anti-inflammatory therapy in the neonatal intensive care unit: present and future. Semin Fetal Neonatal Med. 2006;11:378–84. doi: 10.1016/j.siny.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Xie X, Zhang J. Trends in the rates of resistance of Ureaplasma urealyticum to antibiotics and identification of the mutation site in the quinilone resistance-determining region in Chinese patients. FEMS Microbiol Lett. 2006;259:181–6. doi: 10.1111/j.1574-6968.2006.00239.x. [DOI] [PubMed] [Google Scholar]
- 16.Ramsey PS, Vaules MB, Vasdev GM, Andrews WW, Ramin KD. Maternal and transplacental pharmacokinetics of azithromycin. Am J Obstret Gynecol. 2003;188:714–8. doi: 10.1067/mob.2003.141. [DOI] [PubMed] [Google Scholar]
- 17.Heikkinen T, Laine K, Neuvonen PJ, Ekblad U. The transplacental transfer of the macrolide antibiotics erythromycin, roxithromycin and azithromycin. BJOG. 2000;107:770–5. doi: 10.1111/j.1471-0528.2000.tb13339.x. [DOI] [PubMed] [Google Scholar]
- 18.Witt A, Sommer EM, Cichna M, et al. Placental passage of clarithromycin surpasses other macrolide antibiotics. Am J Obstet Gynecol. 2003;188:816–9. doi: 10.1067/mob.2003.171. [DOI] [PubMed] [Google Scholar]
- 19.Olsen KM, San Pedro G, Gann LP, Gubbins PO, Halinski DM, Campbell GD., Jr Intrapulmonary pharmacokinetics of azithromycin in healthy volunteers given five oral doses. Antimicrob Agents Chemother. 1996;40:2582–5. doi: 10.1128/aac.40.11.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodvold KA, Danziger LH, Gotfried MH. Steady-state plasma and bronchopulmonary concentrations of intravenous levofloxacin and azithromycin in healthy adults. Antimicrob Agents Chemother. 2003;47:2450–7. doi: 10.1128/AAC.47.8.2450-2457.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ulrich M, Albers C, Moller JG, et al. Moxifloxacin and azithromycin, but not amoxicillin protect human respiratory epithelial cells against streptococcus pneumoniae in vitro when administered up to 6 h after challenge. Antimicrob Agents Chemother. 2005;49:5119–22. doi: 10.1128/AAC.49.12.5119-5122.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suarez-Mier G, Giguere S, Lee EA. Pulmonary disposition of erythromycin, azithromycin, and clarithromycin in foals. J Vet Pharmacol Ther. 2007;30:109–15. doi: 10.1111/j.1365-2885.2007.00833.x. [DOI] [PubMed] [Google Scholar]
- 23.McDonald HM, Brocklehurst P, Gordon A. Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database Syst Rev. 2007:CD000262. doi: 10.1002/14651858.CD000262.pub3. (1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morency AM, Bujold E. Comment on pregnancy outcome after early detection of bacterial vaginosis. Eur J Obstet Gynecol Reprod Biol. 2006;128:40–5. doi: 10.1016/j.ejogrb.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 25.Swadpanich U, Lumbiganon P, Prasertcharoensook W, Laopaiboon M. Antenatal lower genital tract infection screening and treatment programs for preventing preterm delivery. Cochrane Database Syst Rev. 2008:CD006178. doi: 10.1002/14651858.CD006178.pub2. (2) [DOI] [PubMed] [Google Scholar]
- 26.Stetzer BP, Mercer BM. Antibiotics and preterm labor. Clin Obstet Gynecol. 2000;43:809–17. doi: 10.1097/00003081-200012000-00011. [DOI] [PubMed] [Google Scholar]
- 27.King JF, Flenady V, Murray L. Prophylactic antibiotics for inhibiting preterm labour with intact membranes. Cochrane Database Syst Rev. 2002:CD000246. doi: 10.1002/14651858.CD000246. (4) [DOI] [PubMed] [Google Scholar]
- 28.Waites KB, Schelonka RB, Xiao L, Grigsby PL, Novy MJ. Congenital and opportunistic infections: ureaplasma species and Mycoplasma hominis. Semin Fetal Neonatal Med. 2009;14:190–99. doi: 10.1016/j.siny.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Grigsby PL, Novy MJ, Adams Waldorf KM, Sadowsky DW, Gravett MG. Choriodecidual inflammation: a harbinger of the preterm labor syndrome. Reprod Sci. 2010;17:85–94. doi: 10.1177/1933719109348025. [DOI] [PubMed] [Google Scholar]
- 30.Gravett MG, Witkin SS, Haluska GJ, Edwards JL, Cook MJ, Novy MJ. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol. 1994;171:1660–7. doi: 10.1016/0002-9378(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 31.Grigsby PL, Novy MJ, Sadowsky DW, et al. Maternal azithromycin therapy for ureaplasma intraamniotic infection delays preterm delivery and reduces fetal lung injury in a primate model. Am J Obstet Gynecol. 2012;207:475.e1–14. doi: 10.1016/j.ajog.2012.10.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Novy MJ, Duffy LB, Axthelm M, et al. Ureaplasma parvum or Mycoplasma hominis as sole pathogens cause chorioamnionitis, preterm delivery and fetal pneumonia in rhesus macaques. Rep Sci. 2009;16:56–70. doi: 10.1177/1933719108325508. [DOI] [PubMed] [Google Scholar]
- 33.Sadowsky DW, Adams KM, Gravett MG, Witkin SS, Novy MJ. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am J Obstet Gynecol. 2006;195:1578–89. doi: 10.1016/j.ajog.2006.06.072. [DOI] [PubMed] [Google Scholar]
- 34.Waites KB, Duffy LB, Schwartz S, Talkington DF. Mycoplasma and ureaplasma. In: Garcia L, editor. Clinical microbiology procedure handbook. 3rd ed. ASM Press; 2010. pp. 3.15.1–3.15.17. [Google Scholar]
- 35.Blanchard A, Hentschel J, Duffy L, Baldus K, Cassell GH. Detection of ureaplasma urealyticum by polymerase chain reaction in the urogenital tracts of adults, in amniotic fluid, and in the respiratory tract of newborns. Clin Infect Dis. 1993;17(Suppl 1):S148–53. doi: 10.1093/clinids/17.supplement_1.s148. [DOI] [PubMed] [Google Scholar]
- 36.Teng LJ, Zheng X, Glass JI, Watson HL, Tsai J, Cassell GH. Ureaplasma urealyticum biovar specificity and diversity are encoded in multiple-banded antigen gene. J Clin Microbiol. 1994;32:1464–9. doi: 10.1128/jcm.32.6.1464-1469.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.D'Argenio DZ, Schumitzky A, Wang X. Los Angeles: Biomedical Simulations Resource; 2009. ADAPT 5 user's guide: pharmacokinetic/pharmacodynamic systems analysis software. [Google Scholar]
- 38.Zithromax (azithromycin for injection), prescribing information. 2011.
- 39. Zithromax (azithromycin tablets and azithromycin for oral suspension), prescribing information, 2009.
- 40.Seeds AE, Kock HC, Myers RE, Stolte LA, Hellegers AE. Changes in rhesus monkey amniotic fluid pH, pCO2, and bicarbonate concentration following maternal and fetal hypercarbia and fetal death in utero. Am J Obstet Gynecol. 1967;97:67–75. doi: 10.1016/0002-9378(67)90594-7. [DOI] [PubMed] [Google Scholar]
- 41.Waites KB, Crouse DT, Cassell GH. Antibiotic susceptibilities and therapeutic options for ureaplasma urealyticum infections in neonates. Ped Infect Dis J. 1992;11:23–9. doi: 10.1097/00006454-199201000-00007. [DOI] [PubMed] [Google Scholar]
- 42.Fresno L, Rodriguez-Gil JE, Rigau T, Pastor J, Rivera del Alamo MM. Modulation of the biochemical composition of amniotic and allantoic fluids as a control mechanism of feline foetal development. Placenta. 2012;33:522–7. doi: 10.1016/j.placenta.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 43.Jauniaux E, Gulbis B, Jurkovic D, Campbell S, Collins WP, Ooms HA. Relationship between protein concentrations in embryological fluids and maternal serum and yolk sac size during human early pregnancy. Hum Reprod. 1994;9:161–6. doi: 10.1093/oxfordjournals.humrep.a138308. [DOI] [PubMed] [Google Scholar]