Abstract
Background. Herpesviruses have been linked to cognitive impairment in older individuals but little is known about the association in the general US population.
Methods. We determined whether cytomegalovirus (CMV) and herpes simplex virus 1 (HSV-1) seropositivity were associated with cognitive impairment among children (aged 6–16 years) and adults aged 20–59 or ≥60 years, using data from the National Health and Nutrition Examination Survey (NHANES) III. Linear and logistic regression models were used to examine the associations between pathogen seropositivity and cognitive impairment.
Results. Among children, HSV-1 seropositivity was associated with lower reading and spatial reasoning test scores (β, −0.69; 95% confidence interval [CI], −1.18 to −.21 and β, −0.82; 95% CI, −1.29 to −.36, respectively). Among middle-aged adults, HSV-1 and CMV seropositivity were associated with impaired coding speed (odds ratio [OR], 1.54; 95% CI, 1.13–2.11, and OR, 1.41; 95% CI, 1.09–1.82, respectively). CMV seropositivity was also associated with impaired learning and recall (OR, 1.43; 95% CI, 1.14–1.80). Among older adults, HSV-1 seropositivity was associated with immediate memory impairment (OR, 3.26; 95% CI, 1.68–6.32).
Conclusions. Future studies examining the biological pathways by which herpesviruses influence cognitive impairment across the life course are warranted.
Keywords: Cognitive impairment, herpes simplex virus-1, cytomegalovirus, lifecourse, NHANES
Cognitive impairment is a major contributor to disease burden among the elderly in the United States, with morbidity expected to increase as the US population ages [1]. The etiology of cognitive decline remains unclear, however; thus, there is a need to identify novel factors that increase risk for cognitive impairment across the life course. Persistent pathogens such as herpes simplex virus (HSV)-1 and cytomegalovirus (CMV) have been linked to several neurological disorders of aging of which cognitive impairment is a major component, including Alzheimer's disease and dementia [2, 3]. These pathogens are capable of infecting the central nervous system [4, 5], where they may exert neurodegenerative effects that negatively affect cognition [6], as evidenced by the adverse neurological and cognitive consequences of HSV encephalitis and congenital CMV infection [7, 8]. Viral replication within the brain may lead to cell death and morphological changes at sites of infection [9]; atrophy and loss of gray matter have been observed in the brains of both individuals with HSV encephalitis [10] and HSV-1–seropositive individuals with comorbid psychiatric disorders [11, 12]. Such changes have been further linked to cognitive impairment among these populations [10–12]. Even in the absence of severe infection, however, herpesviruses undergo subclinical reactivation, triggering the release of proinflammatory cytokines [13], which are also hypothesized to affect cognitive impairment via neuroinflammatory pathways [14].
Although more than one-third of the US population is seropositive for HSV-1 and/or CMV by early childhood [15, 16], previous studies examining the association between herpesviruses and cognitive impairment have been largely limited to middle-aged and older populations with psychiatric disorders and/or from specific geographic regions [11, 12, 17–29]. Thus, little is known about the association between herpesviruses and cognitive impairment in the general US population, or whether these pathogens affect cognition beginning early in life, setting the stage for poor cognitive trajectories over the life course. Therefore, the present study aims to examine the association between CMV and HSV-1 seropositivity and cognitive impairment among children (aged 6–16 years), middle-aged adults (aged 20–59 years), and elderly adults (aged ≥60 years) in the US population.
MATERIALS AND METHODS
Ethics Statement
This study was deemed not regulated by the University of Michigan Institutional Review Board.
Study Population
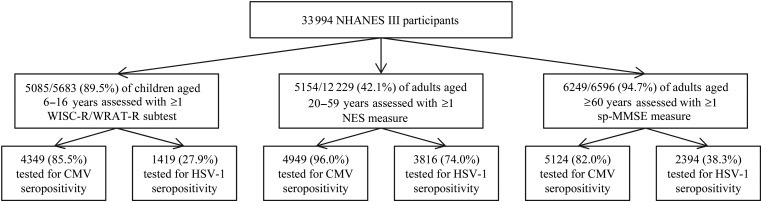
The National Health and Nutrition Examination Survey (NHANES) III was a cross-sectional, multistage, stratified probability survey conducted from 1988 to 1994 on the health and nutrition status of the US civilian, noninstitutionalized population [30]. Participants provided informed consent at the time of enrollment. The derivation of our study sample is shown in Figure 1. Of the 33 994 individuals interviewed, 5085 of 5683 (89.5%) children 6–16 years were assessed with the Wechsler Intelligence Scale for Children–Revised (WISC-R) and/or the Wide Range Achievement Test–Revised (WRAT-R), and 5154 of 12 229 (42.1%) adults aged 20–59 years were assessed with the Neurobehavioral Evaluation System during the mobile examination. In addition, 6249 of 6596 (94.7%) adults aged ≥60 years were assessed for ≥1 measure from the short portable Mini-Mental State Examination (sp-MMSE) during the interview or mobile/home examination. Serum samples used for pathogen testing were collected at the mobile or home examination, with blinding as to whether cognitive testing was performed, within 4 weeks of the interview. Of children assessed for cognitive function, 4349 (85.5%) were tested for CMV and 1419 (27.9%) for HSV-1 seropositivity (children aged 12–16 years only). Among adults aged 20–59 years, 4949 (96.0%) of those assessed for cognitive function were also tested for CMV, and 3816 (74.0%) for HSV-1 seropositivity. Among adults aged ≥60 years assessed for cognitive function, 5124 (82.0%) were also tested for CMV and 2394 (38.3%) for HSV-1 seropositivity (individuals enrolled in phase 1 only).
Figure 1.
Derivation of study sample from the National Health and Nutrition Examination Survey (NHANES) III, 1988–1994. Abbreviations: CMV, cytomegalovirus; HSV-1, herpes simplex virus-1; NES, Neurobehavioral Evaluation System; sp-MMSE, short portable Mini-Mental State Examination WISC-R, Wechsler Intelligence Scale for Children–Revised; WRAT-R, Wide Range Achievement Test–Revised.
Laboratory Analyses
Serum samples were tested for HSV-1 seropositivity by immunodot assay using the glycoprotein gG-1 antigen [15]. CMV immunoglobulin G (IgG) was measured by using an enzyme-linked immunosorbent assay (Quest International) [16]. If the value was near the cutoff, the test was confirmed with a second enzyme-linked immunosorbent assay (bioMerieux). If the 2 assays disagreed, an immunofluorescence assay (Bion International) was performed [16]. Individuals with equivocal or indeterminate results were excluded from the analyses.
Cognitive Function Measures
Children aged 6–16 years were assessed with the WISC-R reading and mathematics tests, which measure reading vocabulary and ability to perform mathematical calculations, and the WRAT-R block design and digit span tests, which measure perceptional/visuospatial reasoning and working memory/attention [31, 32]. Raw scores from all examinations were scaled to allow comparisons between the WISC-R and WRAT-R components [33]. Continuous scores were utilized, with higher scores indicating superior performance.
A random subset of all adults aged 20–59 years were assessed with the Neurobehavioral Evaluation System [34], including the Simple Reaction Time Test (SRTT) measuring motor response speed, the Symbol-Digit Substitution Test (SDST) measuring coding speed and attention, and Serial Digit Learning Test (SDLT; trials to criterion and total errors) measuring attention, learning, and recall [34]. For all measures, lower values indicate superior performance. Continuous scores for the SRTT, SDST, and SDLT were not normally distributed. Consistent with other studies [35], individuals were dichotomized into the upper quartile of reaction time or number of trials/errors (ie, impaired) versus the 3 lowest quartiles of reaction time or number of trials/errors (ie, not impaired).
Adults aged ≥60 years were assessed for immediate/delayed memory impairment and impaired serial subtraction with tests from the sp-MMSE [36]. Participants were asked to recall 3 words and 6 details from a short story at 2 time points and were assigned a point for each word and detail successfully recalled (range, 0–9). Individuals who scored <5 of 9 points at the first time point and <4 of 9 at the second time point were categorized as having immediate and delayed memory impairment, respectively, consistent with previous studies [37]. Participants also participated in a serial subtraction test in which they were asked to subtract $3 from $20 over 5 trials. As in other studies, individuals making any error in subtraction were categorized as impaired, and those making no errors were categorized as not impaired [37].
Covariates
Covariates of interest included age, sex, race/ethnicity, country of origin, census region, smoke exposure (children only) or smoking status (adults only), poverty-income ratio [PIR] (children only), and educational level (family reference person for children, individual for adults). Age was calculated from date of birth at the date of interview and was treated as continuous. Sex was self-reported as male or female. Race/ethnicity was derived from self-reported race and ethnicity, and individuals were categorized as non-Hispanic white, non-Hispanic black, Mexican American, or other. Country of origin was derived from place of birth, and individuals were categorized as born in the United States, Mexico, or other. Census region of residence was categorized as Northeast, Midwest, South, or West. Smoke exposure among children was based on household smoke exposure, and individuals were categorized as having a smoker present in the household or no smoker present. Smoking status in adults was based on self-reported smoking history, and individuals were categorized as never, former, or current smokers. Self-reported years of education of the family reference person among children and for the individual among adults was categorized as <high school, high school, or any college. The PIR (ie, ratio of a family's income to their appropriate threshold income based on household size) level was categorized into 1 of 3 categories: low (0.000–1.300), medium (1.301–3.500), or high (≥3.501).
Statistical Analyses
Statistical analyses were performed using SAS version 9.2 (SAS Institute) survey commands, with appropriate weights, strata, and clusters to account for the complex study design of NHANES III [38]. Bivariate associations between CMV and HSV-1 seropositivity, cognitive measures, and hypothesized confounders [15, 16, 39, 40] were estimated for each age group. Covariates were included in adjusted models if they were hypothesized a priori to be relevant confounders in the associations between pathogens and cognitive impairment, were associated with pathogen seropositivity and cognitive measures in bivariate analyses (data not shown), and were not hypothesized to be on the causal pathway.
Multiple linear regression models were used to examine the crude and adjusted associations between pathogen seropositivity and WISC-R/WRAT-R scores among children, and logistic regression models were used to examine the crude and adjusted associations between pathogen seropositivity and cognitive impairment among middle-aged and elderly adults. Fully adjusted models controlled for age, sex, race/ethnicity, education level (family or individual), PIR, country of origin, census region and/or smoke exposure or status based on covariates being considered relevant confounders in each model. Adjusted models included those with nonmissing values for all covariates included in the model.
RESULTS
Descriptive statistics (weighted) for children aged 6–16 years tested for cognitive measures and CMV (n = 4349) and aged 12–16 years tested for HSV-1 (n = 1419) serostatus are shown in Supplementary Table 1. The mean age ± standard error [SE] among children tested for cognitive function and CMV was 11.10 ± 0.10 years, 48.5% were female, 66.1% reported non-Hispanic white race/ethnicity, 32.0% were in the lowest PIR category, 24.3% had a family educational level of less than high school, and 38.4% were CMV seropositive. Among children tested for cognitive function and HSV-1, the mean age ± SE was 14.01 ± 0.06 years, 47.2% were female, 65.7% reported non-Hispanic white race/ethnicity, 30.9% were in the lowest PIR category, 24.8% had a family educational level of less than high school, and 42.0% were HSV-1 seropositive. The mean reading, mathematics, block design, and digit span scores ± SE were 8.56 ± 0.11, 8.18 ± 0.12, 9.50 ± 0.10, and 8.69 ± 0.08, respectively, among those tested for CMV and 8.51 ± 0.19, 8.58 ± 0.17, 9.19 ± 0.14, and 8.37 ± 0.14, among those tested for HSV-1.
Among the 4949 adults aged 20–59 years tested for cognitive function and CMV seropositivity, the mean age ± SE was 36.97 ± 0.23 years, 51.5% were female, 76.4% reported non-Hispanic white race/ethnicity, 20.3% had less than a high school education, and 56.3% were CMV seropositive (see Supplementary Table 2). Among the 3816 adults aged 20–59 years who were tested for cognitive function and HSV-1 seropositivity, the mean age was 35.89 ± 0.40 years, 50.4% were female, 75.9% reported non-Hispanic white race/ethnicity, 20.4% had less than a high school education, and 66.0% were HSV-1 seropositive (see Supplementary Table 2).
Of the 5124 adults aged ≥60 years who were tested for cognitive function and CMV seropositivity, the mean age ± SE was 70.12 ± 0.23 years, 56.0% were female, 84.6% reported non-Hispanic white race/ethnicity, 40.9% had less than a high school education, and 86.0% were CMV seropositive (see Supplementary Table 3). Among these individuals, 5.4% had immediate memory impairment, 6.7% had delayed memory impairment, and 22.4% had serial subtraction impairment. Among the 2394 adults aged ≥60 years who were tested for cognitive function and HSV-1 seropositivity, the mean age ± SE was 69.58 ± 0.21 years, 56.0% were female, 86.8% reported non-Hispanic white race/ethnicity, 42.1% had less than a high school education, and 86.9% were seropositive for HSV-1. Among these individuals, 5.0% had immediate memory impairment, 6.4% had delayed memory impairment, and 22.2% had serial subtraction impairment (see Supplementary Table 3).
Results from models examining the association between HSV-1 and CMV seropositivity and WISC-R/WRAT-R scores among children are shown in Tables 1 and 2. HSV-1–seropositive children scored 0.69 (95% confidence interval [CI], −1.18 to −.21) points lower on the reading examination and 0.82 (95% CI, −1.29 to −.36) points lower on the block design examination, than HSV-1 seronegative children in models adjusted for age, sex, race/ethnicity, country of origin, PIR, family educational level, and smoke exposure. Tables 3 and 4 show the relationship between CMV and HSV-1 seropositivity and impairment on the SRTT, SDST, and SDLT (trials to criterion/total errors), among adults aged 20–59 years. HSV-1 seropositivity was associated with impaired reaction time on the SDST (odds ratio [OR], 1.54; 95% CI, 1.13–2.11), after adjustment for age, sex, race/ethnicity, country of origin, educational level, and smoking status. CMV seropositive adults had 1.41 (95% CI, 1.09–1.82) times greater odds of impaired reaction time on the SDST compared with seronegative adults after adjustment for age, sex, race/ethnicity, country of origin, and educational level. CMV seropositivity was also associated with total errors in the highest quartile on the SDLT in fully adjusted models (OR, 1.43; 95% CI, 1.14–1.80). Among adults aged ≥60 years, HSV-1 seropositivity was also associated with immediate memory impairment (OR, 3.26; 95% CI, 1.68–6.32), with adjustment for age, sex, race/ethnicity, educational level, and country of origin (see Table 5).
Table 1.
Associations Between CMV and HSV-1 Seropositivity and WISC-R Scores Among Children Aged 6–16 or 12–16 Years in the NHANES III, 1988–1994
| Seropositivity | Mathematics Scores |
Reading Scores |
||
|---|---|---|---|---|
| Model 1a | Model 2b | Model 1a | Model 2b | |
| β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | |
| CMV | −0.41 (−.77 to −.05)c | 0.07 (−.28 to .41) | −0.38 (−.73 to −.03)c | 0.23 (−.10 to .56) |
| HSV-1 | −1.76 (−2.34 to −1.18)c | −0.59 (−1.28 to .10) | −1.88 (−2.47 to −1.30)c | −0.69 (−1.18 to −.21)c |
Abbreviations: CI, confidence interval; CMV, cytomegalovirus; HSV-1, herpes simplex virus 1; NHANES, National Health and Nutrition Examination Survey; WISC-R, Wechsler Intelligence Scale for Children–Revised.
a Model 1 for CMV and mathematics scores (n = 4343), CMV and reading scores (n = 4326), HSV-1 and mathematics scores (n = 1418), and HSV-1 and reading scores (n = 1409), unadjusted.
b Model 2 for CMV and mathematics scores (n = 3978), CMV and reading scores (n = 3961), HSV-1 and mathematics scores (n = 1287), and HSV-1 and reading scores (n = 1278), adjusted for age, sex, race/ethnicity, country of origin, poverty-income ratio, family educational level, smoke exposure (HSV-1 models only), and census region (CMV mathematics model only).
c P< .05.
Table 2.
Associations Between CMV and HSV-1 Seropositivity and WRAT-R Scores Among Children Aged 6–16 or 12–16 Years in NHANES III, 1988–1994
| Seropositivity | Block Design |
Digit Span |
||
|---|---|---|---|---|
| Model 1a | Model 2b | Model 1a | Model 2b | |
| β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | |
| CMV | −0.49 (−.86 to −.12)c | 0.03 (−.34 to .41) | −0.16 (−.53 to .21) | 0.19 (−.13 to .51) |
| HSV-1 | −1.54 (−2.10 to −.99)c | −0.82 (−1.29 to −.36)c | −0.66 (−1.14 to −.19)c | −0.04 (−.63 to .55) |
Abbreviations: CMV, cytomegalovirus; HSV-1, herpes simplex virus-1; NHANES, National Health and Nutrition Examination Survey; WRAT-R, Wide Range Achievement Test–Revised.
a Model 1 for CMV and block design (n = 4303), CMV and digit span (n = 4299), HSV-1 and block design (n = 1393), and HSV-1 and digit span (n = 1393), unadjusted.
b Model 2 for CMV and block design (n = 3938), CMV and digit span (n = 3934), HSV-1 and block design (n = 1262), and HSV-1 and digit span (n = 1262), adjusted for age, sex, race/ethnicity, country of origin, poverty-income ratio, family educational level, smoke exposure (HSV-1 models only), and census region (CMV block design model only).
c P < .05.
Table 3.
Associations Between CMV and HSV-1 Seropositivity and SRTT and SDST Impairment Among Adults Aged 20–59 Years in NHANES III, 1988–1994
| Seropositivity | SRTT |
SDST |
||
|---|---|---|---|---|
| Model 1a | Model 2b | Model 1a | Model 2b | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| CMV | 1.54 (1.20–1.97)c | 1.06 (.81–1.37) | 3.14 (2.52–3.91)c | 1.41 (1.09–1.82)c |
| HSV-1 | 1.57 (1.24–1.98)c | 1.21 (.97–1.53) | 2.79 (2.18–3.57)c | 1.54 (1.13–2.11)c |
Abbreviations: CI, confidence interval; CMV, cytomegalovirus; HSV-1, herpes simplex virus-1; NHANES, National Health and Nutrition Examination Survey; OR, odds ratio; SDST, Symbol-Digit Substitution Test; SRTT, Simple Reaction Time Test.
a Model 1 for CMV and SRTT (n = 4929), CMV and SDST (n = 4869), HSV-1 and SRTT (n = 3792), and HSV-1 and SDST (n = 3748), unadjusted.
b Model 2 for CMV and SRTT (n = 4891), CMV and SDST (n = 4831), HSV-1 and SRTT (n = 3765), and HSV-1 and SDST (n = 3720), adjusted for age, sex, race/ethnicity, country of origin, educational level, and smoking status (HSV-1 and SDST model only).
c P < .05.
Table 4.
Associations Between CMV and HSV-1 Seropositivity and Impairment on the SDLT: Trials to Criterion and Total Errors, Among Adults Aged 20–59 Years in NHANES III, 1988–1994
| Seropositivity | SDLT: Trials to Criterion |
SDLT: Total Errors |
||
|---|---|---|---|---|
| Model 1a | Model 2b | Model 1a | Model 2b | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| CMV | 2.42 (2.02–2.89)c | 1.20 (.98–1.48) | 2.82 (2.33–3.41)c | 1.43 (1.14–1.80)c |
| HSV-1 | 1.68 (1.30–2.17)c | 0.98 (.74–1.31) | 1.75 (1.38–2.22)c | 1.03 (.80–1.33) |
Abbreviations: CI, confidence interval; CMV, cytomegalovirus; HSV-1, herpes simplex virus-1; NHANES, National Health and Nutrition Examination Survey; OR, odds ratio; SDLT, Serial Digit Learning Test.
a Model 1 for CMV and SDLT trials to criterion and total errors (n = 4760) and HSV-1 and SDLT trials to criterion and total errors (n = 3660), unadjusted.
b Model 2 for CMV and SDLT trials to criterion and total errors (n = 4725) and HSV-1 and SDLT trials to criterion and total errors (n = 3636), adjusted for age, sex, race/ethnicity, country of origin, educational level, and census region (CMV and SDLT trials to criterion model only).
c P < .05.
Table 5.
Associations Between CMV and HSV-1 Seropositivity and Cognitive Impairment Among Adults Aged ≥60 Years in NHANES III, 1988–1994
| Seropositivity | Immediate Memory Impairment |
Delayed Memory Impairment |
Serial Subtraction Impairment |
|||
|---|---|---|---|---|---|---|
| Model 1a | Model 2b | Model 1a | Model 2b | Model 1a | Model 2b | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| CMV | 2.12 (1.31–3.41)c | 1.22 (.71–2.11) | 2.39 (1.55–3.70)c | 1.35 (.86–2.11) | 2.43 (1.77–3.32)c | 1.33 (.91–1.93) |
| HSV-1 | 5.33 (2.74–10.38)c | 3.26 (1.68–6.32)c | 2.26 (1.35–3.80)c | 1.41 (.78–2.53) | 2.17 (1.49–3.14)c | 1.41 (.91–2.17) |
Abbreviations: CI, confidence interval; CMV, cytomegalovirus; HSV-1, herpes simplex virus-1; NHANES, National Health and Nutrition Examination Survey; OR, odds ratio.
a Model 1 for CMV and immediate memory (n = 4806), CMV and delayed memory (n = 4928), CMV and serial subtraction (n = 5087), HSV-1 and immediate memory (n = 2205), HSV-1 and delayed memory (n = 2290), and HSV-1 and serial subtraction (n = 2367), unadjusted.
b Model 2 for CMV and immediate memory (n = 4781), CMV and delayed memory (n = 4902), CMV and serial subtraction (n = 5057), HSV-1 and immediate memory (n = 2190), HSV-1 and delayed memory (n = 2277), and HSV-1 and serial subtraction (n = 2351), adjusted for age, sex, race/ethnicity, educational level, country of origin (HSV-1, immediate memory and serial subtraction models only), and census region (CMV models only).
c P < .05.
DISCUSSION
To our knowledge, this is the first study to examine the association between HSV-1 and CMV seropositivity and multiple cognitive measures among different age groups in a large, representative US sample. We found that HSV-1 seropositivity was associated with impaired reading vocabulary and visuospatial processing among children, slower coding speed in middle-aged adults, and immediate memory impairment among older adults, suggesting that this pathogen may have life course effects on cognition. In contrast, CMV seropositivity was only associated with slower coding speed and impaired learning and recall among middle-aged adults. Importantly, if herpesviruses are causally associated with cognitive impairment, interventions focused on prevention and/or treatment of these pathogens, especially early in life, could serve to improve trajectories of cognitive function across the life course.
The adverse neurological and cognitive sequelae of congenital CMV infection and neonatal herpes infection of the central nervous system are well established [7, 8], but the effects of herpesviruses on cognitive impairment in childhood, outside the context of severe infection, are relatively unknown. We found HSV-1 seropositivity was associated with impaired reading vocabulary and visuospatial processing among children, which may represent deficits in working memory [41]. Bilbo and Frank [14] hypothesized that immune activation in early life may influence cognitive development via neuroinflammatory pathways not only in childhood but also across the life course, as early exposure to infection may program individuals for an exaggerated immune response, which continues to interfere with neural processes related to cognition over time. These proposed pathways are compatible with herpesvirus physiology in healthy hosts because the virus particles persist in a latent state and may reactivate over time, triggering production of proinflammatory cytokines [13]. If immune activation is a key predictor of cognitive function, individuals who acquire herpesviruses earlier in life and undergo reactivation more often may be at greater risk for cognitive impairment across the life course. Given the strong social patterning of age of acquisition, seroprevalence of and immune response to herpesviruses beginning in childhood [42, 43], these pathogens may contribute to social disparities in cognition, educational attainment and social mobility across the life course [44].
Among middle-aged individuals, both HSV-1 and CMV seropositivity were associated with slower coding speed, indicative of impairments in attention, which may affect cognitive efficiency and reflect short-term and working memory deficits [45, 46]. CMV seropositivity was also associated with more total errors on the SDLT, indicative of impaired learning and recall. We are aware of only 1 previous study examining the association between herpesviruses and cognitive function among middle-aged individuals without psychiatric disorders [19]. In contrast to our findings, Dickerson et al [19] found that seropositivity for HSV-1 was associated with delayed memory impairment, but not immediate memory impairment or deficits in visuospatial/constructional, language, or attention indices, among 240 healthy individuals (mean age, 33.7 years). Numerous other studies conducted among middle-aged adults with psychiatric disorders [11, 12, 17, 18, 20–24], have identified an association between HSV-1 seropositivity and deficits in a variety of cognitive domains including immediate memory, attention and visuospatial/constructional indices, processing and psychomotor speed, vigilance, and verbal and working memory [11, 12, 17, 18, 20–24]. CMV seropositivity, however, was only found to be associated with impaired working memory and psychomotor speed [20]. It has been hypothesized that HSV-1 infection may be particularly detrimental for cognition among those with psychiatric disorders that may also cause damage to the brain [11, 12], which may explain the associations previously observed in these populations. Future studies are needed, examining the association between herpesviruses and multiple measures of cognitive impairment among those with and without comorbid psychiatric disorders and incorporating neuroimaging to examine the role of morphological brain changes in these relationships.
Among adults aged ≥60 years, HSV-1 seropositivity remained significantly associated with immediate memory impairment after confounder adjustment, but neither herpesvirus was associated with delayed memory impairment or impaired serial subtraction in fully adjusted models. Studies that have examined the association between both HSV-1 and CMV seropositivity and cognitive impairment among older adults have yielded mixed results [25–29]. For example, Aiello et al [26] found that increased CMV IgG antibody titer level was associated with cognitive decline, as measured by MMSE score, but not with impaired episodic memory among elderly Latinos (mean age, 70,3 years). Neither CMV or HSV-1 seropositivity, nor HSV-1 IgG antibody level were associated with cognitive measures in the study by Aiello et al [26].
In contrast, a cross-sectional study by Mathei et al [27] found no association between CMV seropositivity or IgG antibody titer level and MMSE score among older adults (mean age, 84.9 years) in Belgium. Gow et al [28], however, found that both CMV seropositivity and IgG antibody titer were associated with general cognitive ability, memory impairment, as measured by the Wechsler Memory Scale–III, processing speed, and National Adult Reading Test scores among Scottish adults (mean age, 70 years). Given that immediate memory is a component of the MMSE and Wechsler Memory Scale–III, it is possible the association between HSV-1 seropositivity and this cognitive domain is driving the findings observed in previous studies. Overall, because previous studies were conducted among cohorts from specific geographic regions and cognitive outcomes have been largely limited to aggregate measures of cognition, studies among older adults that include multiple measures of cognitive impairment are needed to clarify the specific cognitive domains associated with herpesviruses in older age.
The mechanisms by which herpesviruses may affect different cognitive domains at different time points across the life course in the general US population are largely unknown but may reflect the differential areas of the brain that are typically affected by each pathogen. For example, studies among schizophrenic populations have shown, compared with those who are HSV-1 seronegative, HSV-1–seropositive individuals have decreased gray matter volumes in the anterior and posterior cingulate gyrus, which is involved in executive function, and dorsolateral prefrontal cortex, which regulates working memory [11, 12, 47]. Subclinical HSV-1 infection may also affect memory through neuroanatomic brain changes similar to those observed among patients recovering from HSV-1 encephalitis and experiencing amnesia [10, 48]. CMV is also known to affect the limbic regions of the brain [49], which regulate emotion, learning, and attention [50]. Age at acquisition and the degree to which individuals reactivate with herpesviruses over time may also play an important role in determining which cognitive domains are affected across the life course. Taken together, future studies should examine the longitudinal associations between herpesviruses, morphological brain changes, and various domains of cognitive function while taking into account age of acquisition and immune response to infection over time to clarify the role that herpesviruses may play in the etiology of cognitive impairment across the life course.
Our study has a few limitations. Power to detect statistically significant associations may have been limited for some models, particularly among those aged ≥60 years, owing to smaller sample sizes for those tested for HSV-1 seropositivity. NHANES III is also a cross-sectional study; thus, the duration of infection and directionality of the association between persistent pathogens and cognitive impairment cannot be determined. However, because approximately one-third of the US population acquires CMV and HSV-1 infection by 12 years of age [15, 16], it is likely that acquisition of these pathogens preceded the development of cognitive impairment observed in middle-aged and older adults. Furthermore, we observed a specific effect of CMV and HSV-1 seropositivity on different cognitive measures across age groups, even after controlling for numerous sociodemographic factors, making it unlikely that the effects observed are due solely to residual confounding by socioeconomic status.
To our knowledge, this is the first study to find an association between HSV-1 and CMV seropositivity and cognitive impairment among individuals of various ages in a large sample representative of the US population. Overall, we found that HSV-1 seropositivity was associated with impaired reading vocabulary and visuospatial processing among children, HSV-1 and CMV seropositivity were associated with impaired attention in middle-aged adults, CMV seropositivity was also associated with learning and recall in middle-aged adults, and HSV-1 seropositivity was associated with immediate memory impairment in older adults. Therefore, if herpesviruses are causally associated with cognitive impairment beginning early in life, interventions focused on prevention and/or treatment of herpesvirus infection, such as vaccines or antiviral drugs, could serve to improve trajectories of cognitive function over time in the general US population. Future studies that examine the longitudinal association between herpesviruses and the development of cognitive impairment over time and aim to identify the biological mechanisms by which these pathogens may affect different cognitive domains across the life course are warranted.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. The authors thank Fuller Torrey, MD for his critical review of the manuscript. During the time this study was conducted, A.M.S. was affiliated with the Department of Epidemiology, Center for Social Epidemiology & Population Health, University of Michigan School of Public Health, Ann Arbor, MI.
Financial support. This work was supported by the National Institute on Aging, National Institutes of Health (grant 1-R01-AG-040115 to A.E.A and J.B.D.) and the Stanley Medical Research Institute (to A. E. A.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Lopez OL. The growing burden of Alzheimer's disease. Am J Manag Care. 2011;17(Suppl 13):S339–45. [PubMed] [Google Scholar]
- 2.Itzhaki RF, Wozniak MA. Herpes simplex virus type 1 in Alzheimer's disease: the enemy within. J Alzheimers Dis. 2008;13:393–405. doi: 10.3233/jad-2008-13405. [DOI] [PubMed] [Google Scholar]
- 3.Lurain NS, Hanson BA, Martinson J, et al. Virological and immunological characteristics of human cytomegalovirus infection associated with Alzheimer disease. J Infect Dis. 2013;208:564–72. doi: 10.1093/infdis/jit210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffiths P. Cytomegalovirus infection of the central nervous system. Herpes. 2004;11(Suppl 2):95A–104. [PubMed] [Google Scholar]
- 5.Schmutzhard E. Viral infections of the CNS with special emphasis on herpes simplex infections. J Neurol. 2001;248:469–77. doi: 10.1007/s004150170155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Chiara G, Marcocci ME, Sgarbanti R, et al. Infectious agents and neurodegeneration. Mol Neurobiol. 2012;46:614–38. doi: 10.1007/s12035-012-8320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elliott SP. Congenital cytomegalovirus infection: an overview. Infect Disord Drug Targets. 2011;11:432–6. doi: 10.2174/187152611797636712. [DOI] [PubMed] [Google Scholar]
- 8.Engman ML, Adolfsson I, Lewensohn-Fuchs I, Forsgren M, Mosskin M, Malm G. Neuropsychologic outcomes in children with neonatal herpes encephalitis. Pediatr Neurol. 2008;38:398–405. doi: 10.1016/j.pediatrneurol.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 9.DeBiasi RL, Kleinschmidt-DeMasters BK, Richardson-Burns S, Tyler KL. Central nervous system apoptosis in human herpes simplex virus and cytomegalovirus encephalitis. J Infect Dis. 2002;186:1547–57. doi: 10.1086/345375. [DOI] [PubMed] [Google Scholar]
- 10.Kapur N, Barker S, Burrows EH, et al. Herpes simplex encephalitis: Long term magnetic resonance imaging and neuropsychological profile. J Neurol Neurosurg Psychiatry. 1994;57:1334–42. doi: 10.1136/jnnp.57.11.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schretlen DJ, Vannorsdall TD, Winicki JM, et al. Neuroanatomic and cognitive abnormalities related to herpes simplex virus type 1 in schizophrenia. Schizophr Res. 2010;118:224–31. doi: 10.1016/j.schres.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Prasad KM, Eack SM, Goradia D, et al. Progressive gray matter loss and changes in cognitive functioning associated with exposure to herpes simplex virus 1 in schizophrenia: a longitudinal study. Am J Psychiatry. 2011;168:822–30. doi: 10.1176/appi.ajp.2011.10101423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glaser R, Kiecolt-Glaser JK. Chronic stress modulates the virus-specific immune response to latent herpes simplex virus type 1. Ann Behav Med. 1997;19:78–82. doi: 10.1007/BF02883323. [DOI] [PubMed] [Google Scholar]
- 14.Bilbo SD, Frank A. Beach award: Programming of neuroendocrine function by early-life experience: A critical role for the immune system. Horm Behav. 2013;63:684–91. doi: 10.1016/j.yhbeh.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schillinger JA, Xu F, Sternberg MR, et al. National seroprevalence and trends in herpes simplex virus type 1 in the United States, 1976-1994. Sex Transm Dis. 2004;31:753–60. doi: 10.1097/01.olq.0000145852.43262.c3. [DOI] [PubMed] [Google Scholar]
- 16.Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. Seroprevalence of cytomegalovirus infection in the United States, 1988-1994. Clin Infect Dis. 2006;43:1143–51. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]
- 17.Dickerson FB, Boronow JJ, Stallings C, Origoni AE, Ruslanova I, Yolken RH. Association of serum antibodies to herpes simplex virus 1 with cognitive deficits in individuals with schizophrenia. Arch Gen Psychiatry. 2003;60:466–72. doi: 10.1001/archpsyc.60.5.466. [DOI] [PubMed] [Google Scholar]
- 18.Dickerson FB, Boronow JJ, Stallings C, et al. Infection with herpes simplex virus type 1 is associated with cognitive deficits in bipolar disorder. Biol Psychiatry. 2004;55:588–93. doi: 10.1016/j.biopsych.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Dickerson F, Stallings C, Sullens A, et al. Association between cognitive functioning, exposure to herpes simplex virus type 1, and the COMT Val158Met genetic polymorphism in adults without a psychiatric disorder. Brain Behav Immun. 2008;22:1103–7. doi: 10.1016/j.bbi.2008.04.156. [DOI] [PubMed] [Google Scholar]
- 20.Shirts BH, Prasad KM, Pogue-Geile MF, Dickerson F, Yolken RH, Nimgaonkar VL. Antibodies to cytomegalovirus and herpes simplex virus 1 associated with cognitive function in schizophrenia. Schizophr Res. 2008;106:268–74. doi: 10.1016/j.schres.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yolken RH, Torrey EF, Lieberman JA, Yang S, Dickerson FB. Serological evidence of exposure to herpes simplex virus type 1 is associated with cognitive deficits in the CATIE schizophrenia sample. Schizophr Res. 2011;128:61–5. doi: 10.1016/j.schres.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 22.Dickerson F, Stallings C, Origoni A, Vaughan C, Khushalani S, Yolken R. Additive effects of elevated C-reactive protein and exposure to herpes simplex virus type 1 on cognitive impairment in individuals with schizophrenia. Schizophr Res. 2012;134:83–8. doi: 10.1016/j.schres.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Watson AM, Prasad KM, Klei L, et al. Persistent infection with neurotropic herpes viruses and cognitive impairment. Psychol Med. 2013;43:1023–31. doi: 10.1017/S003329171200195X. [DOI] [PubMed] [Google Scholar]
- 24.Gerber SI, Krienke UJ, Biedermann NC, et al. Impaired functioning in euthymic patients with bipolar disorder–HSV-1 as a predictor. Prog Neuropsychopharmacol Biol Psychiatry. 2012;36:110–6. doi: 10.1016/j.pnpbp.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Strandberg TE, Pitkala KH, Linnavuori KH, Tilvis RS. Impact of viral and bacterial burden on cognitive impairment in elderly persons with cardiovascular diseases. Stroke. 2003;34:2126–31. doi: 10.1161/01.STR.0000086754.32238.DA. [DOI] [PubMed] [Google Scholar]
- 26.Aiello AE, Haan M, Blythe L, Moore K, Gonzalez JM, Jagust W. The influence of latent viral infection on rate of cognitive decline over 4 years. J Am Geriatr Soc. 2006;54:1046–54. doi: 10.1111/j.1532-5415.2006.00796.x. [DOI] [PubMed] [Google Scholar]
- 27.Mathei C, Vaes B, Wallemacq P, Degryse J. Associations between cytomegalovirus infection and functional impairment and frailty in the BELFRAIL cohort. J Am Geriatr Soc. 2011;59:2201–8. doi: 10.1111/j.1532-5415.2011.03719.x. [DOI] [PubMed] [Google Scholar]
- 28.Gow AJ, Firth CM, Harrison R, Starr JM, Moss P, Deary IJ. Cytomegalovirus infection and cognitive abilities in old age. Neurobiol Aging. 2013;34:1846–52. doi: 10.1016/j.neurobiolaging.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 29.Katan M, Moon YP, Paik MC, Sacco RL, Wright CB, Elkind MS. Infectious burden and cognitive function: the northern Manhattan study. Neurology. 2013;80:1209–15. doi: 10.1212/WNL.0b013e3182896e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Center for Health Statistics. Hyattsville, MD: National Center for Health Statistics; 1994. Plan and operation of the Third National Health and Nutrition Examination Survey, 1988-94. [PubMed] [Google Scholar]
- 31.Jastak S. The Wide Range Achievement Test: revised administration manual. Wilmington, DE: Jastak Associated; 1984. [Google Scholar]
- 32.Wechsler D. Manual for intelligence scale, for children: revised. New York, NY: The Psychological Corporation; 1974. [Google Scholar]
- 33.National Center for Health Statistics. National Health and Nutrition Survey III: cognitive testing for children MEC interviewer manual. Hyattsville, MD: National Center for Health Statistics; 1989. [Google Scholar]
- 34.National Center for Health Statistics. National Health and Nutrition Survey III: neurological testing procedure manual. Hyattsville, MD: National Center for Health Statistics; 1993. [Google Scholar]
- 35.Hailpern SM, Melamed ML, Cohen HW, Hostetter TH. Moderate chronic kidney disease and cognitive function in adults 20 to 59 years of age: Third National Health And Nutrition Examination Survey (NHANES III) J Am Soc Nephrol. 2007;18:2205–13. doi: 10.1681/ASN.2006101165. [DOI] [PubMed] [Google Scholar]
- 36.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state": a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 37.Noble JM, Borrell LN, Papapanou PN, Elkind MS, Scarmeas N, Wright CB. Periodontitis is associated with cognitive impairment among older adults: analysis of NHANES-III. J Neurol Neurosurg Psychiatry. 2009;80:1206–11. doi: 10.1136/jnnp.2009.174029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.National Center for Health Statistics. Hyattsville, MD: National Center for Health Statistics; 1996. Analytic and reporting guidelines: the Third National Health and Nutrition Examination Survey, NHANES III (1988-94) [Google Scholar]
- 39.Dowd JB, Aiello AE, Alley DE. Socioeconomic disparities in the seroprevalence of cytomegalovirus infection in the US population: NHANES III. Epidemiol Infect. 2009;137:58–65. doi: 10.1017/S0950268808000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee Y, Back JH, Kim J, et al. Systematic review of health behavioral risks and cognitive health in older adults. Int Psychogeriatr. 2010;22:174–87. doi: 10.1017/S1041610209991189. [DOI] [PubMed] [Google Scholar]
- 41.Nevo E, Breznitz Z. Assessment of working memory components at 6 years of age as predictors of reading achievements a year later. J Exp Child Psychol. 2011;109:73–90. doi: 10.1016/j.jecp.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 42.Dowd JB, Zajacova A, Aiello A. Early origins of health disparities: burden of infection, health, and socioeconomic status in U.S. children. Soc Sci Med. 2009;68:699–707. doi: 10.1016/j.socscimed.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Colugnati FA, Staras SA, Dollard SC, Cannon MJ. Incidence of cytomegalovirus infection among the general population and pregnant women in the United States. BMC Infect Dis. 2007;7:71. doi: 10.1186/1471-2334-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Osler M, Avlund K, Mortensen EL. Socio-economic position early in life, cognitive development and cognitive change from young adulthood to middle age. Eur J Public Health. 2013;23:974–80. doi: 10.1093/eurpub/cks140. [DOI] [PubMed] [Google Scholar]
- 45.Brebion G, Smith MJ, Gorman JM, Malaspina D, Sharif Z, Amador X. Memory and schizophrenia: Differential link of processing speed and selective attention with two levels of encoding. J Psychiatr Res. 2000;34:121–7. doi: 10.1016/s0022-3956(99)00050-3. [DOI] [PubMed] [Google Scholar]
- 46.Rypma B, Berger JS, Prabhakaran V, et al. Neural correlates of cognitive efficiency. Neuroimage. 2006;33:969–79. doi: 10.1016/j.neuroimage.2006.05.065. [DOI] [PubMed] [Google Scholar]
- 47.Prasad KM, Shirts BH, Yolken RH, Keshavan MS, Nimgaonkar VL. Brain morphological changes associated with exposure to HSV1 in first-episode schizophrenia. Mol Psychiatry. 2007;12:105–13. doi: 10.1038/sj.mp.4001915. [DOI] [PubMed] [Google Scholar]
- 48.Gitelman DR, Ashburner J, Friston KJ, Tyler LK, Price CJ. Voxel-based morphometry of herpes simplex encephalitis. Neuroimage. 2001;13:623–31. doi: 10.1006/nimg.2000.0734. [DOI] [PubMed] [Google Scholar]
- 49.Hanshaw J. In: Infectious diseases of the fetus and newborn infant. Remington J, Klein J, editors. Vol. 107. Philadelphia, PA: WB Saundrers; 1976. [Google Scholar]
- 50.Lavenex P, Banta Lavenex P. Building hippocampal circuits to learn and remember: Insights into the development of human memory. Behav Brain Res. 2013;254:8–21. doi: 10.1016/j.bbr.2013.02.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.