An acquired immune deficiency due to IFNγ autoantibodies was diagnosed in a 78-year-old Japanese man with treatment-refractory disseminated nontuberculous mycobacterial infection. In addition to standard antimycobacterial therapy, he was successfully treated with rituximab to eliminate B cells and thereby the autoantibody. Subsequently, he obtained a sustained remission from infection.
Keywords: nontuberculous mycobacteria, immune deficiency, interferon gamma, rituximab
Abstract
An acquired immune deficiency due to interferon gamma (IFN-γ) autoantibodies was diagnosed in a 78-year-old Japanese man with treatment-refractory disseminated nontuberculous mycobacterial infection. In addition to standard antimycobacterial therapy, he was successfully treated with rituximab to eliminate B cells and thereby the autoantibody. Subsequently, he obtained a sustained remission from infection.
Persons with immunodeficiency due to defects of the interleukin 12 (IL-12) and interferon gamma (IFN-γ) pathways can develop disseminated infection with nontuberculous mycobacteria (NTM). Such immunodeficiency syndromes can be heritable or due to acquired autoantibodies to IFN-γ [1]. Disseminated NTM infections in persons with anti-IFN-γ autoantibodies tend to be recalcitrant to treatment. Therapies directed at the autoantibody may supplement traditional antibiotics [2]. Herein, we describe an elderly man with persistent disseminated Mycobacterium abscessus infection due to the presence of autoantibodies to IFN-γ treated successfully with rituximab.
Case Report
A 78-year-old Japanese man from Guam presented with progressive cervical lymphadenopathy, cough, arthralgias, and weakness. Past medical history was notable for atherosclerosis, type 2 diabetes mellitus, chronic kidney disease, and previously treated localized prostate cancer. Notable laboratory findings included an erythrocyte sedimentation rate (ESR) of 112 mm/hour and a negative human immunodeficiency virus (HIV) antibody test. High-resolution chest computed tomography showed pulmonary fibrosis in a pattern typical of usual interstitial pneumonia. Cervical lymph node biopsy revealed multifocal abscesses with paracortical hyperplasia, reactive plasmacytosis, diffuse histiocytic infiltrates, and Epstein-Barr virus (EBV)–positive lymphocytes. Lymph node culture was positive for Mycobacterium chelonae–abscessus group. He was treated with azithromycin, imipenem-cilastatin, and tobramycin for 3.5 months with clinical improvement, then continued on azithromycin alone.
Within a few weeks, he developed axillary lymphadenopathy, neck pain, and a blistering rash on his hands. Axillary lymph node biopsy revealed nonnecrotizing granulomas with culture positive for M. abscessus. Magnetic resonance imaging (MRI) of the cervical spine showed C4–C6 diskitis and osteomyelitis. Skin biopsy was consistent with leukocytoclastic vasculitis. ESR was 122 mm/hour, antineutrophil cytoplasmic antibodies were negative, and there was no evidence of primary systemic vasculitis. Bone marrow was hypercellular with myeloid preponderance without granulomas or malignant cells. We suspected an acquired defect in the IL-12/IFN-γ pathway, and a comprehensive immunologic evaluation was initiated. He was treated with azithromycin, imipenem-cilastatin, tobramycin, and moxifloxacin with improvement in symptoms and resolution of MRI findings over several months, after which he continued taking azithromycin and moxifloxacin.
Over the next 2.5 years, he had 2 more recurrences of intrathoracic and axillary lymphadenopathy associated with exacerbation of leukocytoclastic vasculitis and elevated ESR. Prednisone was given for treatment of the leukocytoclastic vasculitis and titrated between 10 and 20 mg/day. During the first recurrence, he was found to have MRI evidence of marrow edema of the distal tibia that did not resolve 3 months later, suggesting infection vs traumatic injury. Multiple biopsies of mediastinal and axillary lymph nodes showed granulomatous and suppurative lymphadenitis, EBV-associated lymphoproliferation, and cultures positive for M. abscessus. His recurrences manifested when antibiotics were discontinued or narrowed, and he would respond to intensified intravenous antimycobacterial regimens. He was ultimately maintained on azithromycin, clofazimine, and prednisone. Prednisone taper was precluded by relapse of his skin disease.
During this period, he was treated for an episode of multidermatomal zoster (VZV), for an acute exacerbation of interstitial lung disease (ILD), and for cytomegalovirus (CMV) found on bronchoalveolar lavage in the setting of residual chest computed tomographic abnormalities (without cytopathic effect). Despite sporadic follow-up dispersed geographically between multiple providers, a diagnosis of anti-IFN-γ autoantibody was made. Rituximab was initiated once his acute infections were treated.
MATERIALS AND METHODS
Samples
Whole blood from patient and healthy controls was collected with informed consent, under an institutional review board–approved protocol. Samples for analysis included peripheral blood mononuclear cells (PBMCs) and plasma.
General Immune Assessment
General immune assessment included evaluation of T, B, and natural killer (NK) cells, PBMC proliferation in response to mitogenic or Toll-like receptor (TLR) stimulation, cytokine production (IL-12, tumor necrosis factor–α, IFN-γ, and interleukin 2), and surface expression of IFN-γ and IL-12 receptors.
Analysis of IFN-γ Neutralizing Activity
To assess patients’ plasma for evidence of IFN-γ neutralizing activity, we used flow cytometric measurement of phosphorylated signal transducer and activator of transcription 1α (pSTAT1α), as previously described [3]. Specificity of the response was determined by evaluation of pSTAT1α in the presence of IFN-α and patients’ plasma, and the identity of the neutralizing component was evaluated by purifying the immunoglobulin fraction from patients’ plasma and then testing the ability of immunoglobulin and immunoglobulin-depleted fractions to inhibit IFN-γ–induced phosphorylation of STAT1α.
Biological Significance of Anti–IFN-γ Antibodies
Normal PBMCs in complete medium (RPMI + 10% AB serum, 1× l-glutamine) were plated in triplicate at 1 × 106 cells/mL and incubated overnight at 37°C with 100 ng/mL IFN-γ or phosphate-buffered saline in the presence or absence of control or patient plasma (1:40 dilution). The cells were washed, infected with Listeria monocytogenes for 1 hour at 37°C, washed again, and cultured with complete media, 100 ng/mL IFN-γ, and 10 μg/mL gentamicin. Four and 7 hours after infection, the supernatant was removed and cells were washed twice and lysed with 500 µL pure distilled water. The lysate was serially diluted, cultured on Luria-Bertani agar plates, and incubated overnight at 37°C. Bacterial colonies were counted, and growth was quantified as the percentage of colonies harvested from IFN-γ–treated cells compared with untreated cells.
Rituximab Dosing
Rituximab was administered according to lymphoma dosing (375 mg/m2) with 100 mg methylprednisolone every 7 days × 4 doses.
RESULTS
Baseline Laboratory Findings
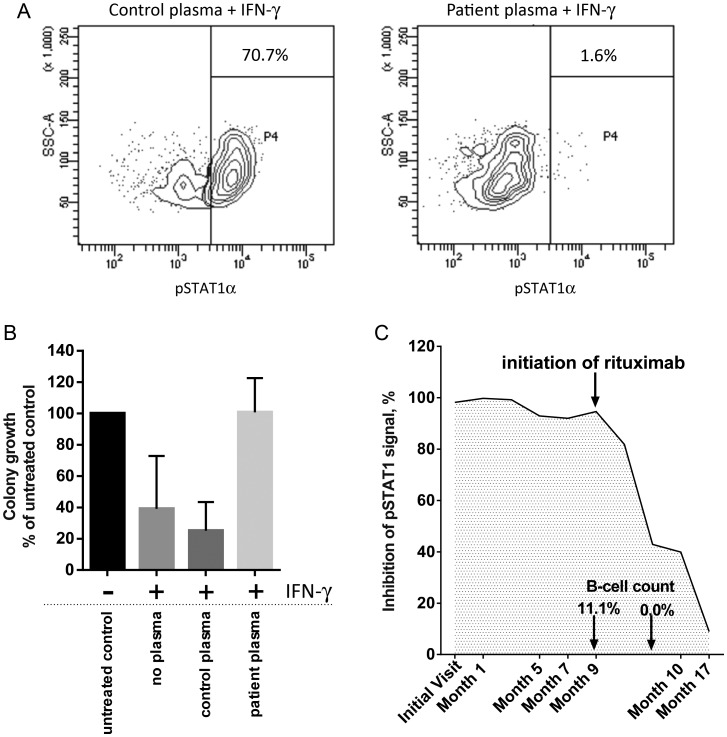
Assessment of lymphocyte percentages and function did not reveal significant abnormalities, with T, B, and NK cells within the expected normal ranges for age, responsive to mitogenic and TLR stimulation, and able to produce cytokines similar to normal controls. Expression of IFN-γ and IL-12 receptors was normal. Suspecting the presence of a neutralizing antibody to IFN-γ, we examined the ability of the patient's plasma to neutralize IFN-γ in vitro. In the presence of patients’ plasma, IFN-γ-stimulated PBMCs showed complete abrogation of STAT1α phosphorylation, indicating that the plasma contained a component that interfered with the IFN-γ signaling pathway (Figure 1A). pSTAT1α inhibition was specific to IFN-γ, as patients’ plasma did not affect phosphorylation of STAT1α in the presence of IFN-α (data not shown). The immunoglobulin fraction purified from plasma identified the component with neutralizing activity to be an antibody. Examination of the plasma of 20 patients with pulmonary NTM infection showed that none of these samples inhibited IFN-γ–induced phosphorylation of STAT1α (data not shown). Finally, we demonstrated that IFN-γ–treated PBMCs were unable to control L. monocytogenes infection in the presence of patients’ plasma (0% reduction in L. monocytogenes growth vs 70% reduction in the presence of normal plasma; Figure 1B).
Figure 1.
A, Flow cytometric measurement of phosphorylated STAT1α (pSTAT1α) in donor peripheral blood mononuclear cells (PBMCs) in the presence of interferon gamma (IFN-γ) and control (left panel) or patient plasma (right panel). Plots are gated on monocytes, and the percentage of pSTAT1α- positive monocytes is shown. B, Relative growth of Listeria monocytogenes in PBMCs. Data are presented as a percentage of the number of colony-forming units of L. monocytogenes isolated from PBMCs without any IFN-γ treatment and are the mean and standard deviation of 3 independent experiments. C, Monitoring neutralization of IFN-γ with patient plasma over time. Initiation of rituximab therapy followed by the loss of IFN-γ inhibition, as well as the elimination of B cells, as indicated. Abbreviations: IFN-γ, interferon gamma; pSTAT1α, phosphorylated signal transducer and activator of transcription 1α; SSC-A, side scattered light.
Clinical Response to Rituximab
Eight months after rituximab therapy, our patient had no systemic symptoms and no evidence of lymphadenitis or leukocytoclastic vasculitis, had reduced his prednisone dose to 4 mg daily, and experienced no side effects.
Post–Rituximab Treatment Laboratory Findings
At the end of the 4-week treatment with rituximab, circulating B cells were effectively eliminated (<1%), and the inhibition of IFN-γ signaling was reduced to approximately 30%–40% of pretreatment level (Figure 1C). Eight months after rituximab treatment was initiated, inhibition of IFN-γ signaling was not only sustained but reduced to approximately 10% of the initial inhibitory level (Figure 1C).
DISCUSSION
More than a decade ago, a clinical syndrome of (1) adult-onset disseminated lymphadenitis caused by rapidly growing NTM with progressive involvement of other organs, (2) coinfection with endemic fungi, herpes viruses, and salmonella, and (3) reactive skin disease was first described in HIV-uninfected patients from Thailand [4, 5]. This syndrome has now been associated with high-titer neutralizing antibodies to IFN-γ [1, 3, 6–11]. Anti– IFN-γ autoantibody syndrome predominantly affects persons of Asian descent and has been linked to certain human leukocyte antigen alleles [12].
Our patient's clinical phenotype suggested that he had an anti–IFN-γ autoantibody. He presented with relapsing lymphadenitis and suspected osteomyelitis due to M. abscessus group (differentiation from Mycobacterium massiliense and Mycobacterium bolletii was not routinely done). He also experienced herpes virus infections with EBV, VZV, and CMV, although the clinical significance of the EBV and CMV is unclear. As opposed to most other reported cases, he was older, was Japanese, had ILD, had less severe extralymphatic NTM infection, and did not experience fungal and bacterial opportunistic infections [1]. Notably, he had severe leukocytoclastic vasculitis that appeared coincident with recrudescence of his NTM infection. Prior reports have described Sweet syndrome, pustular psoriasis, exanthematous pustulosis, and erythema nodosum as reactive skin diseases [1].
Our patient demonstrated presence of an anti-IFN-γ autoantibody with significant neutralizing activity. In addition to abrogating the pSTAT1α response in vitro, we demonstrated that the autoantibody abolished IFN-γ–induced bactericidal ability of human PBMCs. These findings support the conclusion that anti–IFN-γ autoantibodies contribute to recalcitrant NTM infection by interfering with the IFN-γ–dependent immune response.
For patients with anti–IFN-γ autoantibody syndrome with disseminated infections, administration of IFN-γ, immune globulin, and plasmapheresis have been reported to be unsuccessful [2, 6, 10]. Browne et al documented a reduction in autoantibody titer, improvement in IFN-γ signaling, and clinical remission in response to rituximab [2]. We observed a similar positive response in our patient at 8 months. Because rise in IFN-γ autoantibody titer and subsequent relapse of infection can occur, we plan to monitor autoantibody levels prospectively in our patient and retreat as necessary.
This case demonstrates that anti–IFN-γ autoantibodies can present late in life with disseminated NTM infection and reactive leukocytoclastic vasculitis. Treatment of disseminated M. abscessus requires aggressive combination antimycobacterial therapy maintained until immune restoration is achieved. Our patient's favorable response to rituximab adds to accumulating evidence that rituximab may be effective treatment for the autoantibody.
Notes
Acknowledgments. The authors thank the staff of The Advanced Diagnostic Laboratories for technical assistance with this study.
Potential conflicts of interest. C. A. C. is a subinvestigator for studies funded by Pharmaxis, Bayer Healthcare, Insmed, and Gilead Sciences. S. G. is currently employed by Teva Respiratory. M. L. W. was formerly employed by Lovelace Respiratory Research Institute. P. A. M. was formerly employed by Amgen. S. K. F. and R. A. have received grant support from the National Institutes of Health. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Browne SK, Burbelo PD, Chetchotisakd P, et al. Adult-onset immunodeficiency in Thailand and Taiwan. New Engl J Med. 2012;367:725–34. doi: 10.1056/NEJMoa1111160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Browne SK, Zaman R, Sampaio EP, et al. Anti-CD20 (rituximab) therapy for anti-IFN-gamma autoantibody-associated nontuberculous mycobacterial infection. Blood. 2012;119:3933–9. doi: 10.1182/blood-2011-12-395707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel SY, Ding L, Brown MR, et al. Anti-IFN-gamma autoantibodies in disseminated nontuberculous mycobacterial infections. J Immunol. 2005;175:4769–76. doi: 10.4049/jimmunol.175.7.4769. [DOI] [PubMed] [Google Scholar]
- 4.Chetchotisakd P, Mootsikapun P, Anunnatsiri S, et al. Disseminated infection due to rapidly growing mycobacteria in immunocompetent hosts presenting with chronic lymphadenopathy: a previously unrecognized clinical entity. Clin Infect Dis. 2000;30:29–34. doi: 10.1086/313589. [DOI] [PubMed] [Google Scholar]
- 5.Chetchotisakd P, Kiertiburanakul S, Mootsikapun P, Assanasen S, Chaiwarith R, Anunnatsiri S. Disseminated nontuberculous mycobacterial infection in patients who are not infected with HIV in Thailand. Clin Infect Dis. 2007;45:421–7. doi: 10.1086/520030. [DOI] [PubMed] [Google Scholar]
- 6.Doffinger R, Helbert MR, Barcenas-Morales G, et al. Autoantibodies to interferon-gamma in a patient with selective susceptibility to mycobacterial infection and organ-specific autoimmunity. Clin Infect Dis. 2004;38:e10–4. doi: 10.1086/380453. [DOI] [PubMed] [Google Scholar]
- 7.Hoflich C, Sabat R, Rosseau S, et al. Naturally occurring anti-IFN-gamma autoantibody and severe infections with Mycobacterium cheloneae and Burkholderia cocovenenans. Blood. 2004;103:673–5. doi: 10.1182/blood-2003-04-1065. [DOI] [PubMed] [Google Scholar]
- 8.Kampitak T, Suwanpimolkul G, Browne S, Suankratay C. Anti-interferon-gamma autoantibody and opportunistic infections: case series and review of the literature. Infection. 2011;39:65–71. doi: 10.1007/s15010-010-0067-3. [DOI] [PubMed] [Google Scholar]
- 9.Kampmann B, Hemingway C, Stephens A, et al. Acquired predisposition to mycobacterial disease due to autoantibodies to IFN-gamma. J Clin Invest. 2005;115:2480–8. doi: 10.1172/JCI19316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koya T, Tsubata C, Kagamu H, et al. Anti-interferon-gamma autoantibody in a patient with disseminated Mycobacterium avium complex. J Infect Chemother. 2009;15:118–22. doi: 10.1007/s10156-008-0662-8. [DOI] [PubMed] [Google Scholar]
- 11.Poulin S, Corbeil C, Nguyen M, et al. Fatal Mycobacterium colombiense/cytomegalovirus coinfection associated with acquired immunodeficiency due to autoantibodies against interferon gamma: a case report. BMC Infect Dis. 2013;13:24. doi: 10.1186/1471-2334-13-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chi CY, Chu CC, Liu JP, et al. Anti-IFN-gamma autoantibodies in adults with disseminated nontuberculous mycobacterial infections are associated with HLA-DRB1*16:02 and HLA-DQB1*05:02 and the reactivation of latent varicella-zoster virus infection. Blood. 2013;121:1357–66. doi: 10.1182/blood-2012-08-452482. [DOI] [PubMed] [Google Scholar]