Abstract
Clinical practice guidelines recommend yearly surveillance mammography for breast cancer survivors, yet many women do not receive this service. The objective of this study was to evaluate factors related to long-term surveillance mammography adherence among breast cancer survivors. We conducted a retrospective cohort study among women ≥18 years, diagnosed with incident stage I or II breast cancer between 1990 and 2008. We used medical record and administrative health plan data to ascertain covariates and receipt of surveillance mammography for up to 10 years after completing breast cancer treatment. Surveillance included post-diagnosis screening exams among asymptomatic women. We used multivariable repeated measures generalized estimating equation regression models to estimate odds ratios (ORs) and robust 95% confidence intervals (CI) to examine factors related to annual receipt of surveillance mammography. The analysis included 3,965 women followed for a median of 6 surveillance years; 79% received surveillance mammograms in year 1 but decreased to 63% in year 10. In multivariable analyses, women were less likely than other women to receive surveillance mammography if they were <40 years or 80+ years of age (compared to 50–59 years), current smokers, had greater co-morbidity, were diagnosed more recently, had stage II cancer or were treated with mastectomy or breast conserving surgery without radiation. Women with outpatient visits during the year to primary care providers, oncologists, or both were more likely to undergo surveillance. In this large cohort study of women diagnosed with early stage invasive breast cancer, we found that important subgroups of women are at high risk for non-adherence to surveillance recommendations, even among younger breast cancer survivors. Efforts should be undertaken to actively engage breast cancer survivors in managing long-term surveillance care.
Keywords: breast cancer, cancer survivors, surveillance, mammography, survivorship care
Introduction
In the US, breast cancer is the most frequently diagnosed cancer in women, with more than 200,000 new cases diagnosed each year [1]. There are an estimated 2.8 million breast cancer survivors living in the US today [1] at risk for recurrence and second primary breast tumors. Regular surveillance mammography among breast cancer survivors reduces breast cancer mortality [2], possibly because second breast cancer events are diagnosed at earlier stages with more favorable prognoses [2]. This reinforces the importance of long-term survivorship care and emphasizes the need for understanding utilization of mammography among survivors.
National guidelines recommend yearly mammographic evaluation for breast cancer survivors [3, 4], yet many women do not undergo surveillance mammography [5–9]. One study reported surveillance rates of 82.1% in the year following breast cancer treatment, which declined to 68.5% by year 4 among women 65 years or older diagnosed with early stage invasive breast cancer [5]. Several factors are associated with lower rates of mammography use in breast cancer survivors, including advanced age, racial/ethnic minority status, higher stage of disease, and receipt of breast conserving surgery without radiation [5, 7, 9–11]. Women with outpatient visits to primary care physicians or oncologists during follow-up are more likely to receive surveillance [5, 10, 11]. However, most studies examining factors related to surveillance mammography have focused on older breast cancer survivors [5, 9–11] or were limited by relatively short follow-up [5, 9–11] and incomplete ascertainment of surveillance procedures [7]. While the risk of recurrence is highest within the first three to five years post-diagnosis [12, 13], second breast cancer events can occur at any time. Thus, it is important to understand patterns of surveillance mammography for a more extended follow-up period. This study has the longest follow-up to date to evaluate factors associated with surveillance mammography among women of all ages diagnosed with early stage invasive breast cancer.
Materials and methods
Study Population
The parent study, COmmonly Used Medications and Breast Cancer Outcomes (COMBO) [14], was conducted within Group Health Cooperative (GH), a nonprofit integrated delivery system in western Washington State and parts of Idaho. GH is located within the reporting region of the western Washington Cancer Surveillance System, a population-based cancer Surveillance, Epidemiology, and End Results (SEER) registry[1]. COMBO included women who were ≥18 years of age, diagnosed with a histologically confirmed incident stage I or II [15] breast cancer between 1990 and 2008, and enrolled for at least 1 year before and after (unless died) the initial breast cancer diagnosis. Women were eligible if they were alive and recurrence free for 120 days after completing surgery for the incident breast cancer [16, 17], giving a sample of 4,216 women. The study was approved by the GH Institutional Review Board.
Data Collection
Data were collected from one year prior to breast cancer diagnosis through the earliest of death, disenrollment from GH (lapse in membership of 90+ days), or end of study (i.e., date of chart abstraction) [18]. Data were collected from medical record review (paper and electronic), SEER, health plan automated administrative databases, and GH’s Breast Cancer Screening Recruitment and Reminder (BSRR) survey.[19] GH’s automated databases include demographics, smoking status, enrollment, inpatient and outpatient encounters including all breast services [20], results of breast services, pharmacy dispensings, and death [21]. We collected information on primary definitive surgery, chemotherapy, radiation treatment and outcomes (i.e., recurrence and second primaries) from medical records. Cancer registry data included year of diagnosis, American Joint Committee on Cancer (AJCC) stage [15] at diagnosis, lymph node status, hormone receptor status, and tumor size.
Surveillance Procedures
We considered the first six months post-diagnosis as the treatment period and excluded all surveillance procedures performed during this time [7, 9, 10]. We evaluated the following sequential 12- month intervals after diagnosis: months 7–18 (year 1), 19–30 (year 2), 31–42 (year 3), 43–54 (year 4), 55–66 (year 5), 67–78 (year 6), 79–90 (year 7), 91–102 (year 8), 103–114 (year 9), 115–126 (year 10).
Procedures such as blood chemistry tests or bone scans in asymptomatic women to look for distant recurrence are not recommended [3, 4]. Our outcome included surveillance procedures for local second breast cancer events, which included asymptomatic mammographic and breast MRI exams. We ascertained exam date and indication designated by the interpreting radiologist from GH administrative databases (1996 forward) and medical charts (prior to 1996). We only included surveillance procedures post-diagnosis where the patient reported no symptoms at the time of the exam and the indication was designated as screening [2, 22]. Surveillance procedures also included all short interval follow-up (SIFU) exams unless the SIFU took place <9 months after a diagnostic exam [2, 22]. A woman was categorized as having received at least one surveillance procedure (yes/no) within each surveillance year. Only 0.7% of surveillance procedures were breast MRI exams; therefore, we refer to surveillance procedures as surveillance mammography.
Covariates
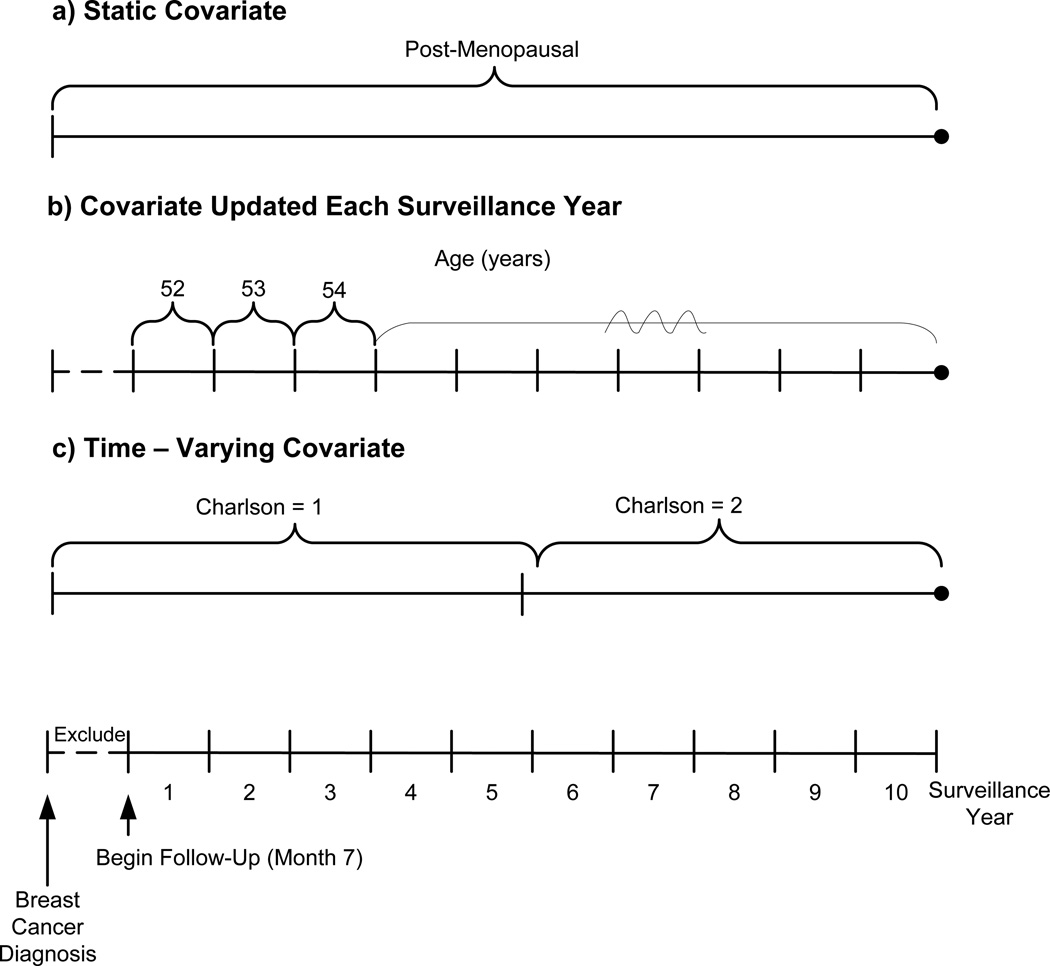
We measured and defined 3 types of covariates: 1) static covariates assessed only once either at pre-diagnosis, diagnosis, or during treatment and remained fixed over time; 2) covariates updated each surveillance year were repeatedly assessed during each of the 10 surveillance years (i.e., variable status did not carry over to the subsequent years); and 3) time-varying covariates assessed throughout the entire study period and varied over time when status changed (i.e., variable status carried over to subsequent years) (Figure 1).
Figure 1. Schematic of covariate types over the entire study period.
- Static covariate: Patient is post-menopausal at diagnosis. She remains categorized as post-menopausal in all surveillance years
- Per surveillance year covariate: Patient’s age = 52 years in year 1. Her age is updated in each subsequent surveillance year
- Time-varying covariate: Patient has Charlson score = 1 at diagnosis; Charlson score = 2 beginning in month 7 of year 5, and no further changes to her Charlson score through year 10. Since she has a new Charlson score for only 5 months of year 5, her Charlson score remains 1 in year 5 and increases to 2 in year 6. She remains Charlson score = 2 for all subsequent surveillance years
Static covariates
We considered the following covariates static: menopausal status, body mass index (BMI), smoking status, breast cancer characteristics (diagnosis year, stage [15], hormone receptor status), and breast cancer treatment including primary surgery (breast conserving or mastectomy), and radiation therapy (Figure 1a). BMI was calculated from the weight and height ascertained from the medical record during the year before diagnosis. If menopausal status was missing or unknown (19%) at diagnosis, we characterized a woman as peri- or pre-menopausal if she was <55 years of age and post-menopausal if she was ≥55 years of age [23].
Covariates updated each surveillance year
We calculated current age by adding one year to the age at diagnosis for each sequential surveillance year (Figure 1b). We captured the type of provider seen (primary care or oncology) during outpatient visits for each surveillance year.
Time-varying covariates
Charlson co-morbidity index score (0, 1, 2+) was calculated using diagnoses for co-morbid conditions captured 12 months before the breast cancer diagnosis and also assessed annually post-diagnosis for changes in score (Figure 1c) [24] Women were only allowed to move from lower to higher Charlson scores (e.g., 0 to 1).
Data Analysis
We used Pearson chi-square tests (categorical) and Wilcoxon rank sum tests (continuous) to compare patient characteristics between women who did and did not receive surveillance mammography within each surveillance year.
For multivariable analyses, we used repeated measures generalized estimating equation (GEE) regression models to estimate odds ratios (ORs) and robust 95% confidence intervals. A logit link based on a binomial distribution with an unstructured correlation covariance was implemented. The GEE method accounts for the correlation among the repeated observations for each subject while also adjusting for different numbers of observations across individuals [25].
Subjects were censored at the earliest of death, disenrollment, diagnosis of a second breast cancer event (SBCE), or 10 years after breast cancer treatment. SBCE was defined as any ductal carcinoma in situ or invasive breast cancers of the ipsilateral (recurrence) or contralateral (second primary) breast or in any regional or distant sites [26]. To ensure women had the opportunity to receive surveillance in a given surveillance year, we required subjects have complete data for that entire surveillance year (i.e., subjects must be alive and not censored). Of the 4,216 women in our sample, we excluded 251 because they did not complete the entire year 1 surveillance interval (108 SBCEs, 66 deaths, 28 end of study, 14 disenrolled), resulting in a final analytic cohort of 3,965 women.
The primary multivariable analysis examined the association between select characteristics and receipt of yearly surveillance mammography over time. The characteristics consisted of known and suspected factors associated with surveillance [5, 7–11, 27]: age, diagnosis year, years of follow-up after treatment, stage[15], hormone receptor status, primary treatment, BMI, smoking status, type of provider seen, menopausal status, and Charlson score. In a sub-group analysis, we stratified the results by age at diagnosis of incident breast cancer (<50, 50–64, 65+ years).
We evaluated the sensitivity of results by excluding the year prior to death, SBCE, or diagnosis of other cancers (including all diagnoses of cancer other than breast) to address the possibility that any differences we observed were influenced by these events. Mode of initial breast cancer detection affects receipt of surveillance mammography[28] therefore in secondary analyses, we added initial mode of detection (screen detected, screen interval-detected, diagnostic-detected, diagnostic interval-detected) to our multivariable model. To compare our results with previous surveillance studies, we repeated analyses in women 65+ years of age at diagnosis and included only 4 years of follow-up. All analyses were performed using Stata/IC 12 software (StataCorp LP, College Station, TX).
Results
Patient characteristics
The majority of women were Caucasian (89%), post-menopausal (73%), non-smokers (86%), had normal BMI (18.5–24.9 kg/m2) (34%), and no co-morbidities (77%) at diagnosis. The mean age at diagnosis was 62.6 years; the majority of cancers was stage I (64%), ER+/PR+ (69%), and treated with breast conserving surgery plus radiation (52%).
Receipt of surveillance
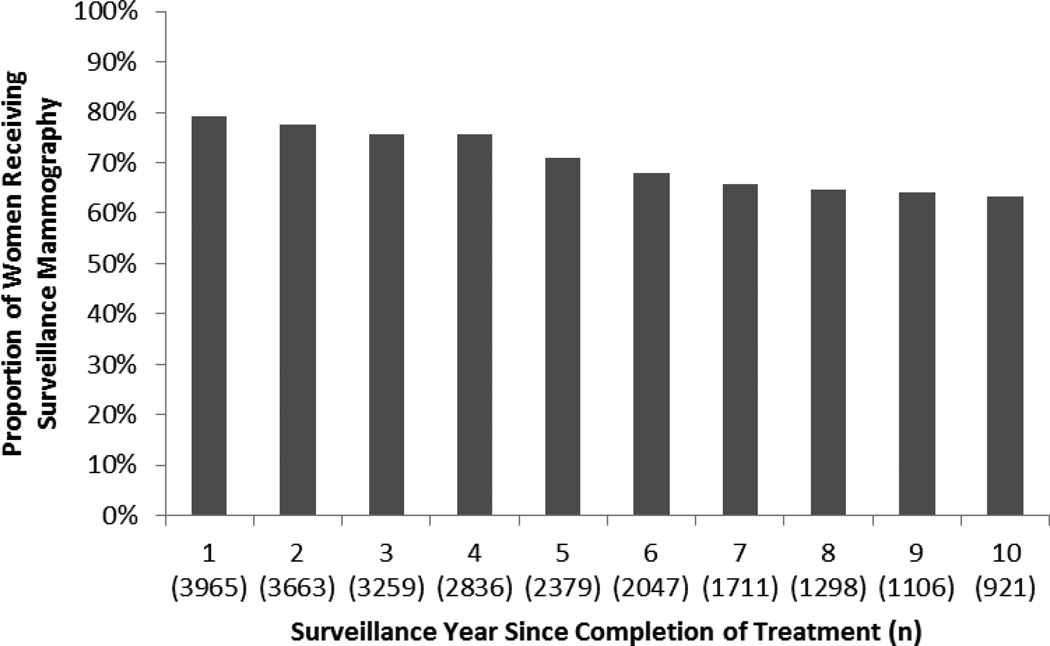
During year 1, 79% of women received surveillance mammography. Receipt of surveillance declined through year 6 to 68% then became relatively stable at 63–66% in years 7–10 (Figure 2). The median number of surveillance mammograms per patient over the 10 year follow-up was 4 (interquartile range 2–6).
Figure 2.
Receipt of yearly surveillance mammographya since completion of breast cancer treatment among women with a history of early stage invasive breast cancerb
a0.7% of surveillance mammography included breast MRI
bFollow-up extended from month 7 post-SEER diagnosis date through 10 years or the earliest of death, disenrollment, or diagnosis of a second breast cancer event (i.e., recurrence or second primary breast cancer)
The number of women remaining in each surveillance year are available in Appendix A. Overall, there were 3,044 (77%) women censored during the 10-year follow-up period (409 SBCEs, 473 deaths, 1,542 end of study, 620 disenrolled). A total of 2,836 (72%) contributed to 4 surveillance years and 1,298 (33%) contributed to 8 surveillance years. The median number of surveillance years per patient was 6 (interquartile range (IQR): 3–9). Median surveillance years among women who experienced a SBCE were 3 (IQR: 1–5) or died during follow-up were 4 (IQR: 2–7).
Factors related to receipt of surveillance
The unadjusted results indicate women who received surveillance mammography in year 1 were slightly younger and non-smokers compared to women not receiving surveillance (Table 1). Women diagnosed with earlier stage breast cancers that were screen-detected, lymph node negative, smaller tumor size, and treated with breast conserving surgery plus radiation were more likely to undergo surveillance mammography in year 1 (all P<0.05). Women diagnosed more recently and those with initial cancers tested for HER-2 were less likely to receive surveillance. Characteristics by subsequent surveillance years are available in Appendix A; patterns of mammography use did not change appreciably from year 1. However, receipt of surveillance in subsequent years was higher among women with Charlson scores of 0 vs. 1+ and initial cancers treated with endocrine therapy vs. no treatment.
Table 1.
Patient characteristics by receipt of surveillance mammography for the first full surveillance year since completion of breast cancer treatment
| Year 1a | |||
|---|---|---|---|
| Surveillance Mammogramb | |||
| No 823 (20.8%) |
Yes 3,142 (79.2%) |
P | |
| Characteristics at Diagnosis of Initial Breast Cancer | |||
| Year of diagnosis | |||
| 1990–1994 | 125 (15.2) | 773 (24.6) | <0.001 |
| 1995–1999 | 152 (18.5) | 962 (30.6) | |
| 2000–2004 | 322 (39.1) | 812 (25.8) | |
| 2005–2008 | 224 (27.2) | 595 (18.9) | |
| Menopausal status | |||
| Peri- or pre-menopausal | 221 (26.9) | 851 (27.1) | 0.89 |
| Post-menopausal | 602 (73.1) | 2291 (72.9) | |
| Race | |||
| Caucasian | 722 (88) | 2780 (88.8) | 0.84 |
| African American | 25 (3.0) | 98 (3.1) | |
| American Indian/Alaska Native | 27 (3.3) | 82 (2.6) | |
| Asian/Pacific Islander | 46 (5.6) | 170 (5.4) | |
| Unknown | 3 | 12 | |
| Education | |||
| High school or less | 86 (24.4) | 323 (23.2) | 0.17 |
| At least some college | 267 (75.6) | 1067 (76.8) | |
| Unknown | 470 | 1752 | |
| Body mass index (kg/m2) | |||
| <18.5 | 17 (2.1) | 45 (1.4) | 0.49 |
| 18.5–24.9 | 277 (33.9) | 1082 (34.5) | |
| 25.0–29.9 | 262 (32.1) | 1030 (32.9) | |
| 30.0–34.9 | 145 (17.7) | 581 (18.5) | |
| 35+ | 116 (14.2) | 395 (12.6) | |
| Unknown | 6 | 9 | |
| Smoking status | |||
| Current | 62 (7.5) | 171 (5.4) | 0.02 |
| Past | 79 (9.6) | 251 (8.0) | |
| Never | 682 (82.9) | 2720 (86.6) | |
| AJCC stage [15] | |||
| I | 477 (58.0) | 2046 (65.1) | <0.001 |
| IIA | 238 (28.9) | 771 (24.5) | |
| IIB | 108 (13.1) | 325 (10.3) | |
| Lymph node status | |||
| Negative | 529 (72.3) | 2174 (77.2) | <0.01 |
| Positive | 203 (27.7) | 641 (22.8) | |
| Unknown | 91 | 327 | |
| ER/PR status | |||
| ER−/PR− | 119 (14.5) | 474 (15.1) | 0.87 |
| ER+/PR− | 69 (8.4) | 288 (9.2) | |
| ER−/PR+ | 14 (1.7) | 43 (1.4) | |
| ER+/PR+ | 579 (70.4) | 2176 (69.3) | |
| ER & PR unknown | 42 (5.1) | 161 (5.1) | |
| Tumor size | |||
| ≤ 2 cm | 580 (70.6) | 2384 (75.9) | <0.01 |
| > 2 cm | 242 (29.4) | 757 (24.1) | |
| Unknown | 1 | 1 | |
| HER2 test result | |||
| Test done | 546 (88.8) | 1404 (76.9) | <0.001 |
| Positive/borderline | 95 (17.4) | 228 (16.2) | 0.09 |
| Negative | 447 (81.9) | 1174 (83.6) | |
| No result | 4 (0.7) | 2 (0.1) | |
| Surgical procedure | |||
| Mastectomy +/− radiation | 376 (45.7) | 1042 (33.2) | <0.001 |
| BCS + radiation | 319 (38.8) | 1759 (56.0) | |
| BCS | 128 (15.6) | 341 (10.9) | |
| Other treatment | |||
| Chemotherapy | 276 (33.5) | 1015 (32.3) | 0.50 |
| Completed course | 240 (87.0) | 901 (88.8) | 0.55 |
| Mode of initial cancer detection | |||
| Screen-detected | 258 (42.4) | 986 (49.9) | <0.01 |
| Screen interval-detected | 80 (13.1) | 247 (12.5) | |
| Diagnostic detected | 253 (41.5) | 674 (34.1) | |
| Diagnostic interval-detected | 18 (3.0) | 68 (3.4) | |
| Unknown | 214 | 1167 | |
| Characteristics Measured Throughout Follow-Up | |||
| Age, yearsc | |||
| Median (IQR) | 64 (30–94) | 64 (31–93) | 0.03 |
| <40 | 29 (3.5) | 67 (2.1) | <0.001 |
| 40–49 | 108 (13.1) | 432 (13.8) | |
| 50–59 | 178 (21.6) | 764 (24.3) | |
| 60–69 | 194 (23.6) | 772 (24.6) | |
| 70–79 | 164 (19.9) | 755 (24.0) | |
| 80+ | 150 (18.2) | 352 (11.2) | |
| Type of provider seenc | |||
| Oncologist only | 16 (1.9) | 69 (2.2) | 0.44 |
| PCP only | 116 (14.1) | 382 (12.2) | |
| Both | 637 (77.4) | 2498 (79.5) | |
| Neither | 54 (6.6) | 193 (6.1) | |
| Charlson co-morbidity index scored | |||
| 0 | 612 (74.4) | 2439 (77.6) | 0.10 |
| 1 | 148 (18.0) | 514 (16.4) | |
| 2+ | 63 (7.7) | 189 (6.0) | |
| Endocrine therapyd,e | 457 (55.5) | 1812 (57.7) | 0.27 |
Abbreviations: SD=standard deviation; IQR=interquartile range; AJCC=American Joint Committee on Cancer; ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2; BCS=breast conserving surgery; PCP=primary care physician
Year 1 is defined as 7–18 months post-diagnosis to account for the initial treatment period
0.7% of surveillance included breast MRI
Characteristic updated each surveillance year
Time-varying characteristic: variable status was ascertained throughout the entire study period; once subjects met the variable definition for use, they were included in that category and remained there until the end of follow-up.
Endocrine therapy users included women who received a dispensing for tamoxifen and/or aromatase inhibitors for the initial breast cancer between her diagnosis date and before her SBCE date for cases or the end of follow-up for non-cases.
In adjusted analyses, surveillance declined with increasing years of follow-up (Table 2). Compared to women 50–59 years of age, those age <40 years or 80+ years had lower odds of receiving surveillance mammography. Current smokers and women with more co-morbidities had lower odds of receiving mammography, independent of age. Women with outpatient visits to primary care providers, oncologists, or both had higher odds of undergoing surveillance than women seeing neither type of provider. Characteristics of the initial breast cancer were also associated with receipt of surveillance mammography. Women diagnosed more recently, those with stage II cancer (significant only for stage IIA) and those who received mastectomy or breast conserving surgery without radiation had lower odds of receiving surveillance compared to respective referent groups of earlier diagnosis years, stage I, and breast conserving surgery plus radiation. In secondary adjusted analyses, we found that women initially diagnosed with breast cancer following an asymptomatic screening mammogram had higher odds of surveillance mammography (OR=1.31, 95% CI 1.15–1.50) compared to women diagnostic-detected.
Table 2.
Adjusted odds ratios and 95% confidence intervals relating patient characteristics to receipt of surveillance mammographya
| Adjusted OR (95% CI)b | |
|---|---|
| Years of follow-upc | 0.90 (0.88–0.91) |
| Year of diagnosis | |
| 1990–1994 | 1.00 (Referent) |
| 1995–1999 | 0.89 (0.78–1.01) |
| 2000–2004 | 0.54 (0.47–0.62) |
| 2005–2008 | 0.53 (0.45–0.63) |
| Menopausal status at diagnosis | |
| Peri- or pre-menopausal | 0.85 (0.72–1.01) |
| Post-menopausal | 1.00 (Referent) |
| Body mass index (kg/m2) at diagnosis | |
| <18.5 | 0.70 (0.47–1.03) |
| 18.5–24.9 | 1.00 (Referent) |
| 25.0–29.9 | 1.03 (0.93–1.15) |
| 30.0–34.9 | 0.92 (0.81–1.05) |
| 35.0+ | 0.86 (0.74–1.00) |
| Smoking status at diagnosis | |
| Current | 0.81 (0.67–0.98) |
| Past | 1.02 (0.84–1.24) |
| Never | 1.00 (Referent) |
| AJCC stage[15] | |
| I | 1.00 (Referent) |
| IIA | 0.86 (0.77–0.96) |
| IIB | 0.90 (0.76–1.06) |
| ER/PR status | |
| ER−/PR− | 1.01 (0.89–1.16) |
| ER+/PR− | 0.90 (0.76–1.06) |
| ER−/PR+ | 0.83 (0.54–1.26) |
| ER+/PR+ | 1.00 (Referent) |
| ER & PR unknown | 0.93 (0.76–1.14) |
| Surgical procedure | |
| Mastectomy +/− radiation | 0.60 (0.54–0.67) |
| BCS + radiation | 1.00 (Referent) |
| BCS | 0.65 (0.56–0.75) |
| Age, (yrs)c | |
| <40 | 0.68 (0.48–0.97) |
| 40–49 | 0.87 (0.75–1.00) |
| 50–59 | 1.00 (Referent) |
| 60–69 | 0.97 (0.84–1.12) |
| 70–79 | 0.95 (0.81–1.12) |
| 80+ | 0.53 (0.44–0.63) |
| Type of provider seenc | |
| Oncologist only | 1.55 (1.16–2.08) |
| PCP only | 1.33 (1.11–1.59) |
| Both | 1.87 (1.56–2.25) |
| Neither | 1.00 (Referent) |
| Charlson co-morbidity index scored | |
| 0 | 1.00 (Referent) |
| 1 | 0.81 (0.74–0.89) |
| 2+ | 0.72 (0.64–0.81) |
Abbreviations: OR=odds ratio; 95% CI=95% confidence interval; AJCC= American Joint Committee on Cancer; PCP=primary care physician; ER=estrogen receptor; PR=progesterone receptor; BCS=breast conserving surgery
0.7% of surveillance mammography includes breast MRI
Adjusted by all variables in the table
Covariate updated each surveillance year
Time-varying covariate: variable exposure was ascertained throughout the entire study period; once the subject met the variable definition for use, she was included in that category and remained there until the end of follow-up
In adjusted analyses stratified by age, we observed similar patterns of surveillance mammography use between the age strata, though not all characteristics associated with receipt of surveillance in the unstratified analysis remained statistically significant (Table 3). Peri- or pre-menopausal women <50 and 50–64 years at diagnosis demonstrated lower odds of receiving surveillance mammography compared to post-menopausal women in their respective age strata. There was suggestion that among women <50 years of age at diagnosis, current and past smokers (vs. never smokers) and those with stage II cancer had higher odds of undergoing surveillance mammography, though confidence intervals included 1.0.
Table 3.
Adjusted odds ratios and 95% confidence intervals relating patient characteristics to receipt of surveillance mammographya, stratified by age at incident breast cancer diagnosis
| Adjusted OR (95% CI)b | |||
|---|---|---|---|
| <50 years (n=724) |
50–64 years (n=1,425) |
65+ years (n=1,816) |
|
| Years of follow-upc | 0.92 (0.89–0.95) | 0.92 (0.90–0.94) | 0.84 (0.82–0.86) |
| Menopausal status at diagnosisd | |||
| Peri- or pre-menopausal | 0.56 (0.33–0.94) | 0.76 (0.58–0.99) | -- |
| Post-menopausal | 1.00 (Referent) | 1.00 (Referent) | -- |
| Body mass index (kg/m2) at diagnosis | |||
| <18.5 | 0.59 (0.17–2.04) | 0.95 (0.45–1.99) | 0.66 (0.41–1.06) |
| 18.5–24.9 | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| 25.0–29.9 | 1.01 (0.78–1.31) | 1.02 (0.83–1.25) | 1.03 (0.89–1.20) |
| 30.0–34.9 | 0.99 (0.70–1.39) | 0.91 (0.72–1.15) | 0.86 (0.72–1.04) |
| 35.0+ | 0.92 (0.66–1.28) | 0.87 (0.69–1.10) | 0.72 (0.56–0.93) |
| Smoking status at diagnosis | |||
| Current | 1.37 (0.84–2.23) | 0.65 (0.50–0.85) | 0.78 (0.55–1.09) |
| Past | 1.26 (0.76–2.10) | 0.85 (0.61–1.18) | 1.07 (0.81–1.40) |
| Never | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| AJCC stage[15] | |||
| I | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| IIA | 1.08 (0.83–1.40) | 0.79 (0.65–0.95) | 0.84 (0.71–1.00) |
| IIB | 1.29 (0.92–1.81) | 0.96 (0.73–1.27) | 0.67 (0.50–0.88) |
| ER/PR status | |||
| ER−/PR− | 0.88 (0.67–1.16) | 0.97 (0.78–1.20) | 1.09 (0.88–1.35) |
| ER+/PR− | 0.62 (0.38–1.00) | 0.94 (0.72–1.22) | 0.94 (0.75–1.18) |
| ER−/PR+ | 0.94 (0.47–1.87) | 0.60 (0.27–1.35) | 1.00 (0.53–1.87) |
| ER+/PR+ | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| ER & PR unknown | 0.90 (0.51–1.61) | 0.92 (0.64–1.32) | 0.97 (0.74–1.28) |
| Surgical procedure Mastectomy +/− | |||
| radiation | 0.53 (0.41–0.68) | 0.54 (0.45–0.65) | 0.68 (0.59–0.79) |
| BCS + radiation | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| BCS | 0.81 (0.59–1.11) | 0.69 (0.54–0.88) | 0.57 (0.46–0.72) |
| Age (yrs)c | |||
| <40 | 0.64 (0.47–0.89) | -- | -- |
| 40–44 | 0.95 (0.74–1.21) | -- | -- |
| 45–49 | 1.00 (Referent) | -- | -- |
| 50–54 | -- | 1.00 (Referent) | -- |
| 55–59 | -- | 0.91 (0.70–1.19) | -- |
| 60–64 | -- | 0.84 (0.63–1.11) | -- |
| 65–69 | -- | -- | 1.00 (Referent) |
| 70–74 | -- | -- | 0.83 (0.69–1.00) |
| 75–79 | -- | -- | 0.61 (0.50–0.74) |
| 80+ | -- | -- | 0.36 (0.29–0.43) |
| Type of provider seenc | |||
| Oncologist only | 1.83 (1.03–3.25) | 1.49 (0.90–2.45) | 1.46 (0.92–2.31) |
| PCP only | 1.73 (1.21–2.49) | 1.18 (0.84–1.66) | 1.30 (1.00–1.68) |
| Both | 2.14 (1.48–3.11) | 1.67 (1.19–2.35) | 1.89 (1.44–2.47) |
| Neither | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| Charlson co-morbidity index scoree | |||
| 0 | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| 1 | 0.69 (0.56–0.87) | 0.87 (0.75–1.03) | 0.84 (0.74–0.95) |
| 2+ | 0.63 (0.38–1.05) | 0.85 (0.68–1.06) | 0.74 (0.63–0.87) |
Abbreviations: OR=odds ratio; 95% CI=95% confidence interval; AJCC= American Joint Committee on Cancer; PCP=primary care physician; ER=estrogen receptor; PR=progesterone receptor; BCS=breast conserving surgery
0.7% of surveillance mammography includes breast MRI
Adjusted by all variables in the table
Covariate updated each surveillance year
There was only one post-menopausal woman ≥ 65 years at diagnosis so we could not assess menopausal status within this age strata
Time-varying covariate: variable exposure was ascertained throughout the entire study period; once the subject met the variable definition for use, she was included in that category and remained there until the end of follow-up
Discussion
Guidelines recommend yearly mammography for breast cancer survivors [3, 4], yet many survivors do not undergo annual surveillance mammography [5–7, 9, 11] with declining adherence over time [5, 11, 27]. We observed clear associations between patient characteristics such as smoking, more co-morbidities, ages <40 or 80+, later stage at diagnosis, and treatment with mastectomy or breast conserving surgery without radiation and underutilization of surveillance. Receipt of surveillance mammograms steadily declined in our study population with each year following initial diagnosis, yet our surveillance rates at comparable follow-up times were generally higher over time than those reported previously. Our study population was younger compared to earlier studies and younger women had higher rates of surveillance over time.
Similar to our study results, another study found that surveillance mammography rates among women 65 years or older diagnosed with early stage invasive breast cancer were 82.1% in year 1 but dropped off to 68.5% within 4 years of completing cancer treatment [5]. Similar patterns were observed in another study of breast cancer survivors age 55 years and older [11]. Onega et al. found slightly higher rates, though 18-month intervals were used instead of 12-month intervals [27]. Women age 65 and older demonstrated surveillance mammography rates of 89.3% at 24 months post-diagnosis and rates remained high at 81.5% at 78 months post-diagnosis [27]. We are the only study to date that evaluated surveillance patterns among breast cancer survivors of all ages, followed for up to 10 years since completion of initial treatment.
Several prior studies examined determinants to explain variability in receipt of surveillance over time [5, 10, 11, 27]. Similar to our findings, factors consistently associated with lower rates of surveillance include advanced age, higher stage of disease, and receipt of breast conserving surgery without radiation [5, 7, 9–11], whereas women with outpatient visits to primary care physicians and oncologists during follow-up are more likely to receive surveillance mammography [5, 10, 11]. Prior studies report that women with high co-morbidity burden are less likely to receive surveillance mammograms [10, 11]. This is supported by our finding that women with higher Charlson scores were less likely to receive surveillance compared to women with no co-morbidities. This may be related to the “sick stopper effect” whereby patients who become increasingly ill, with a greater number of competing co-morbidities, forego preventive care [10, 29]. Women with breast cancers initially identified by screening while asymptomatic (screen-detected) are more likely to undergo surveillance mammography compared to those diagnosed once symptoms develop (diagnostic-detected) [28]. Our findings from secondary analyses support that women initially diagnosed with screen-detected cancers were more likely to undergo surveillance mammography during follow-up. No prior studies have evaluated surveillance patterns among women <40 years of age. We determined women <40 years had lower rates of surveillance during follow-up. These women were more likely to have diagnostic-detected, stage II initial cancers compared to women >40 years, both of which are associated with reduced receipt of surveillance mammograms after diagnosis [5, 7, 10, 11, 27, 28]. When we restricted our analyses to women 65+ years at diagnosis and included only the first 4 years after completion of treatment, our findings were similar to those reported previously [5, 10, 11, 27].
In our study, we had access to complete longitudinal data on surveillance mammograms for up to 10 years, which is unavailable in most research settings. We were able to evaluate patient symptoms reported at the time of the mammographic exam as well as the indication designated by the radiologist to differentiate surveillance from diagnostic exams [20]. GH’s automated databases, link to SEER, and medical charts provide detailed and unbiased access to medical information on demographics, breast cancer characteristics, treatment, medical encounters, co-morbidities, SBCEs, and surveillance history and allowed for time-varying adjustment of covariates. We evaluated a large, covered population with stable membership, minimal co-pays and co-insurance for preventive services such as mammography, and who receive almost all of their care within the system.
Our study is not without limitations. We evaluated a single health plan with an insured population with access to complete medical care so estimates may be lower in populations with less access to medical services or the uninsured. The majority of our population was White (89%) with no co-morbidities (77%) at diagnosis of the incident breast cancer. Our findings may not be generalizable to women who are African American or those with co-morbid medical conditions, where receipt of surveillance is likely lower.[10, 11] Our results predominantly pertain to surveillance mammography and rates may be different for other modalities such as MRI. We did not exclude women who had prophylactic mastectomy after their initial breast cancer diagnosis so our surveillance rates may be underestimated since these women aren’t recommended for surveillance mammography. However, we estimate this to be <1% of women in our study. Contrary to Keating et al. [10], we found that women diagnosed more recently were less likely to undergo surveillance, despite adjusting for years of follow-up. It is possible that more mammograms are classified as diagnostic rather than screening, even if for surveillance, in more recent years. Follow-up care intensity and decision about whether to seek preventive services such as mammography is complex. We did not capture provider recommendations, patient beliefs, or patient attitudes regarding follow-up care, nor could we assess why women choose not to receive surveillance care. Furthermore, we did not address when to stop undergoing surveillance and we did not identify subgroups of women who appropriately may not benefit from continued surveillance because of competing co-morbid conditions or poor prognosis. We did, however, censor on SBCE because receipt of surveillance may dramatically change with the diagnosis of a SBCE. Censoring women who develop a SBCE minimizes misclassification of diagnostic or treatment-related imaging as a surveillance procedure.
In summary, yearly surveillance mammography declines over time after initial early stage cancer treatment. Our results suggest that factors influencing receipt of surveillance mammography among breast cancer survivors include: age, stage and treatment of the initial breast cancer, smoking status, co-morbidity, contact with primary care and oncologists. These findings were consistent across different age strata and reinforce the need for active engagement of all breast cancer survivors in long-term follow-up care, especially among women at highest risk for non-adherence to surveillance recommendations. Our findings can be used to help identify women at highest risk for non-adherence to surveillance recommendations which can inform researchers and providers about where efforts are most needed to improve surveillance adherence. Further research is needed to better understand patient attitudes and behaviors toward survivorship care, with an emphasis on surveillance.
Supplementary Material
Acknowledgements
This manuscript was supported by grant numbers CA120562 (Boudreau), CRTG-03-024-01 (Buist), and CA093772 (Silliman). Part of the data collection was supported by a grant from the National Cancer Institute: U01CA63731, PI: Buist).
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2008, National Cancer Institute. [Accessed 11 June 2011];2011 http://seer.cancer.gov/csr/1975_2008/.
- 2.Lash TL, Fox MP, Buist DS, et al. Mammography surveillance and mortality in older breast cancer survivors. J Clin Oncol. 2007;25:3001–3006. doi: 10.1200/JCO.2006.09.9572. [DOI] [PubMed] [Google Scholar]
- 3.Carlson RW, Allred DC, Anderson BO, et al. Invasive breast cancer. J Natl Compr Canc Netw. 2011;9:136–222. doi: 10.6004/jnccn.2011.0016. [DOI] [PubMed] [Google Scholar]
- 4.Khatcheressian JL, Hurley P, Bantug E, et al. Breast cancer follow-up and management after primary treatment: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:961–965. doi: 10.1200/JCO.2012.45.9859. [DOI] [PubMed] [Google Scholar]
- 5.Field TS, Doubeni C, Fox MP, et al. Under utilization of surveillance mammography among older breast cancer survivors. J Gen Intern Med. 2008;23:158–163. doi: 10.1007/s11606-007-0471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersen MR, Urban N. The use of mammography by survivors of breast cancer. Am J Public Health. 1998;88:1713–1715. doi: 10.2105/ajph.88.11.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geller BM, Kerlikowske K, Carney PA, et al. Mammography surveillance following breast cancer. Breast Cancer Res Treat. 2003;81:107–115. doi: 10.1023/A:1025794629878. [DOI] [PubMed] [Google Scholar]
- 8.Lash TL, Silliman RA. Medical surveillance after breast cancer diagnosis. Med Care. 2001;39:945–955. doi: 10.1097/00005650-200109000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Schapira MM, McAuliffe TL, Nattinger AB. Underutilization of mammography in older breast cancer survivors. Med Care. 2000;38:281–289. doi: 10.1097/00005650-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Keating NL, Landrum MB, Guadagnoli E, et al. Factors related to underuse of surveillance mammography among breast cancer survivors. J Clin Oncol. 2006;24:85–94. doi: 10.1200/JCO.2005.02.4174. [DOI] [PubMed] [Google Scholar]
- 11.Doubeni CA, Field TS, Ulcickas Yood M, et al. Patterns and predictors of mammography utilization among breast cancer survivors. Cancer. 2006;106:2482–2488. doi: 10.1002/cncr.21893. [DOI] [PubMed] [Google Scholar]
- 12.Demicheli R, Biganzoli E, Boracchi P, et al. Recurrence dynamics does not depend on the recurrence site. Breast Cancer Res. 2008;10:R83. doi: 10.1186/bcr2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Retsky M, Demicheli R, Hrushesky WJ. Does surgery induce angiogenesis in breast cancer? Indirect evidence from relapse pattern and mammography paradox. Int J Surg. 2005;3:179–187. doi: 10.1016/j.ijsu.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Wirtz HS, Buist DS, Gralow JR, et al. Frequent Antibiotic Use and Second Breast Cancer Events. Cancer Epidemiol Biomarkers Prev. 2013 doi: 10.1158/1055-9965.EPI-13-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. 6th edn. New York: Springer; 2002. [Google Scholar]
- 16.Chubak J, Buist DS, Boudreau DM, et al. Breast cancer recurrence risk in relation to antidepressant use after diagnosis. Breast Cancer Res Treat. 2008;112:123–132. doi: 10.1007/s10549-007-9828-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geiger AM, Thwin SS, Lash TL, et al. Recurrences and second primary breast cancers in older women with initial early-stage disease. Cancer. 2007;109:966–974. doi: 10.1002/cncr.22472. [DOI] [PubMed] [Google Scholar]
- 18.Calip GS, Boudreau DM, Loggers ET, et al. Changes in adherence to statins and subsequent lipid profiles during and following breast cancer treatment. Breast Cancer Res Treat. 2013;138:225–233. doi: 10.1007/s10549-013-2424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taplin SH, Thompson RS, Schnitzer F, et al. Revisions in the risk-based Breast Cancer Screening Program at Group Health Cooperative. Cancer. 1990;66:812–818. doi: 10.1002/1097-0142(19900815)66:4<812::aid-cncr2820660436>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 20.Group Health Research Institute. Group Health Breast Cancer Surveillance Registry. [Accessed 9 July 2013];2012 http://www.grouphealthresearch.org/surveillanceproject/. [Google Scholar]
- 21.Saunders KW, Davis RL, Stergachis A. Group Health Cooperative. In: Strom BL, editor. Pharmacoepidemiology. 4th edn. West Sussex: John Wiley and Sons; 2005. pp. 223–239. [Google Scholar]
- 22.Houssami N, Abraham LA, Miglioretti DL, et al. Accuracy and outcomes of screening mammography in women with a personal history of early-stage breast cancer. JAMA. 2011;305:790–799. doi: 10.1001/jama.2011.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phipps AI, Ichikawa L, Bowles EJ, et al. Defining menopausal status in epidemiologic studies: A comparison of multiple approaches and their effects on breast cancer rates. Maturitas. 2010;67:60–66. doi: 10.1016/j.maturitas.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 25.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 26.Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. 2007;25:2127–2132. doi: 10.1200/JCO.2006.10.3523. [DOI] [PubMed] [Google Scholar]
- 27.Onega T, Weiss J, Diflorio R, et al. Evaluating surveillance breast imaging and biopsy in older breast cancer survivors. Int J Breast Cancer. 2012;2012:347–646. doi: 10.1155/2012/347646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buist DS, Abraham LA, Barlow WE, et al. Diagnosis of second breast cancer events after initial diagnosis of early stage breast cancer. Breast Cancer Res Treat. 2010;124:863–873. doi: 10.1007/s10549-010-1106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson LA, Nelson JC, Benson P, et al. Functional status is a confounder of the association of influenza vaccine and risk of all cause mortality in seniors. Int J Epidemiol. 2006;35:345–352. doi: 10.1093/ije/dyi275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.