Adding telaprevir to pegylated interferon plus ribavirin for treatment of acute genotype 1 hepatitis C virus infection in HIV-infected men significantly decreased treatment duration to 12 weeks and improved sustained virologic response rates to 84%.
Keywords: acute HCV, treatment, HIV infection, men who have sex with men, telaprevir
Abstract
Background. There is an international epidemic of hepatitis C virus (HCV) infection among human immunodeficiency virus (HIV)–infected men who have sex with men. Sustained virologic response (SVR) rates with pegylated interferon and ribavirin treatment are higher in these men during acute HCV than during chronic HCV, but treatment is still lengthy and SVR rates are suboptimal.
Methods. We performed a pilot study of combination therapy with telaprevir, pegylated interferon, and ribavirin in acute genotype 1 HCV infection in HIV-infected men. Men who were treated prior to the availability of, or ineligible for, telaprevir were the comparator group. The primary endpoint was SVR12, defined as an HCV viral load <5 IU/mL at least 12 weeks after completing treatment.
Results. In the telaprevir group, 84% (16/19) of men achieved SVR12 vs 63% (30/48) in the comparator group. Among men with SVR, median time to undetectable viral load was week 2 in the telaprevir group vs week 4 in the comparator group, and 94% vs 53% had undetectable viral loads at week 4. Most patients (81%) who achieved SVR in the telaprevir group received ≤12 weeks of treatment and there were no relapses after treatment. The overall safety profile was similar to that known for telaprevir-based regimens.
Conclusions. Incorporating telaprevir into treatment of acute genotype 1 HCV in HIV-infected men halved the treatment duration and increased the SVR rate. Larger studies should be done to confirm these findings. Clinicians should be alert to detect acute HCV infection of HIV-infected men to take advantage of this effective therapy and decrease further transmission in this epidemic.
(See the Editorial Commentary by Zeremski et al on pages 880–2.)
Hepatitis C virus (HCV) chronically infects an estimated 5.2 million people in the United States and 170 million people worldwide [1]. However, as most initial (“acute”) infections are completely asymptomatic, newly infected people are rarely identified. The importance of finding these newly HCV-infected people during the acute phase was made clear in the seminal paper by Jaeckel et al [2] that showed a nearly 100% sustained virologic response (SVR) rate using just 24 weeks of interferon alone, an SVR rate many times higher than that of chronically infected patients at that time, and with just half the duration of interferon [3]. We are now faced with an entirely new group of patients who are becoming HCV infected in the international epidemic of sexually transmitted HCV infection among human immunodeficiency virus (HIV)–infected men who have sex with men (MSM). Published cure rates after treatment of acute HCV in these men, using pegylated interferon (peg-IFN) plus ribavirin (RBV) for 24–48 weeks’ duration, are not as good as those of Jaeckel et al [2], ranging from 53% to 83% [4–15], but are clearly better than the 27%–40% success rates in treatment of chronic HCV in HIV-infected men [16, 17]. Still, even with 48 weeks of treatment in many of these studies of acute HCV, fewer than two-thirds of patients achieved SVR, leaving a large proportion of these men uncured, with the possibility of experiencing rapidly advancing liver disease [18–21] and of infecting others and further propagating the epidemic. With the commercial availability of telaprevir (TVR) in the United States, we hypothesized that its potent activity against genotype 1 HCV would allow us to further shorten the treatment period while also improving the SVR rate. We therefore undertook a study of a 12-week treatment course with a combination of TVR, peg-IFN, and RBV in HIV-infected MSM with newly acquired genotype 1 HCV.
METHODS
HIV-infected MSM suspected to have newly acquired HCV infection were referred to a practice (D.S.F.) within the Mount Sinai Medical Center through a network of HIV providers in the New York City area (New York Acute Hepatitis C Surveillance Network) as part of a broad investigation of newly acquired HCV infection among HIV-infected MSM [18–20, 22–24]. Written informed consent was obtained with approval of the Mount Sinai Institutional Review Board in accordance with the Helsinki Declaration of 1975, as revised in 2000, and the men were then screened for eligibility for the TVR treatment protocol. The eligibility criteria were (1) new alanine aminotransferase (ALT) elevation to >150 U/mL; (2) genotype 1 HCV viremia first detected within 6 months of the referral; and (3) enrollment between July 2011 and September 2012. The exclusions were (1) non–genotype 1 HCV; (2) antiretroviral (ARV) regimens contraindicated with TVR that could not be changed without jeopardizing HIV control; and (3) insurance that did not cover TVR. Reinfection after previous SVR was not an exclusion. All ineligible men were offered treatment with peg-IFN plus RBV (without TVR); those with genotype 1 HCV were included in the comparator group.
To ensure we would not treat men who had a reasonable chance of spontaneous clearance, those whose first measured HCV viral load (VL) was <4 log10 IU/mL or who had a ≥2 log10 IU/mL drop in VL in the first 4 weeks of observation had further repeated measurements of ALT and HCV VL drawn every 2 weeks for 12 weeks before initiating treatment.
The triple therapy regimen was TVR 750 mg by mouth every 8 hours, each dose taken with food containing at least 20 g of fat; plus RBV (standard weight-based dosing) by mouth in 1 or 2 daily doses; plus peg-IFN 180 μg subcutaneously weekly. Treatment duration was 12 weeks, assuming an HCV VL of <5 IU/mL at week 4 (rapid virologic response [RVR]). Treatment was stopped if HCV VL was >1000 IU/mL at week 4. Those whose ARV regimen contained efavirenz took TVR at a dose of 1125 mg by mouth every 8 hours. SVR12, defined as HCV VL <5 IU/mL at least 12 weeks after completing treatment, was the primary study endpoint. Secondary endpoints were time to HCV VL <5 IU/mL, proportion with RVR, and SVR24.
The allowed ARV medications for men treated with TVR were tenofovir, emtricitabine, lamivudine, raltegravir, ritonavir-boosted atazanavir, rilpivirine, and efavirenz; all others were excluded. Regimens containing excluded ARV medications were changed to allowed ARV medications as soon as possible after enrollment; changes were allowed up until the day of HCV treatment initiation.
HIV-infected MSM with acute genotype 1 HCV infection who were treated with the standard dual regimen of peg-IFN plus weight-based RBV between March 2008 and September 2012 were the comparator group. Response-guided therapy was used to determine duration of total treatment with peg-IFN + RBV based on the week of treatment that the HCV VL was first <5 IU/mL: (1) by week 6, 24 weeks; (2) between weeks 8 and 12, 36–48 weeks; and (3) after week 12, 60 to 72 weeks.
The HCV antibody test we used was a third-generation HCV enzyme immunoassay version 2.0 (Abbott Laboratories). The HCV VL tests were COBAS AmpliPrep/COBAS TaqMan HCV Test (Roche Diagnostics), lower limit of quantification (LLOQ) 43 IU/mL, lower limit of detection (LLOD) 7 IU/mL for genotype 1; and transcription-mediated amplification (TMA; Quest Diagnostics), both LLOQ and LLOD 5 IU/mL. All values below LLOQ in the real-time polymerase chain reaction (PCR) assay were confirmed using the TMA assay using the same blood sample. VL levels between LLOQ and LLOD in the real-time PCR assay are also referred to as unquantifiable, target detected (UTD), and VL levels below LLOD as unquantifiable, target not detected (UTND), as recently recommended [25]. The HCV genotype test was INNO-LiPAssay (Bayer Diagnostics). The HIV VL test was COBAS AmpliPrep/COBAS TaqMan HIV-1 Test, version 2.0 (Roche Diagnostics), LLOQ 20 copies/mL. IL28B single-nucleotide polymorphism rs12979860 C and T alleles were determined using an allele-specific primer extension assay measured with the Luminex LX 200 instrument (Molecular Pathology Laboratory, Mount Sinai Medical Center) or by real-time PCR with allele-specific TaqMan probes (LabCorp Raritan or Quest Diagnostics). As the IL28B polymorphism data were not yet published at the time we first started treating patients in the comparator group, this result is not available for 6 men.
RESULTS
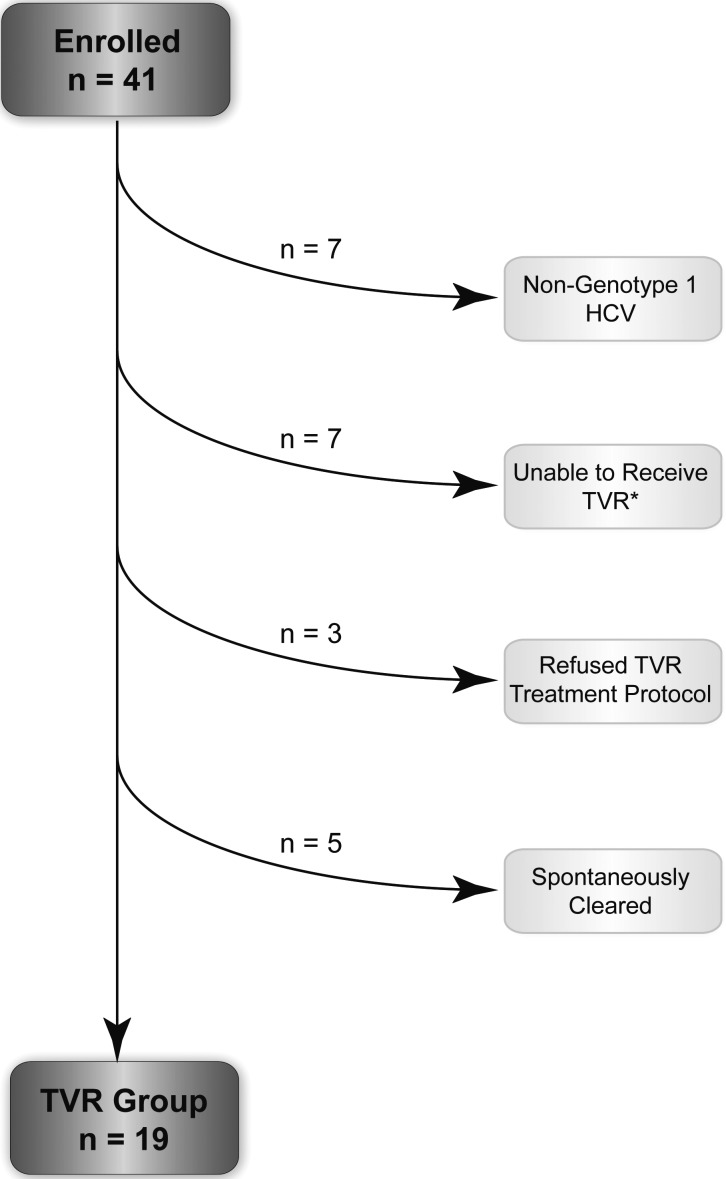
We evaluated 41 HIV-infected MSM with acute HCV at the Mount Sinai Medical Center between July 2011 and September 2012 for enrollment in the study. Thirty-seven (90%) were referred due to asymptomatic elevation of ALT found at routine HIV screening visits; 4 were jaundiced. Seven with non–genotype 1 HCV were excluded. Seven were unable to receive TVR; 6 had insurance that did not cover TVR, and 1 was receiving an ARV regimen that could not be changed without jeopardizing HIV control. These 7 were treated with peg-IFN + RBV and included in the comparator group. Three refused the treatment protocol; 2 refused all treatment and progressed to chronic infection, and 1 requested and received treatment immediately without monitoring for spontaneous clearance. Five spontaneously cleared HCV during the observation period (Figure 1). Of the 19 eligible men with acute genotype 1 HCV, 17 had primary HCV infection and 2 had reinfection after successful treatment (SVR24) with peg-IFN + RBV.
Figure 1.
Flowchart of disposition of human immunodeficiency virus–infected men with acute hepatitis C infection after enrollment. *All 7 men who were unable to receive telaprevir were treated as part of the comparator group. Abbreviations: HCV, hepatitis C virus; TVR, telaprevir.
We treated these 19 eligible men with TVR + peg-IFN + RBV (Table 1). Sixteen of 19 (84%) completed treatment with an HCV VL of <5 IU/mL (end of treatment response [ETR]). All 16 with ETR had SVR12, and all had SVR24; there were no relapses after ETR (Table 2).
Table 1.
Baseline Demographics of Telaprevir and Comparator Groups
| Characteristic | Telaprevir Group (n = 19) | Comparator Group (n = 48) |
|---|---|---|
| Age, y, median (IQR) | 44 (37–49) | 42 (34–45) |
| Race/ethnicity, No. (%) | ||
| White | 16 (84) | 28 (58) |
| Black | 1 (5) | 10 (21) |
| Hispanic | 2 (10) | 10 (21) |
| Duration of HIV, y, median (IQR) | 8 (4–12) | 9 (3–14) |
| CD4 count, cells/µL, median (IQR) | 680 (503–745) | 546 (364–764) |
| HCV genotype, No. (%) | ||
| 1a | 17 (89) | 43 (90) |
| 1b | 2 (11) | 5 (10) |
| IL28B genotype, No.a (%) | ||
| CC | 12 (63) | 18 (42) |
| CT + TT | 7 (37) | 25 (58) |
Abbreviations: HCV, hepatitis C virus; HIV, human immunodeficiency virus; IQR, interquartile range.
a IL28B genotype is available for 42 of the 48 acute HCV infections in the comparator group.
Table 2.
Characteristics and Treatment Results of HIV-Infected Men Who Have Sex With Men With Acute Hepatitis C Virus Infection Who had SVR12 in the Telaprevir and Comparator Groups
| Characteristic | Telaprevir Group SVR12 (n = 16) | Comparator Group SVR12 (n = 30) |
|---|---|---|
| Race/ethnicity, No. (%) | ||
| White | 15/16 (94) | 16/28 (57) |
| Black | 0/1 (0) | 6/10 (60) |
| Hispanic | 1/2 (50) | 8/10 (80) |
| HCV genotype, No. (%) | ||
| 1a | 15/17 (88) | 25/43 (58) |
| 1b | 1/2 (50) | 5/5 (100) |
| IL28B genotype, No.a (%) | ||
| CC | 12/12 (100) | 12/17 (71) |
| CT + TT | 4/7 (57) | 12/25 (48) |
| Time to HCV VL <5 IU/mL, TMA, wk, median (IQR) | 2 (1–3) | 4 (3–9) |
| Time to HCV VL <43 ND (UTND), real-time PCR, wk, median (IQR) | 3 (1–4) | NA |
| First HCV VL UTND in TMA but not real-time PCR assay, No.b (%) | 8/13 (62) | NA |
| RVR achieved, No. (%) | 15/16 (94) | 16/30 (53) |
Abbreviations: HCV VL, Hepatitis C viral load; IQR, interquartile range; NA, not applicable; ND, not detected; PCR, polymerase chain reaction; RVR, rapid virological response (UTND at week 4); SVR12, sustained virologic response at 12 weeks after completing treatment; TMA, transcription-mediated amplification; UTND, unquantifiable, target not detected.
a IL28B genotype was available for 24 of 30 men with SVR12 in the comparator group (see Table 1).
b Complete set of weekly HCV VL data were available for 13 of 16 men with SVR12 in the telaprevir group.
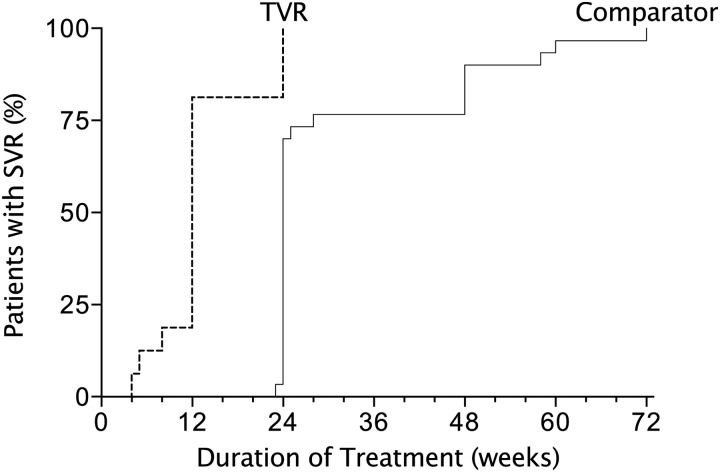
Six men with SVR12 received either shorter or longer treatment courses than the 12-week protocol. Three were successfully treated with shorter courses than 12 weeks: 2 discontinued all treatment due to adverse events after 4 and 5 weeks, and 1 stopped treatment after 8 weeks at his request. Three men received an additional 12 weeks of peg-IFN + RBV after the 12 weeks of triple therapy: 2 at the request of the men, and 1 due to slow initial VL decline (HCV VL <5 IU/mL at week 6 rather than week 4). Nonetheless, these treatment courses were much shorter than in those treated with the standard dual regimen of peg-IFN + RBV, which is described below (Figure 2).
Figure 2.
Duration of treatment in men with sustained virologic response at 12 weeks after treatment (SVR12) treated with telaprevir (n = 16; dashed line) or without telaprevir (pegylated interferon plus ribavirin only; n = 30; solid line). The cumulative percentage of men with SVR12 is shown by duration of treatment for each treatment group. Abbreviations: SVR, sustained virologic response; TVR, telaprevir.
Three (15%) men failed treatment. In 2 (genotype 1b, white, IL28B CT; and genotype 1a, black, IL28B TT), their viremia was never suppressed. The third (genotype 1a, Hispanic, IL28B CT) had HCV VL <5 IU/mL at week 3 with continued suppression through week 10 but had breakthrough viremia at week 12.
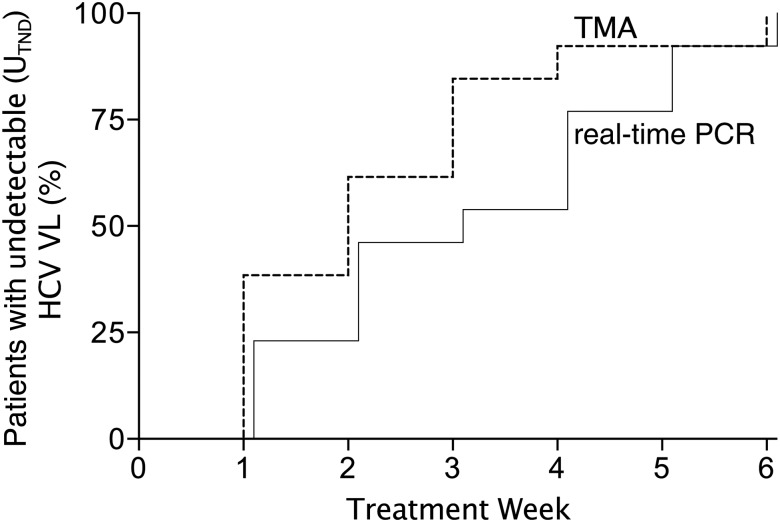
To assess the importance of having an undetectable VL (UTND) vs detectable but not quantifiable VL (UTD) at week 4 in determining the duration of therapy [26, 27], we investigated the concordance between 2 commercially available VL assays with different sensitivities, TMA and a Roche real-time PCR assay. Thirteen men with subsequent SVR12 had weekly HCV VL measurements until VL UTND in both assays, and 8 (62%) had discordant results: VL UTND using the more sensitive TMA assay occurred 1–2 weeks before the less sensitive real-time PCR assay (Table 2, Figure 3). As a result, 2 men did not have a VL UTND with the real-time PCR assay at the week 4 treatment visit but nonetheless were treated successfully with 12 weeks of triple therapy.
Figure 3.
Time to undetectable (target not detected) hepatitis C virus (HCV) viral load (VL) among men with sustained virologic response at 12 weeks after treatment (SVR12) comparing transcription-mediated amplification (TMA; dashed line) and real-time polymerase chain reaction (PCR; solid line) assays. Thirteen of the 16 men who had SVR12 had weekly HCV VL measurements using both TMA and real-time PCR assays. The cumulative percentage of men with undetectable HCV VL is shown by time for the 2 assays. Abbreviations: HCV VL, hepatitis C virus viral load; PCR, polymerase chain reaction; TMA, transcription-mediated amplification; UTND, unquantifiable, target not detected.
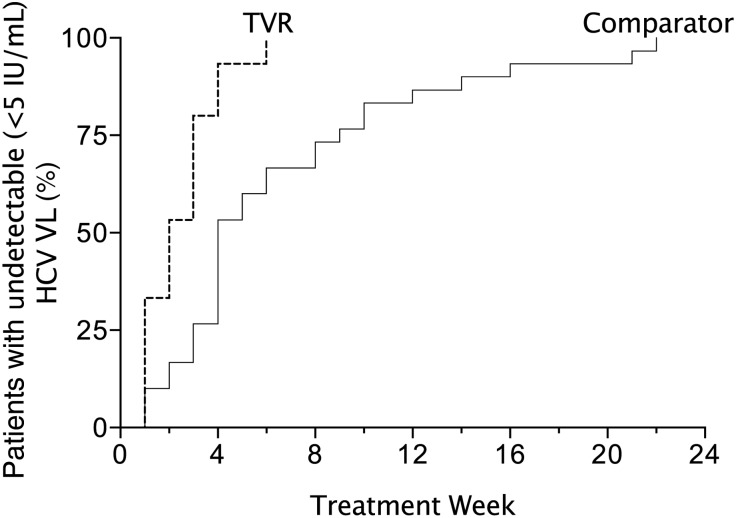
In the comparator group, we treated 48 men with acute HCV with the standard dual regimen of peg-IFN + RBV between March 2008 and January 2012 (Table 1), including 7 who could not receive TVR, as described above. Forty-six had primary HCV infection and 2 had reinfection after successful treatment (SVR24) with peg-IFN + RBV. SVR12 was achieved in 30 (63%) men. Twenty-three (77%) were treated for 23–28 weeks and 7 (23%) were treated for ≥48 weeks (Table 2, Figure 2). Time to HCV VL < 5 IU/mL was much longer than in men with SVR12 in the TVR group (Figure 4). Among the 18 (38%) with treatment failure, 10 never suppressed viremia, mostly with null responses; 3 had virological rebound during treatment after a period of complete virologic suppression; and 5 had virological relapse after the end of therapy. All 5 who had virological relapse had stopped their treatment at 24–33 weeks without completing the 36- to 48-week treatment recommended to them based on the response-guided treatment algorithm.
Figure 4.
Time to undetectable (target not detected) hepatitis C virus (HCV) viral load (VL) among men with sustained virologic response at 12 weeks after treatment (SVR12) treated with telaprevir (n = 16; dashed line) or without telaprevir (pegylated interferon plus ribavirin only; n = 30; solid line). The cumulative percentage of men with SVR12 who had undetectable HCV VL (transcription-mediated amplification assay, <5 IU/mL) is shown by time for each treatment group. Abbreviations: HCV VL, hepatitis C virus viral load; TVR, telaprevir.
Safety
Nine (47%) men had their ARV regimens changed during the observation period to allow use of TVR. None had loss of HIV viral suppression during the treatment or 12-week posttreatment periods.
Two men discontinued treatment early due to adverse events. One had his treatment discontinued after 4 weeks due to severe anemia (hemoglobin 6.6 g/dL) requiring transfusion, and in the 3 weeks after treatment he developed zoster and then skin abscess on his lower neck requiring hospitalization for drainage. Of note, less than one year before starting HCV treatment, he had received chemotherapy for stage 4 non-Hodgkin lymphoma, the duration of which had been reduced due to anemia and leukopenia. The other participant discontinued treatment after 5 weeks due to general intolerance of interferon. Both achieved SVR12.
Hemoglobin decline to <10 g/dL occurred in only 1 other man, and was managed by reduction in RBV dose. Two others had reduction in RBV dose as hemoglobin levels declined toward 10 g/dL. All 3 achieved SVR12. Generalized or solely localized rectal pruritus was reported by 18 (95%) of men, but only 2 (11%) developed a rash, in both cases mild.
Two men had rarer adverse events associated with interferon. One developed progressive numbness in his arms to his elbows and in his legs to his knees that resolved within 12 weeks after completing treatment. The other had an existing diagnosis of Raynaud disease and developed polyarticular arthralgias and prepatellar bursitis [28]. The latter responded to oral antibiotics and had resolved before the end of treatment, and the former improved significantly but remained more symptomatic than he had been before treatment.
DISCUSSION
Almost a decade after the first reports of treatment of acute HCV in HIV-infected men [4, 29], there is still no clear consensus about whether the duration of peg-IFN + RBV treatment should be 24 weeks or 48 weeks [7, 11, 12, 15]. Our response-guided therapy protocol when using peg-IFN + RBV without TVR allowed most men to receive only 24 weeks of dual therapy, but some who had a very slow virologic response needed 48 weeks or more. Even using response-guided therapy, our treatment success rate was only 63%. Adding TVR, however, cut the median time to complete suppression of viremia in half, increased the SVR rate to 84%, and significantly reduced the total duration of treatment to only 12 weeks in most men. The triple regimen was well tolerated by most, largely due to the much shorter exposure to peg-IFN.
Although these results are promising, this study has limitations. The study was performed in a single clinical practice, was small in size, and was not randomized. It did not address treatment duration in the TVR group and was not powered to detect superiority over the comparator group. It should be noted, however, that the currently accepted standard of treatment of these patients with peg-IFN + RBV was quickly adapted from just a few studies that were mostly done in single clinical practices, were small in size, were not randomized, and were not powered for statistical comparisons to other treatment protocols. Moreover, the benefits of adding TVR to peg-IFN + RBV in treating chronic HCV infection are established, both in increasing SVR rate in HIV-infected patients [30], and in decreasing treatment duration in most HIV-uninfected patients [31, 32].
Our study raises a few interesting questions about the role of the IL28B genotypes in acute HCV infection. The IL28B CC genotype appeared to be overrepresented in the TVR group (63%) compared to surveys of the general population without HCV infection [33] or in studies of patients with chronic HCV infection [34]. Patients presenting with acute HCV infection, however, are not representative of either the general population or those with chronic HCV infection, and available data suggest that the IL28B CC genotype is disproportionately higher in patients diagnosed with symptomatic or asymptomatic acute HCV infection. Studies in patients of European descent entering treatment for acute HCV have found the CC genotype in 63% (75 of 120) [35], 60% (59 of 98) [36], 58% (18 of 32) [37], and 47% (25 of 74) [38] of patients. Our TVR group was racially more similar to the European studies than what might be expected from New York City, probably accounting for some of the difference in the distribution of IL28B genotypes compared to our comparator group, which was closer to the expected racial mixture for New York City.
Nonetheless, the higher proportion of the CC genotype in our TVR-treated patients vs our comparator group could be in part responsible for the higher success rate that we achieved. Some with the CC genotype whom we treated might have spontaneously cleared, and there may be an advantage imparted by the CC genotype in the treatment of acute HCV as has been demonstrated in chronic HCV. We believe these considerations did not result in a skewing of our results, however. First, the spontaneous clearance rates of 12% in this study and of approximately 15% in our larger overall cohort (further data not shown) are similar to the very low rates in other studies of acute HCV infections in HIV-infected men [9, 10, 13, 15, 39]. We also allowed the standard initial 12-week observation period for spontaneous clearance, especially for those with large early virological declines, although this observation period was recently shown to be of no benefit in symptomatic patients with the CC genotype [36].
Second, the limited available evidence suggests that any increase in SVR rate associated with the CC genotype when using peg-IFN + RBV to treat acute HCV infection in HIV-infected men is small, with an SVR rate of 71% (25 of 35) in the CC genotype compared to 59% (23 of 39) in the CT + TT genotypes [38]. Our experience with using peg-IFN + RBV was similar, with the numerically higher SVR rate of 72% compared to 48% in those with CC and CT + TT genotypes, respectively. Furthermore, the strongest result from our study, the reduction in treatment duration to 12 weeks (or fewer) from 24 weeks (or more) with the addition of TVR, cannot be explained by the distribution of the IL28B genotypes in the TVR group. Taken together, the available data support that the IL28B genotype distribution in our study is approximately what would be expected in the group of men studied and would therefore not account for both the higher SVR rate and especially the much shorter 12-week treatment duration compared to our comparator group and similar cohorts in the published literature.
In conclusion, we treated 19 HIV-infected MSM with acute genotype 1 HCV infection with TVR + peg-IFN + RBV and achieved SVR12 in 84%, most strikingly with just 12 weeks or fewer of total therapy. Larger studies should be done to confirm these findings. Nonetheless, the addition of TVR appears to overall improve the treatment of acute genotype 1 HCV infection in HIV-infected men. IFN-free regimens will be available in just a few years that will cure most and eventually all HIV-infected patients with chronic HCV infection. This study, the first to test a direct-acting anti-HCV drug for acute HCV, is proof of principle that short courses for acute HCV using these soon-to-be-available new drugs should be evaluated quickly. But we believe that strong efforts should be made now to find and treat the men in this expanding sexually transmitted HCV epidemic using the currently available treatments including TVR, rather than waiting for these new treatments, echoing the call for “test and treat” in HIV [40] and similarly in HCV treatment in injection drug users [41], to try both to prevent liver disease in these men and to prevent further HCV infections and control this epidemic.
Notes
Acknowledgments. We thank the patients who enthusiastically participated in this clinical research.
New York Acute Hepatitis C Surveillance Network: Bisher Akil, MD; Juan Bailey, MD; Paul Bellman, MD; Daniel Bowers, MD; Krisczar Bungay, MD; Susanne Burger, MD; Ward Carpenter, MD; Robert Chavez, MD; Rita Chow, MD; Robert Cohen, MD; Patrick Dalton, MD; John Dellosso, MD; Adrian Demidont, DO; Stephen Dillon, MD; Eileen Donlon, NP; Terry Farrow, MD; Donald Gardenier, NP; Rodolfo Guadron, NP; Stuart Haber, MD; Lawrence Higgins, DO; Lawrence Hitzeman, MD; Ricky Hsu, MD; Shirish Huprikar, MD; Victor Inada, MD; Sneha Jacob, MD; Livette Johnson, MD; Barbara Johnston, MD; Donald Kaminsky, MD; Oscar Klein, MD; Jeffrey Kwong, NP; Jose Lares-Guia, MD; Eric Leach, NP; Randy Levine, MD; Irina Linetskaya, MD; Larisa Litvinova, MD; Amisha Malhotra, MD; William Mandell, MD; Martin Markowitz, MD; Gal Mayer, MD; Eddie Meraz, NP; Erik Mortensen, NP; Michel Ng, NP; Joseph Olivieri, MD; Charles Paolino, DO; Punyadech Photangtham, MD; George Psevdos, MD; Anita Radix, MD; Steven Rapaport, MD; Gabriela Rodriguez-Caprio, MD; William Shay, MD; Nirupama Somasundaram, NP; Lembitu Sorra, MD; Alicia Stivala, NP; Richie Tran, MD; Antonio Urbina, MD; Rona Vail, MD; Francis Wallach, MD; Wen Wang, MD; Susan Weiss, NP; and Melissa Wiener, MD.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (AI-50449 to D. S. F.), the National Institute on Drug Abuse (DA-031095 to A. D. B.), and the National Institute of Diabetes and Digestive and Kidney Diseases (DK-090317 to A. D. B.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Chak E, Talal AH, Sherman KE, Schiff ER, Saab S. Hepatitis C virus infection in USA: an estimate of true prevalence. Liver Int. 2011;31:1090–101. doi: 10.1111/j.1478-3231.2011.02494.x. [DOI] [PubMed] [Google Scholar]
- 2.Jaeckel E, Cornberg M, Wedemeyer H, et al. Treatment of acute hepatitis C with interferon alfa-2b. N Engl J Med. 2001;345:1452–7. doi: 10.1056/NEJMoa011232. [DOI] [PubMed] [Google Scholar]
- 3.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–82. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 4.Gilleece YC, Browne RE, Asboe D, et al. Transmission of hepatitis C virus among HIV-positive homosexual men and response to a 24-week course of pegylated interferon and ribavirin. J Acquir Immune Defic Syndr. 2005;40:41–6. doi: 10.1097/01.qai.0000174930.64145.a9. [DOI] [PubMed] [Google Scholar]
- 5.Luetkemeyer A, Hare CB, Stansell J, et al. Clinical presentation and course of acute hepatitis C infection in HIV-infected patients. J Acquir Immune Defic Syndr. 2006;41:31–6. doi: 10.1097/01.qai.0000191281.77954.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dominguez S, Ghosn J, Valantin MA, et al. Efficacy of early treatment of acute hepatitis C infection with pegylated interferon and ribavirin in HIV-infected patients. AIDS. 2006;20:1157–61. doi: 10.1097/01.aids.0000226956.02719.fd. [DOI] [PubMed] [Google Scholar]
- 7.Vogel M, Nattermann J, Baumgarten A, et al. Pegylated interferon-alfa for the treatment of sexually transmitted acute hepatitis C in HIV-infected individuals. Antivir Ther. 2006;11:1097–101. [PubMed] [Google Scholar]
- 8.Matthews GV, Hellard M, Haber P, et al. Characteristics and treatment outcomes among HIV-infected individuals in the Australian Trial in Acute Hepatitis C. Clin Infect Dis. 2009;48:650–8. doi: 10.1086/596770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schnuriger A, Dominguez S, Guiguet M, et al. Acute hepatitis C in HIV-infected patients: rare spontaneous clearance correlates with weak memory CD4 T-cell responses to hepatitis C virus. AIDS. 2009;23:2079–89. doi: 10.1097/QAD.0b013e328330ed24. [DOI] [PubMed] [Google Scholar]
- 10.Piroth L, Larsen C, Binquet C, et al. Treatment of acute hepatitis C in human immunodeficiency virus-infected patients: the HEPAIG study. Hepatology. 2010;52:1915–21. doi: 10.1002/hep.23959. [DOI] [PubMed] [Google Scholar]
- 11.Lambers FA, Brinkman K, Schinkel J, et al. Treatment of acute hepatitis C virus infection in HIV-infected MSM: the effect of treatment duration. AIDS. 2011;25:1333–6. doi: 10.1097/QAD.0b013e3283480144. [DOI] [PubMed] [Google Scholar]
- 12.Obermeier M, Ingiliz P, Weitner L, et al. Acute hepatitis C in persons infected with the human immunodeficiency virus (HIV): the “real-life setting" proves the concept. Eur J Med Res. 2011;16:237–42. doi: 10.1186/2047-783X-16-5-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomson EC, Fleming VM, Main J, et al. Predicting spontaneous clearance of acute hepatitis C virus in a large cohort of HIV-1-infected men. Gut. 2011;60:837–45. doi: 10.1136/gut.2010.217166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garg S, Taylor LE, Grasso C, Mayer KH. Prevalent and incident hepatitis C virus infection among HIV-infected men who have sex with men engaged in primary care in a Boston community health center. Clin Infect Dis. 2013;56:1480–7. doi: 10.1093/cid/cit054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Webster DP, Wojcikiewicz T, Keller M, et al. Spontaneous clearance and treatment of acute hepatitis C infection in HIV-positive men with 48 weeks of interferon-alpha and ribavirin. Int J STD AIDS. 2013;24:179–83. doi: 10.1177/0956462412472317. [DOI] [PubMed] [Google Scholar]
- 16.Chung RT, Andersen J, Volberding P, et al. Peginterferon alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med. 2004;351:451–9. doi: 10.1056/NEJMoa032653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torriani FJ, Rodriguez-Torres M, Rockstroh JK, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med. 2004;351:438–50. doi: 10.1056/NEJMoa040842. [DOI] [PubMed] [Google Scholar]
- 18.Fierer DS, Uriel AJ, Carriero DC, et al. Liver fibrosis during an outbreak of acute hepatitis C virus infection in HIV-infected men: a prospective cohort study. J Infect Dis. 2008;198:683–6. doi: 10.1086/590430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fierer DS, Mullen MP, Dieterich DT, Fiel MI, Branch AD. Early-onset liver fibrosis due to primary hepatitis C virus infection is higher over time in HIV-infected men. Clin Infect Dis. 2012;55:887–8. doi: 10.1093/cid/cis538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fierer DS, Dieterich DT, Fiel MI, et al. Rapid progression to decompensated cirrhosis, liver transplant, and death in HIV-infected men after primary hepatitis C virus infection. Clin Infect Dis. 2013;56:1038–43. doi: 10.1093/cid/cis1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bottieau E, Apers L, Van Esbroeck M, Vandenbruaene M, Florence E. Hepatitis C virus infection in HIV-infected men who have sex with men: sustained rising incidence in Antwerp, Belgium, 2001-2009. Euro Surveill. 2010;15:19673. [PubMed] [Google Scholar]
- 22.Cox AL, Page K, Bruneau J, et al. Rare birds in North America: acute hepatitis C cohorts. Gastroenterology. 2009;136:26–31. doi: 10.1053/j.gastro.2008.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fierer DS, Khudyakov Y, Hare B, et al. Molecular epidemiology of incident HCV infection in HIV-infected MSM in the US vs infections in Europe and Australia [abstract 112]. 18th Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2011. [Google Scholar]
- 24.Fierer DS, Uriel AJ, Carriero DC, et al. Sexual transmission of hepatitis C virus (HCV) among HIV-infected men who have sex with men (MSM), New York City, 2005-2010. JAMA. 2011;306:1194–6. [Google Scholar]
- 25.Wedemeyer H, Jensen DM, Godofsky E, Mani N, Pawlotsky JM, Miller V. Recommendations for standardized nomenclature and definitions of viral response in trials of hepatitis C virus investigational agents. Hepatology. 2012;56:2398–403. doi: 10.1002/hep.25888. [DOI] [PubMed] [Google Scholar]
- 26.Harrington PR, Zeng W, Naeger LK. Clinical relevance of detectable but not quantifiable hepatitis C virus RNA during boceprevir or telaprevir treatment. Hepatology. 2012;55:1048–57. doi: 10.1002/hep.24791. [DOI] [PubMed] [Google Scholar]
- 27.Lontok E, Mani N, Harrington PR, Miller V. Closing in on the target: sustained virologic response in hepatitis C virus genotype 1 infection response-guided therapy. Clin Infect Dis. 2013;56:1466–70. doi: 10.1093/cid/cit025. [DOI] [PubMed] [Google Scholar]
- 28.Burke CC, Martel-Laferriere V, Dieterich DT. Septic bursitis, a potential complication of protease inhibitor use in hepatitis C virus. Clin Infect Dis. 2013;56:1507–8. doi: 10.1093/cid/cit090. [DOI] [PubMed] [Google Scholar]
- 29.Vogel M, Bieniek B, Jessen H, et al. Treatment of acute hepatitis C infection in HIV-infected patients: a retrospective analysis of eleven cases. J Viral Hepat. 2005;12:207–11. doi: 10.1111/j.1365-2893.2005.00580.x. [DOI] [PubMed] [Google Scholar]
- 30.Sulkowski MS, Sherman KE, Dieterich DT, et al. Combination therapy with telaprevir for chronic hepatitis C virus genotype 1 infection in patients with HIV: a randomized trial. Ann Intern Med. 2013;159:86–96. doi: 10.7326/0003-4819-159-2-201307160-00654. [DOI] [PubMed] [Google Scholar]
- 31.McHutchison JG, Everson GT, Gordon SC, et al. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med. 2009;360:1827–38. doi: 10.1056/NEJMoa0806104. [DOI] [PubMed] [Google Scholar]
- 32.Sherman KE, Flamm SL, Afdhal NH, et al. Response-guided telaprevir combination treatment for hepatitis C virus infection. N Engl J Med. 2011;365:1014–24. doi: 10.1056/NEJMoa1014463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas DL, Thio CL, Martin MP, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798–801. doi: 10.1038/nature08463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 35.Beinhardt S, Aberle JH, Strasser M, et al. Serum level of IP-10 increases predictive value of IL28B polymorphisms for spontaneous clearance of acute HCV infection. Gastroenterology. 2012;142:78–85.e2. doi: 10.1053/j.gastro.2011.09.039. [DOI] [PubMed] [Google Scholar]
- 36.Deterding K, Gruner N, Buggisch P, et al. Delayed versus immediate treatment for patients with acute hepatitis C: a randomised controlled non-inferiority trial. Lancet Infect Dis. 2013;13:497–506. doi: 10.1016/S1473-3099(13)70059-8. [DOI] [PubMed] [Google Scholar]
- 37.Grebely J, Hellard M, Applegate T, et al. Virological responses during treatment for recent hepatitis C virus: potential benefit for ribavirin use in HCV/HIV co-infection. AIDS. 2012;26:1653–61. doi: 10.1097/QAD.0b013e3283553719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nattermann J, Vogel M, Nischalke HD, et al. Genetic variation in IL28B and treatment-induced clearance of hepatitis C virus in HIV-positive patients with acute and chronic hepatitis C. J Infect Dis. 2011;203:595–601. doi: 10.1093/infdis/jiq098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grebely J, Feld JJ, Applegate T, et al. Plasma interferon-gamma-inducible protein-10 (IP-10) levels during acute hepatitis C virus infection. Hepatology. 2013;57:2124–34. doi: 10.1002/hep.26263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Das M, Chu PL, Santos GM, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One. 2010;5:e11068. doi: 10.1371/journal.pone.0011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin NK, Vickerman P, Grebely J, et al. HCV treatment for prevention among people who inject drugs: modeling treatment scale-up in the age of direct-acting antivirals [epub 28 March] Hepatology. 2013 doi: 10.1002/hep.26431. doi:10.1002/hep.26431. [DOI] [PMC free article] [PubMed] [Google Scholar]