Outpatient parenteral antibiotic therapy (OPAT) is a mainstay of current medical therapy. We developed a 30-day readmission prediction model comprised of age, prior admissions, resistant organisms, and aminoglycoside use. Future work should target OPAT patients at high risk of readmission.
Keywords: OPAT, readmission, aminoglycoside, drug-resistant organisms, predictive model
Abstract
Background. Factors associated with readmission for patients prescribed outpatient parenteral antibiotic therapy (OPAT) at hospital discharge have not been definitively identified. The study aim was to develop a model of 30-day readmissions for OPAT patients.
Methods. A database comprising 782 OPAT patients treated between 2009 and 2011 at a single academic center was created. Variables collected included patient demographics, comorbidities, infections, and antibiotic classes. Final model discrimination was assessed using the c-statistic, and calibration was examined graphically.
Results. Mean patient age was 58 years (range, 18–95 years), 43% were women, and the most common diagnoses were bacteremia (24%), osteomyelitis (20%), and pyelonephritis (13%). The unplanned 30-day readmission rate was 26%. The leading indications for readmission were non–infection related (30%), worsening infection (29%), and new infection (19%). The final regression model consisted of age (odds ratio [OR], 1.09 per decade; 95% confidence interval [CI], 0.99–1.21), aminoglycoside use (OR, 2.33; 95% CI, 1.17–4.57), resistant organisms (OR, 1.57; 95% CI, 1.03–2.36), and number of prior hospital discharges without intravenous antibiotics in the past 12 months (OR, 1.20 per prior admission; 95% CI, 1.09–1.32). The c-statistic was 0.61 and the highest-risk quintile of patients had almost a 3-fold higher rate of readmission compared to the lowest.
Conclusions. Patients prescribed OPAT are at risk for readmission. A subgroup of patients at especially high risk can be identified using easily obtainable clinical characteristics at the time of hospital discharge. More intensive interventions to prevent OPAT readmissions should be targeted and tested with those at highest risk.
Outpatient intravenous antibiotic therapy (OPAT) allows patients to receive intravenous treatment at home rather than remaining hospitalized for the duration of their therapy. Since its inception >30 years ago, OPAT has grown to serve approximately 250 000 persons in the United States annually [1]. Multiple studies have demonstrated that OPAT is safe, effective, cost-saving, and preferred by patients over inpatient care [2–7].
Despite the benefits of OPAT, the delivery of potentially toxic therapies outside the acute hospital setting has the potential for complications including hospital readmissions. Thirty-day readmissions are currently receiving particularly intense scrutiny and have been proposed as healthcare quality markers. Patient outcomes with OPAT could be further improved if rates of unplanned readmissions could be predicted and reduced. However, to our knowledge, no predictive models for readmissions have been developed to date for OPAT patients.
The aim of this study was to identify patient and healthcare system factors that might be associated with an increased risk of readmission for patients discharged on OPAT and to develop a predictive model of all-cause unplanned 30-day hospital readmission for these patients.
METHODS
Participants
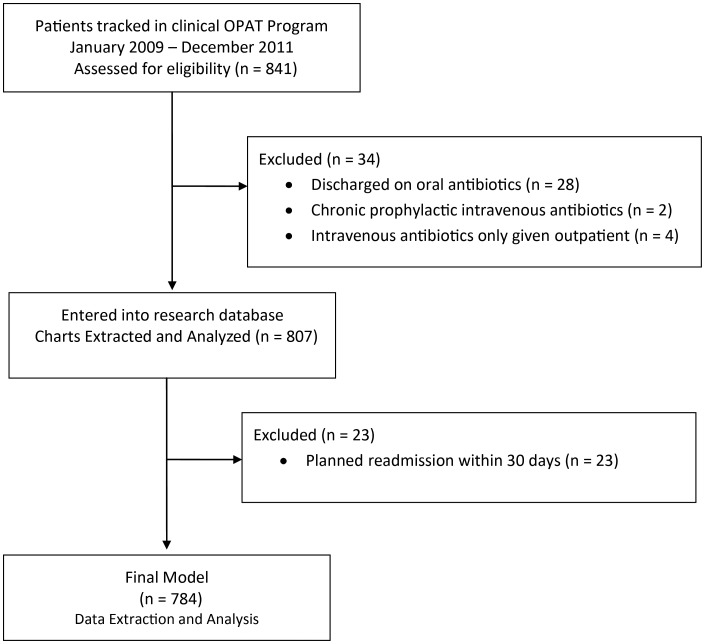
The OPAT Research Cohort comprises 782 patients aged 18 years and older who were discharged from Tufts Medical Center (Boston, Massachusetts) with intravenous antibiotics and followed in the center's clinical OPAT program from January 2009 to December 2011 (Figure 1). Patients who initiated intravenous antibiotics as outpatients, or who were prescribed intravenous antibiotics as chronic suppression, were not included in the study. Patients discharged with intravenous antibiotics who (at the discretion of the primary physician) were not followed in the clinical OPAT program were also excluded. This research was approved by the Institutional Review Board of Tufts Medical Center/Tufts University Health Sciences Campus.
Figure 1.
Flow diagram. Abbreviation: OPAT, outpatient parenteral antibiotic therapy.
OPAT Program
The Tufts Medical Center clinical OPAT program was developed in 2008, based on published OPAT guidelines [1], and formally began in 2009. Its aim is to improve the transitions from inpatient to outpatient settings for patients discharged with intravenous antibiotics. Patients enter the OPAT program starting with inpatient infectious disease (ID) consultation, which is strongly encouraged for all discharges with parenteral antibiotic therapy. More than 90% of patients discharged with intravenous antibiotic therapy are followed in the OPAT program. After hospital discharge, patients are followed in a single ID clinic by an ID specialist. An OPAT administrator provides care coordination among visiting nurses, outside laboratories, and outpatient specialty infusion pharmacists. Patients are seen within 1–2 weeks of hospital discharge, with future visit frequency determined by their attending outpatient ID physician. Weekly laboratory studies (eg, complete blood count, liver enzymes, serum creatinine, blood urea nitrogen, serum creatine phosphokinase, drug peak/trough levels) are obtained depending on antibiotic(s) utilized, in accordance with published guidelines [1]. Additional laboratory and imaging studies are added at the discretion of the attending physician.
Data Collection Activities
Patient data were abstracted from medical charts into a secure electronic relational database using REDCap (Research Electronic Data Capture) [8]. Data collected included sociodemographic factors (age, social support, insurance status), measures of healthcare utilization (length of stay, prior hospital admissions for any cause over past 12 months), ID diagnoses, specific antibiotics used, type of intravenous access, primary service, past medical history, and comorbidities at time of hospital admission.
Study Design
The study utilized a retrospective cohort design. The index admission was defined as the first episode of hospitalization resulting in discharge with OPAT, occurring from 1 January 2009 to 31 December 2011. Each participant's records were examined to ensure that they had no prior admissions resulting in discharge with OPAT. The primary outcome was 30-day readmission, which was defined as unplanned hospitalization, at the study institution, for any cause, within 30 days of the index admission's discharge date.
Variables
Patient's age (years), hospital length of stay (days), and number of prior non-OPAT admissions was recorded. Intravenous antibiotics were recoded and analyzed by antibiotic class. History of any drug-resistant organisms included methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, or gram-negative bacteria with expanded-spectrum β-lactamases. Categories of infectious diagnoses were based on previously published work [7]. Diagnoses were not exclusive—that is, a patient with endocarditis and vertebral osteomyelitis would be scored with 2 diagnoses. Primary bacteremia was scored as a separate diagnosis. Each diagnosis was entered into the model as a binary variable. A modified Charlson comorbidity score was calculated for each patient [9]. A history of immunosuppression was recorded and was defined as history of any of following: human immunodeficiency virus/AIDS, cancer, solid organ transplant, or bone marrow transplantation. Vascular access was categorized as PICC (peripherally inserted central catheter) vs other (ie, tunneled catheter, dialysis catheter, dialysis fistula, or port). Primary clinical team was also recorded and categorized as surgery, medicine, or ID. Outcome data and readmission diagnoses were manually abstracted from charts.
Statistical Analysis
Descriptive characteristics were summarized using means and standard deviations for normally distributed variables and medians and interquartile ranges for skewed data. Clinical and demographic characteristics were compared between readmitted and nonreadmitted patients using the Student t, Wilcoxon rank-sum, Pearson χ2, and Fisher exact tests, as appropriate. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using logistic regression. Variables achieving statistical significance P < .2 were candidates for the multivariable model. Age was included in all multivariable models by a priori decision since advancing age has been shown to be significantly associated with hospital readmissions [10, 11]. Candidate variables were selected for final model inclusion using backwards selection with Akaike information criterion (AIC) [12]. Although Charlson comorbidity score and site of postacute care (ie, home vs facility) did not meet predetermined statistical significance, they were also included in the final model as a sensitivity analysis because of their potential clinical significance to readmissions. These variables were also subjected to backward selection by AIC. Variable selection was followed by analysis of leverage and influence points as well as accounting for covariate overestimation [13]. The final model's performance was characterized using c-statistic [14], calibration curves, and Hosmer-Lemeshow goodness-of-fit [15]. Four subjects were removed for high influence by Cook's distance [16]. A bootstrapping algorithm was used for internal validation of the model [17]. Statistical analyses used R Statistical Program, version 2.13.1 (updated 22 July 2011, copyright R Foundation, from http://www.r-project.org).
RESULTS
Study Population Characteristics
Study inclusion and exclusion details are presented in Figure 1. Demographic and clinical characteristics of the cohort are shown in Table 1, and antibiotics and diagnoses in Table 2. The mean age of the study population was 58 years (range, 18–95 years), and 43% were women. The primary discharge diagnoses were bacteremia (24%), osteomyelitis (20%), pyelonephritis (13%), and intra-abdominal infections (11%). Discharge diagnoses were not exclusive. Patients discharged home had a similar rate of readmission as patients sent to a facility (ie, skilled nursing facility or rehabilitation; 28% vs 24%, P = .24).
Table 1.
Demographic and Clinical Characteristics of the Study Cohort (N = 782)
| Characteristic | Readmitted (n = 207) |
Not Readmitted (n = 575) |
P Value | ||
|---|---|---|---|---|---|
| Demographics | |||||
| Age, y, mean (SD) | 61 | (15) | 58 | (16) | .06 |
| Male sex | 123 | 59% | 326 | 57% | .50 |
| Insurance | .71 | ||||
| Medicare | 93 | 45% | 245 | 43% | |
| Medicaid | 25 | 12% | 77 | 13% | |
| Private | 89 | 43% | 248 | 43% | |
| Self-pay | 0 | 0% | 5 | 1% | |
| Support status | .07 | ||||
| Lives alone, no support | 12 | 6% | 41 | 7% | |
| Lives alone, has support | 40 | 19% | 99 | 17% | |
| Lives with adult | 130 | 63% | 397 | 69% | |
| Missing | 25 | 12% | 38 | 7% | |
| Site of care | |||||
| Home | 117 | 56% | 296 | 51% | .24 |
| Facility (rehabilitation, skilled nursing facility) | 90 | 44% | 276 | 49% | |
| Comorbidities | |||||
| Charlson comorbidity score, median (IQR) | 2 | (1–3) | 2 | (0–3) | .34 |
| Diabetes mellitus without complications | 40 | 19% | 100 | 17% | .23 |
| Diabetes mellitus with complications | 31 | 15% | 64 | 11% | |
| No diabetes | 136 | 66% | 411 | 71% | |
| Renal disease, no dialysis | 38 | 18% | 92 | 16% | .36 |
| Renal disease, dialysis | 19 | 9% | 40 | 7% | |
| No renal disease | 150 | 72% | 443 | 77% | |
| History of drug-resistant organism | 44 | 21% | 86 | 15% | .04 |
| Immunocompromised | 52 | 25% | 180 | 31% | .10 |
| Healthcare utilization | |||||
| Oral antibiotic in addition to intravenous | 50 | 24% | 126 | 22% | .51 |
| Length of stay, d, median (IQR) | 7 | (4–10) | 6 | (4–10) | .68 |
| Prior admissions in past 12 mo, mean (SD) | 1.5 | (2.2) | 0.9 | (1.5) | <.001 |
| Peripherally inserted central catheter | 181 | 88% | 486 | 85% | .257 |
| Inpatient service | .160 | ||||
| Medicine | 97 | 47% | 233 | 41% | |
| Surgery | 75 | 36% | 213 | 37% | |
| Infectious disease ward | 35 | 17% | 129 | 22% | |
Data are presented as No. (%) unless otherwise specified.
Abbreviations: IQR, interquartile range; SD, standard deviation.
Table 2.
Antimicrobials and Infectious Disease Diagnoses of Study Cohort
| Antimicrobial/Diagnosis | Readmitted n = 207, No., % |
Not Readmitted n = 575, No., % |
P Value | Odds Ratio | 95% Confidence Interval | ||
|---|---|---|---|---|---|---|---|
| Antibiotic class | |||||||
| Cephalosporins | 53 | 26% | 146 | 25% | .95 | 1.01 | .70–1.45 |
| Carbapenems | 42 | 20% | 107 | 19% | .60 | 1.11 | .74–1.65 |
| Antistaphylococcal β-lactams | 21 | 10% | 83 | 14% | .12 | 0.67 | .39–1.09 |
| Fluoroquinolones | 21 | 10% | 42 | 7% | .20 | 1.43 | .81–2.46 |
| Daptomycin | 11 | 5% | 30 | 5% | .96 | 1.02 | .48–2.01 |
| Aminoglycoside | 16 | 8% | 23 | 4% | .03 | 2.01 | 1.02–3.86 |
| Synthetic nucleoside analogue antivirals | 9 | 4% | 28 | 5% | .76 | 0.89 | .39–1.84 |
| Antipseudomonal β-lactams | 7 | 3% | 24 | 4% | .62 | 0.80 | .32–1.80 |
| Azole antifungals | 11 | 5% | 18 | 3% | .15 | 1.74 | .35–1.74 |
| Metronidazole | 9 | 4% | 19 | 3% | .49 | 1.33 | .56–2.21 |
| Infectious disease diagnosis | |||||||
| Bacteremia | 61 | 29% | 129 | 22% | .04 | 1.44 | 1.01–2.06 |
| Osteomyelitis or septic arthritis of native joint | 39 | 19% | 120 | 21% | .53 | 0.88 | .58–1.31 |
| Pyelonephritis or urinary tract infection | 34 | 16% | 69 | 12% | .11 | 1.44 | .91–2.23 |
| Intra-abdominal | 22 | 11% | 64 | 11% | .84 | 0.95 | .56–1.56 |
| Endocarditis | 26 | 13% | 52 | 9% | .15 | 1.44 | .87–2.36 |
| Pneumonia | 27 | 13% | 47 | 8% | .04 | 1.68 | 1.01–2.77 |
| Cellulitis | 12 | 6% | 49 | 9% | .21 | 0.66 | .33–1.23 |
| Prosthetic joint infection | 14 | 7% | 45 | 8% | .62 | 0.85 | .44–1.55 |
| Sepsis | 10 | 5% | 28 | 5% | .98 | 0.99 | .45–2.01 |
| Central nervous system | 7 | 3% | 29 | 5% | .33 | 0.66 | .26–1.45 |
| Cardio/vascular device | 11 | 5% | 23 | 4% | .43 | 0.48 | .62–2.75 |
| Epidural abscess | 7 | 3% | 18 | 3% | .86 | 1.08 | .42–2.53 |
Antibiotics used in <20 subjects are not included in the table (No.): penicillin (19), echinocandins (19), trimethoprim-sulfamethoxazole (18), amoxicillin/ampicillin group (16), oral vancomycin (15), macrolides (14), linezolid (11), oral rifamycins (11), tigecycline (6), tetracycline group (4), amphotericins (1), atovaquone (1), macrodantin (1), and nonabsorbed oral antifungals (1). Infectious diseases diagnoses occurring in < 20 subjects are not included in the table (No.): septic shock (12), otolaryngeal (9), invasive fungal infection (9), diarrhea (5), myositis (3), babesiosis (1), Whipple disease (1), source unknown (1).
Twenty-six percent (207/782) of subjects were readmitted within 30 days. Readmitted subjects were slightly older (61 years vs 58 years, P = .06), had more previous hospital admissions in the past 12 months (1.5 vs 0.9, P < .001), and had a higher proportion with prior isolation of drug-resistant organisms (21% vs 15%, P = .037) compared to those not readmitted. As shown in Table 2, subjects receiving aminoglycosides had 2-fold higher crude odds of readmission compared with subjects who were not given this drug class. Immunosuppression was less likely to have been diagnosed among readmitted patients (25% vs 31%, P = .10). Median length of stay of index hospitalization was similar between groups (6 days for subjects subsequently readmitted, 7 days for not readmitted; interquartile range, 4–10 days for both). Reasons for readmission are presented in Table 3; the most common indications were non–ID related (30%), worsening infection (30%), and new infection (22%). Patients could have >1 reason for readmission.
Table 3.
Reasons for 30-Day Readmission (n = 207)
| Reason for 30-Day Readmission | No. (%) |
|---|---|
| Not related to infection | 63 (30) |
| Infection worsening | 62 (30) |
| New infection | 48 (22) |
| Adverse reaction to drug | 30 (14) |
| Intravenous line complication | 20 (10) |
| Missing | 4 (2) |
| Diarrhea | 2 (1) |
Multivariable Model
The following variables met our a priori inclusion criteria of significance level P < .2 and therefore were included in the initial multivariable model: age, support status, drug-resistant organism, history of immunosuppression, number of prior non-OPAT admissions in past 12 months, inpatient service, 3 antibiotic classes (antistaphylococcal β-lactams, fluoroquinolones, aminoglycosides) and 5 diagnosis categories (bacteremia, cellulitis, endocarditis, pneumonia, pyelonephritis). For patients prescribed aminoglycosides at discharge, nearly half (6/16) were readmitted for medication side effects (4 acute renal insufficiency, 1 rash, 1 both rash and neutropenia). Of the remainder, 4 were noninfectious causes, 3 were PICC related, and 3 were due to infections.
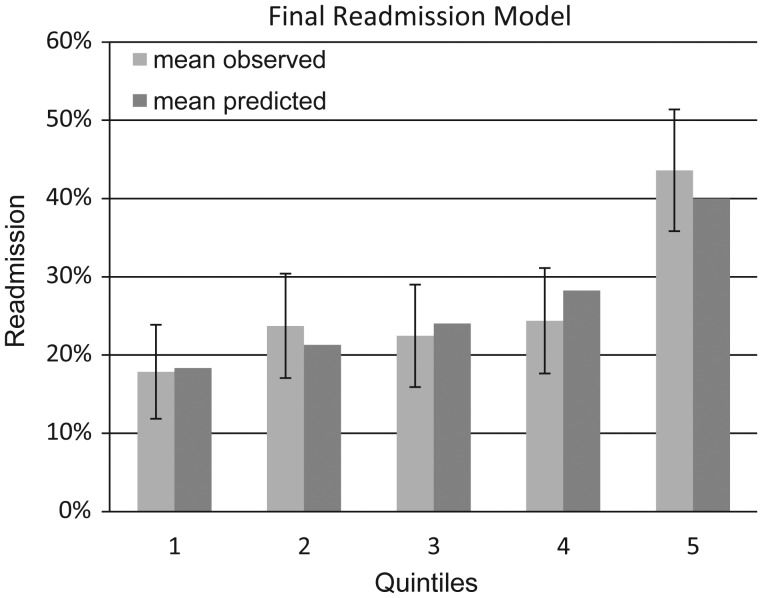
The final regression model consisted of age (OR, 1.09 per decade; 95% CI, .99–1.21), aminoglycoside use (OR, 2.33; 95% CI, 1.17–4.57), resistant organisms (OR, 1.57; 95% CI, 1.03–2.36), and number of prior hospital discharges without intravenous antibiotics in the past 12 months (OR, 1.20 per prior admission; 95% CI, 1.09–1.32) (Table 4). The Charlson comorbidity score was forced into the final model but was removed due to statistical nonsignificance (OR, 1.01; 95% CI, .94–1.1). The c-statistic for the final model was 0.61, and after internal validation the corrected c-statistic was 0.60. As shown in Figure 2, the highest-risk quintile of patients had nearly a 3-fold higher rate of readmission (observed 43.6% [95% CI, 35.8%–51.4%], predicted 40.0%) compared to the lowest-risk quintile (observed 17.8% [95% CI, 11.8%–23.9%], predicted 18.3%). In addition, calibration curves in Figure 2 do not show a pattern of either over- or underestimation. Hosmer-Lemeshow test result for goodness-of-fit was χ2 = 5.5 (P = .7), indicating that the data fit the model well [15].
Table 4.
Adjusted Odds Ratios and Sensitivity Analyses in the Final Model
| Multivariable Model |
Sensitivity Analysis 1 |
Sensitivity Analysis 2 |
|||||
|---|---|---|---|---|---|---|---|
| Final Model Variable | Odds Ratio | 95% Confidence Interval | P Value | Odds Ratio | % Change From Original | Odds Ratio | % Change From Original |
| Age, per 10 y | 1.09 | .99–1.21 | .10 | 1.10 | 0.9% | 1.10 | 0.9% |
| Aminoglycoside | 2.33 | 1.17–4.57 | .01 | 2.24 | −4.1% | 1.95 | −16.5% |
| Drug-resistant organisms | 1.57 | 1.03–2.36 | .03 | 1.46 | −6.8% | 1.36 | −13.2% |
| Prior admissions | 1.20 | 1.09–1.32 | <.001 | 1.17 | −2.4% | 1.09 | −9.1% |
Sensitivity analysis 1: Eliminate 78 subjects from model who never had follow-up at study institution. Sensitivity analysis 2: Change outcome of 78 subjects to “yes readmission” who never had follow-up at study institution (in case they were readmitted elsewhere).
Figure 2.
Collaboration curve. Final readmission model: age, prior non–outpatient parenteral antibiotic therapy admissions in past 12 months, aminoglycoside use, history of drug-resistant organisms. Error bars show 95% confidence intervals for observed readmission rates.
Sensitivity Analysis
To address the potential threat of outcome misclassification bias due to loss to follow-up (ie, patients potentially readmitted to a hospital other than Tufts Medical Center being classified as nonreadmissions), we reviewed subsequent medical services for all subjects who were not readmitted within 30 days (n = 575). Seventy-eight of 575 (14%) subjects had no additional services performed at Tufts Medical Center following hospital discharge. The 78 subjects did not differ significantly from the remaining cohort in terms of age, sex, aminoglycoside use, or length of their index hospital stay. As shown in Table 4, either removing the 78 subjects from analysis or reclassifying all 78 subjects as readmissions did not meaningfully change the final model ORs.
DISCUSSION
Our findings emphasize the need to study OPAT readmissions. Readmission rates were high in this study (26%), which is comparable to some other studies of OPAT patients [2, 18–21]. Our subjects were medically complex, with one-quarter diagnosed with bacteremia.
Four factors, readily obtainable at time of hospital admission as well as discharge, were found to be associated with a higher rate of readmission: age, history of drug-resistant organisms, prior hospitalizations in past 12 months, and aminoglycoside use. Age and prior admission were described as being important in a number of models of readmission prediction [22]. Interestingly, age was less significantly associated with other factors, implying that underlying disease severity, microbial virulence, and antibiotic toxicity may overcome the usual association of age in the OPAT setting. Although the Charlson score has been validated for predicting mortality, we did not find that it was associated with 30-day readmissions for OPAT, further illustrating the challenge of finding both clinically and statistically significant associations with readmissions in the OPAT population. Unlike other studies of readmissions [22], we did not find associations between proxy variables for socioeconomic status (eg, insurance type, lack of social supports). In our system, a patient is cleared for home parenteral therapy by the combined efforts of an ID physician, a case management nurse, and a home infusion nurse. This process may already weed out those patients at risk for readmission due to socioeconomic factors. Aminoglycoside use and a history of drug-resistant organisms are novel risks for OPAT readmission that have not been previously identified. The history of drug resistant-organisms seems an intuitive risk factor for OPAT readmission, as does the use of aminoglycosides. The association of aminoglycoside use with readmission could reflect the toxicity of these agents or the higher disease severity present in patients treated with aminoglycosides compared to those not treated with this antibiotic class. Finding these new factors suggests that improvement in prediction for readmission may depend critically on identifying influential clinical factors for specific subpopulations, rather than anticipating that a single readmission model might work for all.
Despite finding these 4 variables significantly associated with readmission, model discrimination was limited. It is notable that in virtually all models of 30-day readmission for other underlying conditions utilizing retrospective data, the level of discrimination varies between 0.60 and 0.77 [22]. However, similar to other readmission studies with fair discrimination, our model also showed a “clinically meaningful gradient” [22] of readmission risk across quintiles, with a nearly 3-fold difference from lowest to highest. Potential causes of the difficulty of predicting readmission in this population may be that OPAT programs enroll patients at similar (high) risk—that is, patients sick enough to require hospitalization and well enough to be discharged with intravenous antibiotics. Finally, because the reasons for readmission are heterogeneous, prediction of this event may be more difficult. Thirty percent of these readmissions were unrelated to an ID diagnosis or complications of antibiotic use.
Study strengths include the capture of planned vs unplanned readmissions, which is not always accomplished in readmission studies [22]. A wide range of patient demographic and clinical variables encountered in everyday clinical practice was also evaluated. The breadth of conditions treated promotes generalizability of this model among hospitalized patients and ensures that this model is relevant to real-world situations. Because subjects were referred to the OPAT program by their inpatient ID consultants, we presume that data on infectious diagnoses and antibiotic treatments were correctly documented in the medical record. Diagnoses and treatment were abstracted from individual patient medical records as opposed to billing or other administrative data, further increasing the accuracy of exposure and covariate classification. All data were originally collected prospectively, thus diminishing the usual risk of recall bias in a retrospective study design.
A limitation of this single center study is restriction of analysis to the first readmission, and we made no analysis of second or subsequent readmissions. However, this restriction ensured better comparisons among subjects in the research cohort and avoided additional potential confounding by downstream events. Another limitation is the possibility of misclassification bias for subjects who did not return for further follow-up to the host institution. However, in sensitivity analyses, removing these subjects from the data set or reclassifying them as readmissions did not meaningfully influence the results. We did not present extensive information on readmissions due to infections, as the diagnoses were extremely broad and did not shed light on the predictive model. The patient case mix and healthcare processes of a tertiary center, including a dedicated ID ward service, may make these results less germane to every hospital setting. Some authors have raised a reasonable concern regarding the use of 30-day readmission as a quality measure [23, 24]. These concerns notwithstanding, there remains intense interest in reducing rates of 30-day readmissions and, in turn, in identifying patients at especially high risk of readmission.
In summary, we developed a predictive model for 30-day unplanned readmissions for patients discharged with OPAT using 4 readily obtainable variables: age, prior hospitalizations in past 12 months, history of drug-resistant organisms, and aminoglycoside use. While discrimination was modest (c-statistic 0.61), it was similar to many published readmission models [22]. This study indicates the high risk of readmission for OPAT patients and the need to develop evidence-based interventions to prevent OPAT readmissions using appropriate risk stratification to ensure that efforts target the highest-risk patients.
Notes
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. E. G. M. is the inaugural recipient of the Francis P. Tally endowed fellowship in infectious diseases, Tufts Medical Center, Boston, Massachusetts. G. M. A., J. K. P., A. R., and R. R. were supported by the National Center for Research Resources (grant number UL1 RR025752), now the National Center for Advancing Translational Sciences, NIH (grant number UL1 TR000073). G. M. A. and A. R. were also supported by the National Cancer Institute (grant number KM1 CA156726). D. M. K. is supported by the NIH (grant numbers UL1TR001064 and KM1CA156726).
Potential conflicts of interest. G. M. A. has worked as a consultant for Coram Infusion Company, was on a speakers bureau for Merck, has a grant from Merck, and produced continuing medical education content funded by Gilead. D. R. S. has grants from Optimer, Cubist, Pfizer, Merck, and Genentech; is on the speakers bureau of Cubist and Optimer; and has worked as a consultant for Genentech, Microbiotix, Merck, Chimerix, Millenium, Genzyme, Seres Health, and AstraZeneca. All other authors report no reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Tice AD, Rehm SJ, Dalovisio JR, et al. Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis. 2004;38:1651–72. doi: 10.1086/420939. [DOI] [PubMed] [Google Scholar]
- 2.Amodeo MR, Clulow T, Lainchbury J, et al. Outpatient intravenous treatment for infective endocarditis: safety, effectiveness and one-year outcomes. J Infect. 2009;59:387–93. doi: 10.1016/j.jinf.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Bernard L, El-Hajj PB, Lotthe A, et al. Outpatient parenteral antimicrobial therapy (OPAT) for the treatment of osteomyelitis: evaluation of efficacy, tolerance and cost. J Clin Pharm Ther. 2001;26:445–51. doi: 10.1046/j.1365-2710.2001.00380.x. [DOI] [PubMed] [Google Scholar]
- 4.Cervera C, Del Rio A, Garcia L, et al. Efficacy and safety of outpatient parenteral antibiotic therapy for infective endocarditis: a ten-year prospective study. Enferm Infecc Microbiol Clin. 2011;29:587–92. doi: 10.1016/j.eimc.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Kunkel MJ. Quality assurance and outcomes in outpatient parenteral antibiotic therapy. Infect Dis Clin North Am. 1998;12:1023–34. doi: 10.1016/s0891-5520(05)70035-0. [DOI] [PubMed] [Google Scholar]
- 6.Le J, San Agustin M, Hernandez EA, Tran TT, Adler-Shohet FC. Complications associated with outpatient parenteral antibiotic therapy in children. Clin Pediatr (Phila) 2010;49:1038–43. doi: 10.1177/0009922810374210. [DOI] [PubMed] [Google Scholar]
- 7.Nathwani D, Tice A. Ambulatory antimicrobial use: the value of an outcomes registry. J Antimicrob Chemother. 2002;49:149–54. doi: 10.1093/jac/49.1.149. [DOI] [PubMed] [Google Scholar]
- 8.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–9. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 10.Boult C, Dowd B, McCaffrey D, Boult L, Hernandez R, Krulewitch H. Screening elders for risk of hospital admission. J Am Geriatr Soc. 1993;41:811–7. doi: 10.1111/j.1532-5415.1993.tb06175.x. [DOI] [PubMed] [Google Scholar]
- 11.Anderson GF, Steinberg EP. Predicting hospital readmissions in the medicare population. Inquiry. 1985;22:251–8. [PubMed] [Google Scholar]
- 12.Burnham KP, Anderson DR. Multimodel inference—understanding AIC and BIC in model selection. Soc Meth Res. 2004;33:261. [Google Scholar]
- 13.Friedman J, Hastie T, Tibshirani R. Sparse inverse covariance estimation with the graphical lasso. Biostatistics. 2008;9:432–41. doi: 10.1093/biostatistics/kxm045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sing T, Sander O, Beerenwinkel N, Lengauer T. ROCR: visualizing classifier performance in R. Bioinformatics. 2005;21:3940–1. doi: 10.1093/bioinformatics/bti623. [DOI] [PubMed] [Google Scholar]
- 15.Hosmer DW, Hosmer T, Le Cessie S, Lemeshow S. A comparison of goodness-of-fit tests for the logistic regression model. Stat Med. 1997;16:965–80. doi: 10.1002/(sici)1097-0258(19970515)16:9<965::aid-sim509>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 16.Cook RD, Weisberg S. Characterizations of an empirical influence function for detecting influential cases in regression. Technometrics. 1980;22:495–508. [Google Scholar]
- 17.Steyerberg EW, Harrell FE, Jr, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54:774–81. doi: 10.1016/s0895-4356(01)00341-9. [DOI] [PubMed] [Google Scholar]
- 18.Cervera C, del Rio A, Garcia L, et al. Efficacy and safety of outpatient parenteral antibiotic therapy for infective endocarditis: a ten-year prospective study. Enferm Infecc Microbiol Clin. 2011;29:587–92. doi: 10.1016/j.eimc.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Chapman AL, Dixon S, Andrews D, Lillie PJ, Bazaz R, Patchett JD. Clinical efficacy and cost-effectiveness of outpatient parenteral antibiotic therapy (OPAT): a UK perspective. J Antimicrob Chemother. 2009;64:1316–24. doi: 10.1093/jac/dkp343. [DOI] [PubMed] [Google Scholar]
- 20.Duncan CJ, Barr DA, Ho A, Sharp E, Semple L, Seaton RA. Risk factors for failure of outpatient parenteral antibiotic therapy (OPAT) in infective endocarditis. J Antimicrob Chemother. 2013;68:1650–4. doi: 10.1093/jac/dkt046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barr DA, Semple L, Seaton RA. Outpatient parenteral antimicrobial therapy (OPAT) in a teaching hospital-based practice: a retrospective cohort study describing experience and evolution over 10 years. Int J Antimicrob Agents. 2012;39:407–13. doi: 10.1016/j.ijantimicag.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 22.Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306:1688–98. doi: 10.1001/jama.2011.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaduganathan M, Bonow RO, Gheorghiade M. Thirty-day readmissions: the clock is ticking. JAMA. 2013;309:345–6. doi: 10.1001/jama.2012.205110. [DOI] [PubMed] [Google Scholar]
- 24.Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355–63. doi: 10.1001/jama.2012.216476. [DOI] [PMC free article] [PubMed] [Google Scholar]