Abstract
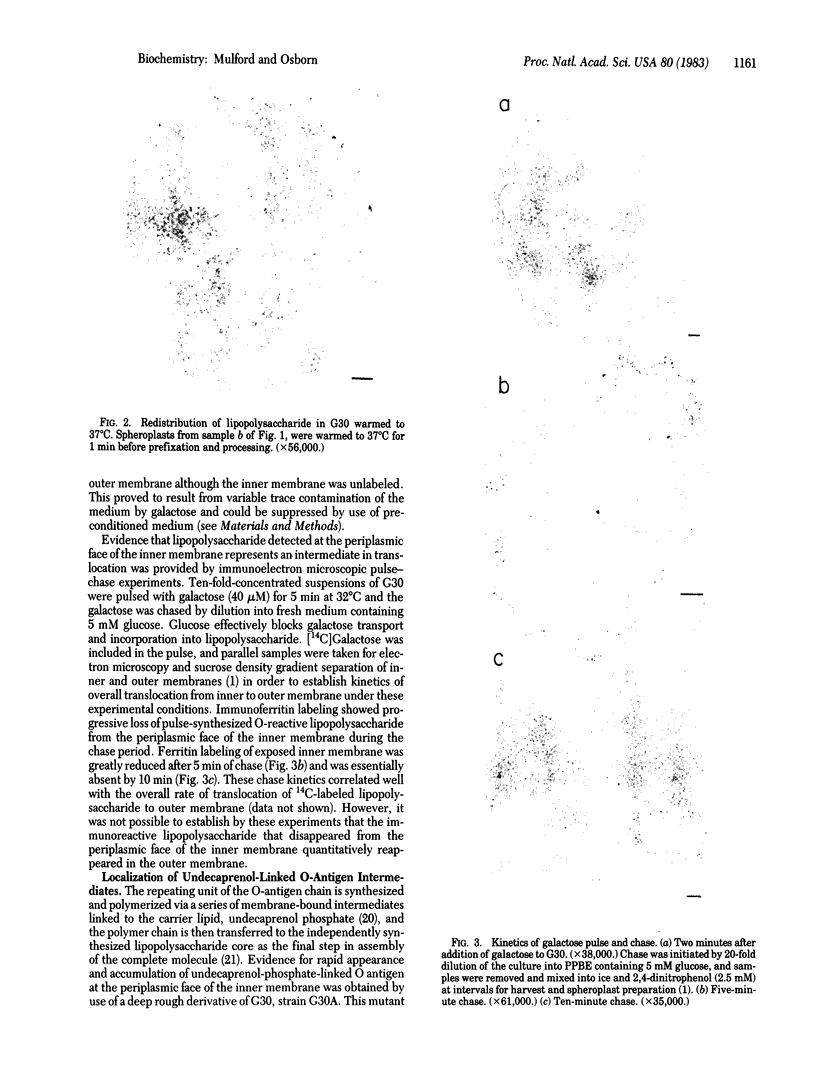
Evidence for transient localization of newly synthesized lipopolysaccharide at the periplasmic face of the inner membrane has been obtained by immunoelectron microscopic techniques. Salmonella typhimurium galE mutants in which O-antigen synthesis is dependent on addition of exogenous galactose were employed, and the distribution and fate of pulse-synthesized O antigen was examined by indirect ferritin labeling with anti-O-antigen IgG of spheroplasts prepared by treatment with lysozyme/EDTA. O-reactive lipopolysaccharide appeared rapidly at the exposed periplasmic face of the inner membrane after addition of galactose and was rapidly depleted upon termination of the pulse. Control experiments showed that secondary redistribution of lipopolysaccharide from outer membrane did not occur under the conditions employed for spheroplast formation and immunolabeling, and the pulse-chase kinetics were consistent with those expected for an intermediate in translocation of lipopolysaccharide to the outer membrane. In addition, undecaprenol-linked O antigen was detectable at the periplasmic face of the inner membrane within 30 sec after addition of galactose to a galE deep rough double mutant, and it accumulated stably in that location. The mutation in synthesis of the lipopolysaccharide core in the deep rough strain prevents transfer of O-antigen chains from undecaprenol phosphate to lipopolysaccharide. The result suggests that attachment of O antigen to lipopolysaccharide occurs on the extracytoplasmic side of the inner membrane and supports the conclusion that lipopolysaccharide is translocated to the outer membrane from the periplasmic, rather than the cytoplasmic, face of the inner membrane.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON J. S., MATSUHASHI M., HASKIN M. A., STROMINGER J. L. LIPID-PHOSPHOACETYLMURAMYL-PENTAPEPTIDE AND LIPID-PHOSPHODISACCHARIDE-PENTAPEPTIDE: PRESUMED MEMBRANE TRANSPORT INTERMEDIATES IN CELL WALL SYNTHESIS. Proc Natl Acad Sci U S A. 1965 Apr;53:881–889. doi: 10.1073/pnas.53.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth S. K., Karnovsky M. J. An ultrastructural staining method for enhancing the size and electron opacity of ferritin in thin sections. J Histochem Cytochem. 1972 Mar;20(3):225–229. doi: 10.1177/20.3.225. [DOI] [PubMed] [Google Scholar]
- Bayer M. E. Areas of adhesion between wall and membrane of Escherichia coli. J Gen Microbiol. 1968 Oct;53(3):395–404. doi: 10.1099/00221287-53-3-395. [DOI] [PubMed] [Google Scholar]
- Endo A., Rothfield L. Studies of a phospholipid-requiring bacterial enzyme. I. Purification and properties of uridine diphosphate galactose: lipopolysaccharide alpha-3-galactosyl transferase. Biochemistry. 1969 Sep;8(9):3500–3507. doi: 10.1021/bi00837a003. [DOI] [PubMed] [Google Scholar]
- Funahara Y., Nikaido H. Asymmetric localization of lipopolysaccharides on the outer membrane of Salmonella typhimurium. J Bacteriol. 1980 Mar;141(3):1463–1465. doi: 10.1128/jb.141.3.1463-1465.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanover J. A., Lennarz W. J. Transmembrane assembly of membrane and secretory glycoproteins. Arch Biochem Biophys. 1981 Oct 1;211(1):1–19. doi: 10.1016/0003-9861(81)90423-9. [DOI] [PubMed] [Google Scholar]
- Kent J. L., Osborn M. J. Properties of the O-specific hapten formed in vivo by mutant strains of Salmonella typhimurium. Biochemistry. 1968 Dec;7(12):4396–4408. doi: 10.1021/bi00852a036. [DOI] [PubMed] [Google Scholar]
- Kulpa C. F., Jr, Leive L. Mode of insertion of lipopolysaccharide into the outer membrane of escherichia coli. J Bacteriol. 1976 Apr;126(1):467–477. doi: 10.1128/jb.126.1.467-477.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacaAlister T. J., Irvin R. T., Costerton J. W. Cell surface-localized alkaline phosphatase of Escherichia coli as visualized by reaction product deposition and ferritin-labeled antibodies. J Bacteriol. 1977 Apr;130(1):318–328. doi: 10.1128/jb.130.1.318-328.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlradt P. F., Golecki J. R. Asymmetrical distribution and artifactual reorientation of lipopolysaccharide in the outer membrane bilayer of Salmonella typhimurium. Eur J Biochem. 1975 Feb 21;51(2):343–352. doi: 10.1111/j.1432-1033.1975.tb03934.x. [DOI] [PubMed] [Google Scholar]
- Mühlradt P. F., Menzel J., Golecki J. R., Speth V. Lateral mobility and surface density of lipopolysaccharide in the outer membrane of Salmonella typhimurium. Eur J Biochem. 1974 Apr 16;43(3):533–539. doi: 10.1111/j.1432-1033.1974.tb03440.x. [DOI] [PubMed] [Google Scholar]
- Mühlradt P. F., Menzel J., Golecki J. R., Speth V. Outer membrane of salmonella. Sites of export of newly synthesised lipopolysaccharide on the bacterial surface. Eur J Biochem. 1973 Jun 15;35(3):471–481. doi: 10.1111/j.1432-1033.1973.tb02861.x. [DOI] [PubMed] [Google Scholar]
- OSBORN M. J., ROSEN S. M., ROTHFIELD L., HORECKER B. L. Biosynthesis of bacterial lipopolysaccharide. I. Enzymatic incorporation of galactose in a mutant strain of Salmonella. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1831–1838. doi: 10.1073/pnas.48.10.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Site of synthesis of lipopolysaccharide. J Biol Chem. 1972 Jun 25;247(12):3973–3986. [PubMed] [Google Scholar]
- Osborn M. J., Rick P. D., Rasmussen N. S. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Translocation and integration of an incomplete mutant lipid A into the outer membrane. J Biol Chem. 1980 May 10;255(9):4246–4251. [PubMed] [Google Scholar]
- Osborn M. J. Structure and biosynthesis of the bacterial cell wall. Annu Rev Biochem. 1969;38:501–538. doi: 10.1146/annurev.bi.38.070169.002441. [DOI] [PubMed] [Google Scholar]
- ROSEN S. M., OSBORN M. J., HORECKER B. L. BIOSYNTHESIS OF BACTERIAL LIPOPOLYSACCHARIDE. 3. CHARACTERIZATION OF THE GALACTOSE INCORPORATION PRODUCT. J Biol Chem. 1964 Oct;239:3196–3200. [PubMed] [Google Scholar]
- ROTHFIELD L., OSBORN M. J., HORECKER B. L. BIOSYNTHESIS OF BACTERIAL LIPOPOLYSACCHARIDE. II. INCORPORATION OF GLUCOSE AND GALACTOSE CATALYZED BY PARTICULATE AND SOLUBLE ENZYMES IN SALMONELLA. J Biol Chem. 1964 Sep;239:2788–2795. [PubMed] [Google Scholar]
- Shands J. W. Localization of Somatic Antigen on Gram-Negative Bacteria by Electron Microscopy. J Bacteriol. 1965 Jul;90(1):266–270. doi: 10.1128/jb.90.1.266-270.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shands J. W. Localization of somatic antigen on gram-negative bacteria using ferritin antibody conjugates. Ann N Y Acad Sci. 1966 Jun 30;133(2):292–298. doi: 10.1111/j.1749-6632.1966.tb52372.x. [DOI] [PubMed] [Google Scholar]
- Snider M. D., Robbins P. W. Synthesis and processing of asparagine-linked oligosaccharides of glycoproteins. Methods Cell Biol. 1981;23:89–100. doi: 10.1016/s0091-679x(08)61493-4. [DOI] [PubMed] [Google Scholar]
- Takamiya H., Batsford S., Gelderblom H., Vogt A. Immuno-electron microscopic localization of lipopolysaccharide antigens on ultrathin sections of Salmonella typhimurium. J Bacteriol. 1979 Oct;140(1):261–266. doi: 10.1128/jb.140.1.261-266.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]