Anal human papillomavirus (HPV) 16 infections were more likely to persist than other high-risk HPV types, and sexual behaviors were strongly associated with its persistence. As HPV-16 is responsible for 90% of anal cancers, prevention should include education around sexual practices.
Keywords: anal, human papillomavirus infection, persistence, women
Abstract
Background. Anal cancer is more common in women than in men, yet little is known about the natural history of human papillomavirus (HPV) in women. The objective was to examine the natural history of anal HPV in heterosexual women.
Methods. Young women participating in an HPV cohort study were seen at 4-month intervals for cervical and anal HPV testing. Time to clearance was estimated using the Kaplan-Meier approach; risks for persistence were assessed using Cox regression models.
Results. Seventy-five women (mean age, 23.5 ± 4.1 years) who tested positive for anal HPV were followed for a mean of 84.5 ± 44.9 months. By 3 years, 82.5% of anal non-16 high-risk (HR) HPV, 82.6% of low-risk (LR) HPV, and 76.2% of HPV-16 infections had cleared. By 3 years, only 36.4% of women had become negative for all HPV types. In the multivariable model, concurrent cervical HPV-16 (P < .001), weekly alcohol use (P = .015), anal touching during sex (P = .045), recent anal sex (P = .04), and no condom use during anal sex (P = .04) were associated with HPV-16 persistence. Greater number of new sex partners (P = .024) and condom use during vaginal sex (P = .003) were associated with clearance. Similar associations were found for clearance in all HR-HPV infections. Only concomitant cervical HPV was associated with non-16 HR-HPV persistence.
Conclusions. The majority of anal HPV infections cleared within 3 years. HPV-16 infections were slower to clear than other HR-HPV infections, consistent with its role in anal cancer. Specific sexual behaviors were associated with persistence, suggesting that education and behavioral interventions may decrease persistence.
Anal cancer is the second most common anogenital (AG) cancer caused by human papillomavirus (HPV) [1]. Compared to cervical cancer, the most common AG cancer, anal cancer is relatively uncommon, occurring at a rate of approximately 1.5 per 100 000 population [2]. In the United States, females have a higher incidence than men. Recent estimates projected that 2250 men and 3980 women would be diagnosed with anal cancer in 2012 [2]. Alarmingly, the rate of invasive anal squamous cell carcinoma among females increased by 2.0% per year between the years 1973 and 2009 [2]. Reasons for this increase are unknown, but sexual practices have likely played a role. Anal intercourse, multiple lifetime sexual partners, and smoking are known risks for anal cancer, and data suggest these practices have increased over time in women [3–6].
Most studies of the natural history of HPV in women have focused on the cervix. By contrast, most investigations of the links between HPV and anal cancer have focused on immunocompromised men infected with human immunodeficiency virus (HIV), and, to a lesser extent, HIV-infected women. The few existing studies focusing on HPV and anal cancer in nonimmunocompromised women have been cross-sectional [7–9], with the exception of a longitudinal investigation of anal HPV infection by Shvetsov et al [10]. These investigators reported a clearance rate of 58% over 1.2 years in college-aged women, similar to that seen in the cervix. Tobacco use, douching, and anal sex were associated with slower clearance. Risk factors for anal cancer include both anal sex and cigarette smoking [4, 11].
The objective of this study was to examine the natural history of anal HPV in heterosexual women who have been followed for at least 3 years, and to examine risk factors associated with HPV persistence in the anus.
MATERIALS AND METHODS
Subject Population
Women selected for this study were participating in the University of California, San Francisco (UCSF) HPV natural history study. Recruitment of these women has been detailed previously [12–15]. In brief, women were required to be sexually active <5 years, aged 13–21 years, nonpregnant, nonimmunocompromised, and have no history of cervical dysplasia. From 1990 to 1994, 908 women were recruited from a state university medical clinic and a Planned Parenthood clinic if they were positive for HPV DNA on cervical screening. A smaller, random group of HPV DNA–negative women were also recruited. In 1999, women who had become cervical HPV DNA negative for >2 years (a minimum of 7 consecutive negative cervical HPV DNA tests at 4-month intervals) were discontinued: 125 (31%) continued in the study after 1999. Between 2000 and 2004, 651 additional women were enrolled from the same sites in a second wave of recruitment. However, women were randomly approached and not recruited based on HPV DNA status. Other inclusion and exclusion criteria, as described above, were applied for entry into the study. This study was approved by the UCSF and San Francisco State University institutional review boards. Women were interviewed on sexual and substance use behaviors and examined at 4-month intervals as detailed previously [13, 14, 16]. Anal sex practice questions included self-reported indicators of anal intercourse, condom use during anal sex, and anal touching using fingers or mouth. Examinations included cervical samples for HPV DNA testing, cytology, and wet mounts for diagnosis of Trichomonas vaginalis, yeast, and bacterial vaginosis [12, 14–16]. Samples for Chlamydia trachomatis and Neisseria gonorrhoeae were obtained at annual visits or if symptoms were present. Lesions suggestive of herpes simplex virus (HSV) were tested by commercial laboratories. Starting in 1991, women were asked but not required to undergo anal HPV testing in addition to the above tests. For inclusion in this analysis, women had to have at least 1 anal sample positive for HPV and at least 3 years of anal samples collected.
HPV Testing
For participants consenting to anal testing, a moistened Dacron swab was placed 2 cm into the anal canal and rotated 3 times, removed, and placed into normal saline. DNA was extracted from anal specimens using a commercial DNA extraction kit (QIAamp MinElute Media, Qiagen, Valencia, California) according to the manufacturer's instructions. HPV DNA typing for anal samples used the polymerase chain reaction (PCR)–based PGMY09/11 primer system as previously described [13, 14]. The linear array assay utilizes amplification of target DNA by PCR from 37 HPV genotypes (6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51, 52, 53, 54, 55, 56, 58, 59, 61, 62, 64, 66, 67, 68, 69, 70, 71, 72, 73, 81, 82, 83, 84, IS39, and CP6108) and human β-globin gene for monitoring sample adequacy. Samples with negative β-globin were reprepped and reamplified by PCR. Eighty of the 1980 samples tested were β-globin negative including repeat analysis. These samples were considered missing data.
Statistical Analysis
Analyses focused on characterizing clearance of single HPV types detected in anal samples, as well as groups of types recognized as high risk (HR) and low risk (LR) for disease progression. The HR group included types 16, 18, 31, 33, 35, 45, 51, 52, 56, 68, 69, 73, and 82; the non-16 HR group included all HR types except 16; the LR group included types 2, 6, 11, 40, 42, 54, 55, 57, 61, 62, 64, 67, 69, 70, 71, 72, 81, 83, 84, and CP6108. Time to clearance for a specific HPV type was defined as the time (in days) between the visit of the first positive anal test inclusive of the baseline visit, and the first of 2 subsequent consecutive negative visits. Infections not meeting this definition of clearance were treated as censored in analyses. Women contributed at most 1 clearance time for infection with a specific type. For groups of types, women were allowed to contribute clearance times for each type detected. The distribution of clearance times was summarized using Kaplan-Meier estimates. Differences in distributions between specific HPV types were evaluated using Wilcoxon test, and differences between HPV groups were tested using Cox regression models with robust standard error estimates to account for within-woman correlations in clearance times.
Cox proportional hazards regression models were used to examine the effects of fixed and time-varying predictors on HPV clearance. Separate models were fitted for clearance of HPV-16, non-16 HR-HPV infections, and all HR-HPV infections. Definition of clearance events and censoring mirror the description given above. Inferences for models for groups of types used robust standard error estimates. Definition for clearance of all HR types was defined as 2 consecutive visits with no HR-HPV DNA detected. Women were allowed to contribute only once in this analysis starting with first visit with HR-HPV DNA detection.
Predictors were initially entered singly in regression models. Variables with marginal associations significant at the ≤10% level were retained for further analyses in multivariable models. Proportionality tests were performed for the models using the Schoenfeld residual approach.
To investigate risk factors for clearance of simultaneous multiple infections with non-16 HR-HPV types detected in a given woman, we conducted additional regression analyses with the clearance event defined as observed clearance of all initially detected infections with such types. Women were allowed to contribute >1 event in these analyses following clearance of all previous qualifying infections. We also performed analyses investigating the representativeness of our sample of 75 women by comparing selected demographic characteristics with the rest of the cohort using χ2 and 2-sample t tests.
RESULTS
Of the 1568 women entered into the natural history study, 204 women had at least 1 anal sample tested for HPV and 122 had at least 1 positive anal HPV test. Of these, 75 women had ≥3 years of anal HPV testing. Table 1 provides descriptive statistics characterizing the 75 women in the analysis sample and the remaining cohort. In comparison to the rest of the cohort (n = 1493), selected women were somewhat older, more likely to have experienced recent anal touching, more likely to drink alcohol weekly, less likely to be a smoker, and more likely to have a cervical HPV-16 and cervical HR-HPV at the time of recruitment. These women also had longer average participation times (138 ± 53 months vs 63.9 ± 55.2 months; P < .001). The mean age of the women at first anal HPV–positive visit was 23.5 ± 4.1 years and the mean number of months of follow-up after the positive test was 84.5 ± 44.9 months with a mean of 16 ± 7 visits.
Table 1.
Comparison of Demographics at Baseline Visit in Patients With Anal Human Papillomavirus to Those of the Entire Cohort
| Characteristic | Anal HPV Cohort | Cohorta | P Value |
|---|---|---|---|
| No. of patients | 75 | 1493 | NA |
| Race | .57 | ||
| White | 31 (41%) | 626 (43%) | |
| Black | 12 (16%) | 175 (12%) | |
| Hispanic | 15 (20%) | 343 (24%) | |
| Asian/Pacific Islander | 13 (17%) | 234 (16%) | |
| Other | 4 (5%) | 73 (5%) | |
| Age, y, mean ± SD | 19.8 ± 1.9 | 19.2 ± 2.4 | .005 |
| Ever had anal sex | 26 (35%) | 390 (26%) | .08 |
| Anal sex in last 4 mo | 12 (16%) | 165 (11%) | .19 |
| Anal touching in last 4 mo | 19 (25%) | 197 (13%) | .003 |
| Smoke cigarettes, current | 7 (9%) | 282 (19%) | .04 |
| Total lifetime sex partners | 6.3 ± 6.0 | 5.6 ± 6.2 | .39 |
| Used condoms during anal sex, mostly/always | 7 (27%)b | 81 (21%) | .46 |
| Drank alcohol at least weekly | 24 (32%) | 318 (21%) | .03 |
| Smoked marijuana at least weekly | 13 (17%) | 225 (15%) | .59 |
| HPV-16 at baseline, cervical | 15 (20%) | 157 (11%) | .03 |
| HR-HPVc at baseline, cervical | 37 (49%) | 440 (29%) | .001 |
Data are presented as No. (%) unless otherwise specified.
Abbreviations: HPV, human papillomavirus; HR, high risk; NA, not applicable; SD, standard deviation.
a Excludes those who tested positive for anal HPV.
b Among those who had anal sex.
c High-risk HPV types included 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82.
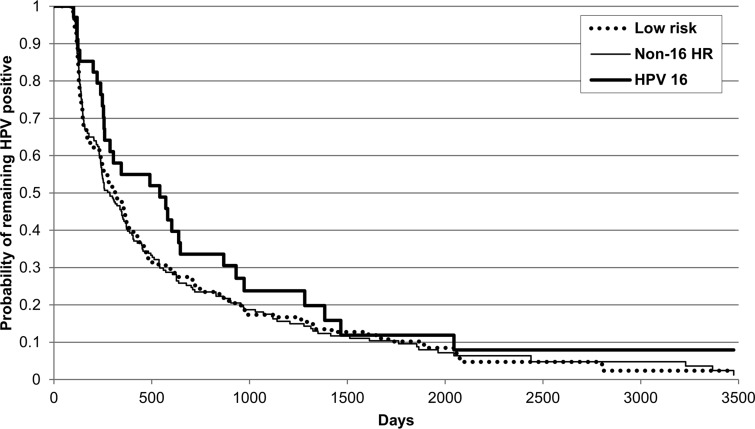
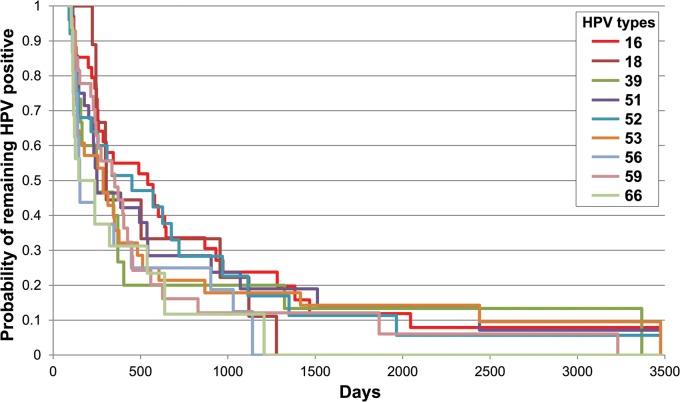
Figure 1 demonstrates the time to clearance of type-specific HR-HPV without HPV-16 (non-16 HR-HPV), LR-HPV types, and HPV-16. HPV-16 showed a trend for slower clearance than did non-16 HR-HPV (P = .09) and LR-HPV (P = .1). By 3 years, 82.5% of non-16 HR-HPV, 82.6% of LR-HPV, and 76.2% of HPV-16 infections had cleared (Table 2). Figure 2 and Table 2 demonstrate time to clearance of the 8 most prevalent HR-HPV types and HPV-18. HPV-16 was shown to clear more slowly than HPV-56 (P = .03) and HPV-66 (P = .01), with a trend for HPV-39 (P = .1). All the other types (18, 51, 52, 53, and 59) cleared at similar rates to HPV-16. No difference was found for clearance of non-16 HR-HPV types if there was coinfection with HPV-16 (P = .84).
Figure 1.
Time to clearance for human papillomavirus (HPV) type 16, non-16 high-risk HPV, and low-risk HPV infections using Kaplan-Meier method. Women could be entered into the analysis more than once. Abbreviations: HPV, human papillomavirus; HR, high risk.
Table 2.
Type-Specific Clearance of Anal Human Papillomavirus
| HPV Type (No.a) | Percentage (No.b) Who Cleared Infection |
|||
|---|---|---|---|---|
| By 1 y | By 2 y | By 3 y | P Valuec | |
| HPV-16 (34) | 45.0 (15) | 66.4 (22) | 76.2 (25) | NA |
| HPV-18 (9) | 55.6 (5) | 66.7 (6) | 77.8 (7) | .82 |
| HPV-39 (15) | 60.0 (9) | 80.0 (12) | 86.7 (13) | .1 |
| HPV-51 (28) | 57.8 (16) | 71.5 (19) | 81 (21) | .22 |
| HPV-52 (26) | 48.6 (12) | 71.7 (17) | 77.4 (18) | .53 |
| HPV-53 (28) | 64.3 (18) | 78.6 (22) | 82.1 (23) | .14 |
| HPV-56 (16) | 68.7 (11) | 75 (12) | 87.5 (14) | .03 |
| HPV-59 (27) | 51.9 (14) | 83.8 (22) | 87.9 (23) | .19 |
| HPV-66 (17) | 68.7 (11) | 88.3 (13) | 88.3 (13) | .01 |
| Non-16 HR-HPV (65)d | 58.1 (114) | 76.5 (147) | 82.5 (157) | .09 |
| Low risk (68)d | 57.2 (110) | 74.8 (142) | 82.6 (155) | .11 |
| Any HR-HPV (68)e | 29.7 (20) | 50.0 (33) | 56.5 (37) | … |
| Any HPV (75)f | 14.8 (11) | 23.1 (17) | 36.4 (26) | … |
Abbreviations: HPV, human papillomavirus; HR, high risk; NA, not applicable.
a The number of patients at the time of the first infection.
b The denominator of percentage is the number of remaining patients at the end of 1 year, 2 years and 3 years, after excluding right-censored cases.
c P value is for differences between HPV-16 and individual HPV type using Wilcoxon test and between non-16 HR-HPV and low risk-HPV using Cox regression model with robust sandwich-variance estimator. No comparisons were made for any HR-HPV and any HPV as HPV-16 was included in these groups.
d Type-specific clearance rates for non-16 HR-HPV and low-risk HPV allowed coinfections with HPV-16 and HR-HPV, respectively.
e Clearance of high-risk HPV type defined as time to 2 consecutive visits negative for all HR-HPV types.
f Clearance of any HPV type defined as time to 2 consecutive visits negative for all HPV types.
Figure 2.
Type-specific clearance for the 8 most common human papillomavirus (HPV) types found in the anus and HPV-18. Women could be entered into analysis more than once. Abbreviation: HPV, human papillomavirus.
Time to Clearance of All HR-HPV Types
We also examined time to clearance of all HR-HPV types defined by 2 consecutive visits negative for all HR-HPV types. We found that overall clearance rates were low with only 56.5% showing no HR-HPV DNA and 36.4% showing no HPV DNA detection by year 3 (Table 2). This underscores the commonness of repeated detections of anal HPV over the course of the study.
Factors Associated With HPV Clearance
Results from marginal and multipredictor regression models for variables associated with anal HPV clearance of type-specific non-16 HR-HPV, HPV-16, and all HR-HPV are summarized in Tables 3 and 4, respectively. No significant deviations from proportional hazards were detected in regression models. Variables associated with slower type-specific non-16 HR-HPV clearance included having a concurrent cervical infection with the same HR-HPV type (P < .001). Concurrent cervical infection with the same HPV type remained significant even after adjusting for anal sex, condom use, number of recent partners, and prevalent infection status at baseline. Prevalent anal infections cleared more slowly than incident infections (hazard ratio = 0.62; 95% confidence interval [CI], .39–.96; P = .03). No other variables were significantly associated with clearance. As this model examined clearance of each HPV type independently, it did not account for multiple-type infections that might clear at different rates. When we performed the additional regression model investigating factors influencing clearance of simultaneous infections with multiple non-16 HR-HPV types (see Materials and Methods), we found very similar results to those shown in Table 3 (data not shown).
Table 3.
Bivariable Associations With Clearancea of Anal Non-16 High-Risk Human Papillomavirus (HPV), HPV-16, and All High-Risk HPV
| Variable | Hazard Ratio (95% CI) |
||
|---|---|---|---|
| Clearance of Type-Specific Non-16 HR-HPVb (n = 227)c | Clearance of HPV-16 (n = 34)d | Clearance of All HR-HPVa (n = 75)e | |
| Age, per y | 1.02 (.97–1.06) | 0.99 (.90–1.08) | 0.96 (.89–1.03)a |
| Concurrent cervical HPV (with same HR type) at visit, yes vs no | 0.08 (.04–.16)** | 0.26 (.09–.75)** | 0.12 (.02–.89)** |
| Concurrent cervical HPV (with otherf HR type) at visit, yes vs no | 0.94 (.63–1.41) | 1.63 (.32–8.30) | 0.09 (.03–.3)*** |
| Previous cervical HPV (with same type) at visit, yes vs no | 0.82 (.50–1.35) | 0.98 (.38–2.54) | 0.70 (.48–1.01)* |
| Total lifetime sex partners, per partner | 1.0 (.98–1.02) | 0.95 (.90–1.01)* | 0.96 (.94–.99)** |
| Recent new sex partnerg, per partner | 1.09 (.96–1.25) | 1.34 (1.04–1.73)** | 0.96 (.80–1.15) |
| Ever had anal sex, yes vs no | 0.96 (.56–1.65) | 0.49 (.21–1.15)* | 0.44 (.24–.80)** |
| Recent anal sexg, yes vs no | 1.37 (.89–2.12) | 0.46 (.41–1.51) | 0.12 (.15–1.17)* |
| Recent sexual anal touchingg, yes vs no | 1.24 (.69–2.23) | 0.31 (.09–1.07)* | 0.56 (.25–1.27) |
| Current cigarette use, yes vs no | 1.52 (.82–2.82) | 1.06 (.29–3.85) | 1.02 (.47–2.21) |
| Weekly alcohol useg, yes vs no | 1.36 (.89–2.09) | 0.32 (.15–.70)** | 0.96 (.55–1.69) |
| Condom use during vaginal intercourseg, any use vs none | 0.85 (.57–1.28) | 3.69 (1.51–8.99)** | 1.69 (.95–2.99)* |
| Nonmonogamousg, yes vs no | 1.14 (.68–1.92) | 1.19 (.41–3.42) | 1.15 (.6–2.2) |
| No anal condom useg, vs no anal sex | 0.96 (.57–1.62) | 0.25 (.06–1.03)* | 0.27 (.06–1.1)* |
| Condom use during anal sexg, any vs no anal sex | 1.72 (.53–5.56) | NE | 0.82 (.11–6.1) |
| Months on oral contraceptivesg, per 6 mo | 1.01 (1.98–1.04) | 0.93 (.86–.99)** | 0.98 (.93–1.02) |
Abbreviations: CI, confidence interval; HPV, human papillomavirus; HR, high risk; NE, not able to estimate because too few cases.
a Clearance defined as time to 2 consecutive visits negative for all HR-HPV types.
b Includes HPV types 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82.
c Number of anal non-16 HR-HPV events qualifying for analysis.
d Number of events with anal HPV-16.
e Number of events with anal HR-HPV.
f In the case of multiple types, “other” was defined only if same type was not found.
g Reported since last visit.
*P < .1; **P < .05; ***P < .001.
Table 4.
Multivariable Analysis for Risks Associated With Clearance of Human Papillomavirus (HPV) Type 16 and All High-Risk HPVa
| Variable | Hazard Ratio (95% CI) |
|
|---|---|---|
| Clearance of HPV-16 | Clearance of All HR-HPVa,b | |
| Concurrent cervical HPV (with same type) at visit, yes vs no | 0.12 (.04–.41)** | NS |
| Concurrent cervical HPV (otherc HR type) at visit, yes vs no | NS | 0.09 (.03–.29)*** |
| Total lifetime sex partners, per partner | NS | 0.96 (.93–.99)** |
| Recent new sex partnerd, per partner | 1.97 (1.09–3.55)** | NS |
| Condom use during vaginal intercourse, yes vs no | e | 1.81 (1.01–3.26)** |
| Weekly alcohol used, yes vs no | 0.28 (.10–.78)** | NS |
| Recent sexual anal touchingd,f, yes vs no | 0.21 (.04–.96)** | NS |
| Ever had anal sex, yes vs no | f | 0.45 (.24–.86)** |
Abbreviations: CI, confidence interval; HPV, human papillomavirus; HR, high risk; NS, not significant.
a Clearance defined as time to 2 consecutive negative tests for all HR-HPV types.
b Includes HPV types 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82.
c In the case of multiple types, “other” was defined only if same type was not found.
d Reported since last visit.
e Because recent new sex partner and condom use during vaginal intercourse were highly correlated, the variable “condom use during vaginal intercourse” was entered separately into the HPV-16 clearance model with concurrent cervical HPV with same type, weekly alcohol use, and recent sexual anal touching excluding recent new sex partners. In the model, condom use during vaginal intercourse remained significant (hazard ratio = 5.68; 95% CI, 1.82–17.7). Associations for the other variables in the model remain similar.
f As the anal sex behavioral variables were highly correlated, each variable was entered separately into the HPV-16 clearance model with concurrent cervical HPV with same type, recent new sex partner, and weekly alcohol use. For ever had anal sex, hazard ratio = 0.35 (95% CI, .12–1.00); for recent anal sex, hazard ratio = 0.28 (95% CI, .08–.94); and for no anal condom use (vs no anal sex), hazard ratio = 0.16 (95% CI, .05–.51). Associations for the other variables remained similar in all the models.
*P < .1; **P < .05; ***P < .001.
Variables displaying inverse marginal associations with HPV-16 clearance included concurrent cervical infection with HPV-16 (P = .013), anal touching during sex (P = .06), ever having anal sex (P = .1), greater number of total lifetime sex partners (P = .07), weekly alcohol use (P = .004), no anal condom use (vs no anal sex) (P = .06), and months on combined hormonal contraception (P = .04). Condom use during vaginal intercourse (P = .004) and having a recent new sex partner (P = .025) were associated with faster clearance. Prevalent infections also cleared more slowly than incident infections (hazard ratio = 0.33; 95% CI, .14–.77; P = .01). No other variables were significant.
In the multipredictor regression model for anal HPV-16 clearance (Table 4), concurrent cervical HPV-16 (P < .001), weekly alcohol use (P = .015), and anal touching during sex (P = .045) were inversely associated with clearance. Greater number of recent new sex partners (P = .024) was associated with enhanced clearance. The model remained similar after adjusting for prevalent infections (data not shown). As condom use and reporting a new sex partner were highly correlated, they were entered separately into the model with concurrent cervical HPV-16, weekly alcohol use, and anal touching. Condom use during vaginal intercourse remained significant (P = .003), and the other variables had similar hazard ratio (Table 4). Anal sex behaviors were also correlated, and thus were also entered separately (Table 4). When excluding anal touching, ever having anal sex (P = .05) remained borderline significant in the model (P = .05). When we entered the other 2 anal sex behavior variables separately, they also remained significant—recent anal sex had a hazard ratio of 0.28 (95% CI, .08–.94; P = .04) and no anal condom use (vs no anal sex) had a hazard ratio of 0.16 (95% CI, .05–.51; P = .04).
Last, among women with HR-HPV, we examined factors associated with clearance of all HR-HPV (Tables 3 and 4). In the bivariable analysis, slower clearance was associated with a cervical HPV detection of the same (P = .04) and other (P < .001) HR type at the visit, a cervical HPV with the same HR type at the previous visit (P = .06), greater number of lifetime partners (P = .016), history of ever having anal sex (P = .008), recent anal sex (P = .09), and no anal condom use (vs no anal sex) (P = .07). Faster clearance was associated with condom use during vaginal intercourse (P = .07). In the multivariable analysis, concurrent cervical with other HR-HPV types present (P < .001), total lifetime number of sex partners (P = .002), and ever having reported anal sex (P = .02) were associated with persistence, and condom use during vaginal intercourse was associated (P = .03) with faster clearance. When anal sex variables were entered separately into the model, excluding ever having anal sex, there was a nonsignificant trend associated with persistence for sexual anal touching (hazard ratio = 0.53; 95% CI, .24–1.2; P = .13) and no association for recent anal sex or anal condom use.
A sensitivity analysis with a stricter definition of clearance using 3 negative consecutive tests yielded similar results (data not shown).
DISCUSSION
We report on the natural history of anal HPV in heterosexual women over a mean of 5 years of follow-up. Although we observed variability in clearance rates between types, the majority of women experienced type-specific clearance of HR- and LR-HPV types during follow-up. Approximately 85% of women cleared LR types and non-16 HR-HPV infections by 3 years. Only 1 other study of anal HPV infection in women is available for comparison to our group [10]. Although their follow-up was much shorter, clearance at 1 year was similar to ours [10]. These clearance rates parallel those reported for cervical HPV infections in young women in our study as well as in others [17, 18]. In contrast, the rate of anal HPV-16 clearance was slower than that reported for the cervix. Only three-quarters of the women cleared anal HPV-16 by 3 years in this cohort. In comparison, we recently reported a 3-year clearance rate for HPV-16 infection of 83.2% (95% CI, 78.3–87.5) for women participating in the same study [19]. This is similar to most other studies [20]. The slower rate noted for HPV-16 in the anus is not surprising as HPV-16 appears to be more important than the other HR-HPV types in anal cancers than in cervical cancers. More than 90% of anal cancers are associated with HPV-16 compared to slightly more than 50% of cervical cancers [21]. In one of our previous publications describing HPV type–specific cervical clearance in this group, cervical HPV-16 was noted to clear more slowly than HPV-6, -18, -66, and -84 but faster than HPV-62 and -68 [17]. No difference was found for HPV-51, -52, -53, and -59. In this study, we had only 6 types with sufficient data to compare and found similar data in that anal HPV-16 was slower to clear than HPV-18 and HPV-51, -52, and -53. The slower clearance rate noted for HPV-16 may be due to its ability to evade the host immune response through dampening of innate and adaptive immune responses [22–24].
Interestingly, one of the strongest predictors of anal HPV-16 persistence was having a concomitant cervical infection with HPV-16. This might suggest that some type of global immune dysregulation is present, resulting in persistence of HPV at both mucosal sites [19–21]. Certainly, having had cervical intraepithelial neoplasia grade III or cervical cancer is a risk for anal cancer [25, 26]. The concurrent HPV-16 cervical infections may also suggest that the cervix is somehow a reservoir for anal HPV infections or vice-versa. Goodman and colleagues [27] demonstrated that a cervical HPV infection often preceded an incident anal infection and vice-versa, concluding that transmission of HPV between the anus and cervix was common and each served as a reservoir for the other. In our study, a preceding cervical HPV-16 detection did not predict persistence at the anus at the following visit, suggesting that the cervix was not a significant reservoir for persistence. On the other hand, cervix-to-anal transmission could have occurred during the 4-month interval. In support of possible cervical reservoirs resulting in repeated infections was the finding that anal touching was associated with anal HPV-16 persistence. Several studies have documented HPV DNA on hands and fingers, which thereby serve as a conduit between the cervix and anus [28, 29].
The association with alcohol use suggests that high-risk behavior plays a significant role in HPV-16 persistence. Anal sex behaviors including anal intercourse, anal touching, and lack of condom use during anal sex were all associated with HPV-16 persistence. Interestingly, having a new sex partner and condom use during vaginal intercourse was associated with HPV-16 clearance. We speculate that both of these suggest that having a new partner reflects a dissociation with the previous partner who was responsible for the constant reexposure. Condom use is more commonly used with new partners than steady partners, thereby reflecting an association with the new partner [30]. Condom use may have also prevented the reexposure from the cervix. As seen with the cervix, prevalent HPV infections were associated with persistence compared to incident infection [31]. This finding has been attributed to the fact that these prevalent infections are already “persistent” and hence reflect some immune dysregulation.
Interestingly, the low rate of visits negative for all HPV underscores the commonness of anal HPV detections. Variables associated with clearance of all HR-HPV found similar associations as that for HPV-16 including concomitant cervical infections, condom use during intercourse, and ever having anal sex. The trend associations for anal touching underscore the importance of these behaviors in anal HPV reinfections. Despite approaching the analysis in several ways, the lack of finding any behavioral associations for non-16 HR-HPV underscores the challenges in examining the natural course of anal HPV infections, specifically when there are multiple events such as incidence, clearance, and coinfections occurring simultaneously.
One of the limitations of this study was the relatively small sample size. In addition, women not consenting to anal testing may have influenced our results in that they were less likely to have higher-risk behaviors or engage in anal sex practices. These women may have cleared HPV faster and had different risks. On the other hand, if women refused testing because they were concerned that their high-risk behavior led to infection, exclusion of this population would also bias our results.
In conclusion, this longitudinal study of anal HPV in women with >5 years of observation demonstrates the commonness of repeated anal HPV detections, with only a third of women with an infection ever becoming negative for all HPV types by 3 years. On the other hand, type-specific clearance mimicked observations shown for cervical infections with >80% clearing by 3 years. The exception was HPV-16, which was slower to clear, consistent with its role in anal cancer. Sexual behavior highly influenced clearance rates, including digital–anal sex. Education and behavioral interventions could decrease rates of HPV-16 persistence and anal cancer.
Notes
Acknowledgments. We thank Anthony Kung for help with manuscript preparation.
Disclaimer. The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. This work was supported by the NIH (grant numbers R37 CA051323 and RC1 AI86051-01) and by the National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH, through UCSF-CTSI (grant number UL1 RR024131).
Potential conflicts of interest. A.-B. Moscicki, MD, has served as an advisory board member to Merck. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Forman D, de Martel C, Lacey CJ, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30(suppl 5):F12–23. doi: 10.1016/j.vaccine.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975–2009 (vintage 2009 populations) Available at: http://seer.cancer.gov/csr/1975_2009_pops09/ . Accessed 14 January 2013.
- 3.Daling JR, Madeleine MM, Johnson LG, et al. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer. 2004;101:270–80. doi: 10.1002/cncr.20365. [DOI] [PubMed] [Google Scholar]
- 4.Frisch M, Glimelius B, van den Brule AJ, et al. Sexually transmitted infection as a cause of anal cancer. New Engl J Med. 1997;337:1350–8. doi: 10.1056/NEJM199711063371904. [DOI] [PubMed] [Google Scholar]
- 5.Tseng HF, Morgenstern H, Mack TM, Peters RK. Risk factors for anal cancer: results of a population-based case-control study. Cancer Causes Control. 2003;14:837–46. doi: 10.1023/b:caco.0000003837.10664.7f. [DOI] [PubMed] [Google Scholar]
- 6.Chandra A, Mosher WD, Copen C, Sionean C. Sexual behavior, sexual attraction, and sexual identity in the United States: data from the 2006–2008 National Survey of Family Growth. Nat Health Stat Rep. 2011;36:1–36. [PubMed] [Google Scholar]
- 7.Castro FA, Quint W, Gonzalez P, et al. Prevalence of and risk factors for anal human papillomavirus infection among young healthy women in Costa Rica. J Infect Dis. 2012;206:1103–10. doi: 10.1093/infdis/jis458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moscicki AB, Durako SJ, Houser J, et al. Human papillomavirus infection and abnormal cytology of the anus in HIV-infected and uninfected adolescents. AIDS. 2003;17:311–20. doi: 10.1097/00002030-200302140-00004. [DOI] [PubMed] [Google Scholar]
- 9.Castor M, da Silva HJ, Gondim Martins DB, de Mello RJ. HPV and precancerous lesions of anal canal in women: systematic review. Int J Colorectal Dis. 2012;27:271–6. doi: 10.1007/s00384-011-1298-1. [DOI] [PubMed] [Google Scholar]
- 10.Shvetsov YB, Hernandez BY, McDuffie K, et al. Duration and clearance of anal human papillomavirus (HPV) infection among women: the Hawaii HPV cohort study. Clin Infect Dis. 2009;48:536–46. doi: 10.1086/596758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frisch M, Glimelius B, Wohlfahrt J, Adami HO, Melbye M. Tobacco smoking as a risk factor in anal carcinoma: an antiestrogenic mechanism? J Natl Cancer Inst. 1999;91:708–15. doi: 10.1093/jnci/91.8.708. [DOI] [PubMed] [Google Scholar]
- 12.Farhat S, Nakagawa M, Moscicki AB. Cell-mediated immune responses to human papillomavirus 16 E6 and E7 antigens as measured by interferon gamma enzyme-linked immunospot in women with cleared or persistent human papillomavirus infection. Int J Gynecol Cancer. 2009;19:508–12. doi: 10.1111/IGC.0b013e3181a388c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moscicki AB, Shiboski S, Broering J, et al. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J Pediatr. 1998;132:277–84. doi: 10.1016/s0022-3476(98)70445-7. [DOI] [PubMed] [Google Scholar]
- 14.Moscicki AB, Shiboski S, Hills NK, et al. Regression of low-grade squamous intra-epithelial lesions in young women. Lancet. 2004;364:1678–83. doi: 10.1016/S0140-6736(04)17354-6. [DOI] [PubMed] [Google Scholar]
- 15.Scott ME, Ma Y, Farhat S, Shiboski S, Moscicki AB. Covariates of cervical cytokine mRNA expression by real-time PCR in adolescents and young women: effects of Chlamydia trachomatis infection, hormonal contraception, and smoking. J Clin Immunol. 2006;26:222–32. doi: 10.1007/s10875-006-9010-x. [DOI] [PubMed] [Google Scholar]
- 16.Moscicki AB, Hills N, Shiboski S, et al. Risks for incident human papillomavirus infection and low-grade squamous intraepithelial lesion development in young females. JAMA. 2001;285:2995–3002. doi: 10.1001/jama.285.23.2995. [DOI] [PubMed] [Google Scholar]
- 17.Moscicki AB, Widdice L, Ma Y, et al. Comparison of natural histories of human papillomavirus (HPV) detected by clinician- and self- sampling. Int J Cancer. 2010;127:1882–92. doi: 10.1002/ijc.25199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moscicki AB, Schiffman M, Burchell A, et al. Updating the natural history of human papillomavirus and anogenital cancers. Vaccine. 2012;30(suppl 5):F24–33. doi: 10.1016/j.vaccine.2012.05.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moscicki AB, Ma Y, Farhat S, et al. Redetection of cervical human papillomavirus type 16 (HPV16) in women with a history of HPV16. J Infect Dis. 2013;208:403–12. doi: 10.1093/infdis/jit175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Insinga RP, Perez G, Wheeler CM, et al. Incident cervical HPV infections in young women: transition probabilities for CIN and infection clearance. Cancer Epidemiol Biomarkers Prev. 2011;20:287–96. doi: 10.1158/1055-9965.EPI-10-0791. [DOI] [PubMed] [Google Scholar]
- 21.Clifford G, Franceschi S, Diaz M, Munoz N, Villa LL. Chapter 3: HPV type-distribution in women with and without cervical neoplastic diseases. Vaccine. 2006;24(suppl 3):S26–34. doi: 10.1016/j.vaccine.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 22.Daud II, Scott ME, Ma Y, Shiboski S, Farhat S, Moscicki AB. Association between toll-like receptors expression and human papillomavirus type 16 persistence. Int J Cancer. 2011;128:879–86. doi: 10.1002/ijc.25400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Sherif AM, Seth R, Tighe PJ, Jenkins D. Quantitative analysis of IL-10 and IFN-gamma mRNA levels in normal cervix and human papillomavirus type 16 associated cervical precancer. J Pathol. 2001;195:179–85. doi: 10.1002/path.929. [DOI] [PubMed] [Google Scholar]
- 24.Bais AG, Beckmann I, Lindemans J, et al. A shift to a peripheral Th2-type cytokine pattern during the carcinogenesis of cervical cancer becomes manifest in CIN III lesions. J Clin Pathol. 2005;58:1096–100. doi: 10.1136/jcp.2004.025072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edgren G, Sparen P. Risk of anogenital cancer after diagnosis of cervical intraepithelial neoplasia: a prospective population-based study. Lancet Oncol. 2007;8:311–6. doi: 10.1016/S1470-2045(07)70043-8. [DOI] [PubMed] [Google Scholar]
- 26.Saleem AM, Paulus JK, Shapter AP, Baxter NN, Roberts PL, Ricciardi R. Risk of anal cancer in a cohort with human papillomavirus-related gynecologic neoplasm. Obstet Gynecol. 2011;117:643–9. doi: 10.1097/AOG.0b013e31820bfb16. [DOI] [PubMed] [Google Scholar]
- 27.Goodman MT, Shvetsov YB, McDuffie K, et al. Sequential acquisition of human papillomavirus (HPV) infection of the anus and cervix: the Hawaii HPV Cohort Study. J Infect Dis. 2010;201:1331–9. doi: 10.1086/651620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Widdice L, Ma Y, Jonte J, et al. Concordance and transmission of human papillomavirus within heterosexual couples observed over short intervals. J Infect Dis. 2013;207:1286–94. doi: 10.1093/infdis/jit018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winer RL, Hughes JP, Feng Q, et al. Detection of genital HPV types in fingertip samples from newly sexually active female university students. Cancer Epidemiol Biomarkers Prev. 2010;19:1682–5. doi: 10.1158/1055-9965.EPI-10-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Catallozzi M, Bell DL, Short MB, Marcell AV, Ebel SC, Rosenthal SL. Does perception of relationship type impact sexual health risk? Sex Transm Dis. 2013;40:473–5. doi: 10.1097/OLQ.0b013e318287bf44. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez AC, Schiffman M, Herrero R, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst. 2010;102:315–24. doi: 10.1093/jnci/djq001. [DOI] [PMC free article] [PubMed] [Google Scholar]