Abstract
Myosin-binding protein C (MyBP-C) is an accessory protein of the myosin filaments of vertebrate striated muscle. In the heart it plays a key role in modulating contractility in response to β-adrenergic stimulation. Mutations in the cardiac isoform (cMyBP-C) are a leading cause of inherited hypertrophic cardiomyopathy. Understanding cMyBP-C function and its role in disease requires knowledge of the structure of the molecule, its organization in the sarcomere and its interactions with other sarcomeric proteins. Here we review the main structural features of this modular, elongated molecule and the properties of some of its key domains. We describe observations suggesting that the bulk of the molecule extends perpendicular to the thick filament, enabling it to reach neighboring thin filaments in the sarcomere. We review structural and functional evidence for interaction of its N-terminal domains with actin and how this may modulate thin filament activation. We also discuss the effects that phosphorylation of cMyBP-C has on some of these structural features, and how this might relate to cMyBP-C function in the beating heart.
Keywords: MyBP-C, cMyBP-C, thick filament, myosin filament, sarcomere, cardiac muscle
1. Introduction
Myosin-binding protein C (MyBP-C) is an important accessory component of vertebrate striated muscle thick filaments. It was first detected as an impurity in skeletal muscle myosin preparations [28], its co-purification with myosin suggesting that it was a myosin-binding protein. Later studies showed that MyBP-C existed in different isoforms: fast and slow skeletal (sometimes present together in the same muscle and even the same sarcomere), and cardiac, where it is named cMyBP-C [9]. In addition to binding to titin and to the light meromyosin (LMM) tail of myosin, anchoring it to the thick filament backbone, MyBP-C can also interact with myosin subfragment 2 (S2), with the myosin regulatory light chain (RLC), and with actin (reviewed in [9,21]). The cardiac isoform can be phosphorylated, and this weakens its S2 and actin interactions, which may contribute to cMyBP-C’s modulation of cardiac contraction in response to β-adrenergic stimulation [3,9,21].
Understanding cMyBP-C function and its role in cardiomyopathy [3,15] requires knowledge of its structure and molecular interactions. Here we review the main structural features of the cMyBP-C molecule, its three-dimensional arrangement in the sarcomere, its interaction with the thin filament, and the structural changes that occur when it is phosphorylated. We also suggest areas for future research.
2. Molecular Structure
MyBP-C is a modular protein of the immunoglobulin (Ig) and fibronectin type 3 (Fn) family, which also includes titin and proteins of the M-line. The skeletal isoforms contain seven Ig and three Fn domains (numbered C1-C10 starting at the N-terminal end), together with a small proline/alanine-rich N-terminal region (the Pro-Ala domain) and a MyBP-C-specific motif (the M-domain) located between the C1 and C2 modules. The cardiac isoform is similar but contains an additional Ig module (C0) N-terminal to the Pro-Ala domain, and a cardiac-specific, unstructured and highly dynamic insert of ~30 residues in the C5 domain (Fig. 1A; [9]). cMyBP-C also contains four serines within the M-domain that can be phosphorylated by several protein kinases [3]. Each Ig and Fn domain is a compact, ~10 kDa, globular structure with dimensions ~ 2 x 3 x 4 nm (Fig. 1B). These domains are connected linearly with an ~ 4nm repeat, creating a molecule ~ 40 nm long and 2–3 nm wide [9], with short linker sequences between some domains (Fig. 1A). Single molecule EM images show that the molecule is very flexible and has sharp hinge points near its middle and its N-terminal end (Fig. 1C; [9]), possibly at the linker between C4 and C5 and in the flexible M-domain.
Figure 1. Structure of cMyBP-C.
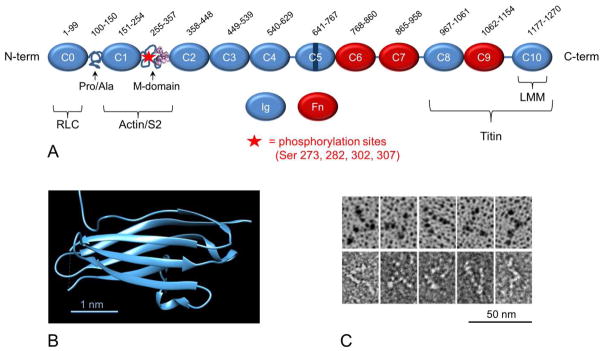
A. Organization of cMyBP-C into Ig- and Fn-like domains (C5 with cardiac specific insert) together with Pro/Ala-rich and M-domains. Brackets indicate regions involved in binding other sarcomeric proteins. Numbers at top specify amino acids in each domain based on UniProt domain annotation (mouse sequence; UniProt. O70468). Lines show linker sequences between domains. B. Ribbon diagram showing β-sandwich structure of the C1 Ig domain (PDB 2V6H). C. EM images of cMyBP-C, shadowed (upper) and negatively stained (lower) (JYM, unpublished).
The C-terminal region is responsible for anchoring MyBP-C to the thick filament backbone. The C10 domain binds strongly to LMM, while domains C8-C10, or possibly a subset of these domains, bind to titin [9]. The rest of the molecule (C0-C7) may extend away from the thick filament backbone. The binding sites for actin, S2 and the myosin RLC are located in the N-terminal region (domains C0-M), furthest from the thick filament anchoring domains. The molecule is long enough to reach neighboring thin filaments (and possibly neighboring thick filaments) while the C-terminus remains anchored on its parent filament [22].
Crystallographic data are available for one of the Ig domains (C1), showing that it consists of a β-sandwich fold typical of the IgI family, its two β-sheets containing 3 and 4 β strands respectively [10] (Fig. 1B). NMR studies have been carried out on multiple Ig and Fn domains of different MyBP-C isoforms, showing a similar overall β-sandwich organization (e.g. [1]).
The ~50 residue Pro-Ala domain appears to be intrinsically disordered [18]; sequence comparisons between species show that the number of proline and alanine residues varies inversely with heart rate, suggesting that the Pro-Ala region might help match contractile speed to cardiac function [36].
The M-domain has a unique structure, similar in the different MyBP-C isoforms, but not found in other proteins. An NMR study suggests that it comprises two subdomains—an N-terminal portion that is flexible and mostly disordered, and a more ordered C-terminal portion containing three α-helices in a three-helix bundle. It has been proposed that this may provide a site for actin-binding [16]. The four regulatory phosphorylation sites in cMyBP-C are located close to each other in the disordered half of the M-domain, at residues S273, S282, S302, and S307 (mouse sequence).
The mechanical properties of different cMyBP-C modules have been studied by atomic force microscopy (AFM). Force-extension curves reveal the presence of a long, extensible segment that becomes stretched at low force before the Ig and Fn domains sequentially unfold [18]. This extensible region is thought to be the M-domain, which is probably disordered based on intrinsic disorder prediction programs, consistent with the NMR data [16]. The compact nature of the M-domain in solution [17] and its extensibility at low force, suggest that it may function as an elastic element, possibly acting as an entropic spring, which may enhance cardiac relaxation rates [18]. The flexibility of the M-domain may enable MyBP-C to bind to multiple target proteins at a distance by a fly-casting mechanism, or to mediate low affinity dynamic interactions (e.g. S2 or actin binding), for example during filament sliding [18]. AFM studies of an expressed N-terminal fragment (C0C3) showed that M-domain phosphorylation significantly decreased the contour length of the fragment [23]. Thus the M-domain, which is extensible in the non-phosphorylated state, appears to adopt a more stable, compact structure upon phosphorylation; this structural change may play a role in the phosphorylation-induced reduction in binding of cMyBP-C to actin and S2 [23].
3. Sarcomeric organization
The location of MyBP-C in the thick filament was first determined by immuno-EM. In skeletal muscle, antibodies labeled 7–9 stripes in the middle one third (the C-zone) of each half A-band [8], the precise number being determined by fiber type [4,5]. The distance between the stripes was 43 nm, equal to the repeat distance of the myosin helix. Thus MyBP-C is located at every third level of myosin heads, which are themselves 14.3 nm apart. Cardiac MyBP-C has been shown to have a similar location, in 9 stripes 43 nm apart in the C-zone [20] (Fig. 2A). This periodic location is probably determined by the binding of MyBP-C’s C-terminal domains to titin, which has an eleven Ig/Fn-domain super-repeat of 43 nm in the C-zone and serves as a template for contractile filament assembly [9,11]. This organization of MyBP-C explains the appearance of stripes that have long been seen in unlabeled A-bands of longitudinal sections (Fig. 2B) and of isolated myofibrils and A-segments [7,14].
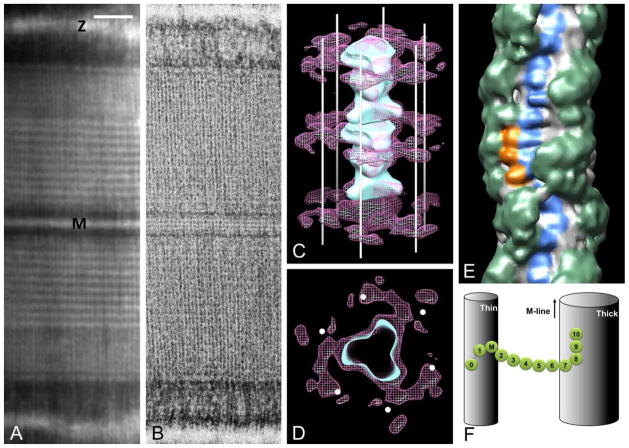
Figure 2. cMyBP-C organization in the sarcomere.
A. Negatively stained mouse cardiac myofibril labeled with antibody to the C0-M region of cMyBP-C, showing nine, white, antibody-labeled stripes in each half A-band. M- and Z-lines marked (KHL, unpublished). B. Negatively stained longitudinal EM cryo-section of rat papillary muscle (aligned with (A) and at same magnification), showing narrow stripes representing unlabeled cMyBP-C (protein white, adapted from [20]). C, D. Electron tomogram of thick filament in longitudinal and transverse views respectively, showing MyBP-C density (pink) extending from thick filament surface (blue, helically organized heads) to neighboring thin filaments (positions indicated with white lines and dots; adapted from [22]). D. 3D reconstruction of mouse cardiac thick filament showing myosin heads (green), titin (blue) and putative MyBP-C C-terminal domains (orange; from [45]). E. Model of cMyBP-C organization, showing linear arrangement of domains C8-C10 and extension of domains C7-C0 to actin filament (cf. C). M-line up in C, E, F. Bar for A, B = 200 nm.
To understand how MyBP-C functions in muscle, its 3-dimensional organization must be determined. Various models have been proposed, each with different consequences for MyBP-C’s potential interactions with other sarcomeric proteins. The narrow axial extent of the MyBP-C stripes suggests that most of the molecule is confined axially, with its long axis either wrapped around or extending perpendicularly from the thick filament. Yeast two-hybrid experiments demonstrated interactions between domains C5 and C8 and between C7 and C10 of cMyBP-C. Based on these interactions it was suggested that the C-terminal region forms a collar around the thick filament backbone [24], possibly serving to strengthen the backbone or regulate myosin head movements. Modeling of muscle X-ray patterns suggests, on the other hand, that the C-terminal domains run axially along the filament surface [38]. Direct evidence for such an arrangement comes from EM of negatively stained cardiac thick filaments [2,45]. 3D reconstructions show three 4-nm domains, running longitudinally, at the level of each cMyBP-C stripe, most likely the thick filament anchoring domains, C8-C10 (Fig. 2E). A longitudinal arrangement of C-terminal domains is also suggested by EM of cardiac myofibrils labeled with domain-specific antibodies to cMyBP-C [19]. Antibodies to the N-terminus and the C5-C7 region label at similar axial positions, while C10 antibodies label 10 nm closer to the M-line, consistent with extension of the three C-terminal domains along the filament surface. A similar conclusion was reached for MyBP-C organization in slow skeletal muscle [5].
In contrast to the C-terminal domains, the N-terminal two thirds of cMyBP-C appear to extend from the thick filament and bind to the neighboring actin filament. Indirect evidence for this comes from X-ray diffraction data [38], while electron tomography of intact skeletal muscle sarcomeres [21,22] shows directly that the narrow stripes in the C-zone arise from an axially confined but laterally extended density that connects thick and thin filaments (Fig. 2C, D). This orientation is supported by the coincidence of the axial positions of antibodies labeling the N-terminus and C5-C7 domains [19]. We conclude that the most likely arrangement of MyBP-C is one in which the three C-terminal domains run along the thick filament surface while the rest of the molecule extends out and may attach to the neighboring thin filament (Fig. 2F).
This arrangement offers a simple explanation for cMyBP-C’s ability to modulate thin filament activity and sliding (see 4. Actin binding). It is harder to explain cMyBP-C’s interaction with S2 and the myosin RLC if these lie on the surface of the thick filament. Such interactions might be possible when myosin heads extend from the thick filament, e.g. during contraction. Alternatively, MyBP-C might interact with S2 and the RLC on a neighboring thick filament, although there is so far no structural evidence for such contacts in intact muscle.
4. Actin binding
Although MyBP-C was first isolated as a myosin-binding protein [28], it soon became clear that it could also bind to F-actin [25] and to regulated thin filaments, containing tropomyosin and troponin [44]. Binding occurs through the N-terminal region, involving primarily the C1 and M domains [6,33,37,39], with a saturating cMyBP-C:actin molar ratio of 1:1. The ability to bind actin appears to be essential for normal cMyBP-C sarcomeric distribution [6]. Binding via the C-terminal half has also been suggested [34], although this has not been confirmed by other laboratories [6], and it is not clear how C-terminal domains, close to the thick filament, could bind to actin in situ. In vitro motility assays demonstrate that cMyBP-C binding to actin slows the sliding of actin filaments over myosin molecules and native thick filaments [30,33,35,39], suggesting that cMyBP-C might act as a regulator of filament sliding in situ.
EM and neutron scattering experiments on F-actin decorated with N-terminal cMyBP-C fragments provide insights into the nature of the actin-cMyBP-C complex. Modeling of neutron scattering data from filaments decorated with C0C2 suggested a structure in which the C0 and C1 domains bind near subdomain 1 (SD1) and the DNase I binding loop of actin [43]. EM reconstructions of C0C3-decorated filaments provide more direct information and suggest that C0 and C1 bind to SD1, while the M-domain bridges over SD2, and C2 and C3 extend above the filament (Fig. 3A) [26]. This arrangement places C0 and C1 in a position on actin SD1 that would appear to interfere with tropomyosin binding in its low Ca2+ (blocking) position [26,29,43] (Fig. 3A). Thus, cMyBP-C could potentially modulate thin filament activity by displacing tropomyosin from its blocking position on actin.
Figure 3. cMyBP-C binding to thin filaments.
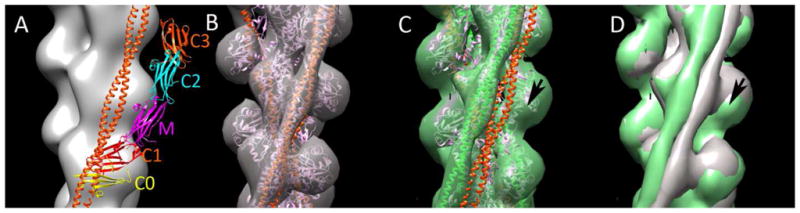
A. Reconstruction of F-actin (gray) showing the putative locations of C0C3 domains (represented by Ig domain atomic structure) in a C0C3-decorated filament. C0 and C1 domains would clash with tropomyosin (orange) in its low-Ca2+ blocking position (adapted from [26]). B. Reconstruction of low Ca2+ thin filament, fitted with actin-tropomyosin atomic model; tropomyosin (orange) in low Ca2+ position. (C) Thin filament decorated with C0C2 under low Ca2+ conditions, showing displacement of tropomyosin from low Ca2+ position in B (orange) towards high Ca2+ position (green). D. Superposition of B and C, highlighting displacement of tropomyosin. Black arrows indicate extra density from proximal end of C0C2. B-D adapted from [27].
Such displacement of tropomyosin has been confirmed by 3D reconstruction of thin filaments decorated with C0C2. When sufficient C0C2 is added to thin filaments under low Ca2+ conditions, tropomyosin is found in its high Ca2+ position (Fig. 3B–D) [27], which would be expected to activate the thin filament. Thin filament activation by binding of N-terminal cMyBP-C fragments has been directly demonstrated by solution kinetics studies [41,42].
Modulation of thin filament activity by cMyBP-C is supported by in vitro motility assays. When cMyBP-C N-terminal fragments are included in assays in which troponin-tropomyosin-regulated thin filaments slide over molecular myosin, motility occurs even under low Ca2+ (relaxing) conditions [33]. Conversely, at high Ca2+ levels, or with F-actin alone, cMyBP-C actually slows filament sliding [33,39]. These results are consistent with a model in which cMyBP-C binding to the thin filament at low Ca2+ displaces tropomyosin towards its high Ca2+ position, thus activating the filament. In contrast, at high Ca2+, or with F-actin alone, the binding of cMyBP-C places a load on the thin filament, reducing its rate of sliding.
Interestingly, displacement of tropomyosin does not occur when a shorter N-terminal fragment (C0C1f, containing C0C1 and the first 17 amino acid residues of the M-domain) binds to the thin filament [27]; this fragment also fails to activate thin filament motility at low Ca2+. Thus regions of the M-domain beyond the first 17 amino acids appear to be required for fully productive binding to thin filaments. Yeast two hybrid and NMR data are consistent with this finding, suggesting that positively charged residues of the M-domain beyond the first seventeen are important to actin binding ability [6,16].
A novel in vitro motility assay, in which F-actin or native thin filaments slide along native cardiac muscle thick filaments, adds to the evidence that binding of cMyBP-C to actin can modulate filament sliding [30,31]. Actin filaments sliding near the tips of the thick filament (where MyBP-C is absent; Fig. 2A) slow abruptly when they enter the C-zone, consistent with cMyBP-C (extending from the thick filament surface) placing a drag on actin sliding. This braking effect disappears when the N-terminus (C0C1f) of cMyBP-C is missing, supporting the view that this region is essential for actin binding. When regulated thin filaments are used in the assay, filament sliding occurs in the C-zone even under low Ca2+ conditions. This supports the motility data described above for molecular myosin, suggesting that cMyBP-C’s ability to modulate thin filament activity is also present when it is incorporated into native thick filaments.
As mentioned previously, cMyBP-C can be phosphorylated at four sites within the M-domain, reducing, but not abolishing, its interaction with actin [3]. Phosphorylated cMyBP-C still binds to thin filaments [37,39,40], but 3D reconstruction shows that this binding does not displace tropomyosin (Mun et al., unpublished data). If cMyBP-C phosphorylation has similar effects in the intact myocardium, it could modulate contraction in two ways: by reducing cMyBP-C’s slowing of filament sliding [30,39,40] and by reducing its activating effect on the thin filament [31]. It appears that cMyBP-C is designed to provide a high level of fine tuning of cardiac contraction.
5. Future directions
Our understanding of MyBP-C structure and function has progressed greatly in the past five years. Involvement of cMyBP-C mutations in dilated and hypertrophic cardiomyopathy has provided major impetus to these studies, and is likely to do so in the future. We have focused here on recent advances in understanding MyBP-C structure and organization, its interaction with thin filaments, and modulation of its function by phosphorylation. Many questions remain concerning the function of this intriguing molecule. (1) Although we have focused on the actin binding effects of its N-terminal domains, this region of cMyBP-C also binds to myosin S2, close to its connection to the myosin heads [12]; as with actin, this interaction is also weakened by cMyBP-C phosphorylation [13]. The N-terminus can also interact with the myosin RLC [32]. What is the function of this N-terminal myosin binding? Can actin and myosin interactions occur simultaneously or does the N-terminus switch between them depending on availability of binding sites or on the state of the muscle? (2) If MyBP-C is confined to one half the length of the thick filament (the two C-zones), and there may interact with only every third level of myosin molecules, how do these limited interactions impact contractility of the sarcomere as a whole? (3) How do MyBP-C organization or interactions change in different physiological states (activation cf. relaxation)? (4) Can MyBP-C binding to actin and/or myosin be demonstrated in vivo? (6) How does MyBP-C affect myosin head organization in the C-zone compared with the P- and D-zones, where it is absent? (7) In what ways are the skeletal MyBP-C isoforms similar to and different from the cardiac isoform?
Answers to these and similar questions should provide many new insights into MyBP-C function in the coming years.
Acknowledgments
This work was supported by National Institutes of Health grants R01 AR034711 and P01 HL059408, and by British Heart Foundation Programme Grant RG/11/21/29335.
References
- 1.Ababou A, Gautel M, Pfuhl M. Dissecting the N-terminal myosin binding site of human cardiac myosin-binding protein C. Structure and myosin binding of domain C2. J Biol Chem. 2007;282:9204–9215. doi: 10.1074/jbc.M610899200. [DOI] [PubMed] [Google Scholar]
- 2.Al-Khayat HA, Kensler RW, Squire JM, Marston SB, Morris EP. Atomic model of the human cardiac muscle myosin filament. Proc Natl Acad Sci U S A. 2013;110:318–323. doi: 10.1073/pnas.1212708110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barefield D, Sadayappan S. Phosphorylation and function of cardiac myosin binding protein-C in health and disease. J Mol Cell Cardiol. 2010;48:866–875. doi: 10.1016/j.yjmcc.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett P, Craig R, Starr R, Offer G. The ultrastructural location of C-protein, X-protein and H-protein in rabbit muscle. J Muscle Res Cell Motil. 1986;7:550–567. doi: 10.1007/BF01753571. [DOI] [PubMed] [Google Scholar]
- 5.Bennett PM, Furst DO, Gautel M. The C-protein (myosin binding protein C) family: regulators of contraction and sarcomere formation? Rev Physiol Biochem Pharmacol. 1999;138:203–234. doi: 10.1007/BFb0119628. [DOI] [PubMed] [Google Scholar]
- 6.Bhuiyan MS, Gulick J, Osinska H, Gupta M, Robbins J. Determination of the critical residues responsible for cardiac myosin binding protein C's interactions. J Mol Cell Cardiol. 2012;53:838–847. doi: 10.1016/j.yjmcc.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Craig R. Structure of A-segments from frog and rabbit skeletal muscle. J Mol Biol. 1977;109:69–81. doi: 10.1016/s0022-2836(77)80046-6. [DOI] [PubMed] [Google Scholar]
- 8.Craig R, Offer G. The location of C-protein in rabbit skeletal muscle. Proc R Soc Lond B Biol Sci. 1976;192:451–461. doi: 10.1098/rspb.1976.0023. [DOI] [PubMed] [Google Scholar]
- 9.Flashman E, Redwood C, Moolman-Smook J, Watkins H. Cardiac myosin binding protein C: its role in physiology and disease. Circ Res. 2004;94:1279–1289. doi: 10.1161/01.RES.0000127175.21818.C2. [DOI] [PubMed] [Google Scholar]
- 10.Govada L, Carpenter L, da Fonseca PC, Helliwell JR, Rizkallah P, Flashman E, Chayen NE, Redwood C, Squire JM. Crystal structure of the C1 domain of cardiac myosin binding protein-C: implications for hypertrophic cardiomyopathy. J Mol Biol. 2008;378:387–397. doi: 10.1016/j.jmb.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 11.Gregorio CC, Granzier H, Sorimachi H, Labeit S. Muscle assembly: a titanic achievement? Curr Opin Cell Biol. 1999;11:18–25. doi: 10.1016/s0955-0674(99)80003-9. [DOI] [PubMed] [Google Scholar]
- 12.Gruen M, Gautel M. Mutations in beta-myosin S2 that cause familial hypertrophic cardiomyopathy (FHC) abolish the interaction with the regulatory domain of myosin-binding protein-C. J Mol Biol. 1999;286:933–949. doi: 10.1006/jmbi.1998.2522. [DOI] [PubMed] [Google Scholar]
- 13.Gruen M, Prinz H, Gautel M. cAPK-phosphorylation controls the interaction of the regulatory domain of cardiac myosin binding protein C with myosin-S2 in an on-off fashion. FEBS Lett. 1999;453:254–259. doi: 10.1016/s0014-5793(99)00727-9. [DOI] [PubMed] [Google Scholar]
- 14.Hanson J, O'Brien EJ, Bennett PM. Structure of the myosin-containing filament assembly (A-segment) separated from frog skeletal muscle. J Mol Biol. 1971;58:865–871. doi: 10.1016/0022-2836(71)90045-3. [DOI] [PubMed] [Google Scholar]
- 15.Harris SP, Lyons RG, Bezold KL. In the thick of it: HCM-causing mutations in myosin binding proteins of the thick filament. Circ Res. 2011;108:751–764. doi: 10.1161/CIRCRESAHA.110.231670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howarth JW, Ramisetti S, Nolan K, Sadayappan S, Rosevear PR. Structural insight into unique cardiac myosin-binding protein-C motif: a partially folded domain. J Biol Chem. 2012;287:8254–8262. doi: 10.1074/jbc.M111.309591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeffries CM, Whitten AE, Harris SP, Trewhella J. Small-angle X-ray scattering reveals the N-terminal domain organization of cardiac myosin binding protein C. J Mol Biol. 2008;377:1186–1199. doi: 10.1016/j.jmb.2008.01.080. [DOI] [PubMed] [Google Scholar]
- 18.Karsai A, Kellermayer MS, Harris SP. Mechanical unfolding of cardiac myosin binding protein-C by atomic force microscopy. Biophys J. 2011;101:1968–1977. doi: 10.1016/j.bpj.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee KH, Sadayappan S, Harris S, Craig R. Determination of MyBP-C orientation in the cardiac sarcomere by immuno-EM. Biophys J. 2013;104:309a. [Google Scholar]
- 20.Luther PK, Bennett PM, Knupp C, Craig R, Padron R, Harris SP, Patel J, Moss RL. Understanding the organisation and role of myosin binding protein C in normal striated muscle by comparison with MyBP-C knockout cardiac muscle. J Mol Biol. 2008;384:60–72. doi: 10.1016/j.jmb.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luther PK, Craig R. Modulation of striated muscle contraction by binding of myosin binding protein C to actin. Bioarchitecture. 2011;1:277–283. doi: 10.4161/bioa.1.6.19341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luther PK, Winkler H, Taylor K, Zoghbi ME, Craig R, Padron R, Squire JM, Liu J. Direct visualization of myosin-binding protein C bridging myosin and actin filaments in intact muscle. Proc Natl Acad Sci U S A. 2011;108:11423–11428. doi: 10.1073/pnas.1103216108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michalek AJ, Howarth JW, Gulick J, Previs MJ, Robbins J, Rosevear PR, Warshaw DM. Phosphorylation modulates the mechanical stability of the cardiac myosin-binding protein C motif. Biophys J. 2013;104:442–452. doi: 10.1016/j.bpj.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moolman-Smook J, Flashman E, de LW, Li Z, Corfield V, Redwood C, Watkins H. Identification of novel interactions between domains of myosin binding protein-C that are modulated by hypertrophic cardiomyopathy missense mutations. Circ Res. 2002;91:704–711. doi: 10.1161/01.res.0000036750.81083.83. [DOI] [PubMed] [Google Scholar]
- 25.Moos C, Mason CM, Besterman JM, Feng IN, Dubin JH. The binding of skeletal muscle C-protein to F-actin, and its relation to the interaction of actin with myosin subfragment-1. J Mol Biol. 1978;124:571–586. doi: 10.1016/0022-2836(78)90172-9. [DOI] [PubMed] [Google Scholar]
- 26.Mun JY, Gulick J, Robbins J, Woodhead J, Lehman W, Craig R. Electron microscopy and 3D reconstruction of F-actin decorated with cardiac myosin-binding protein C (cMyBP-C) J Mol Biol. 2011;410:214–225. doi: 10.1016/j.jmb.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mun JY, Previs MJ, Yu H, Gulick J, Tobacman LS, Previs SB, Robbins J, Warshaw DM, Craig R. Myosin-binding protein C displaces tropomyosin to activate cardiac thin filaments and governs their speed by an independent mechanism. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1316001111. Under revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Offer G, Moos C, Starr R. A new protein of the thick filaments of vertebrate skeletal myofibrils. Extraction, purification and characterization. J Mol Biol. 1973;74:653–676. doi: 10.1016/0022-2836(73)90055-7. [DOI] [PubMed] [Google Scholar]
- 29.Orlova A, Galkin VE, Jeffries CM, Egelman EH, Trewhella J. The N-terminal domains of myosin binding protein C can bind polymorphically to F-actin. J Mol Biol. 2011;412:379–386. doi: 10.1016/j.jmb.2011.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Previs MJ, Beck PS, Gulick J, Robbins J, Warshaw DM. Molecular mechanics of cardiac myosin-binding protein C in native thick filaments. Science. 2012;337:1215–1218. doi: 10.1126/science.1223602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Previs MJ, Yu H, Beck PS, Gulick J, Robbins J, Warshaw DM. Dephosphorylated cardiac myosin-binding protein c (cMyBP-C) activates native cardiac thin filaments within the C-zone of native cardiac thick filaments. Biophys J. 2013;104:186a. [Google Scholar]
- 32.Ratti J, Rostkova E, Gautel M, Pfuhl M. Structure and interactions of myosin-binding protein C domain C0: cardiac-specific regulation of myosin at its neck? J Biol Chem. 2011;286:12650–12658. doi: 10.1074/jbc.M110.156646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Razumova MV, Shaffer JF, Tu AY, Flint GV, Regnier M, Harris SP. Effects of the N-terminal domains of myosin binding protein-C in an in vitro motility assay: Evidence for long-lived cross-bridges. J Biol Chem. 2006;281:35846–35854. doi: 10.1074/jbc.M606949200. [DOI] [PubMed] [Google Scholar]
- 34.Rybakova IN, Greaser ML, Moss RL. Myosin binding protein C interaction with actin: characterization and mapping of the binding site. J Biol Chem. 2011;286:2008–2016. doi: 10.1074/jbc.M110.170605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saber W, Begin KJ, Warshaw DM, VanBuren P. Cardiac myosin binding protein-C modulates actomyosin binding and kinetics in the in vitro motility assay. J Mol Cell Cardiol. 2008;44:1053–1061. doi: 10.1016/j.yjmcc.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaffer JF, Harris SP. Species-specific differences in the Pro-Ala rich region of cardiac myosin binding protein-C. J Muscle Res Cell Motil. 2009;30:303–306. doi: 10.1007/s10974-010-9207-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaffer JF, Kensler RW, Harris SP. The myosin-binding protein C motif binds to F-actin in a phosphorylation-sensitive manner. J Biol Chem. 2009;284:12318–12327. doi: 10.1074/jbc.M808850200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Squire JM, Luther PK, Knupp C. Structural evidence for the interaction of C-protein (MyBP-C) with actin and sequence identification of a possible actin-binding domain. J Mol Biol. 2003;331:713–724. doi: 10.1016/s0022-2836(03)00781-2. [DOI] [PubMed] [Google Scholar]
- 39.Weith A, Sadayappan S, Gulick J, Previs MJ, VanBuren P, Robbins J, Warshaw DM. Unique single molecule binding of cardiac myosin binding protein-C to actin and phosphorylation-dependent inhibition of actomyosin motility requires 17 amino acids of the motif domain. J Mol Cell Cardiol. 2012;52:219–227. doi: 10.1016/j.yjmcc.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weith AE, Previs MJ, Hoeprich GJ, Previs SB, Gulick J, Robbins J, Warshaw DM. The extent of cardiac myosin binding protein-C phosphorylation modulates actomyosin function in a graded manner. J Muscle Res Cell Motil. 2012;33:449–459. doi: 10.1007/s10974-012-9312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White HD, Belknap B, Harris SP. Activation and inhibition of F-actin and cardiac thin filaments by the N-terminal domains of cardiac myosin binding protein C. Biophys J. 2013;104:158a–159a. [Google Scholar]
- 42.White HD, Harris S. Activation of cardiac thin filaments by N-terminal domains of cardiac myosin binding protein C. Biophys J. 2012;102:435a. [Google Scholar]
- 43.Whitten AE, Jeffries CM, Harris SP, Trewhella J. Cardiac myosin-binding protein C decorates F-actin: Implications for cardiac function. Proc Natl Acad Sci U S A. 2008;105:18360–18365. doi: 10.1073/pnas.0808903105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamamoto K. The binding of skeletal muscle C-protein to regulated actin. FEBS Lett. 1986;208:123–127. doi: 10.1016/0014-5793(86)81545-9. [DOI] [PubMed] [Google Scholar]
- 45.Zoghbi ME, Woodhead JL, Moss RL, Craig R. Three-dimensional structure of vertebrate cardiac muscle myosin filaments. Proc Natl Acad Sci U S A. 2008;105:2386–2390. doi: 10.1073/pnas.0708912105. [DOI] [PMC free article] [PubMed] [Google Scholar]