Summary
People with epilepsy often experience long-term cognitive dysfunction and other neurological deficits, including memory loss, learning disabilities, and neurobehavioral disorders, which may exhibit a progressive course correlating with worsening seizure control. Furthermore, one-third of epilepsy patients have seizures that are intractable to all available treatments. Thus, novel therapies for seizures and the neurological comorbidities of epilepsy are desperately needed. As most current treatments are merely “symptomatic” therapies that suppress seizures, recently epilepsy researchers have realized the critical need for novel therapeutic strategies targeting the underlying mechanisms of epileptogenesis and seizure-related brain injury. Yet to date, few such “anti-epileptogenic” therapies have emerged or are even in developmental stages. Although many seizure medications modulate the functional or physiological activity of neurons, a relatively unexplored therapeutic strategy for epilepsy are methods for stabilizing the structure of neurons. Human pathological studies and animal models of epilepsy demonstrate obvious structural abnormalities in dendrites of neurons, which could contribute to neuronal dysfunction, epileptogenesis, and cognitive/neurological deficits in epilepsy patients. This dendritic injury may be caused by activity-dependent breakdown of cytoskeletal elements, such as actin. Mechanistically-targeted approaches to limit seizure-related structural changes in dendrites may represent a novel therapeutic strategy for treating epilepsy and its complications.
Keywords: seizures, epilepsy, epileptogenesis, brain injury, cognitive deficits, dendrite, spine, actin
Introduction
In addition to the immediate detrimental consequences of seizures, people with epilepsy often also experience long-term cognitive dysfunction and other neurological deficits. In particular, memory loss, learning disabilities, and behavioral problems are especially common in epilepsy patients, and often exhibit a progressive course, frequently correlating with worsening seizure control [1–5]. Furthermore, about one-third of all patients with epilepsy have seizures that are intractable to all currently-available treatments and that may escalate in frequency and severity [6–8]. There are multiple biological, environmental, and psychosocial factors that may contribute to progressive neurological dysfunction in epilepsy patients. From the biological perspective, increasing attention has focused on the role of underlying epileptogenic mechanisms in the brain, as well as the seizures themselves, in directly causing brain injury and leading to neurological deficits in epilepsy patients. Thus, understanding mechanisms of epileptogenesis and seizure-induced brain injury is critical for devising novel therapies that can potentially prevent or reverse the detrimental neurocognitive consequences of seizures and retard progressive epileptogenesis.
Despite the recent proliferation of medications and other treatments available for epilepsy, the current state of epilepsy therapies developed to date has been characterized by significant limitations and disappointments. First, given the high prevalence of epilepsy (~1% worldwide), the one-third of patients with intractable epilepsy represents an enormous population of patients. While about a dozen new seizure medications have become available over the past 15 years and may offer more favorable side effect profiles than older drugs, all these medications have had minimal effect in reducing the number of patients with intractable seizures [9–12]. In addition, even in patients who respond well to medication, treatment with current “antiepileptic” drugs does not appear to alter the natural history of epilepsy or improve ultimate prognosis. For example, long-term outcomes, such as chance of chronic seizure remission, are not significantly improved by treatment with seizure medication [13–15]. Thus, most current “antiepileptic” drugs are probably not truly anti-epileptic or anti-epileptogenic in nature, but are simply symptomatic treatments that suppress seizures (anti-seizure) without affecting the underlying mechanisms of epileptogenesis and brain injury. Finally, very little can be offered epilepsy patients in terms of specifically treating the other comorbidities of epilepsy, such as cognitive and learning deficits, which may be as disabling as the seizures themselves [16].
The realization of the limitations of currently available epilepsy treatments has produced a recent paradigm shift in strategies for developing new therapies for epilepsy. Rather than treating the end-stage symptoms of seizures, over the past decade epilepsy researchers have increasingly discussed and promoted the concept of targeting underlying brain mechanisms of epileptogenesis and injury that causes epilepsy and its complications to develop [17–19]. On one extreme, this “anti-epileptogenic” or neuroprotective approach could be applied to patients at risk for epilepsy (e.g. severe traumatic brain injury) to interrupt the process of epileptogenesis and completely prevent the development of epilepsy and its comorbidities in the first place. In patients with newly diagnosed epilepsy, “disease-modifying” therapy could limit or reverse the progression of epilepsy, the emergence of intractability, and the development of cognitive and other neurological deficits.
Despite this modern trend focusing on developing anti-epileptogenic or disease-modifying therapies for epilepsy, unfortunately to date, no such novel therapies have yet become available, or are even in clinical trials. However, there is promise for substantial progress in this area in the near future, based on recent advances in our understanding of mechanisms of epileptogenesis and seizure-induced brain injury, such as related to neuronal death, glial activation (astrogliosis), neurogenesis, axonal sprouting, and changes in gene expression (especially of ion channels, neurotransmitter receptors, or other proteins directly affecting neuronal excitability). These biological mechanisms identify potential novel targets for therapeutic intervention. Most current seizure medications directly regulate the functional or physiological activity of neurons, such as by modulating ion channels or neurotransmitter receptors. In contrast, rather than focusing on the functional activity of neurons, a relatively unexplored therapeutic strategy for epilepsy is stabilizing the structure of neurons. Human pathological studies and animal models of epilepsy demonstrate obvious structural abnormalities in dendrites of neurons, which could contribute to neuronal dysfunction, epileptogenesis, and other cognitive/neurological deficits in epilepsy patients. On a mechanistic level, this dendritic injury is likely mediated by activity-dependent breakdown of cytoskeletal elements, such as actin. Thus, mechanistically-targeted approaches to limit seizure-induced structural changes in dendrites may represent a novel therapeutic strategy for treating epilepsy and its complications. In the remainder of this article, I will review the evidence for structural abnormalities in dendrites in epilepsy, the functional implications and molecular mechanisms of this dendritic injury, and rational therapeutic strategies for preventing these dendritic changes.
Normal Dendritic Structure, Function, and Plasticity
The dendrites of neurons have long been recognized to serve the critical role of the basic postsynaptic structure that receives synaptic signals from other neurons during synaptic transmission. In addition to this elementary function of transmitting electrical signals from the synapse to the decision-making cell body of the neuron, in recent years more detailed information has emerged about the complex regulation and mechanisms of dendritic structure, function, and plasticity. Rather than acting as one, uniform entity, dendrites are typically compartmentalized into many individual processing units. In many neurons, such as principal neurons in the mammalian neocortex and hippocampus, the structural basis for this compartmentalization occurs in the form of dendritic spines, small knob-like protuberances that extend out from dendritic branches. Dendritic spines usually receive synaptic contact from a single presynaptic terminal and are the major site of input for glutamatergic synapses, the primary type of excitatory synapse in the brain. While spines serve as the initial conduits for transmitting signals from the synapse to the dendrite and ultimately cell body of the neuron, instead of simply transferring the original message in a faithful manner, spines also have the capability of modulating the original synaptic signal [20]. Postsynaptic potentials can be altered electrically via structural variability in passive membrane properties and active regulation by voltage-gated ion channels within spines [21]. In addition to their electrical properties, spines may also serve as localized biochemical compartments for modulating receptor expression and activating intracellular signaling pathways and second messengers, such as calcium [22–26].
Given the unique ability of dendritic spines to modulate synaptic signaling, much attention has focused on the role of dendritic spines in synaptic plasticity and mechanisms of learning and memory [27,28]. A fundamental hypothesis of dendritic spine function has been that the formation of new spines or a change in existing spine morphology occurs during learning and these spine changes, especially when associated with new or strengthened synaptic connections, serves as the anatomical locus of memory storage. This hypothesis is now supported by several lines of evidence. First, modulation of dendritic spine morphology and number has been demonstrated in cellular models of learning and synaptic plasticity. This is seen most clearly in studies of long-term potentiation (LTP), a synaptic model of learning in which a long-lasting increase in synaptic efficacy occurs following high-frequency electrical stimulation of presynaptic inputs onto postsynaptic neurons. In both pathological studies and live time-lapse imaging of dendrites in animal models, induction of LTP causes a rapid increase in dendritic spine number or expansion of spine size [29–34]. Furthermore, behavioral studies in animals have documented changes in dendritic spines as a result of learning. The number of dendritic spines, and the corresponding number of synapses, increase in critical brain regions with learning following different animal conditioning paradigms [35–42]. In addition, abnormalities in dendritic spines and synapses have been frequently found in different syndromic and non-syndromic forms of mental retardation in people, again suggesting a role of spines in cognitive function and learning [43–49].
Dendritic Abnormalities in Epilepsy
With the known effects of synchronous electrical activity on dendritic spines, such as with high-frequency electrical stimulation to induce LTP, it would not be surprising if dendritic structure were also affected by seizures, which similarly consist of highly synchronized electrical activity. Given the probable involvement of dendritic spines in synaptic plasticity and learning, abnormalities in dendritic spines could also represent a possible structural substrate and mechanistic basis for seizure-induced brain injury and cognitive deficits in epilepsy. Furthermore, dendritic dysfunction has been increasingly implicated in mechanisms of epileptogenesis itself. Consistent with these ideas, there is strong evidence for abnormalities in dendritic structure in human epilepsy and animal models.
Evidence for dendritic abnormalities in human epilepsy is primarily derived from pathological analysis of brain specimens resected as part of surgical treatment of patients with intractable epilepsy. Such pathological specimens obtained from or near the region of the epileptic focus in neocortex or hippocampus have revealed a number of abnormalities in dendrites, but most commonly demonstrate a loss of dendritic spines [50,51]. Dendritic spine loss is commonly seen in hippocampal pyramidal neurons and dentate granule cells in patients with temporal lobe epilepsy and may occur in isolation or associated with varicose swelling of the dendritic branches [52–56]. Similar findings of spine loss and dendritic swelling have also been documented in pyramidal neurons of neocortex, including sites distant from the primary epileptogenic focus [57]. Other less common dendritic abnormalities that have been described in both neocortical and hippocampal epilepsy include changes in dendritic length, shape, and branching patterns, as well as a focal increase in dendritic spines [55–59].
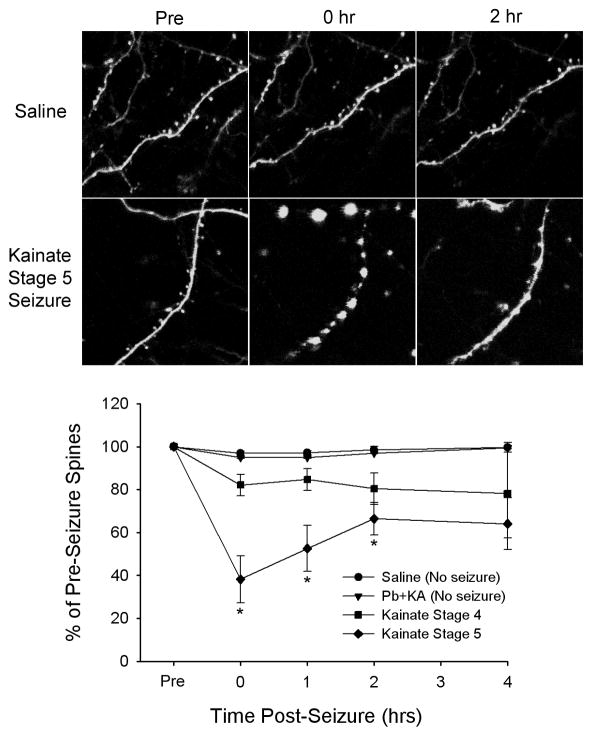
While human pathological studies are usually limited by problems with confounding factors and lack of good control specimens, well-controlled studies have demonstrated analogous findings of dendritic injury in animal models of epilepsy. Similar to the clinical findings, a loss of dendritic spines and varicose swelling of dendrites is frequently found in histological sections obtained from rats that had acute seizures or chronic epilepsy induced in vivo by various methods, such as convulsant drugs or electrical kindling [60–65], although rarely an increase in dendrites or spines has been reported [66–68]. Furthermore, spine loss and other dendritic changes can also occur with in vitro seizure models involving epileptiform bursting in brain slice-cultures [69–72]. While previous studies have utilized fixed-tissue methods to give isolated, static views of dendritic injury, recently modern microscopy methods have directly visualized seizure-related dendritic injury with time-lapse imaging in living animals in vivo [73–75]. These in vivo time-lapse studies have demonstrated a remarkable evolution of dendritic injury acutely following seizures, first with a transient beading of dendrites that resolves quickly within a couple hours after a seizure, followed by a more persistent loss of dendritic spines (Fig. 1).
Figure 1.
Seizures cause acute dendritic injury in mice in vivo. Dendrites of cortical neurons expressing green-fluorescent protein were imaged directly by multiphoton microscopy in vivo under control conditions and before and immediately after seizures. In control (saline-injected) mice or mice given phenobarbital with kainate (Pb+KA) to suppress seizures, there were minimal structural changes in dendrites and spines over a several hour period. In mice given kainate to induce seizures, seizures for 30 minutes caused acute, evolving changes in dendrites, depending on the severity (stage) of the seizures. In severe stage 5 seizures, there was often an immediate beading of dendrites with complete loss of spines. The dendritic beading would usually resolve within two hours of seizure termination with an accompanying partial recovery of spines, but a residual, longer-term loss of spines persisted. Modified with permission from [75]; Copyright 2007 by the Society for Neuroscience.
Although evidence from both human epilepsy and animal models strongly supports the hypothesis that dendritic abnormalities occur in epilepsy, the functional, behavioral, and clinical consequences of these dendritic changes are not as well documented. While the animal studies have shown that seizures can directly induce dendritic injury, the converse role of these dendritic abnormalities in promoting epileptogenesis is not as clearly established. It seems probable that dendritic injury could be epileptogenic and enhance the likelihood of future seizures by disrupting the normal, finely-tuned balance between excitatory and inhibitory networks in the brain, especially if inhibitory circuits are more affected. On the other hand, it is also possible that a loss of dendritic spines and synapses could actually be beneficial in suppressing seizures, by inhibiting synaptic transmission and preventing the propagation of seizure activity. In terms of cognitive deficits and other neurological comorbidities of epilepsy, it is rational to conclude that the dendritic injury and loss of spines most commonly documented in epilepsy, especially in hippocampal and other relevant cortical areas, should predispose to learning problems and other cognitive deficits. It is more difficult to explain the functional significance of increased dendritic branching and spines that has occasionally been reported, but this could represent a compensatory response to brain injury. Clearly, additional studies are needed to define more specifically the behavioral and functional effects of dendritic changes in epilepsy.
Mechanisms of Dendritic Injury
Assuming that the documented structural abnormalities in dendrites cause adverse consequences in epilepsy patients, a novel, rational therapeutic strategy for epilepsy would be to attempt to stabilize dendritic structure and thus prevent dendritic injury. Before such a therapeutic approach can be pursued, an understanding of the underlying biological mechanisms causing these dendritic changes is necessary. Identification of the molecular substrates and cellular signaling pathways mediating and regulating dendritic architecture may reveal new therapeutic targets for preventing or reversing deleterious structural changes in dendrites. Although mechanistic information about dendritic injury in epilepsy has just recently begun to be explored, helpful clues and rational hypotheses can be derived from more established data related to mechanisms of structural plasticity in dendrites under physiological conditions. It is reasonable to hypothesize that the same mechanisms that account for normal synaptic plasticity might also mediate pathophysiological changes in dendrites with epilepsy, but in a more extreme, disregulated, or opposing fashion.
The molecular mechanisms mediating the previously-mentioned plasticity of dendritic spines with long-term potentiation have been well-characterized. Formation of new dendritic spines or changes in existing spine morphology during LTP likely results from modulation of the filamentous actin cytoskeleton of dendrites [76–80]. Actin is a major structural protein of dendrites that is highly concentrated in dendritic spines and can exist in a stable polymerized filamentous form (F-actin) or a soluble, depolymerized monomeric form (G-actin). F-actin forms complex filamentous networks that provide structural support and stability for dendrites, whereas conversion between F-actin and G-actin may allow for structural plasticity. Actin polymerization can be regulated directly by actin-binding proteins, such as cofilin and profilin, which in turn are controlled by a number of upstream signaling pathways, in particular a series of kinases and phosphatases, such as PAK and LIM kinases and calcineurin and slingshot phosphatases, that can be activated by neuronal and synaptic activity [81–83]. Physiological forms of neuronal activation, such as during LTP, have been shown to regulate actin polymerization in a complex, tightly-regulated manner, and may lead to increases in F-actin [76–78] or depolymerization of F-actin [79,80] depending on the situation. Overall, it is likely that LTP causes an initial transient phase of actin depolymerization, which allows for structural plasticity and motility leading to new spine formation or changes in spine morphology, followed by subsequent polymerization of F-actin, which results in long-term stabilization of these dendritic changes and consolidation of LTP [80].
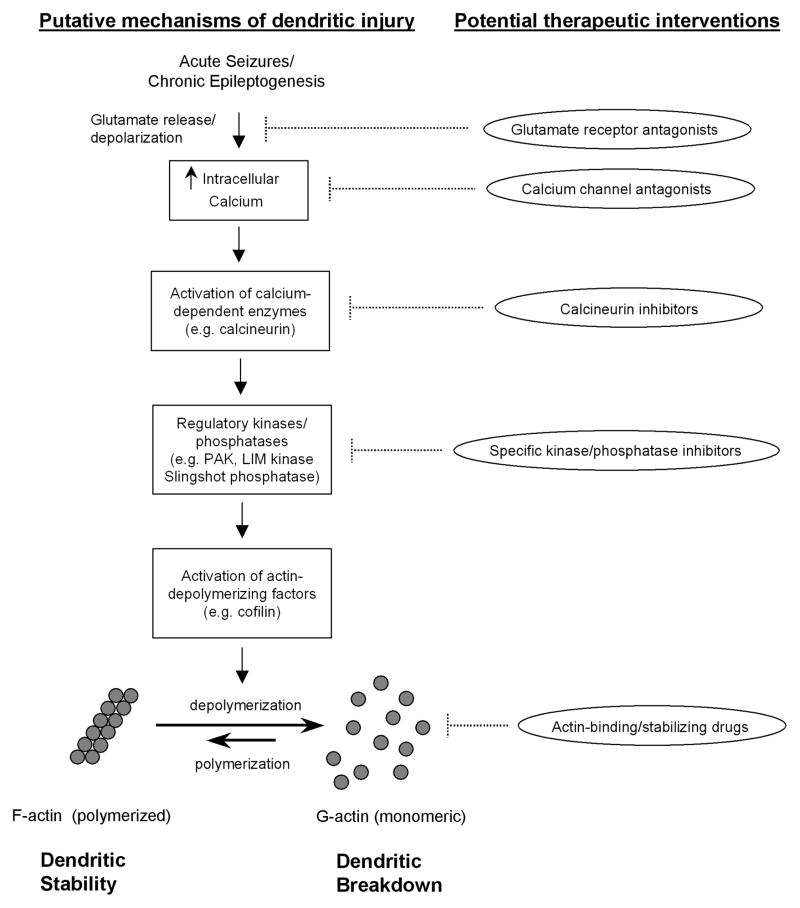
Analogous to activity-dependent regulation of the actin cytoskeleton with synaptic plasticity in LTP under physiological conditions, similar mechanisms could also be involved in epilepsy, but may be activated in an extreme or inappropriate manner leading instead to pathological changes in dendritic structure. It is reasonable to hypothesize that abnormal cellular and molecular processes that cause epileptogenesis, or are activated by seizure themselves, could trigger mechanisms leading to complete breakdown of the normal actin cytoskeleton and resulting dendritic injury and loss of spines. In recent years, some data from animal models have started to accumulate to support this hypothesis. It is well-established that acute seizures cause massive activation of glutamate receptors and calcium influx, and chronic epileptogenesis is also associated with dysregulation of calcium-dependent processes, such as within dendritic spines [84,85]. Recent evidence suggests that specific calcium-activated enzymes, such as calcineurin, are activated in animal models of epilepsy [86]. Furthermore, actin and actin-associated proteins are regulated by acute seizures or during chronic epileptogenesis [75,87,88]. In particular, seizures lead to acute depolymerization of filamentous actin, which could directly account for structural changes in dendrites [75]. Thus, a logical mechanistic scheme mediating dendritic injury in epilepsy could involve dysregulation of intracellular calcium within spines, activation of a cascade of calcium-dependent phosphatases and kinases, and depolymerization of F-actin, with resultant breakdown of the actin cytoskeleton of dendrites (Fig. 2).
Figure 2.
Putative signaling pathways and molecular mechanisms mediating dendritic injury in epilepsy. Acute seizures and chronic epileptogenesis may be associated with increased intracellular calcium in dendrites due to neuronal depolarization and excessive glutamate receptor and calcium channel activation. Elevated intracellular calcium may activate calcium-dependent enzymes, such as calcineurin, triggering a cascade of downstream kinases and phosphatases. These regulatory kinases (e.g. PAK and LIM kinase) and phosphatases (e.g. Slingshot) control the activity of actin binding proteins and depolymerizing/polymerizing factors due to changes in phosphorylation states. Ultimately, activation of actin-depolymerizing factors, such as cofilin, can cause depolymerization of filamentous actin (F-actin), leading to the breakdown of the actin cytoskeleton of dendrites. From a therapeutic standpoint, multiple steps along this pathway could be targeted to prevent dendritic injury, although the specificity and safety of such interventions are uncertain.
While actin is a major cytoskeletal protein in dendrites, there are other structural or regulatory proteins that could also be involved in epilepsy-related dendritic abnormalities. For example, myosin has recently been shown to regulate dendritic spine morphology [89]. Furthermore, in addition to intracellular elements controlling dendritic architecture, proteins in the extracellular matrix, such as the matrix metalloproteinases, can also affect remodeling on dendritic spines [90,91]. Future research should determine whether these or other cytoskeletal and regulatory proteins are critically involved in dendritic injury in epilepsy.
Although defining mechanisms of dendritic injury in epilepsy may reveal interesting basic insights into activity-dependent synaptic plasticity, from a clinical standpoint the ultimate goal is to use this knowledge to develop novel, more effective treatments for epilepsy patients. Similar to most other prospects for anti-epileptogenic or disease-modifying therapies, clearly this process is still in its infancy with regard to developing methods for modulating dendritic structure. However, there is already some preliminary evidence to suggest that targeting dendritic stucture could be an effective strategy. At least in animal models of dendritic injury following acute seizures, pharmacological inhibition of upstream signaling pathways regulating actin polymerization is neuroprotective against seizure-induced dendritic injury. In particular, the calcineurin inhibitor, FK506, which may also have neuroprotective properties in other neurological disorders [92,93], is able to prevent changes in actin-binding proteins that cause actin depolymerization and correspondingly avert dendritic beading and spine loss that usually results from the seizures [75]. These experiments at least provide “proof-of-principle” that stabilizing dendritic structure pharmacologically represents a viable therapeutic strategy for epilepsy. A number of other drugs targeting different portions of the actin pathway, including direct actin-binding agents, could also be similarly tested (Fig. 2), although the specificity and safety of currently available pharmacological agents could be significant concerns. Furthermore, many additional experiments are needed to determine the functional and clinical benefits of this type of approach, as well as devising practical methods for applying it to patients.
Expert Commentary
Given the enormous burden of intractable seizures and neurocognitive deficits in epilepsy patients, clearly novel therapies for epilepsy and its comorbidities are needed that are more than just symptomatic treatments, but that target underlying processes of epileptogenesis and seizure-related brain injury. Successful development of such disease-modifying therapies not only depends on identifying the relevant causal mechanisms to modulate, but also requires that these interventions not result in serious adverse effects. Unfortunately to date, no such therapy for epilepsy has emerged, or even appears close to clinical development beyond the basic science lab. As supported by the evidence reviewed in this article, the proposed approach of stabilizing dendritic structure holds much promise. Most available therapies for epilepsy modulate neuronal function, physiology, and excitability and are able to suppress seizures in many patients, but again do not appear to alter the underlying course or prognosis of epilepsy or address the neurocognitive aspects of epilepsy. In contrast, a completely different therapeutic strategy focusing on synaptic structure and integrity may have a better chance of having true “anti-epileptogenic” properties and preventing brain injury that causes neurocognitive deficits.
On the other hand, there are a number of potential limitations and pitfalls with adopting this novel therapeutic approach targeting structural stability of neurons. First of all, while it makes intrinsic sense that dendritic beading and spine loss are not beneficial for the brain, it’s not clear that preventing these structural changes will have anti-epileptogenic effects. As mentioned above, it is also possible that the decrease in synaptic transmission and connectivity occurring with dendritic injury could actually retard seizure generation or spread, though it seems just as likely that such disruption of finely-tuned neuronal networks would promote seizures. With specific regard to actin, the potentially complicated effects of regulating actin-based motility is exemplified in a recent study that showed that while stabilizing actin with actin-binding drugs helped to inhibit seizures in the short-term, both actin depolymerizing and stabilizing agents resulted in a long-term increase in neuronal excitability and seizures in rats [94]. Thus, there is likely a delicate balance between actin polymerization and depolymerization, with excessive changes in either direction potentially causing undesirable effects. Since direct actin-binding drugs could have strong neurological and non-neurological toxicity, it might be safer to first explore the utility of upstream signaling modulators, such as calcineurin inhibitors, which have already been used in people for other clinical purposes usually without serious neurological side effects.
Even if stabilizing dendritic structure does not have beneficial effects for epileptogenesis, there is still potential for this strategy in limiting seizure-related brain injury that may cause neurocognitive deficits in epilepsy patients. Given the importance of dendritic spines in synaptic plasticity and putative memory mechanisms, it is likely that dendritic injury contributes to learning problems and other neurological issues, and preventing this injury could have beneficial effects for epilepsy patients. But again, there could be a fine balance between protecting against dendritic injury and causing adverse effects, as excessive stabilization of dendritic structure could unintentionally impede normal synaptic plasticity and thus have detrimental effects on cognition and learning. In addition, as actin and other structural components of dendrites are localized ubiquitously in a variety of cell types throughout the nervous system and rest of the body, the potential for widespread neurological and systemic side effects is a significant concern. Thus, in order to maximize efficacy while minimizing potential side effects, there may be only selected situations or specific patient populations at risk, in which these protective drugs may be indicated. In a plausible scenario, actin-stabilizing drugs could be administered to patients in the acute setting following a significant brain injury (e.g. severe head trauma) or during status epilepticus to prevent initial dendritic damage from occurring, but then be withdrawn as the patient starts to show signs of recovery from the initial event, in order to prevent long-term adverse effects.
Overall, the therapeutic strategy of stabilizing dendritic structure offers some enticing advantages and promise, but a number of issues need to be addressed before such an approach could be seriously considered for clinical use. While it appears that actin-stabilizing drugs may offer neuroprotection against epilepsy-related dendritic injury in some situations, these same drugs could theoretically have detrimental effects on the brain and non-neurological systems under other conditions, especially with long-term use. Future studies, as outlined below, need to define the specific factors and circumstances dictating when the benefits of these therapies would outweigh the risks.
Five-year View
Much additional work will be required over the next five years to determine whether targeting dendritic structure represents a feasible strategy to pursue for clinical applications. At this point, most of this work will need to continue to be pursued on the basic research level, primarily in animal models, before considering clinical trials. First, as mentioned above, while there is already promising data to indicate that stabilizing actin can prevent dendritic injury, the long-term behavioral and functional consequences of this protective effect are not known. Thus, additional studies need to be done in animal models to determine whether preventing dendritic injury actually translates into functionally meaningful improvements in either the development of epilepsy or behavioral/learning deficits in rodents. Second, although preliminary evidence suggests that calcineurin inhibitors, such as FK506, are efficacious for preventing dendritic injury, similar basic research studies testing other drugs that modulate different parts of the relevant cell signaling pathways (Fig. 2) may find drugs with better efficacy or fewer side effects. Finally, even if promising drugs are identified that appear to be efficacious, more detailed studies in animals will first need to be performed to determine optimal methods of drug delivery, pharmacokinetics, safety, and potential toxicities of the drugs. Of course, there is always some uncertainty about the relevance of animal studies to people. Nevertheless, if these steps can be accomplished, it is conceivable that initial clinical trials could then be proposed in epilepsy patients, especially for drugs, such as calcineurin inhibitors, which are already FDA approved for other indications.
Even if the therapeutic strategy of stabilizing dendritic structure ultimately proves to be ineffective or infeasible, other parallel approaches for identifying therapeutic targets focusing on other mechanisms of epileptogenesis and seizure-induced brain injury should continue. In the ideal scenario, within a five-year time frame a number of potential therapies that exert true “anti-epileptogenic” actions or protect against seizure-induced brain injury would be identified and ready to proceed to clinical trials in epilepsy patients.
Key Issues.
Many patients with epilepsy have intractable seizures and neurocognitive deficits.
As most currently-available treatments for epilepsy are symptom-based only, novel therapeutic strategies targeting underlying mechanisms of epileptogenesis and seizure-induced brain injury are needed.
Structural abnormalities in dendrites (spine loss and dendritic beading) are commonly observed in human pathological studies and animal models of epilepsy and may contribute to progressive neurological dysfunction and cognitive deficits.
Specific cell signaling pathways and regulatory molecules leading to the breakdown of cytoskeletal proteins, such as actin, are involved in the mechanistic basis of dendritic injury.
While a number of issues need to be defined related to efficacy and safety of potential drugs, mechanistically-targeted therapeutic strategies for stabilizing the dendritic cytoskeleton may represent a novel treatment for epilepsy and its complications.
Acknowledgments
The author receives grant support from the National Institutes of Health (K02NS045583, R01NS056872) and the Tuberous Sclerosis Alliance.
Footnotes
Financial Disclosures
The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Dodrill CB. Progressive cognitive decline in adolescents and adults with epilepsy. Prog Brain Res. 2002;135:399–407. doi: 10.1016/S0079-6123(02)35037-4. [DOI] [PubMed] [Google Scholar]
- 2.Elger CE, Helmstaedter C, Kurthen M. Chronic epilepsy and cognition. Lancet Neurol. 2004;3:663–677. doi: 10.1016/S1474-4422(04)00906-8. [DOI] [PubMed] [Google Scholar]
- 3.Helmstaedter C. Effects of chronic epilepsy on declarative memory systems. Prog Brain Res. 2002;135:439–453. doi: 10.1016/S0079-6123(02)35041-6. [DOI] [PubMed] [Google Scholar]
- 4.Williams J. Learning and behavior in children with epilepsy. Epilepsy Behav. 2003;4:107–111. doi: 10.1016/s1525-5050(03)00024-6. [DOI] [PubMed] [Google Scholar]
- 5.Austin JK, Dunn DW. Progressive behavioral changes in children with epilepsy. Prog Brain Res. 2002;135:419–427. doi: 10.1016/S0079-6123(02)35039-8. [DOI] [PubMed] [Google Scholar]
- 6.Kwan P, Brodie MJ. Refractory epilepsy: mechanisms and solutions. Expert Rev Neurother. 2006;6:397–406. doi: 10.1586/14737175.6.3.397. [DOI] [PubMed] [Google Scholar]
- 7.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 8.Berg AT, Vickrey BG, Testa FM, et al. How long does it take for epilepsy to become intractable? A prospective investigation. Ann Neurol. 2006;60:73–79. doi: 10.1002/ana.20852. [DOI] [PubMed] [Google Scholar]
- 9.Walker MC, Sander JW. The impact of new antiepileptic drugs on the prognosis of epilepsy: seizure freedom should be the ultimate goal. Neurology. 1996;46:912–914. doi: 10.1212/wnl.46.4.912. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt D. The clinical impact of new antiepileptic drugs after a decade of use in epilepsy. Epilepsy Res. 2002;50:21–32. doi: 10.1016/s0920-1211(02)00065-7. [DOI] [PubMed] [Google Scholar]
- 11.Wilby J, Kainth A, Hawkins N, et al. Clinical effectiveness, tolerability and cost-effectiveness of newer drugs for epilepsy in adults: a systematic review and economic evaluation. Health Technol Assess. 2005;9:1–157. doi: 10.3310/hta9150. [DOI] [PubMed] [Google Scholar]
- 12.Perucca E, French J, Bialer M. Development of new antiepileptic drugs: challenges, incentives, and recent advances. Lancet Neurol. 2007;6:793–804. doi: 10.1016/S1474-4422(07)70215-6. [DOI] [PubMed] [Google Scholar]
- 13.Temkin NR, Dikmen SS, Wilensky AJ, et al. A randomized, double-blind study of phenytoin for the prevention of post-traumatic seizures. N Engl J Med. 1990;323:497–502. doi: 10.1056/NEJM199008233230801. [DOI] [PubMed] [Google Scholar]
- 14.Mussico M, Beghi E, Solari A, Viani F. Treatment of first tonic-clonic seizure does not improve the prognosis of epilepsy. First Seizure Trial Group (FIRST Group) Neurology. 1997;49:991–998. doi: 10.1212/wnl.49.4.991. [DOI] [PubMed] [Google Scholar]
- 15.Marson A, Jacoby A, Johnson A, et al. Immediate versus deferred antiepileptic drug treatment for early epilepsy and single seizures: a randomized controlled trial. Lancet. 2005;365:2005–2013. doi: 10.1016/S0140-6736(05)66694-9. [DOI] [PubMed] [Google Scholar]
- 16.Devinsky O. Therapy for neurobehavioral disorders in epilepsy. Epilepsia. 2004;45 (Suppl 2):34–40. doi: 10.1111/j.0013-9580.2004.452003.x. [DOI] [PubMed] [Google Scholar]
- 17.Loscher W, Schmidt D. New horizons in the development of antiepileptic drugs: innovative strategies. Epilepsy Res. 2006;69:183–272. doi: 10.1016/j.eplepsyres.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stefan H, Lopes da Silva FH, Loscher W, et al. Epileptogenesis and rational therapeutic strategies. Acta Neurol Scand. 2006;113:139–155. doi: 10.1111/j.1600-0404.2005.00561.x. [DOI] [PubMed] [Google Scholar]
- 19.Dichter MA. Models of epileptogenesis in adult animals available for antiepileptogenesis drug screening. Epilepsy Res. 2006;68:31–35. doi: 10.1016/j.eplepsyres.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 20.Nimchinsky EA, Sabatini BL, Svoboda K. Structure and function of dendritic spines. Ann Rev Physiol. 2002;64:313–353. doi: 10.1146/annurev.physiol.64.081501.160008. [DOI] [PubMed] [Google Scholar]
- 21.Tsay D, Yuste R. On the electrical function of dendritic spines. TINS. 2004;27:77–83. doi: 10.1016/j.tins.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Koch C, Zador A. The function of dendritic spines – devices subserving biochemical rather than electrical compartmentalization. J Neurosci. 1993;13:413–422. doi: 10.1523/JNEUROSCI.13-02-00413.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuzaki M, Ellis-Davis GC, Nemoto T, Miyashita Y, Iino M, Kasai H. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuste R, Majewska A, Holthoff K. From form to function: calcium compartmentalization in dendritic spines. Nat Neurosci. 2000;3:653–659. doi: 10.1038/76609. [DOI] [PubMed] [Google Scholar]
- 25.Majewska A, Brown E, Ross J, Yuste R. Mechanisms of calcium decay kinetics in hippocampal spines: role of spine calcium pumps and calcium diffusion through the spine neck in biochemical compartmentalization. J Neurosci. 2000;20:1722–1734. doi: 10.1523/JNEUROSCI.20-05-01722.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noguchi J, Matsuzaki M, Ellis-Davies GC, Kasai H. Spine-neck geometry determines NMDA receptor-dependent Ca2+ signaling in dendrites. Neuron. 2005;46:609–622. doi: 10.1016/j.neuron.2005.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Segal M. Dendritic spines and long-term plasticity. Nature Rev Neurosci. 2005;6:277–284. doi: 10.1038/nrn1649. [DOI] [PubMed] [Google Scholar]
- 28.Carlise HJ, Kennedy MB. Spine architecture and synaptic plasticity. TINS. 2005;28:182–187. doi: 10.1016/j.tins.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Desmond NL, Levy WB. Synaptic interface surface area increases with long-term potentiation in the hippocampal dentate gyrus. Brain Res. 1988;453:308–314. doi: 10.1016/0006-8993(88)90171-0. [DOI] [PubMed] [Google Scholar]
- 30.Trommald M, Hulleberg G, Andersen P. Long-term potentiation is associated with new excitatory spine synapses on rat dentate granule cells. Learn Mem. 1996;3:218–228. doi: 10.1101/lm.3.2-3.218. [DOI] [PubMed] [Google Scholar]
- 31.Maletic-Savatic M, Malinow R, Svoboda K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science. 1999;283:1923–1926. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- 32.Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- 33.Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lang C, Barco A, Zablow L, Kandel ER, Siegelbaum SA, Zakharenko SS. Transient expansion of synaptically connected dendritic spines upon induction of hippocampal long-term potentiation. Proc Natl Acad Sci USA. 2004;47:16665–16670. doi: 10.1073/pnas.0407581101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moser MB, Trommald M, Andersen P. An increase in dendritic spine density on hippocampal CA1 pyramidal cells following spatial learning in adult rats suggests the formation of new synapses. Proc Natl Acad Sci USA. 1994;91:12673–12675. doi: 10.1073/pnas.91.26.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Airey DC, Kroodsma DE, DeVoogd TJ. Differences in the complexity of song tutoring cause differences in the amount learned and in dendritic spine density in a songbird telencephalic song control nucleus. Neurobiol Learn Mem. 2000;73:274–281. doi: 10.1006/nlme.1999.3937. [DOI] [PubMed] [Google Scholar]
- 37.Leuner B, Falduto J, Shors TJ. Associative memory formation increases the observation of dendritic spines in the hippocampus. J Neurosci. 2003;23:659–665. doi: 10.1523/JNEUROSCI.23-02-00659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knafo S, Grossman Y, Barkai E, Benshalom G. Olfactory learning is associated with increased spine density along apical dendrites of pyramidal neurons in the rat piriform cortex. Eur J Neurosci. 2001;13:633–638. doi: 10.1046/j.1460-9568.2001.01422.x. [DOI] [PubMed] [Google Scholar]
- 39.Knafo S, Ariav G, Barkai E, Libersat F. Olfactory learning-induced increase in spine density along the apical dendrites of CA1 hippocampal neurons. Hippocampus. 2005;14:819–825. doi: 10.1002/hipo.10219. [DOI] [PubMed] [Google Scholar]
- 40.Lowndes M, Stewart MG. Dendritic spine density in the lobus parolfactorius of the domestic chick is increased 24 h after one-trial passive avoidance training. Brain Res. 1994;654:129–136. doi: 10.1016/0006-8993(94)91578-4. [DOI] [PubMed] [Google Scholar]
- 41.Leuner B, Falduto J, Shors TJ. Associative memory formation increases the observation of dendritic spines in the hippocampus. J Neurosci. 2003;23:659–665. doi: 10.1523/JNEUROSCI.23-02-00659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hongpaisan J, Alkon DL. A structural basis for enhancement of long-term associative memory in single dendritic spines regulated by PKC. Proc Natl Acad Sci USA. 2007;104:19571–19576. doi: 10.1073/pnas.0709311104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaufmann WE, Moser HW. Dendritic anomalies in disorders associated with mental retardation. Cerebral Cortex. 2000;10:981–991. doi: 10.1093/cercor/10.10.981. [DOI] [PubMed] [Google Scholar]
- 44.Purpura DP. Dendritic spine ‘dysgenesis’ and mental retardation. Science. 1974;186:1126–1128. doi: 10.1126/science.186.4169.1126. [DOI] [PubMed] [Google Scholar]
- 45.Huttenlocher PR. Dendritic development in neocortex of children with mental defect and infantile spasms. Neurology. 1974;24:203–210. doi: 10.1212/wnl.24.3.203. [DOI] [PubMed] [Google Scholar]
- 46.Marin-Padilla M. Pyramidal cell abnormalities in the motor cortex of a child with Down syndrome. A Golgi study. J Comp Neurol. 1976;167:63–81. doi: 10.1002/cne.901670105. [DOI] [PubMed] [Google Scholar]
- 47.Takashima S, Becker DL, Armstrong DL, Chan FW. Abnormal neuronal development in the visual cortex of the human fetus and infant with Down’s syndrome A quantitative and qualitative Golgi study. Brain Res. 1981;225:1–21. doi: 10.1016/0006-8993(81)90314-0. [DOI] [PubMed] [Google Scholar]
- 48.Armstrong D, Dunn JK, Antalffy B, Triveldi R. Selective dendritic alterations in the cortex of Rett syndrome. J Neuropathol Exp Neurol. 1995;54:195–201. doi: 10.1097/00005072-199503000-00006. [DOI] [PubMed] [Google Scholar]
- 49.Hinton VJ, Brown WT, Wisniewski K, Rudelli RD. Analysis of neocortex in three males with the fragile X syndrome. Am J Med Genet. 1991;41:289–294. doi: 10.1002/ajmg.1320410306. [DOI] [PubMed] [Google Scholar]
- 50.Swann JW, Al-Noori S, Jiang M, Lee CL. Spine loss and other dendritic abnormalities in epilepsy. Hippocampus. 2000;10:617–625. doi: 10.1002/1098-1063(2000)10:5<617::AID-HIPO13>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 51.Wong M. Modulation of dendritic spines in epilepsy: cellular mechanisms and functional implications. Epilepsy & Behav. 2005;7:569–577. doi: 10.1016/j.yebeh.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 52.Scheibel ME, Crandall PH, Scheibel AB. The hippocampal-dentate complex in temporal lobe epilepsy. Epilepsia. 1974;15:55–80. doi: 10.1111/j.1528-1157.1974.tb03997.x. [DOI] [PubMed] [Google Scholar]
- 53.Belichenko PV, Dahlstrom A. Studies on the 3-dimensional architecture of dendritic spines and varicosities in human cortex by confocal laser scanning microscopy and Lucifer yellow microinjections. J Neurosci Methods. 1995;57:55–61. doi: 10.1016/0165-0270(94)00125-z. [DOI] [PubMed] [Google Scholar]
- 54.Isokawa M, Levesque MF. Increased NMDA responses and dendritic degeneration in human epileptic hippocampal neurons in slices. Neurosci Lett. 1991;132:212–216. doi: 10.1016/0304-3940(91)90304-c. [DOI] [PubMed] [Google Scholar]
- 55.Blumcke I, Zuschratter W, Schewe JC, et al. Cellular pathology of hilar neurons in Ammon’s horn sclerosis. J Comp Neurol. 1999;414:437–453. doi: 10.1002/(sici)1096-9861(19991129)414:4<437::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 56.von Campe G, Spencer DD, Lanerolle NC. Morphology of dentate granule cells in the human epileptogenic hippocampus. Hippocampus. 1997;7:472–488. doi: 10.1002/(SICI)1098-1063(1997)7:5<472::AID-HIPO4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 57.Multani P, Myers RH, Blume HW, Schomer DL, Sotrel A. Neocortical dendritic pathology in human partial epilepsy: a quantitative Golgi study. Epilepsia. 1994;35:728–736. doi: 10.1111/j.1528-1157.1994.tb02503.x. [DOI] [PubMed] [Google Scholar]
- 58.Belichenko PV, Sourander P, Malmgren K, et al. Dendritic morphology in epileptogenic cortex from TRPE patients, revealed by intracellular Lucifer Yellow microinjection and confocal laser scanning microscopy. Epilepsy Res. 1994;18:233–247. doi: 10.1016/0920-1211(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 59.Isokawa M. Preservation of dendrites with the presence of reorganized mossy fiber collaterals in hippocampal dentate granule cells in patients with temporal lobe epilepsy. Brain Res. 1997;744:339–343. doi: 10.1016/S0006-8993(96)01067-0. [DOI] [PubMed] [Google Scholar]
- 60.Isokawa M. Remodeling dendritic spines in the rat pilocarpine model of temporal lobe epilepsy. Neurosci Lett. 1998;258:73–76. doi: 10.1016/s0304-3940(98)00848-9. [DOI] [PubMed] [Google Scholar]
- 61.Jiang M, Lee CL, Smith KL, Swann JW. Spine loss and other persistent alterations of hippocampal pyramidal cell dendrites in a model of early-onset epilepsy. J Neurosci. 1998;18:8356–8368. doi: 10.1523/JNEUROSCI.18-20-08356.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Willmore LJ, Ballinger WE, Jr, Boggs W, Sypert GW, Rubin JJ. Dendritic alterations in rat isocortex within an iron-induced chronic epileptic focus. Neurosurgery. 1980;7:142–146. doi: 10.1227/00006123-198008000-00005. [DOI] [PubMed] [Google Scholar]
- 63.Nishizuka M, Okada R, Seki K, Arai Y, Ilizuka R. Loss of dendritic synapses in the medial amygdala associated with kindling. Brain Res. 1991;552:351–355. doi: 10.1016/0006-8993(91)90104-4. [DOI] [PubMed] [Google Scholar]
- 64.Gonzalez-Burgos I, Lopez-Vazquez MA, Beas-Zarate C. Density, but not shape, of hippocampal dendritic spines varies after a seizure-inducing acute dose of monosodium glutamate in rats. Neurosci Lett. 2004;363:22–24. doi: 10.1016/j.neulet.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 65.Ampuero E, Dagnino-Subiabre A, Sandoval R, et al. Status epilepticus induces region-specific changes in dendritic spines, dendritic length and TrkB protein content of rat brain cortex. Brain Res. 2007;1150:225–228. doi: 10.1016/j.brainres.2007.02.089. [DOI] [PubMed] [Google Scholar]
- 66.Bundman MC, Pico RM, Gall CM. Ultrastructural plasticity of the dentate gyrus granule cells following recurrent limbic sesizures: I Increase in somatic spines. Hippocampus. 1994;4:601–610. doi: 10.1002/hipo.450040510. [DOI] [PubMed] [Google Scholar]
- 67.Suzuki F, Makiura Y, Guilhem D, Sorensen J-C, Onteniente B. Correlated axonal sprouting and dendritic spine formation during kainate-induced neuronal morphogenesis in the dentate gyrus of adult mice. Exp Neurol. 1997;145:203–213. doi: 10.1006/exnr.1997.6469. [DOI] [PubMed] [Google Scholar]
- 68.Spigelman I, Yan XX, Obenaus A, Lee EYS, Wasterlain CG, Ribak CE. Dentate granule cells form novel basal dendrites in a rat model of temporal lobe epilepsy. Neurosci. 1998;86:109–120. doi: 10.1016/s0306-4522(98)00028-1. [DOI] [PubMed] [Google Scholar]
- 69.Muller M, Gahwiler BH, Rietschin L, Thompson SM. Reversible loss of dendritic spines and altered excitability after chronic epilepsy in hippocampal slice cultures. Proc Natl Acad Sci USA. 1993;90:257–261. doi: 10.1073/pnas.90.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thompson SM, Fortunato C, McKinney RA, Muller M, Gahwiler BH. Mechanisms underlying the neuropathological consequences of epileptic activity in the rat hippocampus in vitro. J Comp Neurol. 1996;372:515–528. doi: 10.1002/(SICI)1096-9861(19960902)372:4<515::AID-CNE2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 71.Zha XM, Green SH, Dailey ME. Regulation of hippocampal synapse remodeling by epileptiform activity. Mol Cell Neurosci. 2005;29:494–506. doi: 10.1016/j.mcn.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 72.Nishimura M, Owens J, Swann JW. Effects of chronic network hyperexcitability on the growth of hippocampal dendrites. Neurobiol Dis. 2008;29:267–277. doi: 10.1016/j.nbd.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mizrahi A, Crowley JC, Shtoyerman E, Katz LC. High-resolution in vivo imaging of hippocampal dendrites and spines. J Neurosci. 2004;24:3147–3151. doi: 10.1523/JNEUROSCI.5218-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rensing NR, Ouyang Y, Yang XF, Yamada KA, Rothman SM, Wong M. In vivo imaging of dendritic spines during electrographic seizures. Ann Neurol. 2005;58:888–898. doi: 10.1002/ana.20658. [DOI] [PubMed] [Google Scholar]
- 75.Zeng LH, Xu L, Rensing NR, Sinatra PM, Rothman SM, Wong M. Kainate seizures cause acute dendritic spine loss and actin depolymerization in vivo. J Neurosci. 2007;27:11604–11613. doi: 10.1523/JNEUROSCI.0983-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fukazawa Y, Saitoh Y, Ozawa F, Ohta Y, Mizuno K, Inokuchi K. Hippocampal LTP is accompanied by enhanced F-actin content with the dendritic spine that is essential for late LTP maintenance in vivo. Neuron. 2003;38:447–460. doi: 10.1016/s0896-6273(03)00206-x. [DOI] [PubMed] [Google Scholar]
- 77.Okamoto KI, Nagai T, Miyawaki A, Hayashi Y. Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nature Neurosci. 2004;7:1104–1112. doi: 10.1038/nn1311. [DOI] [PubMed] [Google Scholar]
- 78.Lin B, Kramar EA, Bi X, Brucher FA, Gall CM, Lynch G. Theta stimulation polymerizes actin in dendritic spines of hippocampus. J Neurosci. 2005;25:2062–2069. doi: 10.1523/JNEUROSCI.4283-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim CH, Lisman JE. A role of actin filament in synaptic transmission and long-term potentiation. J Neurosci. 1999;19:4314–4324. doi: 10.1523/JNEUROSCI.19-11-04314.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ouyang Y, Wong M, Capani F, et al. A transient decrease in F-actin may be necessary for translocation of proteins into dendritic spines. Eur J Neurosci. 2005;22:2995–3005. doi: 10.1111/j.1460-9568.2005.04521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Halpain S, Hipolito A, Saffer L. Regulation of F-actin stability in dendritic spines by glutamate receptors and calcineurin. J Neurosci. 1998;18:9835–9844. doi: 10.1523/JNEUROSCI.18-23-09835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Meng Y, Zhang Y, Tregoubov V, et al. Abnormal spine morphology and enhanced LTP in LIMK-1 knockout mice. Neuron. 2002;35:121–133. doi: 10.1016/s0896-6273(02)00758-4. [DOI] [PubMed] [Google Scholar]
- 83.Chen LY, Rex CS, Casale MS, Gall CM, Lynch G. Changes in synaptic morphology accompany actin signaling during LTP. J Neurosci. 2007;27:5363–5372. doi: 10.1523/JNEUROSCI.0164-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Delorenzo RJ, Sun DA, Deshpande LS. Cellular mechanisms underlying acquired epilepsy: the calcium hypothesis of the induction and maintenance of epilepsy. Pharmacol Ther. 2005;105:229–266. doi: 10.1016/j.pharmthera.2004.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McNamara JO, Huang YZ, Leonard AS. Molecular signaling mechanisms underlying epileptogenesis. Sci STKE Science Signaling. 2006;10:356. doi: 10.1126/stke.3562006re12. [DOI] [PubMed] [Google Scholar]
- 86.Kurz JE, Sheets D, Parsons JT, Rana A, Delorenzo RJ, Churn SB. A significant increase in both basal and maximal calcineurin activity in the rat pilocarpine model of status epilepticus. J Neurochem. 2001;78:304–315. doi: 10.1046/j.1471-4159.2001.00426.x. [DOI] [PubMed] [Google Scholar]
- 87.Roth SU, Sommer C, Mundel P, Kiessling M. Expression of synaptopodin, an actin-associated protein, in the rat hippocampus after limbic epilepsy. Brain Path. 2001;11:169–181. doi: 10.1111/j.1750-3639.2001.tb00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ferhat L, Esclapez M, Represa A, Fattoum A, Shirao T, Ben-Ari Y. Increased levels of acidic calponin during dendritic spine plasticity after pilocarpine-induced seizures. Hippocampus. 2003;12:845–858. doi: 10.1002/hipo.10136. [DOI] [PubMed] [Google Scholar]
- 89.Ryu J, Liu L, Wong TP, et al. A critical role for myosin IIb in dendritic spine morphology and synaptic function. Neuron. 2006;49:175–182. doi: 10.1016/j.neuron.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 90.Szklarczyk A, Lapinska J, Rylski M, McKay RD, Kaczmarek L. Matrix metalloproteinase-9 undergoes expression and activation during dendritic remodeling in adult hippocampus. J Neurosci. 2002;22:920–930. doi: 10.1523/JNEUROSCI.22-03-00920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bilousova TV, Rusakov DA, Ethell DW, Ethell IM. Matrix metalloproteinase-7 disrupts dendritic spines in hippocampal neurons through NMDA receptor activation. J Neurochem. 2006;97:44–56. doi: 10.1111/j.1471-4159.2006.03701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Klettner A, Herdegen T. FK506 and its analogs – therapeutic potential for neurological disorders. Curr Drug Targets CNS Neurol Disord. 2003;2:153–162. doi: 10.2174/1568007033482878. [DOI] [PubMed] [Google Scholar]
- 93.Macleod MR, O’Collins T, Horky LL, Howells DW, Donnan GA. Systemic review and metaanalysis of the efficacy of FK506 in experimental stroke. J Cereb Blood Flow Metab. 2005;25:713–721. doi: 10.1038/sj.jcbfm.9600064. [DOI] [PubMed] [Google Scholar]
- 94.Sierra-Paredes G, Orieoro-Garcia T, Nunez-Rodriguez A, Vazquez-Lopez A, Sierra-Marcuno G. Seizures induced in vivo by latruculin a and jasplakinolide microperfusion in the rat hippocampus. J Mol Neurosci. 2006;28:151–160. doi: 10.1385/JMN:28:2:151. [DOI] [PubMed] [Google Scholar]