Abstract
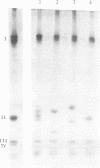
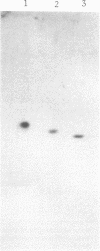
Photosystem I reaction center was isolated from the cyanobacterium Mastigocladus laminosus. It contained four different subunits with molecular masses (as determined by sodium dodecyl sulfate gels) of about 70,000 (subunit I), 16,000 (subunit II), 11,000 (subunit III), and 10,000 (subunit IV) daltons. The purified reaction center contained about 100 chlorophyll a molecules per P700; however, they could be readily depleted down to about 50 chlorophyll a per P700 without loss in the photochemical activities. The reaction center was active in cytochrome c photooxidation, but the photooxidation of an acidic cytochrome, like the Euglena cytochrome 552, required the presence of cations. The purified reaction center was found to be similar in several respects to the photosystem I reaction centers from higher plants and, especially, to the one isolated from green algae. Subunit I appeared on sodium dodecyl sulfate gels in the same position and possessed the same shape of an apparent double band as the corresponding subunits I of green plants and of algae. Subunits I and II of photosystem I reaction centers from Mastigocladus, higher plants, and green algae showed immunological crossreactivity. This observation might serve as biochemical evidence for the common evolution of the photosystem I reaction centers. In higher plants and green algae subunit II is a product of cytoplasmic ribosomes and therefore, a high degree of homology should have been preserved upon transfer of its gene from the prokaryote to the nucleus of the eukaryotes.
Keywords: photosynthesis, immunological crossreactivity, evolution, oxidation-reduction
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bengis C., Nelson N. Purification and properties of the photosystem I reaction center from chloroplasts. J Biol Chem. 1975 Apr 25;250(8):2783–2788. [PubMed] [Google Scholar]
- Bengis C., Nelson N. Subunit structure of chloroplast photosystem I reaction center. J Biol Chem. 1977 Jul 10;252(13):4564–4569. [PubMed] [Google Scholar]
- Binder A., Bachofen R. Isolation and characterization of a coupling factor I ATPase of the thermophilic blue-green alga (cyanobacterium) Mastigocladus laminosus. FEBS Lett. 1979 Aug 1;104(1):66–70. doi: 10.1016/0014-5793(79)81086-8. [DOI] [PubMed] [Google Scholar]
- Clayton R. K., Haselkorn R. Protein components of bacterial photosynthetic membranes. J Mol Biol. 1972 Jul 14;68(1):97–105. doi: 10.1016/0022-2836(72)90265-3. [DOI] [PubMed] [Google Scholar]
- Douglas M. G., Butow R. A. Variant forms of mitochondrial translation products in yeast: evidence for location of determinants on mitochondrial DNA. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1083–1086. doi: 10.1073/pnas.73.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt E., Hauska G. A cytochrome f/b6 complex of five polypeptides with plastoquinol-plastocyanin-oxidoreductase activity from spinach chloroplasts. Eur J Biochem. 1981 Jul;117(3):591–595. doi: 10.1111/j.1432-1033.1981.tb06379.x. [DOI] [PubMed] [Google Scholar]
- Junge W., Schaffernicht H., Nelson N. On the mutual orientation of pigments in photosystem I particles from green plants. Biochim Biophys Acta. 1977 Oct 12;462(1):73–85. doi: 10.1016/0005-2728(77)90190-6. [DOI] [PubMed] [Google Scholar]
- Kagawa Y. Reconstitution of the energy transformer, gate and channel subunit reassembly, crystalline ATPase and ATP synthesis. Biochim Biophys Acta. 1978 Sep 21;505(1):45–93. doi: 10.1016/0304-4173(78)90008-3. [DOI] [PubMed] [Google Scholar]
- Klein S. M., Vernon L. P. Composition of a photosystem I chlorophyll protein complex from Anabaena flos-aquae. Biochim Biophys Acta. 1977 Mar 11;459(3):364–375. doi: 10.1016/0005-2728(77)90038-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ludwig B. Heme aa3-type cytochrome c oxidases from bacteria. Biochim Biophys Acta. 1980 Dec;594(2-3):177–189. doi: 10.1016/0304-4173(80)90008-7. [DOI] [PubMed] [Google Scholar]
- Ludwig B., Schatz G. A two-subunit cytochrome c oxidase (cytochrome aa3) from Paracoccus dentrificans. Proc Natl Acad Sci U S A. 1980 Jan;77(1):196–200. doi: 10.1073/pnas.77.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullet J. E., Burke J. J., Arntzen C. J. Chlorophyll proteins of photosystem I. Plant Physiol. 1980 May;65(5):814–822. doi: 10.1104/pp.65.5.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nechushtai R., Nelson N. Photosystem I reaction centers from Chlamydomonas and higher plant chloroplasts. J Bioenerg Biomembr. 1981 Dec;13(5-6):295–306. doi: 10.1007/BF00743207. [DOI] [PubMed] [Google Scholar]
- Nelson N., Deters D. W., Nelson H., Racker E. Partial resolution of the enzymes catalyzing photophosphorylation. 8. Properties of isolated subunits of coupling factor 1 from spinach chloroplasts. J Biol Chem. 1973 Mar 25;248(6):2049–2055. [PubMed] [Google Scholar]
- Nelson N., Neumann J. Interaction between ferredoxin and ferredoxin nicotinamide adenine dinucleotide phosphate reductase in pyridine nucleotide photoreduction and some partial reactions. I. Inhibition of ferredoxin nicotinamide adenine dinucleotide phosphate reductase by ferredoxin. J Biol Chem. 1969 Apr 10;244(7):1926–1931. [PubMed] [Google Scholar]
- Newman P. J., Sherman L. A. Isolation and characterization of photosystem I and II membrane particles from the blue-green alga, Synechococcus cedrorum. Biochim Biophys Acta. 1978 Aug 8;503(2):343–361. doi: 10.1016/0005-2728(78)90193-7. [DOI] [PubMed] [Google Scholar]
- Okamura M. Y., Steiner L. A., Feher G. Characterization of reaction centers from photosynthetic bacteria. I. Subunit structure of the protein mediating the primary photochemistry in Rhodopseudomonas spheroides R-26. Biochemistry. 1974 Mar 26;13(7):1394–1403. doi: 10.1021/bi00704a013. [DOI] [PubMed] [Google Scholar]
- Olson J. M. The evolution of photosynthesis. Science. 1970 Apr 24;168(3930):438–446. doi: 10.1126/science.168.3930.438. [DOI] [PubMed] [Google Scholar]
- Rott R., Nelson N. Purification and immunological properties of proton-ATPase complexes from yeast and rat liver mitochondria. J Biol Chem. 1981 Sep 10;256(17):9224–9228. [PubMed] [Google Scholar]
- Sone N., Ohyama T., Kagawa Y. Thermostable single-band cytochrome oxidase. FEBS Lett. 1979 Oct 1;106(1):39–42. doi: 10.1016/0014-5793(79)80690-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]