Abstract
Cocaine remains one of the most addictive substances of abuse and one of the most difficult to treat. Although increasingly sophisticated experimental and technologic advancements in the last several decades have yielded a large body of clinical and preclinical knowledge on the direct effects of cocaine on the brain, we still have a relatively incomplete understanding of the neurobiological processes that occur when drug use is discontinued. The goal of this manuscript is to review both clinical and preclinical data related to abstinence from cocaine and discuss the complementary conclusions that emerge from these different levels of inquiry. This commentary will address observed alterations in neural function, neural structure, and neurotransmitter system regulation that are present in both animal models of cocaine abstinence and data from recovering clinical populations. Although these different levels of inquiry are often challenging to integrate, emerging data discussed in this commentary suggest that from a structural and functional perspective, the preservation of cortical function that is perhaps the most important biomarker associated with extended abstinence from cocaine.
Keywords: cocaine, abstinence, neuroimaging, addiction, white matter, glutamate
A. Introduction
Chronic cocaine use is a seemingly intractable public health problem worldwide. Whether cocaine is snorted, injected, or smoked as crack, users often suffer serious negative consequences to their health, social relationships, as well as severe economic hardships. Although there have been many efforts to develop effective treatments, whether pharmacological or cognitive and behavioral, rates of relapse continue to be alarmingly high. Moreover, these relapse rates continue to be among the highest of all illegal drugs (Vocci, 2007). One substantial obstacle to the discovery of successful treatment approaches has been our rather incomplete understanding of the neurobiological processes that naturally occur when drug use is discontinued (likely best modeled in animals) as well as any unique features of the small population of addicts that are able to successfully abstain from cocaine for extended periods of time. Without a more complete picture of these structural and functional neuroadaptations, it is difficult to direct effective strategies towards targets with the greatest potential for promoting abstinence and reducing harm.
To understand the natural neural adaptations that follow discontinuation of drug use as well as neurological features that promote successful abstinence in humans, it is first necessary to understand the changes that directly result from cocaine exposure. Decades of robust molecular, genetic, cellular, and neural systems level studies have provided important insights in this area. One important approach that has been used in both human and animal models of chronic cocaine use is neuroimaging. This approach encompasses a wide range of in vivo and in vitro techniques capable of assessing neural function and structure, such as positron emission tomography (PET), functional magnetic resonance imaging (fMRI), diffusion tensor imaging, tissue morphometry, metabolic mapping, and receptor autoradiography, among others. Not only do these approaches have the advantage of being able to sample multiple brain regions simultaneously, but many in vivo approaches can be applied to human drug users and animal models alike providing for substantial translation and cross-validation of findings. Here, we focus on the insights and perspectives that imaging approaches have contributed to the issues that surround the long-term neural adaptations that follow discontinuation of cocaine use after chronic abuse and dependence.
Although there are many unanswered questions, this brief commentary will consider two fundamental questions about abstinence from continued cocaine use that we believe neuroimaging studies can in part address:
To what extent do the neurostructural and functional abnormalities that accompany chronic cocaine use either improve or persist following discontinuation of cocaine?
Are there patterns of neural function or structure that can be used as predictors of successful abstinence when given the choice to use?
A broad perspective is required in order to address these questions. In this commentary we examine complementary insights from clinical addiction research and preclinical animal models of drug use. When considered together these data give us a deeper understanding of the neurofunctional and structural adaptations that are present in both early and extended periods of abstinence.
B. Imaging the brain of cocaine abstainers: clinical research
As with many psychiatric diseases, the neuropathology present in cocaine-dependent individuals is not restricted to a single brain region, a single cell type, or a single neurotransmitter system. Rather substance dependence is frequently associated with disruptions in at least three major systems that contribute to behavior - limbic processing, cognition, and basic motor control. These systems span both cortical and subcortical regions of the brain and therefore are vulnerable not only to pathology in a local population of cells, but also in the white matter tracts that connect these regions.
Additionally, just as addiction is not limited to one spatially distinct disruption, there is also an important temporal component to the addiction process. That is, addiction exists on a continuum that likely extends from a vulnerable, drug-naïve individual that casually uses a drug, to an individual that becomes dependent, attempts abstinence and, typically, relapses. While several research groups have isolated traits that predict better than average treatment outcomes in cocaine users (Kampman et al., 2002; Poling et al., 2007; Sinha et al., 2007) there are still no FDA approved medications for cocaine dependence. Moreover, relapse rates are among the highest of all illegal drugs (Vocci, 2007).
Longitudinal studies of neural activity during this continuum are very difficult to perform in substance-dependent individuals for pragmatic reasons (e.g. identifying vulnerable individuals, loss to follow-up due to frequent changes in phone numbers, living arrangements, lack of transportation). There is, however, a growing body of research that has tried to address these questions. In this review we will discuss several studies which have investigated individuals at each stage of this continuum. In order to determine whether patterns of brain activity predict treatment success or relapse, however, it is important to first understand common structural and functional abnormalities present in the brain of a cocaine dependent individual.
Beyond the striatum: Altered activity in the prefrontal cortex of users and abstainers
Cocaine’s primary mechanism of action in the brain involves binding to the dopamine transporter which is highly concentrated in the basal ganglia (or striatum). Dopamine disruption in the striatum has been robustly studied in animal models of cocaine use and in several human imaging studies. Additionally however, many highly-cited human neuroimaging studies have revealed significantly lower rates of functional activity in the frontal cortex of cocaine users relative to non-drug using controls. This ‘hypofrontality’ was first documented in PET imaging studies which measured baseline glucose metabolism throughout the brain of cocaine users (Goldstein et al., 2004; Goldstein and Volkow, 2002; Volkow et al., 1991a; Volkow et al., 1992; Volkow et al., 2005).
Volkow and colleagues were also among the first to demonstrate that, in addition to a lower metabolic rate of glucose utilization, both currently active and recently abstinent cocaine users have lower levels of dopamine D2 receptors in both frontal and limbic regions of the cortex (Volkow et al., 1993). Baseline cerebral blood flow (CBF) is also significantly lower in chronic cocaine users compared with non-drug using controls, in the prefrontal and temporal cortices (Goldstein and Volkow, 2002; Holman et al., 1993; Strickland et al., 1993; Volkow et al., 1988).
Although many studies have assessed alterations in cognitive function of cocaine abusers after the cessation of drug use (Bolla et al., 2004; Bolla et al., 2003; Gottschalk et al., 2001), few studies have directly addressed the question of the persistence or potential changes in these abnormalities over the course of abstinence. One of the first and only longitudinal studies in this field was done by Volkow and colleagues (1991). They demonstrated that cerebral metabolism in the basal ganglia and ventral prefrontal cortex of cocaine abusers was elevated above control levels during the first week of abstinence (Volkow et al., 1991b). After 1 to 6 weeks of abstinence however, these acutely elevated cerebral metabolic rates had decreased. These decreases persisted in a subset of subjects tested again after 3 months, suggesting that many neurofunctional abnormalities persist after extended abstinence from cocaine.
It is yet unclear however, if more protracted periods of abstinence (greater than 6 months) are associated with better affective and neurofunctional outcomes. As demonstrated in methamphetamine abstainers (Wang et al., 2004), significant neocortical recovery may not occur until several months after abstinence begins. Emerging data from several laboratories, including our own, suggests that individuals who are able to maintain abstinence for a long period of time may in fact have higher levels of frontal cortex activity. A recent functional MRI study by Connolly and colleagues (2012) demonstrated that during a response inhibition task, individuals that had been abstinent from cocaine for 10–25 months (long term) had significantly higher blood oxygen level dependent (BOLD) signal in the prefrontal cortex during a response inhibition task than shorter term abstainers (1–5 weeks). Furthermore, whereas current cocaine users typically have lower prefrontal activity than controls during this task, both groups of successful abstainers had significantly greater prefrontal activity than controls (Connolly et al., 2012).
Extending these task-based findings to measurements of baseline neural activity, a preliminary study from our group has demonstrated that longer-term abstainers have significantly higher rates of baseline glucose metabolism in the frontal cortex than shorter term abstainers. For this FDG-PET study we enrolled 23 former cocaine users that were currently active participants in community-based outpatient and inpatient treatment programs, as well as 14 age-matched controls with no history of psychiatric illness or substance dependence. The former cocaine users in this study had been abstinent from cocaine for up to 14 months. For preliminary analysis these 23 individuals were divided into groups of short term (1–5 weeks), middle term (1–5 months), and long term (10–14 months) abstinence, similar to the groups reported by Connolly et al (2012). Preliminary analysis of these data demonstrates that, consistent with the original PET studies in this area, short term abstainers have lower neural activity in both the frontal cortex and subcortical areas relative to the matched controls (Figure 1). Similar to the aforementioned functional MRI results, the individuals that had maintained abstinence for 10 or more months had significantly higher rates neural activity (as measured by baseline glucose metabolism) in the frontal cortex relative to shorter term abstainers and age, gender, and education matched controls. The functional activity in the subcortical areas, however, did not vary as a function of length of abstinence. While these data are preliminary, cross-sectional, and from a limited sample, the results complement Connolly et al. (2012) and suggest that long-term abstinence from cocaine in humans may be more related to neural activity in the frontal cortex rather than the subcortical areas.
Figure 1.

Preliminary data demonstrating alterations in cerebral glucose metabolism in among a cohort of former cocaine users living in a residential treatment facility (n = 23) relative to age, gender and education matched controls (n = 14). These individuals had been abstinent for either less than 1 month (short-term, n = 6), between 1–5 months (middle-term, n = 10), or 10–20 months (long-term, n = 7). The colors superimposed on the gray-scale template indicate the areas of significant increases (red colormap, t-values) and decreases (blue colormap, t-values) in regional glucose metabolism in these subgroups relative to age, gender, and education matched controls (p<0.05, corrected clusters). The numbers above the images indicate the location of the coronal section in a standardized human template (Montreal Neurologic Institute).
When considering the relative role of the frontal cortex versus subcortical areas on the maintenance of abstinence, it is important to acknowledge that nearly all behaviors in the human repertoire are the result of complex interactions among neural systems which span multiple brain regions. The mesolimbic and mesocortical dopamine systems, for example, differentially contribute to motivational and cognitive aspects of cocaine dependence and relapse. Whereas they both depend on projections from the prefrontal cortex to subcortical areas, these circuits are both anatomically and functionally segregated, with the mesolimbic systems receiving more input from the medial prefrontal cortex and the mesocortical circuit receiving input from the lateral prefrontal cortex.
Several studies have now documented specific disruptions of baseline frontal-striatal circuitry in cocaine users (Gu et al., 2010; Hanlon et al., 2011b; Ma et al., 2010).
Although there are multiple ways to measure functional connectivity in the brain, one method that is actively being used in the addiction literature is resting-state BOLD imaging. By combining this technique with sophisticated data modeling and analysis it is possible to isolate combinations of neural regions which oscillate together, potentially aberrantly in substance users. Through this technique several groups have demonstrated that, at baseline, cocaine users have less functional connectivity within the mesolimbic dopamine system relative to controls. Furthermore, lower network connectivity among these limbic regions is correlated with longer histories of cocaine use (Gu et al., 2010; Ma et al., 2010). These disruptions in cortical-subcortical connectivity appear to be present after short periods (1–2 weeks) of abstinence (Kelly et al., 2011). There are however, currently no longitudinal investigations of resting state connectivity through the course of dependence and abstinence. Additionally, while the most pronounced differences in neural structure between cocaine users and controls tend to be in cortical areas, Barros-Loscertales and colleagues (2011) recently demonstrated that cocaine-dependent men have significantly lower gray matter volumes in the striatum, compared to controls (Barros-Loscertales et al., 2011).
The survivor effect? Altered neural structure among active users and abstainers
Finally, in addition to aberrant patterns of functional activity observed in cocaine users and abstainers, there is a growing body of evidence demonstrating structural pathology in the brains of cocaine users which may not be present among successful abstainers (Bartzokis et al., 2000; Fein et al., 2002; Liu et al., 1998; Moeller et al., 2005). Magnetic resonance imaging studies among cocaine users consistently report smaller volumes and lower tissue density in the prefrontal cortex of cocaine users relative to non-drug using controls, which may be correlated with length of use (O’Neill et al., 2001). Bartzokis and colleagues (2002) investigated white matter volume in a large cohort of cocaine dependent individuals and demonstrated that cocaine dependent individuals did not have the same age-related increases in white matter volume observed in non-drug using controls. These data suggest that there may be an arrested development of white matter among users (Bartzokis et al., 2002). Franklin et al. (2002) were the first to demonstrate lower density of gray matter in cocaine users using voxel-based morphometry. They reported lower gray matter density in the insula cortex, medial orbitofrontal cortex, superior temporal cortex, and right anterior cingulate (Franklin et al., 2002). Sim et al. (2007) recently reported lower white matter density in the right cerebellum and lower gray matter density in the premotor cortex, temporal cortex, frontal cortex, left thalamus, and cerebellum in current cocaine users (Sim et al., 2007). Given that neural structure is largely inherited and is sensitive to many other environmental stressors that coexist in chronic cocaine users (such as alcohol abuse, chronic hypertension, perinatal stress), however, it is difficult to interpret these data. An innovative study by Ersche and colleagues (2012), provided some insight into the potential heritability of these neurostructural abnormalities (Ersche et al., 2012). They investigated gray matter tissue density and white matter integrity among 50 sibling pairs (one cocaine dependent, one with no history of drug dependence), and 50 unrelated healthy controls. Relative to the controls, the sibling pairs (both the user and non-user) had region specific differences in gray matter density in multiple brain regions that are implicated in addiction (e.g. lower gray matter density in the posterior insula and higher density in the caudate). Between the siblings, the stimulant dependent individual had significantly lower tissue density in the vicinity of the orbitofrontal cortex. These data suggest that while some of the alterations in neural structure observed in chronic cocaine users may be related to an endophenotype that was inherited, the drug-using sibling may have significantly lower gray matter than the non-drug using sibling in the orbitofrontal cortex, a brain region critical to the motivational and compulsive aspects of addiction (Volkow & Fowler, 2000).
The relationship between cocaine abstinence and neural tissue integrity however, is unclear and has not been studied in a longitudinal manner. Matochik et al. (2003) demonstrated that individuals abstinent from cocaine for approximately 20 days had lower gray matter density in the cingulate gyrus, lateral prefrontal cortex, and medial and lateral aspects of the orbitofrontal cortex than controls (Matochik et al., 2003). A study of polydrug abusers that reported abstinence from cocaine for approximately 4 years also demonstrated lower gray matter volume in the orbitofrontal cortex compared with controls (Tanabe et al., 2009). Whereas these studies suggest that in both short and longer tem abstinence, cocaine users have significantly lower tissue density in the orbitofrontal cortex, a recent study by our group demonstrated individuals that are able to remain abstinent for more than 30 days have greater gray and white matter density in many other cortical areas than current users or rigorously matched controls (Hanlon et al., 2011a).
One interpretation of these data is that that structural abnormalities associated with chronic cocaine use may be reversed with extended abstinence. Elevated gray and white matter integrity is well documented in individuals abstaining from alcohol. In these individuals, lower gray and white matter volumes recover to baseline levels after a several weeks of abstinence (Gazdzinski et al., 2005a; Gazdzinski et al., 2005b; Pfefferbaum et al., 1995; Pfefferbaum et al., 1998). Gray matter density also increases during methamphetamine abstinence in the striatum, nucleus accumbens, and parietal cortex (Jernigan et al., 2005). Positron emission tomography studies have revealed elevated glucose metabolism throughout the cortex of methamphetamine abstainers with the greatest increase (≥20%) in the parietal cortex (Berman et al., 2008; London et al., 2004).
Although the mechanism through which gray matter density increases is unclear, it is possible that, in the absence of chronic stimulation by cocaine, dendritic spine density is able to increase in the prefrontal cortex and/or the afferent axons are able to arborize, creating a greater density of local connections. A likely mechanism through which white matter density may increase following discontinuation of cocaine is through the maturation of oligodendrocytes which are sensitive to levels of both glutamate (Gallo et al., 1996) and dopamine (Bongarzone et al., 1998; Howard et al., 1998) (via AMPA and D2/D3 receptors). While chronic cocaine use is associated with lower levels of myelin, in abstinence these immature oligodendrocytes are likely able to develop thereby increasing the integrity and density of the myelin sheaths in the brain.
Alternately, however, beyond basic neurobiological explanations, it is possible that elevated neural tissue density among long-term abstainers may be a “survivor effect.” That is, the lack of differences in neural structure observed in longer term abstainers is because these individuals represent a unique sub-population of drug users that have a greater level of frontal cortical integrity at the beginning of abstinence. This elevated cortical integrity in brain regions involved in self-control may enable them to maintain abstinence for a longer time. This ‘survivor’ effect may also explain the elevated levels of frontal glucose metabolism that have been observed in these long term abstainers (Connolly et al., 2012).
Considered together, there are several themes that emerge from this research
First, in chronic users functional and structural abnormalities in the brain extend beyond dopamine-rich subcortical areas to the glutamate and GABA-rich areas of the prefrontal and temporal cortices which in turn project to the striatum. Second, individuals that remain abstinent for several months likely have higher levels of functional and structural integrity in these cortical areas – possibly a “survivor effect.” Finally third, the high levels of individual variability in substance abuse treatment success and relapse may be associated with neural endophenotypes and variations in the integrity of the prefrontal cortex. While these endophenotypes may increase one’s likelihood of using stimulant drugs (as in the study of Ersche et al., 2012), they may alternatively increase the likelihood that an individual will remain abstinent (as in the study of Connolly et al., 2012).
Although these three themes in the human imaging literature provide a unique window into the neurobiologic ‘fingerprint’ of addiction and abstinence, there are multiple aspects of human substance abuse research which make it very challenging to examine these themes with more rigorous scientific detail. From a clinical perspective, cocaine dependent individuals often have psychiatric comorbidities including depression and post-traumatic stress disorder, and often abuse other legal and illegal drugs. It is also very difficult to perform longitudinal studies on human cocaine users. This challenge results in most of the studies of individuals abstaining from cocaine to be limited to the scope of the typical 1–4 week timeframe of a treatment programs.
C. Investigating the consequences of abstinence from cocaine: preclinical research
There is no question that studies in human drug abusers, current and abstaining, can provide the best evidence about the course of this disorder. Among others, the ability to obtain verbal reports of the feelings engendered by the drug, factors that lead to drug use, the sources of drug craving, or the factors that lead to the motivation to quit, are among some of the obvious advantages of studies in human users. But there are many questions that cannot be readily answered, especially some of the questions posed here about abstinence. The interpretation of studies of human drug abusers is limited by such factors as the highly variable drug histories among the participants, current and prior history of legal and illegal drug use other than cocaine, and frequently co-morbid psychiatric conditions including depression and post-traumatic stress disorder. Another issue that is often limiting is the considerable differences in inclusion criteria for drug use and abstinence, as well as the reliance on self-reported drug use history. Longitudinal studies of abstinence are further hampered by the socioeconomic challenges faced by many of these individuals including lack of steady housing, adequate nutrition, and access to mental health care services necessary to fund treatment. But from the perspective of understanding structural and functional brain changes associated with abstinence, an important problem is distinguishing between those effects produced by the cessation of cocaine use and those effects that predate the initiation of drug use. This is a question best addressed by preclinical studies in animal models.
With animal models, precise control over drug experience in terms of duration of exposure, total intake, and use of other drugs can be carefully controlled. Systematic manipulation of these and other variables can ensure that the results are attributable to the variables in question and provide a framework for mechanistic studies. Another important advantage is the use of well-matched control groups in preclinical studies. It is often much more difficult to match subjects in human studies on key demographic variables. This is an important strength of animal models that is frequently overlooked. Finally, studies of the neurobiological consequences of abstinence in animal models have the important advantage of ensuring abstinence from both the drug in question and any other drug.
Animal models of drug self-administration have proven to be valid predictors of multiple aspects of human drug abuse (Johanson and Fischman, 1989; Schuster and Johanson, 1981) and have allowed us to investigate the neurochemistry of use and abstinence more precisely than is possible in human cocaine abusers. Furthermore, animal models enable us to identify behavioral, pharmacological, and neurobiological variables that mediate cocaine use and abuse. These include decreases in dopamine D2 receptors (Beveridge et al., 2009; Nader et al., 2002b), alterations in dopamine release dynamics (Bradberry, 2000; Bradberry et al., 2000; Wheeler and Carelli, 2009), white matter impairments (Nielsen et al., 2012), and disruptions of glutamate signaling (Kalivas, 2008; Wolf, 2010), as well as changes in a myriad of other systems. While studies in humans have demonstrated differences between cocaine users and healthy controls on many of these same measures, animal models are able to uniquely describe the neuroadaptations that are a direct result of drug exposure rather than a consequence of differences that predate drug use, as is a common caveat in clinical research.
But are such neuroadaptations permanent even with the cessation of cocaine exposure? Does the cessation of drug use lead to a restoration of structure and function disrupted during exposure? Again, these are questions where animal models can provide important insights into the process and consequences of abstinence by allowing for the systematic manipulation of environmental variables, ensuring the absence of cocaine use, removing the complications that come from other legal and illicit drug use, and avoiding the confounds of co-morbid psychiatric disorders.
Is there evidence for recovery? Dopamine systems
One of the most robust findings from imaging studies of cocaine dependent individuals, and certainly among the most studied in cocaine abusers as well as in animal models, is the dysregulation of the dopamine system. Dopamine D2 receptors have been shown consistently to be significantly lower in cocaine dependent individuals (Martinez et al., 2004; Volkow et al., 1993), whereas the levels of dopamine transporters have been found to be elevated (Malison et al., 1998; Mash et al., 2002; Staley et al., 1994) compared to healthy controls. These differences in the dopamine system are accompanied by decreases in stimulated dopamine release, again as measured with PET (see (Volkow et al., 1997) and (Martinez et al., 2007)). Data from investigations in animal models largely corroborate these findings (Letchworth et al., 2001; Mateo et al., 2005; Nader et al., 2002a; Nader et al., 2006), suggesting that the alterations are a consequence of drug use, rather than any pre-existing conditions.
Rodent studies have provided strong evidence for dysregulation of the dopamine system following abstinence. Samuvel et al. (2008), for example, investigated the molecular mechanisms of DAT regulation following 3 weeks abstinence from cocaine self-administration and found significantly higher uptake of dopamine in the caudate putamen and nucleus accumbens, higher surface expression of DAT and decreased serine phosphorylation in the caudate-putamen (Samuvel et al., 2008). Similarly Jones and her colleagues demonstrated that 7 days after the cessation of binge cocaine self-administration, basal levels of dopamine were reduced in the nucleus accumbens, as was electrically and cocaine stimulated release in this same brain region (Mateo et al., 2005). Although longer withdrawal periods were not tested, these data support significant functional dysregulation of the dopamine system during the early stages of abstinence.
Studies in nonhuman primates have shown similar dysregulation in the early stages of withdrawal. Elevations in the concentrations of dopamine D1 receptors evident after chronic cocaine self-administration remained after cessation of drug use. But, 30 days after exposure to cocaine was discontinued, the densities of dopamine D1 receptors were higher throughout both the dorsal and ventral striatum to levels well beyond the already elevated concentrations resulting from cocaine self-administration experience (Figure 2; (Beveridge et al., 2009). The levels of dopamine transporters followed a very similar pattern. However, if the period of drug withdrawal was extended to 90 days, there was evidence of a return to control levels in both systems. These findings strongly suggest that alterations in dopamine systems associated with cocaine exposure are not necessarily permanent and that with extended abstinence may be reregulated to more normal levels of functioning.
Figure 2.
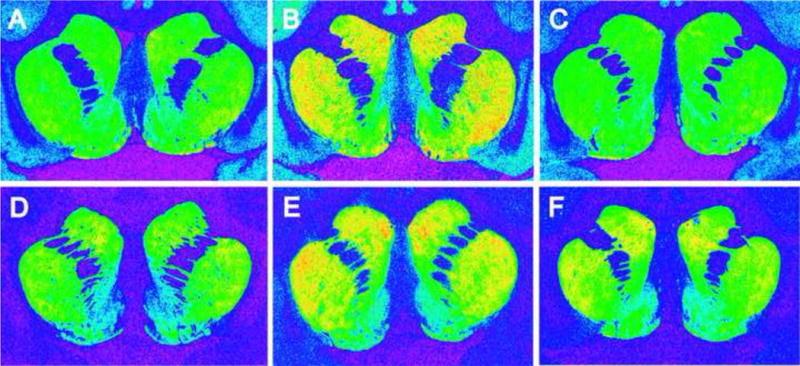
Representative autoradiograms of [3H]SCH 23390 binding to dopamine D1 receptors (top panel) and [3H]WIN 35,428 binding to dopamine transporters (bottom panel) in coronal sections of rhesus monkey striatum. Panels A and D: control animal responding for food-reinforcement. Panels B and E: cocaine self-administration animal with 30 days abstinence. Panels C and F: cocaine self-administration animal with 90 days abstinence (adapted from Beveridge et al, 2009).
In human cocaine abusers, the reductions in D2 receptor concentrations persist into abstinence (Volkow et al., 1993). It is difficult to draw conclusions about the duration of this effect, however, since all of the participants had relapsed by the end of a few months. Furthermore, it is possible that lower D2 receptor concentrations may have predated drug use. In animal models where greater control over environmental and pharmacological variables can be exercised, Nader and colleagues have reported that D2 receptor availability following limited (less than a month) cocaine exposure in nonhuman primates, recovered to control levels after only 1–3 weeks. However, this was not the case when longer periods (12 months) of exposure to cocaine self-administration were investigated. Here, recovery was found in only 60% of the monkeys within 3 months, whereas in the other 40% there was no evidence of recovery even after as long as a year after the cessation of cocaine use. These data emphasize the significance of the exposure period prior to the discontinuation of drug use. It is likely that because of the relatively short periods of exposure used in many studies using animal models, the intensity and duration of many neuroadaptations may be underestimated.
The critical element in these studies, however, is the range of individual differences in the degree of normalization of dopamine systems, particularly in those studies that considered long durations of withdrawal from cocaine use. Martinez and co-workers (2011) have recently shown that among treatment seeking cocaine abusers better treatment outcomes were associated with higher levels of dopamine stimulated release as measured with PET (Martinez et al., 2011). Whether the higher dopamine transmission was due to recovery of the system following drug cessation or a “survivor” effect as described earlier, dopamine transmission may provide an important marker for successful abstinence.
Does the Structure of the Brain Change?
Mirroring findings in human cocaine abusers, a number of studies have reported that cocaine exposure produces significant changes in the structure of gray and white matter. Terry Robinson and Bryan Kolb, along with their colleagues, have shown that there are significant modifications in the architecture of dendrites and dendritic spines, particularly in the prefrontal cortex and nucleus accumbens, of rats after exposure to psychostimulants including both cocaine and amphetamines (Robinson and Kolb, 1999). Repeated exposure to cocaine, whether administered contingently, or non-contingently by the experimenter, produced increased spine density of medium spiny neurons in the accumbens and pyramidal cells in the medial prefrontal cortex (Robinson et al., 2001). These structural changes in dendritic organization have been shown to persist for up a month following the cessation of cocaine self-administration (Robinson and Kolb, 1999) and for as long as 3.5 months after amphetamine exposure (Li et al., 2003). There is some evidence, however, that these cocaine-associated changes may be reversible after longer periods of abstinence (Kolb et al., 2003). The observation that alterations in neural spine density following extended cocaine exposure may return to baseline levels only after extended periods of abstinence, is consistent with the pattern observed in the white matter of cocaine users – namely, as mentioned above, lower white matter integrity observed in chronic users is present in short term abstainers but not in individuals that have been abstinent for several months (Hanlon et al., 2011a). In the case of human abstainers, many of the cognitive deficits observed in active users and short term abstainers are also not present in long-term abstainers (Hanlon et al., 2011a).
Several investigations have also documented changes in the integrity of white matter in the corpus callosum of rats exposed to chronic cocaine (Ma et al., 2009; Narayana et al., 2009). Four weeks of cocaine exposure via minipump resulted in significant changes in fractional anisotropy in the splenium of the corpus callosum, as measured with diffusion tensor imaging. This was accompanied by decreases in myelin basic protein (Narayana et al., 2009). Preliminary findings from our lab have demonstrated that prolonged cocaine exposure in rhesus monkeys was associated with reductions in both myelin basic protein and proteolipid protein (Smith et al, 2011, 2012). However, the decreases in this study were restricted to more anterior regions of the corpus callosum, more consistent with those observed in humans, as described earlier in this review. More recent studies have addressed the question of the persistence of these changes by measuring DNA methylation of genes for transcription factors that regulate myelination in rats following 14 days of cocaine self-administration. These investigators report that within 24 hours of drug cessation there is an increase in methylation, followed by a hypomethylation observed 30 days after withdrawal from cocaine self-administration (Nielsen et al., 2012). These findings highlight the importance of the time after drug withdrawal as a critical factor in determining the nature of the adaptations. Early in the withdrawal phase, the changes observed are most likely to be the direct result of compensatory actions in response to drug removal. While later in the withdrawal phase, the changes are much more likely to represent more stable persistent alterations in brain structure.
Whether these structural changes result in altered functional and behavioral outcomes is the key question that remains to be investigated further. Although very preliminary, studies in our laboratory have begun to address the question of persistent alterations in basal levels of functional activity, as assessed with the 2DG method, following the cessation of cocaine use in nonhuman primate models of cocaine exposure. In these studies functional activity was measured in a neutral environment not associated with the availability of drug or other reinforcers. Persistent reductions in basal activity were observed in the striatum and prefrontal cortex as long as 30 days after the cessation of drug use. Of interest, the functional activity within the prefrontal cortex of these monkeys was highly variable as measured 30 days after the last cocaine exposure. This variability may reflect a different temporal course of recovery in some animals as compared to others, reminiscent of the differential restoration of dopamine systems observed by Nader and his colleagues (Nader et al., 2006).
It is tempting to speculate that these animals with higher basal rates of glucose metabolism in the prefrontal cortex may be most likely to exhibit greater behavioral recovery, based on findings described earlier in human cocaine abusers. Those able to remain abstinent for long periods of time (10 months or greater) appeared to have greater cortical functional activity, again suggesting that this may be an important indicator of successful abstinence. This translational line of research however, is clearly preliminary and needs further investigation. Nonetheless, the value of these studies in animal models is clearly the identification of the molecular and cellular events that lead to the changes which, in the long term, may result in the development of strategies to reverse, compensate or prevent such deficits that can have a very profound impact on cognitive function.
D. Can We Bridge Preclinical and Clinical Research?
Throughout this commentary we have mentioned several ways in which well-controlled experiments in animal models of addiction can provide valuable interpretations for functional and structural findings from clinical research (i.e. extended exposure to cocaine leading to variability in the recovery of the dopamine rich striatum, and oligodendrocyte maturation as an explanation for elevated white matter in abstinence). There are at least two other promising lines of research however that we would like to mention as models for bridging preclinical and clinical research:
Investigating the role of BDNF
One of the ways in which animal models have helped our clinical understanding of cocaine addiction is the identification of the important role of brain derived neurotrophic factor (BDNF). While it has been shown to play a role in various forms of plasticity (Black, 1999; Bramham and Messaoudi, 2005), BDNF is also involved in the survival of dopamine neurons (Hyman et al., 1991). BDNF knockout mice have reduced locomotor responses to cocaine and diminished cocaine-induced conditioned place preference, compared to wild-type littermates (Hall et al., 2003). Subsequently it was shown that infusions of BDNF into the medial prefrontal cortex attenuate the reinstatement of cocaine-seeking (Berglind et al., 2007) and prevent cocaine-induced alterations in nucleus accumbens glutamate levels (Berglind et al., 2009). Interestingly, levels of BDNF in the ventral tegmental area, amygdala and nucleus accumbens have been shown to progressively increase following withdrawal from cocaine self-administration (Grimm et al., 2003), suggesting that regulation of the protein may continue to occur even after the cessation of drug use. Moreover, elevated BDNF expression occurred in parallel with an enhanced response to cocaine cues (the „incubation of craving’ phenomenon). These data suggest that changes in BDNF levels may result directly in changes in behavior.
These rodent data led clinical researchers to investigate whether BDNF levels were altered in human cocaine users (Angelucci et al., 2007; D’Sa et al., 2011; Jiang et al., 2009). D’Sa et al (2011) conducted a study in 3-week abstinent inpatient cocaine-dependent users and prospectively followed them up to 90 days following discharge. While on the inpatient unit, they reported an elevation in serum BDNF levels compared with healthy controls. Interestingly, higher serum levels of BDNF predicted a shorter time to relapse following discharge from the inpatient unit, and greater amount of cocaine used. The authors, and others, posited, therefore, that BDNF may act as a biomarker that may predict relapse to cocaine use (D’Sa et al., 2011; McGinty and Mendelson, 2011). These clinical data in turn encouraged additional research using animal models. This ‘reverse translational’ approach has resulted in a greater understanding the molecular events involved in BDNF regulation following cocaine exposure. Sadri-Vakili and colleagues have shown recently, for example, that cocaine self-administration in rodents resulted in elevated levels of BDNF mRNA in the striatum and medial prefrontal cortex. This increase was associated with cocaine-induced alterations in chromatin remodeling, including histone acetylation (Sadri-Vakili et al., 2010; Schmidt et al., 2012).
Imaging neurochemistry non-invasively
Positron emission tomography is clearly the primary means through which we are currently able to translate findings in animal models of chronic cocaine use and abstinence (often in non-human primates) to clinical populations through various radioligands. Unfortunately however, PET in human users is fairly invasive (requiring that addicts are given a dose of radiation and an arterial or venous line), expensive, and only available at research sites with rapid access to the radioligands. Recently however, there have been significant developments in a non-invasive, widely available MRI technique, magnetic resonance spectroscopy (MRS). This technique uses the properties of magnetic resonance to probe the neurochemical composition of the tissue being examined and is routinely used in academic hospitals to investigate tissue composition. While its reliability initially was limited to markers of neural health, inflammation and energy metabolism, such as creatine, myo-inositol, and N-acetylaspartate, it is now possible to isolate levels of glutamate and GABA (Licata and Renshaw, 2010) and can be applied to non-human primates as well as rodent models of addiction. Although it has not been a primary area of focus in this commentary, both glutamate and GABA are very important components to the maintenance of cocaine taking and are disrupted during abstinence. There have been a number of recent reviews detailing many of the changes in this system in animal models of addiction (Kalivas and Volkow, 2011; Wolf, 2010).
Several early MRS studies demonstrated that cocaine users have 30% less GABA in the prefrontal cortex (Ke et al., 2004) and 23% less GABA in the occipital cortex than controls (Hetherington et al., 2000). Yang and colleagues (2009) at the National Institute on Drug Abuse were the first to demonstrate that cocaine users have significantly lower glutamate/creatine ratios in the anterior cingulate cortex than controls, and that this was related to length of use (Yang et al., 2009). Schmaal and colleagues (2012) administered N-acetylcysteine (which blocks cocaine reinstatement in rodents (Zhou and Kalivas, 2008)), to cocaine users and demonstrated that it normalized the elevated glutamate/creatine ratios in these users. Although it is difficult to rectify the results of these two studies, they both suggest that altered glutamate function is not restricted to the nucleus accumbens in human addicts. Investigating glutamate and GABA concentrations in human cocaine users however, is still in its infancy. Future research in this area holds tremendous potential for translating many of the basic science results of altered glutamate and GABA activity to new, potentially patient-tailored treatment options for human cocaine users.
E. Conclusions
Here, we have presented a collection of findings (by no means intended to be exhaustive) from the perspectives of clinical and preclinical science in an attempt to answer two questions about the cessation of cocaine use after a prolonged history of drug use: 1) whether the neuroadaptations that result from cocaine exposure can reverse with abstinence, and 2) whether there are markers or neural signatures that can predict recovery. We have addressed these questions through examples from both clinical and preclinical research.
On the surface, much of what we now know about the process of recovery from human cocaine abusers and animal models may seem to be worlds apart. Human studies address the disease as it exists, but can be difficult to interpret because there is little knowledge of pre-drug conditions, polydrug abuse and psychiatric co-morbidities. Animal studies can be systematic and carefully control variables, but do not adequately model the pattern of human drug use or the route of administration of the drug and frequently employ very short drug exposure. While human neuroimaging studies provide us with a lens that can only assess activity in relatively large areas of the brain, preclinical studies focus on transmitter systems that elegantly resolve alterations that occur in pre and postsynaptic densities at the synaptic level. While clinical research on abstinence frequently uses “time to relapse” as a dependent measure, preclinical research typically reintroduces cocaine at a predetermined time point and uses behavior as the dependent measure. Other studies have attempted to examine the persistence of some cellular and molecular processes and how these change with time. Despite these seemingly independent lines of inquiry, there are more and more opportunities for convergence, such as through the role of BDNF, white matter depletion and restoration, and finally through evolving techniques which allow us to non-invasively quantify neurotransmitter levels in addicts that are able to successfully abstain from cocaine.
While we have focused on the alterations in brain structure, neurotransmitter system regulation, and functional activity in the brains of cocaine users and abstainers, we have not addressed the critical behavioral hallmarks of the addiction and recovery process. From impulsivity to craving, cognitive set-shifting to measures of self-control, there is a very large body of research which has characterized behavioral phenotypes of addiction in both human users and in non-human primates following exposure to cocaine (Beveridge et al., 2008; Garavan and Hester, 2007; Stalnaker et al., 2009). As we attempt to bridge the neurobiological findings from clinical and preclinical studies of cocaine use and abstinence, it will be important to integrate the literature on behavioral patterns that predict successful recovery. Through the integration of these components we will be much more likely to generate individually-tailored therapies, both pharmacologic and behavioral, for those that find themselves on this continuum of addiction, from vulnerable adolescents to treatment-seeking individuals that recurrently relapse.
Along these lines, perhaps the most important difference between clinical and preclinical investigations of cocaine abstinence is the inability to address the motivation to remain drug free
Animal models of abstinence cannot effectively model the motivation for discontinuing drug use and therefore, cannot mimic the human situation. In animal models, the cessation of drug use is imposed on the animal. This would be analogous to being placed onto a locked ward with no access to drug. In contrast, the motivation of human cocaine users to quit generally stems from fear of negative consequences to their health, welfare, or because of the presence of stronger non-drug reinforcers in the environment (e.g., contingency management). In either case, the intrinsic motivation of drug users to quit and remain abstinent is not modeled in most animal studies. Volition and self-control are not generally considered in animal models. Similarly, neither forced drug withdrawal or extinction procedures mimic the human situation. So as with animal models of other disorders, it is important to consider their limitations along with their strengths and focus on the aspects of the neurobiology that are relevant.
Indeed some attempt has been made using animal models to introduce an element of choice during self-administration (Banks and Negus, 2012; Griffiths et al., 1975; Johanson, 1975; Nader and Woolverton, 1991). These studies typically employ a paradigm in which two levers are presented simultaneously. Responses on one lever result in drug delivery while responding on the other results in delivery of an alternate reinforcer (such as food or sweetened water). These data have provided important insights; for example, some animals prefer to respond on the food-associated lever versus cocaine-associated lever when the magnitude of the alternate reinforcer was sufficient to decrease the potency of the positive reinforcing effects of cocaine (Lenoir et al., 2007; Nader and Woolverton, 1991). These data suggest that, as with humans, self-administration can be attenuated by increasing the value of alternate positive reinforcers (contingency management).
Finally, with regard to biomarkers that can predict successful recovery from cocaine abuse, we propose here that it is preservation of cortical function that is perhaps the most important predictor. This is supported by the lack of cortical structural and functional deficits in those cocaine abusers that have been able to remain abstinent for long periods of time, often beyond a year at the time of testing (references). We have argued that these individuals may represent a unique sub-population of drug users that have been able to remain abstinent because of greater structural cortical integrity and resulting function. Those that exhibit greater degrees of structural and functional cortical damage are far less likely to be able to muster the resources to remain abstinent for even brief periods of time. Longitudinal studies will be necessary to confirm this hypothesis, but increasing numbers of studies that have been focused on the biology of successful recovery support this idea. While clinical studies will be key, preclinical studies can provide important insights into the essential processes that are both necessary and for identification of targets for treatment.
Highlights.
Clinical and preclinical research both reveal neural adaptations cocaine abstinence
Functional and structural changes occur in the cortex as well as subcortical dopamine systems
The integrity of the prefrontal cortex is likely the best hallmark of extended abstinence
There are some uniquely human aspects of abstinence that are difficult to model in abstinence
Acknowledgments
Funding for this work was provided by National Institutes of Health grants K01DA027756, DA09085 and DA06634. The authors received no compensation from other external organizations related to this manuscript and declare no competing financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angelucci F, Gruber SH, El Khoury A, Tonali PA, Mathe AA. Chronic amphetamine treatment reduces NGF and BDNF in the rat brain. Eur Neuropsychopharmacol. 2007;17:756–762. doi: 10.1016/j.euroneuro.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Banks ML, Negus SS. Preclinical Determinants of Drug Choice under Concurrent Schedules of Drug Self-Administration. Adv Pharmacol Sci. 2012;2012:281768. doi: 10.1155/2012/281768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros-Loscertales A, Garavan H, Bustamante JC, Ventura-Campos N, Llopis JJ, Belloch V, Parcet MA, Avila C. Reduced striatal volume in cocaine-dependent patients. Neuroimage. 2011;56:1021–1026. doi: 10.1016/j.neuroimage.2011.02.035. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Edwards N, Bridge P, Mintz J. Brain maturation may be arrested in chronic cocaine addicts. Biol Psychiatry. 2002;51:605–611. doi: 10.1016/s0006-3223(02)01315-x. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Edwards N, Rapoport R, Wiseman E, Bridge P. Age-related brain volume reductions in amphetamine and cocaine addicts and normal controls: implications for addiction research. Psychiatry Res. 2000;98:93–102. doi: 10.1016/s0925-4927(99)00052-9. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, See RE, Fuchs RA, Ghee SM, Whitfield TW, Jr, Miller SW, McGinty JF. A BDNF infusion into the medial prefrontal cortex suppresses cocaine seeking in rats. Eur J Neurosci. 2007;26:757–766. doi: 10.1111/j.1460-9568.2007.05692.x. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, Whitfield TW, Jr, LaLumiere RT, Kalivas PW, McGinty JF. A single intra-PFC infusion of BDNF prevents cocaine-induced alterations in extracellular glutamate within the nucleus accumbens. J Neurosci. 2009;29:3715–3719. doi: 10.1523/JNEUROSCI.5457-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman SM, Voytek B, Mandelkern MA, Hassid BD, Isaacson A, Monterosso J, Miotto K, Ling W, London ED. Changes in cerebral glucose metabolism during early abstinence from chronic methamphetamine abuse. Mol Psychiatry. 2008;13:897–908. doi: 10.1038/sj.mp.4002107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge TJ, Gill KE, Hanlon CA, Porrino LJ. Review. Parallel studies of cocaine-related neural and cognitive impairment in humans and monkeys. Philos Trans R Soc Lond B Biol Sci. 2008;363:3257–3266. doi: 10.1098/rstb.2008.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge TJ, Smith HR, Nader MA, Porrino LJ. Abstinence from chronic cocaine self-administration alters striatal dopamine systems in rhesus monkeys. Neuropsychopharmacology. 2009;34:1162–1171. doi: 10.1038/npp.2008.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge TJR, Smith HR, Nader MA, Porrino LJ. Persistent elevations in the density of group II mGluRs in the striatum of nonhuman primates following abstinence from chronic cocaine self-administration; Society for Neuroscience Annual Meeting; San Diego, CA. 2010. [Google Scholar]
- Black IB. Trophic regulation of synaptic plasticity. J Neurobiol. 1999;41:108–118. [PubMed] [Google Scholar]
- Bolla K, Ernst M, Kiehl K, Mouratidis M, Eldreth D, Contoreggi C, Matochik J, Kurian V, Cadet J, Kimes A, Funderburk F, London E. Prefrontal cortical dysfunction in abstinent cocaine abusers. J Neuropsychiatry Clin Neurosci. 2004;16:456–464. doi: 10.1176/appi.neuropsych.16.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, Funderburk FR, Ernst M. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage. 2003;19:1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongarzone ER, Howard SG, Schonmann V, Campagnoni AT. Identification of the dopamine D3 receptor in oligodendrocyte precursors: potential role in regulating differentiation and myelin formation. J Neurosci. 1998;18:5344–5353. doi: 10.1523/JNEUROSCI.18-14-05344.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradberry CW. Acute and chronic dopamine dynamics in a nonhuman primate model of recreational cocaine use. J Neurosci. 2000;20:7109–7115. doi: 10.1523/JNEUROSCI.20-18-07109.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradberry CW, Barrett-Larimore RL, Jatlow P, Rubino SR. Impact of self-administered cocaine and cocaine cues on extracellular dopamine in mesolimbic and sensorimotor striatum in rhesus monkeys. J Neurosci. 2000;20:3874–3883. doi: 10.1523/JNEUROSCI.20-10-03874.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Connolly CG, Foxe JJ, Nierenberg J, Shpaner M, Garavan H. The neurobiology of cognitive control in successful cocaine abstinence. Drug Alcohol Depend. 2012;121:45–53. doi: 10.1016/j.drugalcdep.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Sa C, Fox HC, Hong AK, Dileone RJ, Sinha R. Increased serum brain-derived neurotrophic factor is predictive of cocaine relapse outcomes: a prospective study. Biol Psychiatry. 2011;70:706–711. doi: 10.1016/j.biopsych.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET. Abnormal brain structure implicated in stimulant drug addiction. Science. 2012;335:601–604. doi: 10.1126/science.1214463. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Meyerhoff DJ. Prefrontal cortical volume reduction associated with frontal cortex function deficit in 6-week abstinent crack-cocaine dependent men. Drug Alcohol Depend. 2002;68:87–93. doi: 10.1016/s0376-8716(02)00110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O’Brien CP, Childress AR. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Gallo V, Zhou JM, McBain CJ, Wright P, Knutson PL, Armstrong RC. Oligodendrocyte progenitor cell proliferation and lineage progression are regulated by glutamate receptor-mediated K+ channel block. J Neurosci. 1996;16:2659–2670. doi: 10.1523/JNEUROSCI.16-08-02659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Hester R. The role of cognitive control in cocaine dependence. Neuropsychol Rev. 2007;17:337–345. doi: 10.1007/s11065-007-9034-x. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Meyerhoff DJ. Temporal dynamics and determinants of whole brain tissue volume changes during recovery from alcohol dependence. Drug Alcohol Depend. 2005a;78:263–273. doi: 10.1016/j.drugalcdep.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Studholme C, Song E, Banys P, Meyerhoff DJ. Quantitative brain MRI in alcohol dependence: preliminary evidence for effects of concurrent chronic cigarette smoking on regional brain volumes. Alcohol Clin Exp Res. 2005b;29:1484–1495. doi: 10.1097/01.alc.0000175018.72488.61. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Leskovjan AC, Hoff AL, Hitzemann R, Bashan F, Khalsa SS, Wang GJ, Fowler JS, Volkow ND. Severity of neuropsychological impairment in cocaine and alcohol addiction: association with metabolism in the prefrontal cortex. Neuropsychologia. 2004;42:1447–1458. doi: 10.1016/j.neuropsychologia.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk C, Beauvais J, Hart R, Kosten T. Cognitive function and cerebral perfusion during cocaine abstinence. Am J Psychiatry. 2001;158:540–545. doi: 10.1176/appi.ajp.158.4.540. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Wurster RM, Brady JV. Discrete-trial choice procedure: effects of naloxone and methadone on choice between food and heroin. Pharmacol Rev. 1975;27:357–365. [PubMed] [Google Scholar]
- Griffiths RR, Bigelow GE, Henningfield JE. Similarities in animals and human drug-taking behavior. Greenwich, CT: JAI Press; 1980. [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, Yang Y. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage. 2010;53:593–601. doi: 10.1016/j.neuroimage.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall FS, Drgonova J, Goeb M, Uhl GR. Reduced behavioral effects of cocaine in heterozygous brain-derived neurotrophic factor (BDNF) knockout mice. Neuropsychopharmacology. 2003;28:1485–1490. doi: 10.1038/sj.npp.1300192. [DOI] [PubMed] [Google Scholar]
- Hanlon CA, Dufault DL, Wesley MJ, Porrino LJ. Elevated gray and white matter densities in cocaine abstainers compared to current users. Psychopharmacology (Berl) 2011a;218:681–692. doi: 10.1007/s00213-011-2360-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Wesley MJ, Stapleton JR, Laurienti PJ, Porrino LJ. The association between frontal-striatal connectivity and sensorimotor control in cocaine users. Drug Alcohol Depend. 2011b;115:240–243. doi: 10.1016/j.drugalcdep.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington HP, Pan JW, Telang F, Pappas N, Volkow N. Reduced brain GABA levels in cocaine abusers. Proc Intern Soc Magn Reson Med. 2000;523 [Google Scholar]
- Holman BL, Mendelson J, Garada B, Teoh SK, Hallgring E, Johnson KA, Mello NK. Regional cerebral blood flow improves with treatment in chronic cocaine polydrug users. J Nucl Med. 1993;34:723–727. [PubMed] [Google Scholar]
- Howard S, Landry C, Fisher R, Bezouglaia O, Handley V, Campagnoni A. Postnatal localization and morphogenesis of cells expressing the dopaminergic D2 receptor gene in rat brain: expression in non-neuronal cells. J Comp Neurol. 1998;391:87–98. doi: 10.1002/(sici)1096-9861(19980202)391:1<87::aid-cne8>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Hyman C, Hofer M, Barde YA, Juhasz M, Yancopoulos GD, Squinto SP, Lindsay RM. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991;350:230–232. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Gamst AC, Archibald SL, Fennema-Notestine C, Mindt MR, Marcotte TD, Heaton RK, Ellis RJ, Grant I. Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am J Psychiatry. 2005;162:1461–1472. doi: 10.1176/appi.ajp.162.8.1461. [DOI] [PubMed] [Google Scholar]
- Jiang X, Zhou J, Mash DC, Marini AM, Lipsky RH. Human BDNF isoforms are differentially expressed in cocaine addicts and are sorted to the regulated secretory pathway independent of the Met66 substitution. Neuromolecular Med. 2009;11:1–12. doi: 10.1007/s12017-008-8051-0. [DOI] [PubMed] [Google Scholar]
- Johanson CE. Pharmacological and environmental variables affecting drug preference in rhesus monkeys. Pharmacol Rev. 1975;27:343–355. [PubMed] [Google Scholar]
- Johanson CE, Fischman MW. The pharmacology of cocaine related to its abuse. Pharmacol Rev. 1989;41:3–52. [PubMed] [Google Scholar]
- Kalivas PW. Addiction as a pathology in prefrontal cortical regulation of corticostriatal habit circuitry. Neurotox Res. 2008;14:185–189. doi: 10.1007/BF03033809. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry. 2011;16:974–986. doi: 10.1038/mp.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampman KM, Volpicelli JR, Mulvaney F, Rukstalis M, Alterman AI, Pettinati H, Weinrieb RM, O’Brien CP. Cocaine withdrawal severity and urine toxicology results from treatment entry predict outcome in medication trials for cocaine dependence. Addict Behav. 2002;27:251–260. doi: 10.1016/s0306-4603(01)00171-x. [DOI] [PubMed] [Google Scholar]
- Ke Y, Streeter CC, Nassar LE, Sarid-Segal O, Hennen J, Yurgelun-Todd DA, Awad LA, Rendall MJ, Gruber SA, Nason A, Mudrick MJ, Blank SR, Meyer AA, Knapp C, Ciraulo DA, Renshaw PF. Frontal lobe GABA levels in cocaine dependence: a two-dimensional, J-resolved magnetic resonance spectroscopy study. Psychiatry Res. 2004;130:283–293. doi: 10.1016/j.pscychresns.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Kelly C, Zuo XN, Gotimer K, Cox CL, Lynch L, Brock D, Imperati D, Garavan H, Rotrosen J, Castellanos FX, Milham MP. Reduced interhemispheric resting state functional connectivity in cocaine addiction. Biol Psychiatry. 2011;69:684–692. doi: 10.1016/j.biopsych.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Gorny G, Li Y, Samaha AN, Robinson TE. Amphetamine or cocaine limits the ability of later experience to promote structural plasticity in the neocortex and nucleus accumbens. Proc Natl Acad Sci U S A. 2003;100:10523–10528. doi: 10.1073/pnas.1834271100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, Serre F, Cantin L, Ahmed SH. Intense sweetness surpasses cocaine reward. PLoS One. 2007;2:e698. doi: 10.1371/journal.pone.0000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letchworth SR, Nader MA, Smith HR, Friedman DP, Porrino LJ. Progression of changes in dopamine transporter binding site density as a result of cocaine self-administration in rhesus monkeys. J Neurosci. 2001;21:2799–2807. doi: 10.1523/JNEUROSCI.21-08-02799.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kolb B, Robinson TE. The location of persistent amphetamine-induced changes in the density of dendritic spines on medium spiny neurons in the nucleus accumbens and caudate-putamen. Neuropsychopharmacology. 2003;28:1082–1085. doi: 10.1038/sj.npp.1300115. [DOI] [PubMed] [Google Scholar]
- Licata SC, Renshaw PF. Neurochemistry of drug action: insights from proton magnetic resonance spectroscopic imaging and their relevance to addiction. Ann N Y Acad Sci. 2010;1187:148–171. doi: 10.1111/j.1749-6632.2009.05143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Matochik JA, Cadet JL, London ED. Smaller volume of prefrontal lobe in polysubstance abusers: a magnetic resonance imaging study. Neuropsychopharmacology. 1998;18:243–252. doi: 10.1016/S0893-133X(97)00143-7. [DOI] [PubMed] [Google Scholar]
- London ED, Simon SL, Berman SM, Mandelkern MA, Lichtman AM, Bramen J, Shinn AK, Miotto K, Learn J, Dong Y, Matochik JA, Kurian V, Newton T, Woods R, Rawson R, Ling W. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psychiatry. 2004;61:73–84. doi: 10.1001/archpsyc.61.1.73. [DOI] [PubMed] [Google Scholar]
- Ma L, Hasan KM, Steinberg JL, Narayana PA, Lane SD, Zuniga EA, Kramer LA, Moeller FG. Diffusion tensor imaging in cocaine dependence: regional effects of cocaine on corpus callosum and effect of cocaine administration route. Drug Alcohol Depend. 2009;104:262–267. doi: 10.1016/j.drugalcdep.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N, Liu Y, Li N, Wang CX, Zhang H, Jiang XF, Xu HS, Fu XM, Hu X, Zhang DR. Addiction related alteration in resting-state brain connectivity. Neuroimage. 2010;49:738–744. doi: 10.1016/j.neuroimage.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malison RT, Best SE, van Dyck CH, McCance EF, Wallace EA, Laruelle M, Baldwin RM, Seibyl JP, Price LH, Kosten TR, Innis RB. Elevated striatal dopamine transporters during acute cocaine abstinence as measured by [123I] beta-CIT SPECT. Am J Psychiatry. 1998;155:832–834. doi: 10.1176/ajp.155.6.832. [DOI] [PubMed] [Google Scholar]
- Martinez D, Carpenter KM, Liu F, Slifstein M, Broft A, Friedman AC, Kumar D, Van Heertum R, Kleber HD, Nunes E. Imaging dopamine transmission in cocaine dependence: link between neurochemistry and response to treatment. Am J Psychiatry. 2011;168:634–641. doi: 10.1176/appi.ajp.2010.10050748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, Huang Y, Cooper TB, Fischman MW, Kleber HD, Laruelle M. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry. 2007;164:622–629. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- Martinez D, Broft A, Foltin RW, Slifstein M, Hwang DR, Huang Y, Perez A, Frankle WG, Cooper T, Kleber HD, Fischman MW, Laruelle M. Cocaine dependence and d2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine-seeking behavior. Neuropsychopharmacology. 2004 Jun;29(6):1190–202. doi: 10.1038/sj.npp.1300420. [DOI] [PubMed] [Google Scholar]
- Mash DC, Pablo J, Ouyang Q, Hearn WL, Izenwasser S. Dopamine transport function is elevated in cocaine users. J Neurochem. 2002;81:292–300. doi: 10.1046/j.1471-4159.2002.00820.x. [DOI] [PubMed] [Google Scholar]
- Mateo Y, Lack CM, Morgan D, Roberts DC, Jones SR. Reduced dopamine terminal function and insensitivity to cocaine following cocaine binge self-administration and deprivation. Neuropsychopharmacology. 2005;30:1455–1463. doi: 10.1038/sj.npp.1300687. [DOI] [PubMed] [Google Scholar]
- Matochik JA, London ED, Eldreth DA, Cadet JL, Bolla KI. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. Neuroimage. 2003;19:1095–1102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- McGinty JF, Mendelson JE. Is brain-derived neurotrophic factor a selective biomarker that predicts cocaine relapse outcomes? Biol Psychiatry. 2011;70:700–701. doi: 10.1016/j.biopsych.2011.08.017. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Dougherty DM, Santos RM, Valdes I, Swann AC, Barratt ES, Narayana PA. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: diffusion tensor imaging. Neuropsychopharmacology. 2005;30:610–617. doi: 10.1038/sj.npp.1300617. [DOI] [PubMed] [Google Scholar]
- Nader MA, Daunais JB, Moore T, Nader SH, Moore RJ, Smith HR, Friedman DP, Porrino LJ. Effects of cocaine self-administration on striatal dopamine systems in rhesus monkeys: initial and chronic exposure. Neuropsychopharmacology. 2002a;27:35–46. doi: 10.1016/S0893-133X(01)00427-4. [DOI] [PubMed] [Google Scholar]
- Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, Ehrenkaufer R, Mach RH. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci. 2006;9:1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- Nader MA, Sinnott RS, Mach RH, Morgan D. Cocaine- and food-maintained responding under a multiple schedule in rhesus monkeys: environmental context and the effects of a dopamine antagonist. Psychopharmacology (Berl) 2002b;163:292–301. doi: 10.1007/s00213-002-1202-3. [DOI] [PubMed] [Google Scholar]
- Nader MA, Woolverton WL. Effects of increasing the magnitude of an alternative reinforcer on drug choice in a discrete-trials choice procedure. Psychopharmacology (Berl) 1991;105:169–174. doi: 10.1007/BF02244304. [DOI] [PubMed] [Google Scholar]
- Narayana PA, Ahobila-Vajjula P, Ramu J, Herrera J, Steinberg JL, Moeller FG. Diffusion tensor imaging of cocaine-treated rodents. Psychiatry Res. 2009;171:242–251. doi: 10.1016/j.pscychresns.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen DA, Huang W, Hamon SC, Maili L, Witkin BM, Fox RG, Cunningham KA, Moeller FG. Forced Abstinence from Cocaine Self-Administration is Associated with DNA Methylation Changes in Myelin Genes in the Corpus Callosum: a Preliminary Study. Front Psychiatry. 2012;3:60. doi: 10.3389/fpsyt.2012.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill J, Cardenas VA, Meyerhoff DJ. Separate and interactive effects of cocaine and alcohol dependence on brain structures and metabolites: quantitative MRI and proton MR spectroscopic imaging. Addict Biol. 2001;6:347–361. doi: 10.1080/13556210020077073. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcohol Clin Exp Res. 1995;19:1177–1191. doi: 10.1111/j.1530-0277.1995.tb01598.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Rosenbloom MJ, Mathalon DH, Lim KO. A controlled study of cortical gray matter and ventricular changes in alcoholic men over a 5-year interval. Arch Gen Psychiatry. 1998;55:905–912. doi: 10.1001/archpsyc.55.10.905. [DOI] [PubMed] [Google Scholar]
- Poling J, Kosten TR, Sofuoglu M. Treatment outcome predictors for cocaine dependence. Am J Drug Alcohol Abuse. 2007;33:191–206. doi: 10.1080/00952990701199416. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Gorny G, Mitton E, Kolb B. Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse. 2001;39:257–266. doi: 10.1002/1098-2396(20010301)39:3<257::AID-SYN1007>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci. 1999;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- Sadri-Vakili G, Kumaresan V, Schmidt HD, Famous KR, Chawla P, Vassoler FM, Overland RP, Xia E, Bass CE, Terwilliger EF, Pierce RC, Cha JH. Cocaine-induced chromatin remodeling increases brain-derived neurotrophic factor transcription in the rat medial prefrontal cortex, which alters the reinforcing efficacy of cocaine. J Neurosci. 2010;30:11735–11744. doi: 10.1523/JNEUROSCI.2328-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuvel DJ, Jayanthi LD, Manohar S, Kaliyaperumal K, See RE, Ramamoorthy S. Dysregulation of dopamine transporter trafficking and function after abstinence from cocaine self-administration in rats: evidence for differential regulation in caudate putamen and nucleus accumbens. J Pharmacol Exp Ther. 2008;325:293–301. doi: 10.1124/jpet.107.130534. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Sangrey GR, Darnell SB, Schassburger RL, Cha JH, Pierce RC, Sadri-Vakili G. Increased brain-derived neurotrophic factor (BDNF) expression in the ventral tegmental area during cocaine abstinence is associated with increased histone acetylation at BDNF exon I-containing promoters. J Neurochem. 2012;120:202–209. doi: 10.1111/j.1471-4159.2011.07571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster CR, Johanson CE. An analysis of drug-seeking behavior in animals. Neurosci Biobehav Rev. 1981;5:315–323. doi: 10.1016/0149-7634(81)90026-9. [DOI] [PubMed] [Google Scholar]
- Sim ME, Lyoo IK, Streeter CC, Covell J, Sarid-Segal O, Ciraulo DA, Kim MJ, Kaufman MJ, Yurgelun-Todd DA, Renshaw PF. Cerebellar gray matter volume correlates with duration of cocaine use in cocaine-dependent subjects. Neuropsychopharmacology. 2007;32:2229–2237. doi: 10.1038/sj.npp.1301346. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fox H, Hong KI, Sofuoglu M, Morgan PT, Bergquist KT. Sex steroid hormones, stress response, and drug craving in cocaine-dependent women: implications for relapse susceptibility. Exp Clin Psychopharmacol. 2007;15:445–452. doi: 10.1037/1064-1297.15.5.445. [DOI] [PubMed] [Google Scholar]
- Smith HR, Wang S, Beveridge TJR, Porrino LJ. Society for Neuroscience Annual Meeting. 2011. Evidence for inflammation in CNS white matter of monkeys following prolonged exposure to cocaine self-administration. [Google Scholar]
- Smith HR, Williams A, Beveridge TJR, Nader MA, Porrino LJ. Society for Neuroscience Annual Meeting. 2012. Disrupted glucose metabolism in central white matter fiber tracts following prolonged cocaine self-administration in nonhuman primates. [Google Scholar]
- Staley JK, Hearn WL, Ruttenber AJ, Wetli CV, Mash DC. High affinity cocaine recognition sites on the dopamine transporter are elevated in fatal cocaine overdose victims. J Pharmacol Exp Ther. 1994;271:1678–1685. [PubMed] [Google Scholar]
- Stalnaker TA, Takahashi Y, Roesch MR, Schoenbaum G. Neural substrates of cognitive inflexibility after chronic cocaine exposure. Neuropharmacology. 2009;56(Suppl 1):63–72. doi: 10.1016/j.neuropharm.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland TL, Mena I, Villanueva-Meyer J, Miller BL, Cummings J, Mehringer CM, Satz P, Myers H. Cerebral perfusion and neuropsychological consequences of chronic cocaine use. J Neuropsychiatry Clin Neurosci. 1993;5:419–427. doi: 10.1176/jnp.5.4.419. [DOI] [PubMed] [Google Scholar]
- Tanabe J, Tregellas JR, Dalwani M, Thompson L, Owens E, Crowley T, Banich M. Medial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individuals. Biol Psychiatry. 2009;65:160–164. doi: 10.1016/j.biopsych.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vocci FJ. Can replacement therapy work in the treatment of cocaine dependence? And what are we replacing anyway? Addiction. 2007;102:1888–1889. doi: 10.1111/j.1360-0443.2007.02014.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wolf AP. Use of positron emission tomography to study cocaine in the human brain. NIDA Res Monogr. 1991a;112:168–179. [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wolf AP, Hitzemann R, Dewey S, Bendriem B, Alpert R, Hoff A. Changes in brain glucose metabolism in cocaine dependence and withdrawal. Am J Psychiatry. 1991b;148:621–626. doi: 10.1176/ajp.148.5.621. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Wolf AP, Dewey SL, Handlesman L. Long-term frontal brain metabolic changes in cocaine abusers. Synapse. 1992;11:184–190. doi: 10.1002/syn.890110303. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Mullani N, Gould KL, Adler S, Krajewski K. Cerebral blood flow in chronic cocaine users: a study with positron emission tomography. Br J Psychiatry. 1988;152:641–648. doi: 10.1192/bjp.152.5.641. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Wong C, Ding YS, Hitzemann R, Swanson JM, Kalivas P. Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction. J Neurosci. 2005;25:3932–3939. doi: 10.1523/JNEUROSCI.0433-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Chang L, Miller E, Sedler M, Hitzemann R, Zhu W, Logan J, Ma Y, Fowler JS. Partial recovery of brain metabolism in methamphetamine abusers after protracted abstinence. Am J Psychiatry. 2004;161:242–248. doi: 10.1176/appi.ajp.161.2.242. [DOI] [PubMed] [Google Scholar]
- Wheeler RA, Carelli RM. Dissecting motivational circuitry to understand substance abuse. Neuropharmacology. 2009;56(Suppl 1):149–159. doi: 10.1016/j.neuropharm.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME. The Bermuda Triangle of cocaine-induced neuroadaptations. Trends Neurosci. 2010;33:391–398. doi: 10.1016/j.tins.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Salmeron BJ, Ross TJ, Xi ZX, Stein EA, Yang Y. Lower glutamate levels in rostral anterior cingulate of chronic cocaine users - A (1)H-MRS study using TE-averaged PRESS at 3 T with an optimized quantification strategy. Psychiatry Res. 2009;174:171–176. doi: 10.1016/j.pscychresns.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Kalivas PW. N-acetylcysteine reduces extinction responding and induces enduring reductions in cue- and heroin-induced drug-seeking. Biol Psychiatry. 2008;63:338–340. doi: 10.1016/j.biopsych.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


