Abstract
The psychostimulant methylphenidate (MPD, Ritalin) is the prescribed drug of choice for treatment of ADHD. In recent years, the diagnosis rate of ADHD has increased dramatically, as have the number of MPD prescriptions. Repeated exposure to psychostimulants produces behavioral sensitization in rats, an experimental indicator of a drug’s potential liability. In studies on cocaine and amphetamine, this effect has been reported to involve the nucleus accumbens (NAc), one of the nuclei belonging to the motive circuit. The aim of this study was to investigate the role of the NAc on the expression of behavioral sensitization as a response to MPD exposure. In the present study, 20 male Sprague-Dawley rats were divided randomly into three groups: an intact control group, a sham operated group, and a NAc bilateral electrical lesion group. Locomotor activity was assessed for the first two hours following 2.5 mg/kg MPD injection, using open field monitoring systems. Recordings were made during six days of continuous MPD administration, and then upon re-challenge with the same dose following three days of washout. Acute MPD exposure elicited an increase in locomotor activity in all three groups. However, the NAc lesion group exhibited significantly increased locomotor activity in comparison to sham and control groups. Chronic MPD did not elicit sensitization in the NAc lesion group, while both sham and control groups did exhibit behavioral sensitization to repetitive MPD administration. These findings suggest that the NAc plays a significant role in eliciting locomotor activity as an acute effect of MPD, and in the expression of sensitization due to chronic MPD exposure.
Keywords: Nucleus accumbens, Ritalin, psychostimulant, behavioral sensitization
Introduction
Psychostimulant medications such as amphetamines (Amph) were used to treat Attention Deficit Hyperactivity Disorder (ADHD) in children since the 1930’s until it was discovered that Amph elicits dependency [63,69]. The psychostimulant methylphenidate (MPD, Ritalin) has since become the prescribed drug of choice for treatment of ADHD [1,28,49,67]. Although the prescribed use of MPD has risen considerably in recent years and non-medical uses of MPD have increased steadily among youths and adults, little is known about its adverse potential.
Psychostimulants such as Amph, cocaine, and MPD exhibit similar pharmacological profiles, but only Amph and cocaine have been extensively researched as potential drugs of abuse [8,29,39,46,52,55,58,68]. Both have consistently exhibited experimental indicators of dependence such as tolerance and behavioral sensitization [10,44,66,68]. Behavioral sensitization is characterized by the progressive augmentation of the drug effect induced by the re-administration of the same dose [3,20,59].
MPD has been reported to induce tolerance [43,80], behavioral sensitization [27,80,81,82], and withdrawal [2,25,47] in rodents. These phenomena were used as an experimental tool to determine whether MPD has the potential to elicit dependency [20,59].
The nucleus accumbens (NAc), one of the nuclei belonging to the motive circuit, is known to play an important role in providing the motivational drive for reward-related behavior [17,55,58,60]. Studies using local injection, electrical lesions, and tissue slices have been used to study the role of the NAc in inducing and expressing behavioral sensitization following chronic exposure to psychostimulants such as cocaine and Amph [12,40]. The above studies have reported that rats with lesions of the NAc experienced attenuated locomotor responses following withdrawal when compared to a sham operated control group. These studies suggest that the expression of sensitization following cocaine and Amph administration is attributed to the NAc [21,57]. Although studies involving the NAc in sensitization to cocaine and Amph have been conducted [12,21,40,57], the role of the NAc in MPD sensitization has not been reported.
This study investigates the role of the NAc in eliciting and expressing the acute and chronic effects of MPD exposure in three groups of animals: intact control, sham operated, and bilateral NAc lesion groups. MPD was administered once daily (2.5 mg/kg) for several days, similar to previous experimental protocol of intact animals [25,78,79,80,81,82]. Rat behavior was assessed using an open field assay [2,25,47,48,79,80,81,82].
Methods
Animals
Twenty Sprague-Dawley rats (Harlan, Indianapolis, IN., USA) weighing from 170–180g at their arrival were housed for three to five days in a sound proof room prior to experimentation. Animals were maintained on a 12 hour light/dark schedule (light on at 06:00). Food and water was provided ad libitum throughout the experiment and the temperature was kept at 21 ±2°C with a relative humidity of 37–42%. Animals were divided randomly into three groups: an intact control group (n=8), a sham operated group (n=5), and a bilateral lesion group (n=7). They were then individually housed in the same room in activity chambers which became their home cages and were allowed to acclimate for an additional 3 days before experimentation began. At the start of recording animals weighed between 200 and 210g.
Drugs
Methylphenidate hydrochloride (MPD) was obtained from Mallinckrodt (Hazelwood, MO) for use in this experiment. In previous dose response experiments [22,25,26,28,80,81,82] it was observed that the dose of 2.5 mg/kg i.p. MPD elicits significant behavioral sensitization. Therefore, this dose was selected for use in this experiment. The MPD dosage was calculated as free-base and dissolved in 0.9% saline. Animals were weighed prior to injection in order to administer the appropriate amount of MPD for each rat’s body weight. All injections were equalized to 0.8 ml in volume with saline. All injections were made at about 07:00, one hour into the light period. Recordings began immediately following injection.
Surgical Procedure
Animals were anesthetized using Pentobarbital (50mg/kg i.p.) and placed in a stereotaxic instrument. An incision was made in the top of the head exposing the skull and two small holes were placed directly over the NAc in each hemisphere using the following coordinates from Paxinos and Watson atlas [54]: 2.0 mm anterior to bregma, 1.5 mm lateral to the midline; 2.0 mm anterior to bregma, 2.2 mm lateral to the midline. Electrodes were made of single stainless steel wires 80 μm in diameter. Bilateral electrolytic lesions were made by applying a current of 2–3 mA for one minute and 30 seconds at depths of 7.4 mm and 7.0 mm in both holes. Following surgery, the skin on the head was closed using wound closing staples. The procedure for the sham operated group was identical except that no current was passed through the electrode. All animals were allowed three days to recover from surgery prior to testing.
Experimental Protocol
Experimentation began after a six to eight day habituation period and lasted for 17 days. Baseline levels of locomotor activity were measured on experimental day one following saline injections for 120 minutes. On experimental day two, animals were separated into three groups: an intact control group (n=8), a sham operated control group (n=5), and a bilateral lesion group (n=7). The sham and lesion groups underwent surgery and were allowed three days to recover from surgery (experimental days 3 to 5). On experimental day six, recording was resumed and saline was re-administered in order to reestablish animal handling/injection controls post-surgery. This was followed by six consecutive days (experimental days 7 to 12) of single 2.5 mg/kg MPD injections in order to record the acute and chronic effects of MPD such as tolerance or sensitization. Recording of locomotive activity was continued on experimental days 13 to 15 without MPD injection (washout period). On experimental day 16, animals were re-challenged with a 2.5 mg/kg dose of MPD to test for sensitization and locomotor activity was recorded (Table 1). All recordings began immediately after injection and lasted for 120 minutes. During the three washout days recordings were done during the same time and for the same duration as previous recording days.
Table 1.
| Group | Experimental Day | ||||||
|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Days 3–5 | Day 6 | Days 7–12 | Days 13–15 | Day 16 | |
| Control | Saline | No treatment | No treatment | Saline | 2.5 mg/kg MPD | Withdrawal/No treatment | 2.5 mg/kg MPD |
| Sham | Saline | Surgery | No treatment | Saline | 2.5 mg/kg MPD | Withdrawal/No treatment | 2.5 mg/kg MPD |
| Lesion | Saline | Surgery | No treatment | Saline | 2.5 mg/kg MPD | Withdrawal/No treatment | 2.5 mg/kg MPD |
Apparatus
Locomotor activity was recorded using open field computerized monitoring systems (AccuScan Instruments, Inc., Columbus, OH), consisting of clear acrylic boxes (40.5 × 40.5 × 31.5 cm) equipped with two levels of 16 infrared beams and motion sensors at 6.0 and 12.5 cm above the floor of the cage. Movement of animals across any beam resulted in the recording of locomotor activity. Activity counts were compiled by Accuscan Analyzer and downloaded to PC every 10 minutes onto OASIS data collection software. In the present study, three locomotor indices were analyzed: horizontal activity (HA), which measured the overall activity in the lower region of the test cage and was used to assess the amount of overall spontaneous activity; total distance (TD), which measures forward ambulation in cm; and number of stereotypic movements (NOS), which counts the number of episodes of purposeless repetitive activity with at least a one second interval between episodes.
Histology
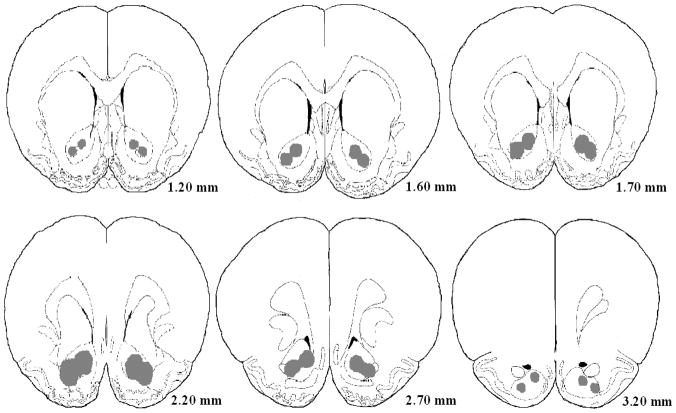
Upon completion of the experiment, animals were overdosed with sodium barbital and perfused intracardially with 10% formaldehyde containing about 3% potassium ferrocyanide. The brains were then removed and placed in 10% formaldehyde for 48 hours to prepare them for sectioning. Brains were then sectioned at 120μm thickness and stained with Cresyl Violet so that lesions could be checked for proper location and size. (Fig. 1)
Fig. 1.
Data Analysis
Locomotor activity counts were collected every 10 minutes for two hours. These recordings were used to produce a sequential temporal graph recording the first 2 hours after injection and a histogram comparing the average sums of 2 hour activity under the temporal graph. For the temporal graph, each 10 minute recording bin was plotted sequentially and the standard error (S.E.) was used to calculate the significance in changes between experimental days using ANOVA and Post hoc analysis with LSD test [24,25,26,47,48,79,82]. Significant changes in two or more consecutive recordings were considered to be significant due to the drug effect. For the 2 hour histogram analysis, the sums of 2 hour activity immediately following injection were used to calculate the average activity level for each test group during the 2 hours. Comparisons were made with ANOVA and Post hoc analysis with LSD test. Significance was set at p<0.05. 5 comparisons were made. (1) Experimental day 1 was compared to experimental day 6 to determine whether the sham and lesion surgeries effected baseline activity levels (see Table 1). (2) Experimental day 6 was compared to experimental day 7 to establish the acute effect of MPD. (3) Experimental day 7 was compared to experimental day 12 to determine whether sensitization, tolerance, or no difference due to MPD treatment had been induced. (4) Experimental day 6 was compared to experimental day 13 to observe possible withdrawal effects. (5) Experimental day 7 was compared to experimental day 16 to determine whether sensitization to chronic treatment with MPD had been expressed following the 3 day washout period.
Results
Effect of saline on baseline activity
Previous studies from our laboratories using similar experimental procedures have determined that single daily saline injections for several weeks in rats induce neither acute nor chronic alterations in locomotor activity recordings, as compared to a non-injected control group [24,25,26,77,81,82]. Since the recording following the first saline injection was similar to all the other saline injection days as well as to the non-injected group, activity recordings for experimental day one following saline injection were used as a baseline control to evaluate the effects of MPD [24,26,77,81].
Effect of sham operation and NAc lesion on baseline activity
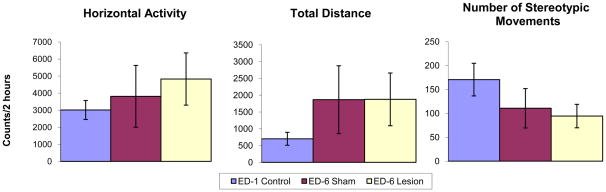
Fig. 2 histograms summarize the baseline activity level during the first 2 hours following saline injection before surgery (experimental day 1) and after NAc sham surgery and NAc electrical lesion (experimental day 6), i.e. 5 days post-surgery, for each group. The locomotor baseline activity levels on experimental day 6 exhibit some differences from experimental day 1, but this fluctuation was not significant (Fig. 2). Therefore, activity recordings from experimental day 6 were used as control recordings to compare the drug effects in sham and NAc lesion groups.
Fig. 2.
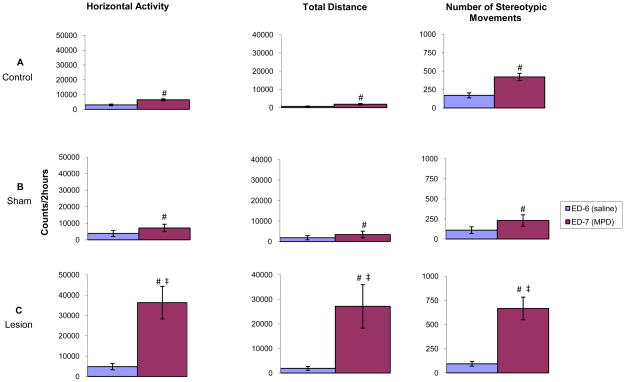
MPD acute effect: comparing experimental day 6 to experimental day 7
Fig. 3 histograms compare and summarize the HA, TD traveled, and NOS activity levels of each group (control, sham, and lesion) during the first 2 hours following MPD treatment on experimental day 7 (initial 2.5 mg/kg MPD) to activity levels of experimental day 6 (saline injection). All three groups show significant (p<0.05; F1, 17=6.87) increases in HA, TD, and NOS in response to a single injection of 2.5 mg/kg MPD (Fig. 3). Following 2.5 mg/kg MPD administration, the NAc lesion group exhibited robust significant (p<0.05; F1, 23=5.01) increases in all three locomotor indices (HA, TD, and NOS) as compared to sham and control groups (Fig. 3). This suggests that NAc lesions result in amplification of the acute response to MPD.
Fig. 3.
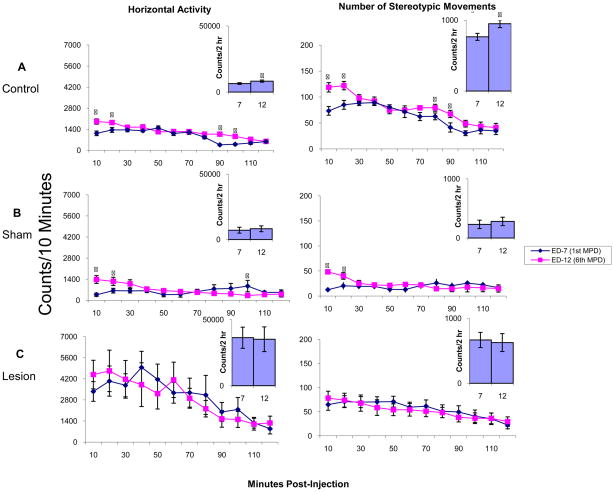
Induction phase: comparing experimental day 7 to experimental day 12
Fig. 4 summarizes the HA and NOS movements in temporal graphs (counts/10 min) and 2 hour histograms (2 hour summation of HA and NOS movements) for each group (control, sham, and lesion), comparing the activity levels during the first 2 hours after MPD administration on experimental day 7 (first day of MPD treatment) with activity levels from experimental day 12 (the 6th day of MPD treatment; Table 1). The control group exhibited a significant (p<0.05; F2, 29=5.23) increase in HA and NOS movements during the initial 20 minutes post-injection as well as at 80–100 minutes post-injection on experimental day 12 compared to experimental day 7. This drug-induced increase in activity is also expressed as a significant (p<0.05; F2,26=4.44) increase in the 2 hour histograms. In the sham group, MPD treatment on experimental day 12 induced a similar significant (p<0.05; F2,26=3.82) increase in activity over the initial 20 minutes post-injection. However, in the 2 hour histogram, this increase was not significant according to the LSD test, but does show an increasing trend. The NAc lesion group showed no significant increase in locomotor activity during any 10 minute count throughout the 2 hours. This was also expressed in the 2 hour histogram as the summation of activity counts was nearly identical for experimental days 12 and 7 in both HA and NOS movements (Fig. 4).
Fig. 4.
Washout phase: comparing experimental day 6 to experimental day 13
Fig. 5 summarizes both the temporal graphs and 2 hour histograms of the control and the NAc lesion groups, comparing the HA, TD traveled, and NOS movements during the initial 2 hours following saline injection given at 07:00 on experimental day 6 (i.e. locomotor activity from 07:00 to 09:00) with activity levels on experimental day 13 (first day of washout) during the 2 hours from 07:00 to 09:00 (the usual time post MPD recording in previous days). The intact control group exhibited significant (p<0.05; F3,48=4.01) increases in activity between 07:00 and 07:50 i.e. for 50 minutes, on experimental day 13 when compared to experimental day 6 (Fig. 5). This increase was also expressed as significant (p<0.05; F3,48=3.86) in the 2 hour histogram. The NAc lesion group exhibited a similar significant (p<0.05; F3,51=3.67) increase in locomotor activity on experimental day 13 compared to experimental day 6 (Fig. 5). This increase in activity on experimental day 13 can be attributed to anticipation or withdrawal from the previous six daily treatments with MPD. However, this increase in activity on the washout days observed in the NAc lesion group were significantly (p<0.05) intensified compared to the increase in activity exhibited by the control group (Fig. 5). Similar observations were observed from during experimental days 14 and 15 (data not shown).
Fig. 5.
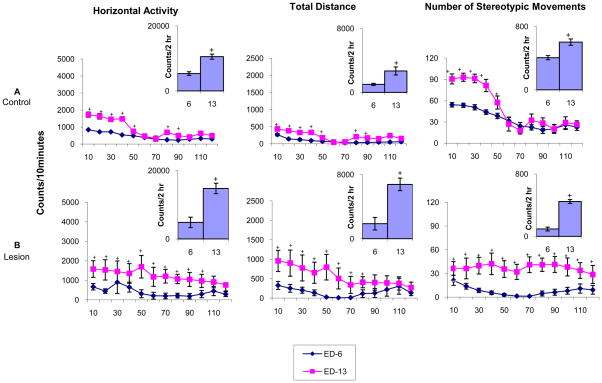
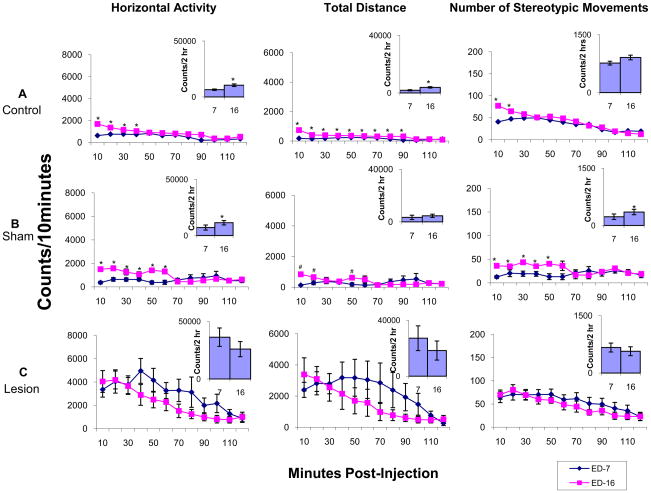
Expression phase: comparing experimental day 7 to experimental day 16
Fig. 6 summarizes the temporal graphs and 2 hour histograms for each group (control, sham, lesion), comparing HA, TD traveled, and NOS movements during the first 2 hours post-MPD (2.5 mg/kg) injection on experimental days 7 (first day of MPD) and experimental day 16 (MPD re-challenge after 3 washout days). The control group exhibited significantly (p<0.05; F2,53=3.65) increased activity throughout the first 40 minutes following 2.5 mg/kg MPD injection on experimental day 16 compared to 2.5mg/kg MPD injection on experimental day 7. This significant increase (p < 0.05; F2,51=3.74) was also expressed in the 2 hour histogram (Fig. 6). Similar observation following 2.5 mg/kg MPD treatment in the sham group was observed, with activity levels remaining significantly (p<0.05; F4 – xy) increased for 50 minutes following injection on experimental day 16 compared to experimental day 7 following MPD injection. This effect was also observed in the 2 hour histogram. However, the NAc lesion group failed to exhibit an increase in activity on experimental day 16 following the 2.5 mg/kg MPD re-challenge versus the 2.5 mg/kg MPD injection on experimental day 7 in both the temporal graph and 2 hour summation. This suggests that NAc lesions prevent the expression of behavioral sensitization.
Fig. 6.
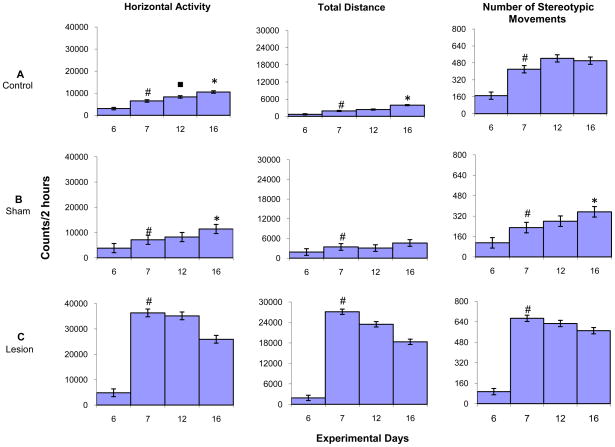
Fig. 7 summarizes the HA, TD traveled, and NOS activity for control, sham, and lesion groups, comparing activity levels during the first two hours following MPD administration on experimental day 6 (saline control) to experimental day 7 (first day of MPD treatment), experimental day 12 (6th day of MPD treatment), and experimental day 16 (MPD re-challenge). Following the initial MPD (2.5 mg/kg) administration (i.e. experimental day 7 compared to experimental day 6), all groups exhibited a significant (p<0.05; F10,85=14.01) increase in HA, TD traveled, and NOS activity, with the NAc lesion group exhibiting an amplified response to a single 2.5 mg/kg dose of MPD. Comparing the activity post MPD injection of experimental day 12 to experimental day 7 (induction phase; Table 1) of the control and sham groups shows an activity increasing trend on all locomotor indices, except for the TD traveled of the sham group. However, the only significant (p<0.05; F10,85=2.82) increase was exhibited by the HA of the control group. The NAc lesion group displayed a decreasing trend on all locomotor indices on experimental day 12 compared to experimental day 7. This observation suggests that NAc lesions modulate the effects of repetitive MPD administration on locomotor activity.
Fig. 7.
Comparing the HA, TD traveled, and NOS activity of experimental day 16 to experimental day 7 (the expression phase; Table 1) shows that the control group displayed a significant (p<0.05; F2,23=3.72) increase in HA and TD traveled, while the sham group exhibited a significant (p<0.05; F2,25=4.34) increase in HA and NOS activity and an increasing trend in TD traveled. The lesion group, however, failed to exhibit any significant increase in activity following the MPD re-challenge dose. This observation shows that repetitive MPD administration elicits behavioral sensitization in intact NAc animals and NAc lesions prevent the expression of the repetitive MPD administration – i.e., the behavioral sensitization (Fig. 7).
Discussion
Methylphenidate exposure has been studied less extensively than other psychostimulants, and the MPD studies report inconsistent observations regarding its adverse effects. Some studies have reported that MPD treatment of youths with ADHD may result in an increased susceptibility to drug abuse disorder [11,45,59,61,62]. Other reports provide evidence alleging that treatment with MPD protects youths from substance abuse later in life [6,76]. In rats, it has also been shown that long-term exposure to caffeine during adolescence induces cross-sensitization to MPD during adulthood [7]. Past data suggests that the inconsistencies in these findings have resulted from a variety of factors, including differences in drug dosage, route of administration, time of administration, age, gender, and animal model used [3,4,20,80].
MPD, like other psychostimulants of abuse, elicits its effect by blocking the activity of DA transporters (DAT) which results in an increase in DA levels within the brain [23,31,73]. This increase in DA and the activation of DA receptors is suggested to be responsible for the behavioral effects, such as sensitization [71]. The process by which an increase in DA levels causes sensitization to psychostimulants is thought to be mediated by the mesocorticolimbic DA system [58,75]. Specifically, the relevant sites high in DA concentrations are the ventral tegmental area (VTA), NAc, prefrontal cortex (PFC), and caudate nucleus (CN) [58]. These structures, collectively known as the motive circuit, are also involved in the neuronal circuitry of motivation and reward [15,18,43,58]. Repeated exposure to psychostimulants has been shown to cause various alterations within the dopaminergic projection from the VTA to the NAc [34,35,75,81]. DA neurons in the VTA that project to the NAc act as a functional interface between the limbic system and motor areas of the brain [16,42]. In studies using local repetitive psychostimulant microinjections within the VTA, it has been reported that animals developed behavioral sensitization [19]. This suggests that the VTA is responsible, at least in part, for the initiation of behavioral sensitization [19,58]. Direct microinjection of psychostimulants into the NAc produced a sensitized motor effect only after rats were pretreated with repeated psychostimulant injections [5]. This suggests that the NAc is involved, at least in part, in the expression of behavioral sensitization [50,58].
Behavioral sensitization is used as an experimental indicator of the potential for a drug to cause dependence [20,25,81]. The goal of this study was to examine the effect of acute and chronic MPD exposure on the locomotor behavior of rats with bilateral electrical lesions made on the NAc.
The main finding of this study is that locomotor baseline activity levels following NAc sham surgery and NAc lesion surgery showed similar activity to intact animals. This indicates that the sham and NAc lesions did not alter baseline locomotor activity. The first injection of MPD (experimental day 7; Table 1) elicited a significant increase in locomotor activity in all three groups (control, sham, and lesion). However, this increase in activity was significantly amplified in NAc lesion animals. This observation is similar to that reported by Weiner et al. (1996) in which animals with electrolytic lesions of the NAc exhibited enhanced acute effects of Amph administration [74]. Similar findings have been obtained following nicotine [37] and cocaine [65] administration in NAc lesion animals. Conversely, it has been reported that NAc lesions do not alter the acute effects of psychostimulants [70]. One likely explanation for this inconsistency is the location of lesion within the NAc. Previous studies report that locomotor response to psychostimulants can be affected by location of lesion within the NAc [9,38,64,74]. Specifically, the NAc core is more involved in locomotor processes such as sensitization than is the NAc shell [13,38]. For this reason, only animals with lesions damaging the NAc core were included in the study, regardless of damage made to the shell. Animals with lesions to both core and shell did not display significant differences in locomotor activity. Future studies using selective chemical lesions and specifying lesion location are necessary to determine functional differences between core and shell in the sensitization mechanism.
Following daily 2.5 mg/kg MPD administration for six consecutive days (experimental days 7–12; table 1), both intact and sham-operated groups exhibited behavioral sensitization while the NAc lesion group failed to exhibit an increase in locomotor activity. This suggests that the NAc plays an important role in the induction of MPD-induced behavioral sensitization. The temporal data of 10 minute bins shows that the animals increased their locomotor activity in the first 20 minutes following injection, whereas in the 2 hour histograms, the data was skewed. The 2 hour histograms showed only an increasing trend in total activity counts from experimental day 7 to experimental day 12, with no significant increase. This observation was expected since MPD has a short time effect, and suggests that temporal graphs are better suited to show the effects of such a short acting drug.
It has been consistently reported that direct chronic microinjection of Amph, cocaine, or morphine into the VTA induces sensitization to these drugs in rats [19,30,33,72]. Other studies have reported that direct injection of Amph into the NAc is sufficient to induce a sensitized response in rats previously chronically injected with psychostimulants, suggesting that the NAc is involved in the induction of behavioral sensitization [5,14,53,56]. More recent studies reported increased locomotor activity following microinjections of p-hydroxyamphetamine directly into the NAc [51]. Although several studies purport that behavioral sensitization originates in the VTA while expression occurs in the NAc, the above observations along with our own suggest that the NAc is also involved in the induction of behavioral sensitization.
Following the 2.5 mg/kg MPD re-challenge (experimental day 16; table 1), the intact control group and sham-operated group exhibited significant increases in locomotion compared to the initial 2.5 mg/kg MPD treatment (experimental day 7), i.e. behavioral sensitization was expressed. The NAc lesion group failed to exhibit an increase in locomotor activity. This observation suggests that the NAc is required for the expression of behavioral sensitization following repetitive MPD administration. Our finding agrees with previous studies using lesions to examine the role of the NAc on the expression of sensitization to Amph and cocaine [32,36,41,70]. In a recent study examining cocaine, animals with electrolytic lesions to the NAc experienced attenuation to cocaine-induced locomotor activity following a re-challenge dose, while a sham-lesioned group remained sensitized [70]. Similar studies using selective chemical lesions of the NAc produced by 6-hydroxydopamine, reported that the destruction of NAc DA nerve terminals abolished both cocaine and Amph-induced locomotor sensitization in rats [32,36,41]. An alternative explanation for this finding is that the exaggerated acute effect of MPD in NAc lesioned animals may have contributed to a ceiling effect, in which additional repetitive MPD administration could not further increase locomotor activity.
During the washout phase, both the intact control group and NAc lesion group exhibited significant increases in locomotor activity on experimental day 13 (first day of washout; table 1) compared to experimental day 6 (saline injection). However, this increase in activity was significantly amplified in lesion animals. This increase in locomotion indicates that the animals were waiting to obtain the drugs, i.e. anticipation or withdrawal as a result of abrupt MPD administration [2,48]. Moreover, this increase in activity in the washout phase indicates that the NAc may be partly involved in attenuation of withdrawal behavior.
Several studies have used lesions to examine the role of the NAc on the expression of sensitization to various psychostimulants such as Amph and cocaine. To our knowledge, there have been no studies examining the role of the NAc on the expression of behavioral sensitization to MPD. In conclusion, the bilateral electrolytic lesions of the NAc had no effect on baseline locomotor activity, but the effect of acute MPD injection was intensified in the NAc lesion animals. Moreover, the induction of behavioral sensitization was prevented in NAc lesion animals. This finding suggests that MPD functions similarly to commonly abused psychostimulants Amph and cocaine. However, more experiments are needed in order to determine whether its risk potential in humans outweighs the benefits obtained from its use in ADHD patients.
Acknowledgments
The authors wish to thank Mallinckrodt, Inc. for the gift of MPD. This study was supported in part by the Pat Rutherford Chair in Psychiatry and NIH R01 DA027222 grant.
Footnotes
Conflict of Interest
The authors declare that they have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Accardo P, Blondis TA. What’s all the fuss about Ritalin? J Pediatr. 2001;138:6–9. doi: 10.1067/mpd.2001.111505. [DOI] [PubMed] [Google Scholar]
- 2.Algahim MF, Yang PB, Wilcox VT, Burau KD, Swann AC, Dafny N. Prolonged methylphenidate treatment alters the behavioral diurnalactivity pattern of adult male Sprague-Dawley rats. Pharmacol Biochem Behav. 2009;92:93–99. doi: 10.1016/j.pbb.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 3.Askenasy E, Taber K, Yang P, Dafny N. Methylphenidate (Ritalin): Behavioral studies in the rat. Intern J Neuroscience. 2007;117:1–38. doi: 10.1080/00207450600910176. [DOI] [PubMed] [Google Scholar]
- 4.Barron E, Yang P, Swann AC, Dafny N. Adolescent and adult male spontaneous hyperactive rats (SHR) respond differently to acute and chronic methylphenidate (Ritalin) Intern J Neuroscience. 2009;119:40–58. doi: 10.1080/00207450802330546. [DOI] [PubMed] [Google Scholar]
- 5.Bell K, Kalivas PW. Context-specific cross sensitization between systemic cocaine and intra-accumbens AMPA infusion in rats. Psychopharmacology. 1996;127:377–383. doi: 10.1007/s002130050101. [DOI] [PubMed] [Google Scholar]
- 6.Biederman J, Wilens T, Mick E, Spencer T, Faraone SV. Pharmacotherapy of attention deficit/hyperactivity disorder reducer risk for substance use disorder. Pediatrics. 1999;104(2):e20. doi: 10.1542/peds.104.2.e20. [DOI] [PubMed] [Google Scholar]
- 7.Boeck CR, Marques VB, Valvassori SS, Constantino LC, Rosa DVF, Lima FF, Romano-Silva MA, Quevedo J. Early long-term exposure with caffeine induces cross-sensitization to methylphenidate with involvement of DARPP-32 in adulthood of rats. Neurochemistry International. 2009;55:318–322. doi: 10.1016/j.neuint.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 8.Bonate P, Swann A, Silverman P. Context-Dependent Cross-Sensitization between Cocaine and Amphetamine. Life Sciences. 1997;60:1–7. doi: 10.1016/s0024-3205(96)00591-7. [DOI] [PubMed] [Google Scholar]
- 9.Boye SM, Grant RJ, Clarke PB. Disruption of dopaminergic neurotransmission in nucleus accumbens core inhibits the locomotor stimulant effects of nicotine and d-amphetamine in rats. Neuropharmacology. 2001;40:792–805. doi: 10.1016/s0028-3908(01)00003-x. [DOI] [PubMed] [Google Scholar]
- 10.Bradberry C. Cocaine sensitization and dopamine mediation of cue effects in rodents, monkeys, and humans: areas of agreement, disagreement, and implications for addiction. Psychopharmacology. 2007;191:705–717. doi: 10.1007/s00213-006-0561-6. [DOI] [PubMed] [Google Scholar]
- 11.Brandon CL, Marinelli M, Baker LK, White FJ. Enhanced reactivity and vulnerability to cocaine following methylphenidate treatment in adolescent rats. Neuropsychopharmacol. 2001;25:651–661. doi: 10.1016/S0893-133X(01)00281-0. [DOI] [PubMed] [Google Scholar]
- 12.Brenhouse H, Montalto S, Stellar J. Electrolytic lesions of a discrete area within the nucleus accumbens shell attenuate the long-term expression, but not early phase, of sensitization to cocaine. Behav Brain Res. 2006;170(2):219–223. doi: 10.1016/j.bbr.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 13.Cadoni C, Solinas M, Chiara GD. Psychostimulant sensitization: differential changes in accumbal shell and core dopamine. Euro Journal of Pharm. 2000;388:69–76. doi: 10.1016/s0014-2999(99)00824-9. [DOI] [PubMed] [Google Scholar]
- 14.Cador M, Bjijou Y, Stinus L. Evidence of a complete independence of the neurobiological substrates for the induction and expression of behavioral sensitization to amphetamine. Neurosci. 1995;65:385–395. doi: 10.1016/0306-4522(94)00524-9. [DOI] [PubMed] [Google Scholar]
- 15.Carr DB, Sesack SR. Hippocampal afferents to the rat prefrontal cortex: synaptic targets and relation to dopamine terminals. J Comp Neurol. 1996;369:1–15. doi: 10.1002/(SICI)1096-9861(19960520)369:1<1::AID-CNE1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 16.Chiara GD, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci. 1988;85(14):5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiara GD. The role of dopamine in drug abuse viewed from the perspective of its role in motivation. Drug Alcohol Depend. 1995;38:95–137. doi: 10.1016/0376-8716(95)01118-i. [DOI] [PubMed] [Google Scholar]
- 18.Christie MJ, Bridge S, James LB, Beart PM. Excitotoxic lesions suggest an aspartatergic projecton from rat medial prefrontal cortex to ventral tegmental area. Brain Res. 1985;333:169–172. doi: 10.1016/0006-8993(85)90140-4. [DOI] [PubMed] [Google Scholar]
- 19.Cornish JL, Kalivas PW. Repeated cocaine administration into the rat ventral tegmental area produces behavioral sensitization to a systemic cocaine challenge. Behav Brain Res. 2001;126:205–209. doi: 10.1016/s0166-4328(01)00239-x. [DOI] [PubMed] [Google Scholar]
- 20.Dafny N, Yang P. The role of age, genotype, sex, and route of acute and chronic administration of methylphenidate: A review of its locomotor effects. Brain Res Bul. 2006;68:393–405. doi: 10.1016/j.brainresbull.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Delfs JM, Schreiber L, Kelley AE. Microinjection of cocaine into the nucleus accumbens elicits locomotor activation in the rat. J Neurosci. 1990;10:303–310. doi: 10.1523/JNEUROSCI.10-01-00303.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eckermann K, Beasley A, Yang P, Gaytan O, Swann A, Dafny N. Methylphenidate sensitization is modulated by valproate. Life Sci. 2001;69:47–57. doi: 10.1016/s0024-3205(01)01095-5. [DOI] [PubMed] [Google Scholar]
- 23.Gatley SJ, Volkow ND, Gifford AN, Fowler JS, Dewey SL, Ding YS, Logan J. Dopamine-transporter occupancy after intravenous doses of cocaine and methylphenidate in mice and humans. Psychopharmacology. 1999;146:93–100. doi: 10.1007/s002130051093. [DOI] [PubMed] [Google Scholar]
- 24.Gaytan O, Ghelani D, Martin S, Swann A, Dafny N. Dose response characteristics of methylphenidate on different indices of rats; locomotor activity at the beginning of the dark cycle. Brain Research. 1996;727:13–21. doi: 10.1016/0006-8993(96)00296-x. [DOI] [PubMed] [Google Scholar]
- 25.Gaytan O, Ghelani D, Martin S, Swann A, Dafny N. Sensitization to locomotor effects of methylphenidate in the rat. Life Sci. 1997;61:101–107. doi: 10.1016/s0024-3205(97)00598-5. [DOI] [PubMed] [Google Scholar]
- 26.Gaytan O, Yang P, Swann A, Dafny N. Diurnal differences in sensitization to methylphenidate. Brain Res. 2000;864:24–39. doi: 10.1016/s0006-8993(00)02117-x. [DOI] [PubMed] [Google Scholar]
- 27.Gaytan O, Sripada S, Dafny N. Blockade of sensitization to methylphenidate by MK-801: partial dissociation from motor effects. Neuropharmacology. 2001;40:298–309. doi: 10.1016/s0028-3908(00)00122-2. [DOI] [PubMed] [Google Scholar]
- 28.Goldman LS, Genel M, Bezman RJ, Slanetz PJ. Diagnosis and treatment of attention deficit hyperactivity disorder in children and adolescents. JAMA. 1998;279:1100–1107. doi: 10.1001/jama.279.14.1100. [DOI] [PubMed] [Google Scholar]
- 29.Henry D, White F. The persistence of behavioral sensitization to cocaine parallels enhanced inhibition of nucleus accumbens neurons. The Journal of Neuroscience. 1995;15:6287–6299. doi: 10.1523/JNEUROSCI.15-09-06287.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hooks MS, Jones GH, Liem BJ, Justice JB., Jr Sensitization and individual differences to IP amphetamine, cocaine, or caffeine following repeated intracranial amphetamine infusions. Pharmacol Biochem Behav. 1992;43:815–823. doi: 10.1016/0091-3057(92)90413-a. [DOI] [PubMed] [Google Scholar]
- 31.John CE, Jones SR. Voltammetric characterization of the effect of monoamine uptake inhibitors and releasers on dopamine and serotonin uptake in mouse caudateputamen and substantia nigra slices. Neuropharmacology. 2007;52:1596–1605. doi: 10.1016/j.neuropharm.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joyce EM, Stinus L, Iversen SD. Effect of injections of 6-OHDA into either nucleus accumbens septi or frontal cortex on spontaneous and drug-induced activity. Neuropharmacology. 1983;22:1141–1145. doi: 10.1016/0028-3908(83)90051-5. [DOI] [PubMed] [Google Scholar]
- 33.Kalivas PW, Weber B. Amphetamine injection into the ventral mesencephalon sensitizes rats to peripheral amphetamine and cocaine. J Pharmacol Exp Ther. 1988;245:1095–1102. [PubMed] [Google Scholar]
- 34.Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- 35.Kalivas PW, Sorg BA, Hooks MS. The pharmacology and neural circuitry of sensitization to psychostimulants. Behav Pharmacol. 1993;4:315–334. [PubMed] [Google Scholar]
- 36.Kelly PH, Iversen SD. Selective 6OHDA-induced destruction of mesolimbic dopamine neurons: abolition of psychostimulant-induced locomotor activity in rats. European Journal of Pharmacology. 1976;40:45–56. doi: 10.1016/0014-2999(76)90352-6. [DOI] [PubMed] [Google Scholar]
- 37.Kelsey JE, Willmore EJ. Electrolytic Lesions of the Nucleus Accumbens Enhance Locomotor Sensitization to Nicotine in Rats. Behavioural Neuroscience. 2006;120:600–611. doi: 10.1037/0735-7044.120.3.600. [DOI] [PubMed] [Google Scholar]
- 38.Kelsey JE, Gerety LP, Guerriero RM. Electrolytic lesions of the nucleus accumbens core (but not the medial shell) and the basolateral amygdala enhance context-specific locomotor sensitization to nicotine in rats. Behav Neurosci. 2009;123:577–588. doi: 10.1037/a0015573. [DOI] [PubMed] [Google Scholar]
- 39.Kim J, Pollak K, Hjelmstad G, Fields H. A single cocaine exposure enhances both opioid reward and aversion through a ventral tegmental area-dependent mechanism. PNAS. 2004;101(15):5664–5669. doi: 10.1073/pnas.0401373101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kolta M, Shreve P, Uretsky N. Effect of pretreatment with amphetamine on the interaction between amphetamine and dopamine neurons in the nucleus accumbens. Neuropharmacology. 1989;28:9–14. doi: 10.1016/0028-3908(89)90060-9. [DOI] [PubMed] [Google Scholar]
- 41.Koob GF, Stinus L, Moal ML. Hyperactivity and hypoactivity produced by lesions to the mesolimbic dopamine system. Behavioural Brain Research. 1981;3:341–359. doi: 10.1016/0166-4328(81)90004-8. [DOI] [PubMed] [Google Scholar]
- 42.Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- 43.Kuczenski R, Segal D. Effects of methylphenidate on extra-cellular dopamine, serotonin, and norepinephrine: comparison with amphetamine. J Neurochem. 1997;68:2032–2037. doi: 10.1046/j.1471-4159.1997.68052032.x. [DOI] [PubMed] [Google Scholar]
- 44.Kuczenski R, Segal D. Locomotor effects of acute and repeated threshold doses of amphetamine and methylphenidate: Relative roles of dopamine and norepinephrine. Journal of Pharmacology and Experimental Therapeutics. 2001;296:876–883. [PubMed] [Google Scholar]
- 45.Lambert NM, Hartsongh CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. J Learn Disabil. 1998;31:533–544. doi: 10.1177/002221949803100603. [DOI] [PubMed] [Google Scholar]
- 46.Laviola G, Wood R, Kuhn C, Francis R, Spear L. Cocaine Sensitization in Periadolescent and Adult Rats. The Journal of Pharmacology and Experimental Therapeutics. 1995;275:345–357. [PubMed] [Google Scholar]
- 47.Lee MJ, Swann AC, Dafny N. Methylphenidate sensitization is prevented by prefrontal cortex lesion. Brain Res. 2008;76:131–140. doi: 10.1016/j.brainresbull.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 48.Lee MJ, Yang PB, Wilcox VT, Burau KD, Swann AC, Dafny N. Does repetitive Ritalin injection produce long-term effects on SK female adolescent rats? Neuropharmacology. 2009;57:201–207. doi: 10.1016/j.neuropharm.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 49.Levin FR, Kleber HD. Attention-deficit hyperactivity disorder and substance abuse: relationships and implications for treatment. Harvard Rev Psychiatry. 1995;2:246–258. doi: 10.3109/10673229509017144. [DOI] [PubMed] [Google Scholar]
- 50.Lu W, Wolf ME. Repeated amphetamine administration alters AMPA receptor subunit expression in rat nucleus accumbens and medial prefrontal cortex. Synapse. 1999;32:119–131. doi: 10.1002/(SICI)1098-2396(199905)32:2<119::AID-SYN5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 51.Onogi H, Hozumi M, Nakagawasai O, Arai Y, Ishigaki S, Sato A, Furuta S, Niijima F, Tan-No K, Tadano T. Central administration of p-hydroxyamphetamine produces a behavioral stimulant effect in rodents: evidence for the involvement of dopaminergic systems. Psychopharmacology (Berl) 2010;208:323–331. doi: 10.1007/s00213-009-1734-x. [DOI] [PubMed] [Google Scholar]
- 52.Partridge B, Schenk S. Context-Independent Sensitization to the Locomotor-Activating Effects of Cocaine. Pharmacology Biochemistry and Behavior. 1999;63:543–548. doi: 10.1016/s0091-3057(99)00013-1. [DOI] [PubMed] [Google Scholar]
- 53.Paulson PE, Robinson TE. Sensitization to systemic amphetamine produces an enhanced locomotor response to a subsquent intra-accumbens amphetamine challenge in rats. Psychopharmacol. 1991;104:140–141. doi: 10.1007/BF02244569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2. Academic Press; Orlando: 1986. [Google Scholar]
- 55.Peoples L, Uzwiak A, Guyette F, West M. Tonic inhibition of single nucleus accumbens neurons in the rat: a predominant but not exclusive firing pattern induced by cocaine self-administration sessions. Neuroscience. 1998;86:13–22. doi: 10.1016/s0306-4522(98)00116-x. [DOI] [PubMed] [Google Scholar]
- 56.Perugini M, Vezina P. Amphetamine administered to the ventral tegmental area sensitizes rats to the locomotor effects of nucleus accumbens amphetamine. J Pharmacol Exp Ther. 1994;270:690–696. [PubMed] [Google Scholar]
- 57.Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pierce R, Kalivas P. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- 59.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 60.Russell V. The nucleus accumbens motor-limbic interface of the spontaneously hypertensive rat as studied in vitro by the superfusion slice technique. Neuroscience & Biobehavioral Reviews. 2000;24:133–136. doi: 10.1016/s0149-7634(99)00056-1. [DOI] [PubMed] [Google Scholar]
- 61.Schenk S, Davidson ES. Stimulant pre-exposure sensitizes rats and humans to the rewarding effects of cocaine. NIDA Res Monogr. 1998;169:56–82. [PubMed] [Google Scholar]
- 62.Schenk S, Izenwasser S. Pretreatment with methylphenidate sensitizes rats to the reinforcing effects of cocaine. Pharmacol Biochem Behav. 2002;72:651–657. doi: 10.1016/s0091-3057(02)00735-9. [DOI] [PubMed] [Google Scholar]
- 63.Segal DS, Mandell AJ. Long-term administration of d-amphetamine: progressive augmentation of motor activity and stereotypy. Pharmacol Biochem Behav. 1974;2:249–255. doi: 10.1016/0091-3057(74)90060-4. [DOI] [PubMed] [Google Scholar]
- 64.Sellings LH, Clarke PB. Segregation of Amphetamine Reward and Locomotor Stimulation between Nucleus Accumbens Medial Shell and Core. The Journal of Neuroscience. 2003;23:6295–6303. doi: 10.1523/JNEUROSCI.23-15-06295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sellings LH, McQuade LE, Clarke PB. Evidence for Multiple Sites within Rat Ventral Striatum Mediating Cocaine-Conditioned Place Preference and Locomotor Activation. J Pharmacol Exp Ther. 2006;317:1178–1187. doi: 10.1124/jpet.105.100339. [DOI] [PubMed] [Google Scholar]
- 66.Seymour CM, Wagner JJ. Simultaneous expression of cocaine-induced behavioral sensitization and conditioned place preference in individual rats. Brain Res. 2008;1213:57–68. doi: 10.1016/j.brainres.2008.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Solanto MV. Neuropsychopharmacological mechanisms of stimulant drug action in attention-deficit hyperactivity disorder: a review and integration. Behav Brain Res. 1998;94:127–152. doi: 10.1016/s0166-4328(97)00175-7. [DOI] [PubMed] [Google Scholar]
- 68.Tang A, Wanchoo SJ, Swann AC, Dafny N. Psychostimulant treatment for ADHD is modulated by prefrontal cortex manipulation. Brain Res Bull. 2009;80:353–358. doi: 10.1016/j.brainresbull.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 69.Tilson HA, Rech RH. Conditioned drug effects and absence of tolerance to d-amphetamine induced motor activity. Pharmacol Biochem Behav. 1973;1:149–153. [Google Scholar]
- 70.Todtenkopf MS, Carreiras R, Melloni RH, Jr, Stellar JR. The dorsomedial shell of the nucleus accumbens facilitates cocaine-induced locomotor activity during the induction of behavioral sensitization. Behavioural Brain Research. 2002;131:9–16. doi: 10.1016/s0166-4328(01)00352-7. [DOI] [PubMed] [Google Scholar]
- 71.Vallone D, Picetti R, Borrelli E. Structure and function of dopamine receptors. Neuroscience and Biobehavioral Reviews. 2000;24:125–132. doi: 10.1016/s0149-7634(99)00063-9. [DOI] [PubMed] [Google Scholar]
- 72.Vezina P, Stewart J. Amphetamine administered to the ventral tegmental area but not to the nucleus accumbens sensitizes rats to systemic morphine: lack of conditioned effects. Brain Res. 1990;516:99–106. doi: 10.1016/0006-8993(90)90902-n. [DOI] [PubMed] [Google Scholar]
- 73.Volkow ND, Wang GJ, Fowler JS, Fischman M, Foltin R, Abumrad NN, Gatley SJ, Logan J, Wong C, Gifford A, Ding YS, Hitzemann R, Pappas N. Methylphenidate and cocaine have similar in vivo potency to block dopamine transporters in the human brain. Life Sci. 1999;65:7–12. doi: 10.1016/s0024-3205(99)00225-8. [DOI] [PubMed] [Google Scholar]
- 74.Weiner I, Gal G, Rawlins JN, Feldon J. Differential involvement of the shell and core subterritories of the nucleus accumbens in latent inhibition and amphetamine-induced activity. Behav Brain Res. 1996;81:123–133. doi: 10.1016/s0166-4328(96)00051-4. [DOI] [PubMed] [Google Scholar]
- 75.White FJ, Hu XT, Henry DJ, Zhang X-F. Neurophysiological alterations in the mesocorticolimbic dopamine system with repeated cocaine administration. In: Hammper RP Jr, editor. The neurobiology of Cocaine. CRC Press; Boca Raton: 1995. pp. 99–119. [Google Scholar]
- 76.Wilens TE, Faraone SV, Biederman J, Gunawardoene S. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later stubstance abuse? A meta-analitic review of the literature. Pediatrics. 2003;111:179–185. doi: 10.1542/peds.111.1.179. [DOI] [PubMed] [Google Scholar]
- 77.Yang P, Swann A, Dafny N. NMDA receptor antagonist disrupts acute and chronic effects of methylphenidate. Physiol Behav. 2000;71:133–145. doi: 10.1016/s0031-9384(00)00318-8. [DOI] [PubMed] [Google Scholar]
- 78.Yang P, Singhal N, Modi G, Swann A, Dafny N. Effects of lithium chloride on induction and expression of methylphenidate sensitization. European Journal of Pharmacology. 2001;426:65–72. doi: 10.1016/s0014-2999(01)01213-4. [DOI] [PubMed] [Google Scholar]
- 79.Yang P, Swann A, Dafny N. Chronic pretreatment with methylphenidate induces cross-sensitzation with amphetamine. Life Sciences. 2003;73:2899–2911. doi: 10.1016/s0024-3205(03)00673-8. [DOI] [PubMed] [Google Scholar]
- 80.Yang PB, Amini B, Swann AC, Dafny N. Strain differences in the behavioral responses of male rats to chronically administered mthylphenidate. Brain Res. 2003;971:139–152. doi: 10.1016/s0006-8993(02)04240-3. [DOI] [PubMed] [Google Scholar]
- 81.Yang P, Swann A, Dafny N. Sensory-evoked potentials recordings from the ventral tegmental area, nucleus accumbens, prefrontal cortex, and caudate nucleus and locomotor activity are modulated in dose-response characteristics by methylphenidate. Brain Research. 2006;1073–1074:164–174. doi: 10.1016/j.brainres.2005.12.055. [DOI] [PubMed] [Google Scholar]
- 82.Yang PB, Swann AC, Dafny N. Chronic administration of methylphenidate produces neurophysiological and behavioral sensitization. Brain Res. 2007;1145:66–80. doi: 10.1016/j.brainres.2007.01.108. [DOI] [PMC free article] [PubMed] [Google Scholar]