Summary
It is shown that AtCLCd negatively regulates Arabidopsis PTI, probably by interacting with the PRR signalling pathway. Its sequence indicates that AtCLCd encodes a chloride/proton exchanger.
Key words: Arabidopsis thaliana, AtCLCd, chloride channel, PAMP-triggered immunity.
Abstract
Chloride channel (CLC) family genes are ubiquitous from prokaryotes to eukaryotes and encode proteins with both channel and transporter activities. The Arabidopsis thaliana genome encodes seven CLC genes, and their products are found in a variety of cellular compartments and have various physiological functions. However, a role for AtCLCs in plant innate immunity has not previously been demonstrated. Here it is reported that AtCLCd is a negative regulator of pathogen-associated molecular pattern (PAMP)-triggered immunity (PTI). T-DNA insertion mutants of AtCLCd exhibited enhanced responses to the elicitor, flg22. The PTI phenotypes of the clcd mutants were rescued by expression of AtCLCd. Overexpression of AtCLCd led to impaired flg22-induced responses. In line with a role for AtCLCd in PTI, the clcd mutants were more resistant to a virulent strain of the bacterial pathogen Pseudomonas syringae pv. tomato DC3000 when spray inoculated, while AtCLCd-overexpressing lines displayed increased susceptibility to this pathogen. Interestingly, flg22 treatment was found to repress the expression of AtCLCd. In addition, its expression was elevated in mutants of the flg22 pattern recognition receptor (PRR) FLS2 and the PRR regulatory proteins BAK1 and BKK1, and reduced in an FLS2-overexpressing line. These latter findings indicate that FLS2 complexes regulate the expression of AtCLCd, further supporting a role for AtCLCd in PTI.
Introduction
Under natural conditions, plants are constantly exposed to harmful pathogens. Plants, being sessile, cannot simply escape from biotic stresses. However, they have evolved a complicated innate immune system to fight pathogen attacks. Their innate immune system generally consists of two layers of defence (Jones and Dangl, 2006). The first, named PAMP-triggered immunity (PTI), is triggered by the recognition of pathogen-associated molecular patterns (PAMPs) by plant cell surface pattern recognition receptors (PRRs). Adapted pathogens can secrete effector proteins into host cells to suppress PTI. The second layer of defence, termed effector-triggered immunity (ETI), originates in the cytoplasm and is triggered directly or indirectly by the recognition of secreted microbial effectors by plant resistance (R) proteins. Thus, activation of plant innate immunity is largely dependent on recognition of ‘non-self’ signals (Robatzek and Saijo, 2008; Boller and He, 2009; Tsuda and Katagiri, 2010).
After perception of PAMPs or effectors, host cells initiate a series of physiological processes, of which the oxidative burst, extracellular alkalinization, and protein phosphorylation are the earliest (Felix et al., 1999; Bauer et al., 2001; Kunze et al., 2004). Extracellular alkalization is caused by ion fluxes across the plasma membrane (Nürnberger et al., 1994; Jabs et al., 1997), indicating that ion channels are activated in response to pathogen attacks. A number of channels have been shown to play a role in plant defence responses (Jeworutzki et al., 2010; Qi et al., 2010; Koers et al., 2011). Anion channels have been shown to be required, especially for PTI. Elicitors or PAMPs such as cryptogein and flagellin can induce massive anion efflux (Wendehenne et al., 2002; Jeworutzki et al., 2010). The production of reactive oxygen species (ROS) is a common early response to PAMPs. Using a pharmacological approach, Colcombet et al. (2009) showed that rapid-type (R-type) anion channels are important in flagellin-induced ROS production in Arabidopsis suspension cells, and Jeworutzki et al. (2010) recorded strong anion currents in mesophyll and root hair cells of Arabidopsis upon PAMP treatment. In addition, inhibition of anion channels impaired the cryptogein-induced cell death in the hypersensitive response (HR) of tobacco suspension cells (Wendehenne et al., 2002). PAMP-triggered plasma membrane anion channel opening was found to be dependent on PRRs and BAK1, suggesting that the anion channels are activated downstream of PRRs (Jeworutzki et al., 2010).
Several gene families encoding anion channels/transporters have been identified in plants. Of these, three families, SLAC1 (slow anion channel 1), ALMT1 (aluminium-activated malate transporter 1), and CLC (chloride channel), have been the most studied (Barbier-Brygoo et al., 2011). Patch–clamp studies on Vicia faba guard cells revealed the presence of R-type and slow-type (S-type) anion channels (Schroeder and Keller, 1992). The S-type channel is encoded by SLAC1 (Vahisalu et al., 2008), and the R-type channel by members of the ALMT transporter family (Meyer et al., 2010). Interestingly, barley powdery mildew was shown to activate host cell S-type anion channels and thereby inhibit light-induced stomatal opening (Koers et al., 2011). Similarly, the Arabidopsis guard cell SLAC1 was found to be necessary for stomatal closure in response to biotic stress (Negi et al., 2008; Saji et al., 2008; Vahisalu et al., 2008; Montillet et al., 2013). Despite the evidence of the involvement of plant anion channels in defence responses, there is so far little direct evidence of the participation of anion channels in innate immunity, and how these channels regulate plant defence responses also remains elusive.
CLC family proteins are present in prokaryotes and eukaryotes and have both channel and transporter activities (Jentsch, 2008; Barbier-Brygoo et al., 2011). The Arabidopsis genome encodes seven AtCLC genes (AtCLCa–AtCLCg) (Hechenberger et al., 1996; Lv et al., 2009), and their products are found in several cellular compartments including the vacuole membrane (AtCLCa, AtCLCb, and AtCLCc) (De Angeli et al., 2007; von der Fecht-Bartenbach et al., 2010), the Golgi apparatus (AtCLCd and AtCLCf), and chloroplast membranes (AtCLCe) (Marmagne et al., 2007; von der Fecht-Bartenbach et al., 2007). AtCLCa, AtCLCb, and AtCLCe are required to maintain normal cellular nitrate levels (De Angeli et al., 2007; von der Fecht-Bartenbach et al., 2010), and AtCLCc participates in both nitrate and chloride homeostasis and regulates stomatal movement and salt tolerance (Jossier et al., 2010). AtCLCd has been proposed to regulate luminal pH in the trans-Golgi network (TGN) (von der Fecht-Bartenbach et al., 2007). However, a role for AtCLCs in plant innate immunity has not previously been demonstrated. In this work, the defence phenotypes of all available AtCLC family mutants were examined and it was found that AtCLCd negatively regulates PTI.
Materials and methods
Plant materials and growth conditions
The T-DNA insertion mutants clcd-1 (SALK_042895), clcd-2 (SALK_052368C), clca (CS857712), clcb (SALK_027349C), clcc (SALK_115644C), clce (SALK_010237), and clcg (SALK_087699) were obtained from the Arabidopsis Biological Resource Center. Homozygous T-DNA insertion lines were screened by PCR and confirmed by reverse transcription–PCR (RT–PCR) using gene-specific primers (see Supplementary Table S1 available at JXB online). fls2 (SALK_141277) was kindly provided by Dr Jianmin Zhou. bak1-5, bkk1-1, and bak1-5/bkk1-1 were kindly provided by Dr Cyril Zipfel. Arabidopsis thaliana wild-type Columbia-0 (Col-0) and mutants were grown in a growth room with 8h light/16h darkness at 75% relative humidity and 22 °C.
Gene constructs and plant transformation
To complement the clcd mutant, the 1.6kb promoter region of AtCLCd and the full-length open reading frame were separately PCR amplified from Arabidopsis Col-0 genomic DNA and cDNA, respectively, and the NOS terminator was then cloned into the binary vector pCB302 (Xiang et al., 1999) to create construct pAtCLCd:AtCLCd. To overexpress AtCLCd, the full-length coding sequence was amplified and cloned downstream of the Cauliflower mosaic virus (CaMV) 35S promoter in the binary vector pCB302-3 (Xiang et al., 1999) to obtain p35S:AtCLCd. To overexpress FLS2, the full-length coding sequence was amplified from cDNA and cloned into binary vector pCAMBIA1300 (Cambia) between the BamHI and SpeI multiple cloning sites to form p35S:FLS2. The primers used for making the constructs are listed in Supplementary Table S1 available at JXB online. All constructs were verified by sequencing and introduced into Agrobacterium tumefaciens strain GV3101 by electroporation. Agrobacterium tumefaciens carrying the constructs was used to transform Arabidopsis Col-0 by floral dip (Clough and Bent, 1998). Transformed Arabidopsis lines were selected on soil by spraying with a 1:1000 dilution of Basta (Bayer CropScience) or on MS (Murashige and Skoog) agar plates supplied with 20mg l–1 hygromycin B.
Semi-quantitative and quantitative real-time RT–PCR
Total RNA was extracted from Arabidopsis leaves with TRIzol reagent (Invitrogen). A sample containing 2 μg of total RNA was treated with DNase I (Invitrogen) and reverse transcribed with M-MLV Reverse Transcriptase (Promega). For semi-quantitative PCR, 25 μl reaction mixtures contained 0.5U of Taq DNA polymerase (MBI, Fermentas), 80ng of cDNA, 200 μM of each dNTP, and 0.2 μM of each primer. PCR parameters were: 3min at 95 °C followed by 28 cycles of 95°C for 30 s, 60 °C for 30 s, and 72 °C for 40 s.
Real-time PCR was performed using TakaRa SYBR Premix Ex Taq following the manufacturer’s instructions, and run in a Bio-Rad CFX96 (Bio-Rad Laboratories). The reaction volume was 20 μl containing 10 μl of SYBR pre-mix, 0.5 μM of each primer, and 20ng of cDNA. A three-step protocol was used: a denaturation program (95 °C for 30 s), an amplification and quantification program repeated 40 times (95 °C for 5 s, 60 °C for 30 s and 72°C for 30 s with the fluorescence measurement), and a melting curve program (55–95 °C, with a 0.5 °C increment each cycle). Each sample was replicated three times. ACTIN2 was used as an internal reference gene, and normalized fold expression was calculated employing CFX Manager Software (Bio-Rad) and the –ΔΔC(t) method. Unless otherwise indicated, result values displayed are relative to wild-type (Col-0) untreated plants, which are set to a relative value of 1. The primers used in semi-quantitative and real-time RT–PCR are listed in Supplementary Table S1 available at JXB online. Data are representative of two to three independent biological experiments.
Bacterial growth assays
Five-week-old plants were spray inoculated with Pseudomonas syringae pv. tomato DC3000 (Pst. DC3000), and bacterial growth in planta was analysed as described by Zipfel et al. (2004). The bacterial suspension contained 2.5×108 (Fig. 4A) or 2.5×106 (Fig. 4B) colony-forming units (cfu) ml–1 in 10mM MgCl2 with 0.01% Silwet L-77. Eight plants of each genotype were used per experiment, and the experiments were repeated at least three times. Bacterial numbers in mutant and transgenic lines were compared with those in Col-0 using a two-tailed Student’s t-test.
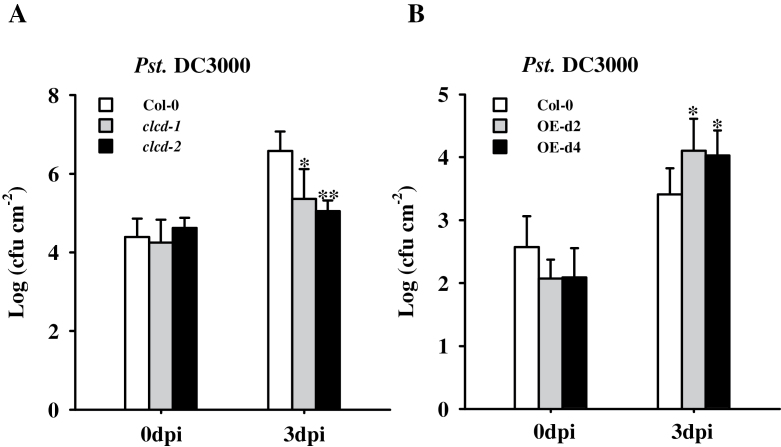
Fig. 4.
Responses of the clcd mutants and AtCLCd-overexpressing lines to the pathogen Pseudomonas syringae. (A and B) Bacterial growth of Pst. DC3000 was measured in Col-0, clcd-1, and clcd-2 (A) or Col-0 and AtCLCd-overexpressing lines OE-d2 and OE-d4 (B) on 0 d and 3 d post spray inoculation (dpi). Bacterial suspensions containing 2.5×108 (A) and 2.5×106 (B) cfu ml–1 were used. Values are mean ±SD (n=8). *P<0.05; **P<0.01 (t-test). cfu, colony-forming units.
ROS burst assays
Flg22 peptide (QRLSTGSRINSAKDDAAGLQIA) and elf18 peptide (acetyl-MSKEKFERTKPHVNVGTI) were synthesized by GenScript Corp. Chitin (Seikagaku) was kindly provided by Dr Morten Petersen. All the above PAMPs were dissolved in sterile water. PAMP-induced ROS production was measured as previously described (Roux et al., 2011; Schwessinger et al., 2011). Briefly, leaf discs (0.125cm2) of 5-week-old plants were incubated overnight in 96-well plates in water, and the water was replaced with 200 μl of a solution containing 10 μg ml–1 peroxidase (Sigma-Aldrich) and 20 μM luminol in the presence of 100nM flg22, 100nM elf18, or 100 μg ml–1 chitin. Luminescence is shown as relative light units (RLUs), measured and calculated using a Berthold Centro LB960 luminometer (Berthhold Technologies).
Callose deposition assays
Leaves of 5-week-old Arabidopsis plants were infiltrated with 1 μM flg22 with a needleless syringe. After 16h, the leaves were cleared, stained with aniline blue as previously described (Hann and Rathjen, 2007), mounted in 50% glycerol, and examined with a UV epifluorescence microscope. The numbers of bright spots (corresponding to callose deposits) per microscopic field of 1mm2 were counted using Image J software (http://imagej.nih.gov/ij/), and 12 microscopic fields were counted per sample.
Root growth assays
Arabidopsis seeds were surface-sterilized and sown on 1/2 MS agar medium. Seeds were stratified at 4 °C for 2 d and grown vertically for 5 d in short-day conditions (8h light/16h dark). Seedlings were then transferred to a new square Petri dish with 1/2 MS agar medium supplemented with different amounts of flg22 peptide. The lengths of the main roots after growth under long-day conditions (16h light/8h dark) were measured with Image J software.
Seedling growth assays
The seedling growth assays were performed as described (Pfund et al., 2004). The fresh weight of seedlings was measured 8 d after flg22 treatment.
Accession numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: Arabidopsis AtCLCa (At5g40890), AtCLCb (At3g27170), AtCLCc (At5g49890), AtCLCd (At5g26240), AtCLCe (At4g35440), AtCLCf (At1g55620), AtCLCg (At5g33280), FRK1 (At2g19190), FLS2 (At5g46330), PR1 (At2g14610), ACTIN7 (At5g09810), and ACTIN2 (At3g18780).
Results
Arabidopsis clcd mutant exhibits enhanced flg22-induced responses
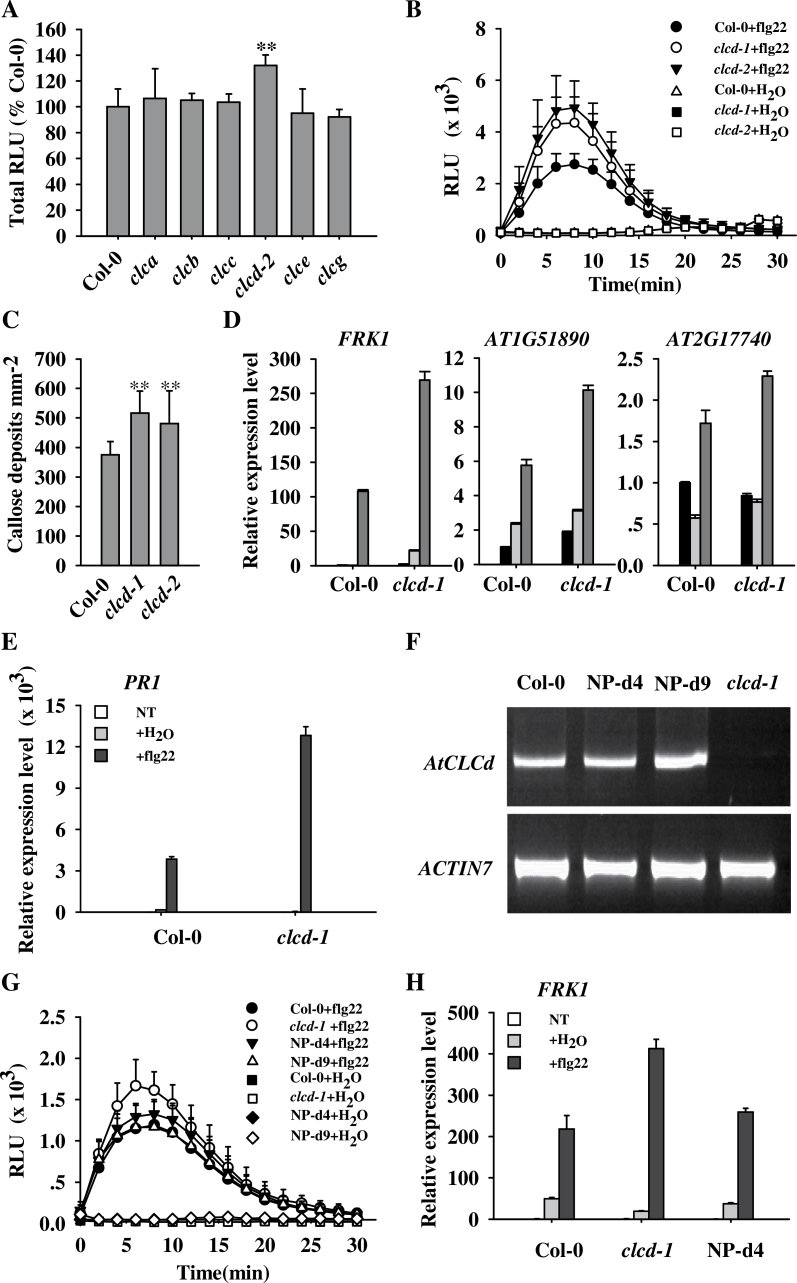
In a search for the anion channel(s) involved in plant defence responses, especially PTI, all available mutants of the Arabidopsis CLC gene family were screened using flg22-induced ROS as an indicator. flg22, a conserved 22 amino acid sequence at the N-terminus of flagellin, is a well-studied PAMP in plant innate immunity (Felix et al., 1999; Bauer et al., 2001). ROS production triggered by flg22 was measured in Arabidopsis T-DNA insertion lines of the AtCLCa, b, c, d, e, and g genes (Supplementary Fig. S1 available at JXB online). Leaf discs from all these lines except the clcd mutant produced similar ROS bursts to those produced by Col-0 (wild-type) Arabidopsis plants (Fig. 1A). However, the flg22-triggered ROS burst was significantly larger in the clcd-2 mutant (Fig. 1A). This result was confirmed with another clcd mutant, clcd-1 (Fig. 1B). The impact of AtCLCd mutations on other temporal responses triggered by flg22 was then examined (Roux et al., 2011). The ROS burst is an early response to PAMPs, whereas callose deposition is a late response, detected in Arabidopsis by aniline blue staining ~16h after flg22 treatment (Boller and Felix, 2009). The flg22-induced callose deposition was also larger in clcd-1 and clcd-2 than in Col-0 (Fig. 1C). These findings indicate that AtCLCd is involved in PTI. Since the clcd-1 and clcd-2 mutants had almost identical phenotypes, to simplify the work, further analyses concentrated on the clcd-1 mutant.
Fig. 1.
PAMP-triggered immunity is enhanced in Arabidopsis clcd mutants. (A) Total ROS production triggered by 100nM flg22 in Arabidopsis leaf discs in relative light units (RLU). Results are expressed as percentages of flg22-treated Col-0. (B) flg22-induced ROS bursts in Col-0, clcd-1, and clcd-2 leaf discs. (C) Callose deposition in Col-0, clcd-1, and clcd-2 leaves after infiltration with 1 μM flg22 (n=22). (D and E) Quantitative real-time PCR analysis of the expression of PTI marker genes (D) and PR1 (E) in Arabidopsis leaves 1h (D) and 24h (E) after infiltration with 1 μM flg22 or water. The samples were measured in triplicate and normalized to ACTIN2. (F) Expression of AtCLCd determined by semi-quantitative RT–PCR in the wild-type (Col-0), AtCLCd complementation lines (NP-d4 and NP-d9), and clcd-1. Levels of ACTIN7 transcripts were used as loading controls. (G) Flg22-induced ROS bursts in Col-0, clcd-1, NP-d4, and NP-d9 in RLU. (H) Quantitative real-time PCR analysis of the expression of the PTI marker gene FRK1 in Col-0, clcd-1, and NP-d4 plants 1h after infiltration with 1 μM flg22 or water. Samples were assayed in triplicate and normalized to ACTIN2. Values are means ±SD. NT, no treatment. **P<0.01 (t-test).
The expression of PAMP-inducible genes in the clcd mutants was next assessed. The expression levels of three different PTI marker genes FRK1, At1g51890, and At2g17740 (He et al., 2006) were measured 1h after infiltration with 1 μM flg22. As shown in Fig. 1D, the induction of all the three PTI marker genes was enhanced in the clcd-1 mutant. Interestingly, the late response gene PR1 was also induced to a strikingly higher level in clcd-1 than in Col-0 (Fig. 1E).
In order to confirm that the phenotype of the clcd mutants is actually caused by mutation in the AtCLCd gene, clcd-1 plants were transformed via A. tumefaciens with pAtCLCd:AtCLCd, a construct carrying the full-length open reading frame of AtCLCd driven by its own promoter (1571bp upstream of the start codon). A number of transgenic plants were obtained, most of which had the wild-type level of AtCLCd expression as checked by semi-quantitative RT–PCR (Fig. 1F). Representative lines homozygous for the rescue construct were used for further phenotypic analysis. The changes in the flg22-triggered ROS burst (Fig. 1G) and in the expression of FRK1 (Fig. 1H) were also rescued by expressing AtCLCd.
In summary, it was shown that mutations in AtCLCd lead to enhanced early and late responses to flg22, suggesting that AtCLCd negatively regulates PTI.
Overexpression of AtCLCd leads to impaired flg22-induced responses
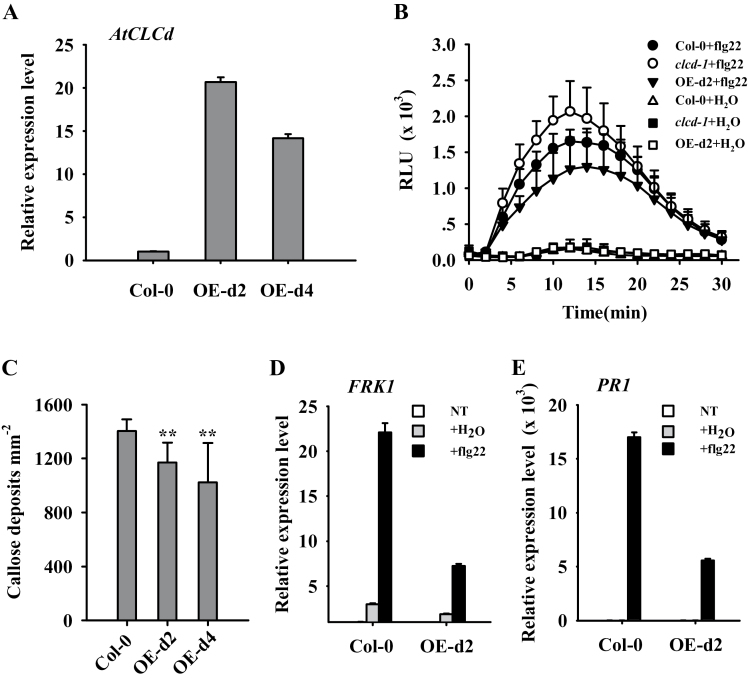
To establish further the role of AtCLCd in PTI, transgenic plants constitutively overexpressing AtCLCd were created (see the Materials and methods). The full-length AtCLCd coding region driven by the CaMV 35S promoter was cloned into a binary vector and introduced into Col-0 plants. Two representative transgenic lines homozygous for the transgene (OE-d2 and OE-d4) with elevated AtCLCd transcript levels (Fig. 2A) were used for detailed phenotypic analyses. ROS bursts (Fig. 2B; Supplementary Fig. S2A available at JXB online) and callose deposition (Fig. 2C) triggered by flg22 were reduced in the AtCLCd-overexpressing lines. In addition, flg22-induced expression of the PTI marker genes FRK1, At1g51890, and At2g17740, and the late response gene PR1 was impaired (Fig. 2D, E; Supplementary Fig. S2B available at JXB online). These data further demonstrate that AtCLCd is a negative regulator of PTI.
Fig. 2.
PAMP-triggered immunity is compromised in the AtCLCd-overexpressing lines. (A) Expression of AtCLCd was determined by quantitative RT–PCR in the wild type (Col-0) and AtCLCd-overexpressing lines (OE-d2 and OE-d4). (B) Flg22-induced ROS bursts in Col-0, clcd-1, and OE-d2 were measured in relative light units (RLU). Values are mean ±SD (n=8). (C) Flg22-induced callose deposition in the leaves of Col-0, OE-d2, and OE-d4 plants. Values are mean ±SD (n=12). **P<0.01 (t-test). (D and E) Quantitative real-time PCR analyses of the expression of FRK1 (D) and PR1 (E) in Col-0 and AtCLCd-overexpressing lines. Samples were assayed in triplicate and normalized to ACTIN2. Error bars indicate the SD. NT, no treatment.
Misexpression of AtCLCd affects PTI responses
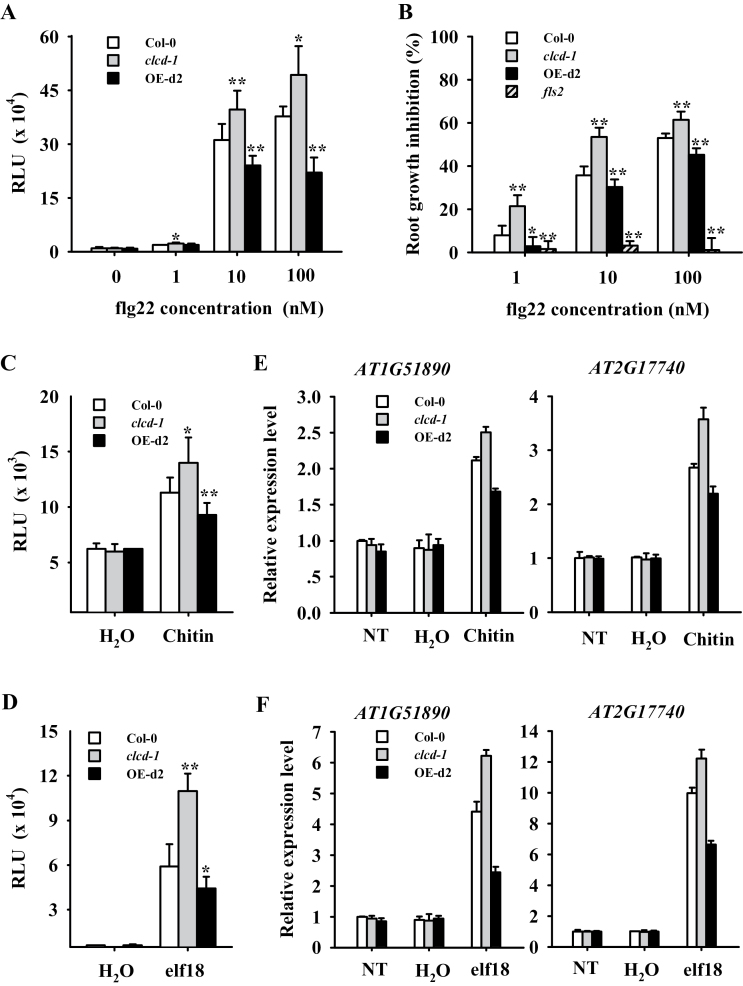
To confirm the regulatory role of AtCLCd in PTI, the phenotypes of AtCLCd-misexpressing plants were investigated in more detail. The responses of the clcd mutants and the AtCLCd-overexpressing lines to various doses of flg22 were first analysed. As shown in Fig. 3A, production of ROS induced by flg22 was increased in the clcd mutant and reduced in the AtCLCd-overexpressing lines compared with the Col-0 wild type. Root growth inhibition is another characteristic effect of flg22 treatment (Boller and Felix, 2009). The clcd-1 seedlings exhibited increased root growth inhibition in the presence of flg22, while root growth was less inhibited by flg22 in the AtCLCd-overexpressing lines (Fig. 3B). However, the most striking difference between the AtCLCd-misexpressing plants and the wild type in their responses to flg22 was observed after treating them with a lower dose (10nM) of the peptide (Fig. 3A, B). It is possible that a saturating dose of flg22 prevents detection of a partial effect of the mutation or of overexpression of AtCLCd. The effect of AtCLCd misexpression on flg22-triggered responses was further revealed by seedling growth inhibition assays (Supplementary Fig. S3 available at JXB online).
Fig. 3.
PAMP-triggered immunity is affected by misexpression of AtCLCd. (A) Total ROS production elicited by different amounts of flg22 in Arabidopsis leaf discs is represented as relative light units (RLU). Results are expressed as percentages of flg22-treated Col-0, and are means ±SD (n=8). (B) Inhibition of primary root growth by different doses of flg22. Results are expressed as percentages of inhibition relative to the untreated control; means ±SD of three independent experiments (n >20). (C and D) Total ROS production induced by chitin or elf18 in Col-0, clcd-1, and OE-d2, measured in RLU. Values are means ±SD (n=8). (E and F) Quantitative RT–PCR analysis of the expression of the PTI marker genes 1h after treatment with chitin and elf18. NT, no treatment. *P<0.05; **P<0.01 (t-test).
Responses of the AtCLCd-misexpressing plants to different PAMPs were next examined. Production of ROS elicited by both chitin and elf18 was significantly enhanced in the clcd mutant, but reduced in the AtCLCd-overexpressing lines (Fig. 3C, D). Accordingly, expression of the PTI marker genes At1g51890 and At2g17740 was also increased in the mutant, but decreased in the overexpressing lines (Fig. 3E, F). In addition, the morphological phenotypes and expression levels of FLS2 (Supplementary Fig. S4 available at JXB online) were not changed in the AtCLCd-misexpressing plants. These results support a general role for AtCLCd in PTI.
Altered bacterial disease resistance in Arabidopsis clcd mutants and AtCLCd-overexpressing plants
PTI plays an important role in basal resistance to bacterial pathogens (Jones and Dangl, 2006), and defects in PTI, due to mutations in the PRR FLS2 and its positive regulator BAK1, result in enhanced susceptibility to the virulent bacterial pathogen Pst. DC3000 upon spray inoculation (Zipfel et al., 2004; Roux et al., 2011). Tests were carried out to determine whether AtCLCd controls disease resistance. Four-week-old clcd-1 and clcd-2 plants were spray inoculated with Pst. DC3000, and growth of the bacterial pathogen in the leaves was assessed. As shown in Fig. 4A, growth of Pst. DC3000 in the leaves of the clcd mutants was reduced, whereas in the AtCLCd-overexpressing lines it was increased (Fig. 4B). These data further support the inhibitory role of AtCLCd in PTI.
Treatment with the PAMP, flg22, represses the expression of AtCLCd
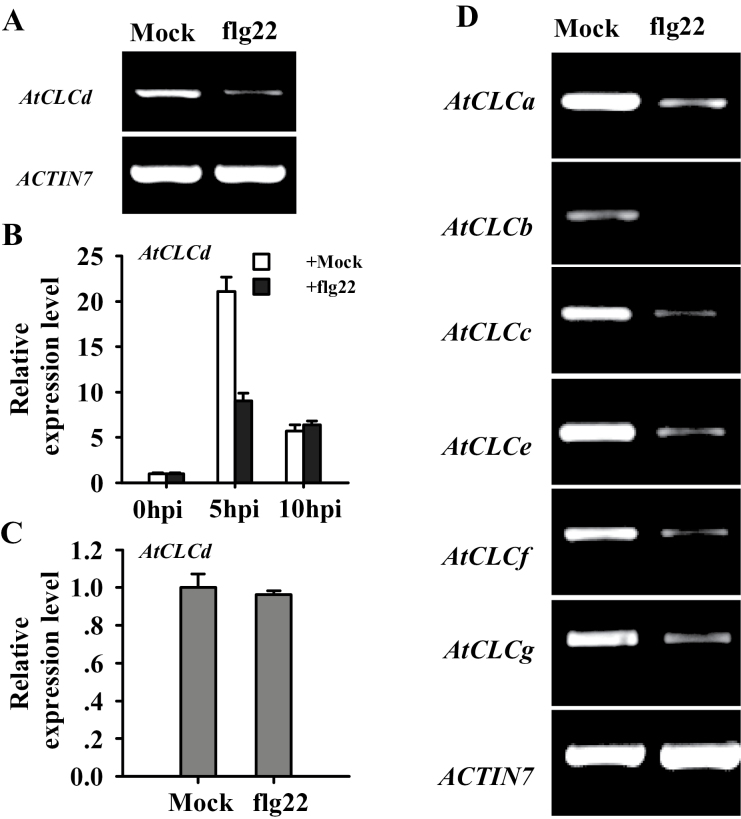
Since AtCLCd negatively regulates PTI, it was of interest to see whether AtCLCd expression was affected by PAMPs. To this end, 4-week-old Col-0 plants grown under short-day conditions were infiltrated with 1 μM flg22 or water (mock). The expression of AtCLCd was reduced by the flg22 treatment (Fig. 5A). Expression of AtCLCd was then examined in more detail (Fig. 5B). Treatment with water (as a control) stimulated the expression of AtCLCd (Fig. 5B), probably due to damage introduced by the infiltration. However, compared with the water-treated plants, the accumulation of AtCLCd mRNA was significantly reduced in the flg22-treated plants at 5h post-treatment (Fig. 5B), further showing that flg22 negatively regulates the expression of AtCLCd. By 10h, expression of AtCLCd was similar in the flg22-treated and water-treated plants. To see whether the repression of AtCLCd by flg22 is dependent on FLS2, expression of AtCLCd was measured in fls2 mutant plants (SALK_141277, Xiang et al., 2008) infiltrated with 1 μM flg22 or water. As shown in Fig. 5C, transcripts of AtCLCd accumulated to similar level in flg22-treated plants and mock-treated plants, indicating that flg22 does not suppress the expression of AtCLCd in the fls2 mutant. Thus, it can be concluded that recognition of flg22 by FLS2 is needed for the inhibition of AtCLCd expression.
Fig. 5.
Treatment with PAMP flg22 inhibits the expression of Arabidopsis CLC family genes. (A) Expression of AtCLCd in water- and flg22-treated wild-type (Col-0) leaves. (B) Accumulation of AtCLCd transcripts in Col-0 leaves 5h and 10h after infiltration of 1 μM flg22 or water. (C) Expression of AtCLCd in fls2 mutant leaves 5h after infiltration of 1 μM flg22 or water. (D) Expression of AtCLC family genes in water- and flg22-treated Col-0 samples 5h after infiltration. Semi-quantitative RT–PCR was performed in (A) and (D). The level of ACTIN7 transcript was used as a loading control. Quantitative RT–PCR was performed in (B) and (C). All samples were assayed in triplicate and normalized to ACTIN2. In (C), the relative expression ratio of AtCLCd transcript was compared with that of water-treated fls2 plants, which are set to a relative value of 1. Error bars indicate the SD. hpi, hours post-infiltration.
Next, the response of all other Arabidopsis CLC family genes to flg22 was examined. Interestingly, all were affected in the same way as AtCLCd (Fig. 5D). It appears therefore that the sensitivity of expression to flg22 may be common to all Arabidopsis CLC genes.
FLS2 signalling complexes regulate the expression of AtCLCd
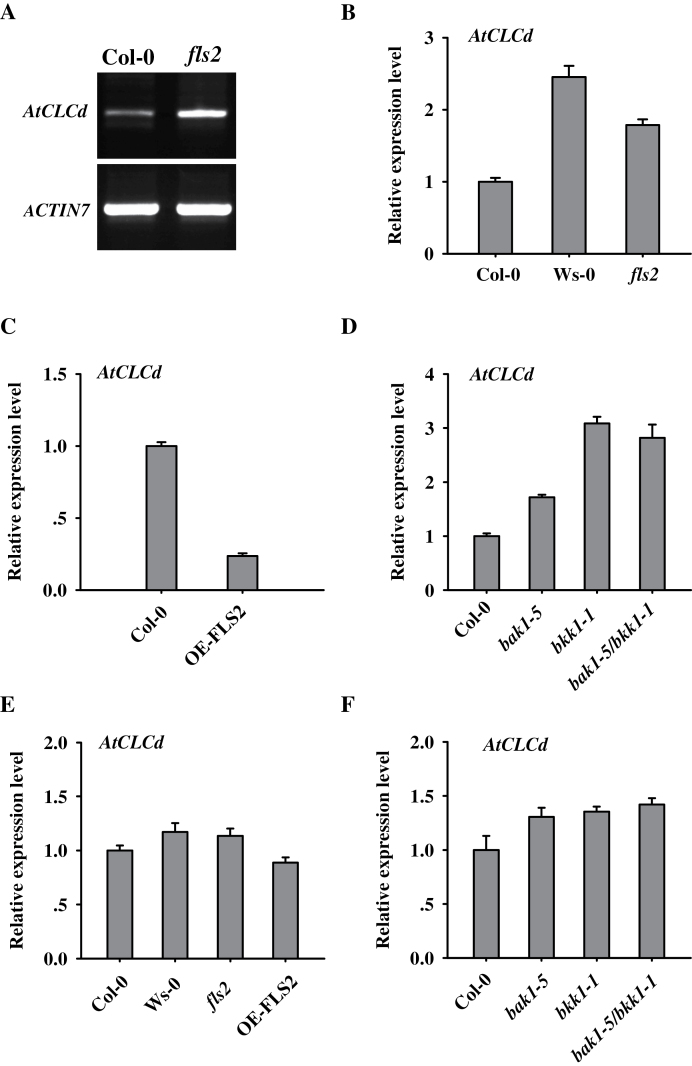
It was noticed that expression of AtCLCd was higher in the fls2 mutant than in Col-0 (Fig. 6A). To confirm this finding, transcript levels in the fls2 mutant and the Wassilewskija (Ws-0) background were compared. The ecotype Ws-0 is a natural fls2 mutant (Gómez-Gómez and Boller, 2000; Zipfel et al., 2004). As shown in Fig. 6B, AtCLCd transcript levels in the Ws-0 background were similar to those in the fls2 mutant and almost 2- to 2.5-fold higher than in Col-0. Moreover, AtCLCd transcript levels were strikingly reduced in the FLS2-overexpressing line (Fig. 6C). These results indicate that PRR FLS2 negatively regulates the expression of AtCLCd.
Fig. 6.
FLS2 and its signalling partners participate in regulating AtCLCd gene expression. (A) Expression of AtCLCd in Col-0 and fls2 mutant leaves analysed by semi-quantitative PCR. The level of ACTIN7 transcript was used as a loading control. (B–D) Expression of AtCLCd determined by quantitative RT–PCR in Col-0, Ws-0, and fls2 plants (B), in Col-0 and an FLS2-overexpressing line (OE-FLS2) (C), and in Col-0, bak1-5, bkk1-1, and bak1-5/bkk1-1 plants (D). (E and F) Expression levels of AtCLCd in Col-0, Ws-0, fls2, OE-FLS2 (E); and bak1-5, bkk1-1, and bak1-5/bkk1-1 (F) 14-day-old seedlings grown under sterile conditions. All samples were assayed in triplicate and normalized to ACTIN2. Error bars indicate the SD.
Arabidopsis somatic embryogenesis receptor-like kinases (SERKs) form complexes with PRRs in a ligand-dependent manner (Monaghan and Zipfel, 2012). BAK1/SERK3 and BKK1/SERK4 are required for FLS2-mediated PTI signalling in Arabidopsis (Roux et al., 2011). Recently, a novel bak1-5 mutant allele was identified in which only PTI was impaired, thereby avoiding the pleiotropic effects of the other bak1 mutations (Schwessinger et al., 2011). Even though the bkk1-1 mutant exhibited wild-type-like responses to flg22, loss of BKK1 further decreased the early and late responses of bak1-5 to flg22 (Roux et al., 2011). The study was thus extended to quantify expression of AtCLCd in bak1-5, bkk1-1, and a bak1-5/bkk1-1 double mutant. As shown in Fig. 6D, transcripts of AtCLCd accumulated to higher levels in the three mutants than in Col-0. Expression of AtCLCd was higher in bkk1-1 than in bak1-5. The combination of the two mutations had no additive effect on expression (Fig. 6D). These findings imply that the FLS2 regulatory proteins BAK1 and BKK1 play a role in the regulation of AtCLCd expression and that they function in the same pathway.
Plants are always exposed to a variety of microbes under non-sterile soil conditions; therefore, AtCLCd expression in sterile seedlings was checked. As shown in Fig. 6E, the accumulation of AtCLCd transcripts did not change significantly in Col-0, Ws-0, fls2, and FLS2-overexpressing plants under sterile conditions. Interestingly, expression of AtCLCd in bak1-5, bkk1-1, and bak1-5/bkk1-1 plants was also significantly reduced under sterile conditions, but was still a little higher than that in wild-type plants (Fig. 6F). These results support that PAMP perception is required for FLS2 to regulate AtCLCd expression.
Taken together, the above results indicate that the FLS2 signalling complex regulates the expression of AtCLCd in Arabidopsis.
Discussion
Changes in cellular anion content are thought to be associated with plant defence responses (De Angeli et al., 2007; Gauthier et al., 2007; Errakhi et al., 2008; Colcombet et al., 2009). Arabidopsis CLC family genes encode putative anion channels (Barbier-Brygoo et al., 2011). However, whether CLC channels participate in plant innate immunity was still unclear. Therefore, T-DNA insertion lines of AtCLCa, b, c, d, e, and g were screened for changes in flg22-induced ROS, and it was found that clcd mutants were unique in displaying enhanced ROS production in response to flg22 (Fig. 1). This and other findings (Figs 2, 3) provide ample evidence that AtCLCd is a negative regulator of PTI.
It was further shown that the PAMP, flg22, represses the expression of AtCLCd (Fig. 5A, B). However, the repression was not seen in a mutant of the flg22 receptor FLS2 (Fig. 5C). This and other findings (Fig. 6) showed that the FLS2 complex is required for maintaining the expression of AtCLCd. Since expression of FLS2 is induced by flg22 (Zipfel et al., 2004), it is possible that the decrease in expression of AtCLCd by flg22 is due in part to an increase of FLS2. Expression of the other Arabidopsis CLC genes was also reduced upon treatment with flg22 (Fig 5D), suggesting that these genes also play a role in PTI. However, ROS production was unchanged in the corresponding mutants (Fig. 1). A possible explanation for that is that there is functional redundancy of these genes. When compared with CLC genes from the monocot, rice, AtCLCd forms its own group, whereas the other AtCLC genes cluster together (von der Fecht-Bartenbach et al., 2010). Analysis of combinations of these mutants may be necessary to elucidate their roles in plant innate immunity.
AtCLCd has been shown to localize to the TGN (von der Fecht-Bartenbach et al., 2007; Lv et al., 2009). Therefore, it is unlikely that it is directly involved in downstream signalling upon PAMP perception, which takes place at the plasma membrane. The yeast genome encodes only one CLC protein, Gef1p, and it regulates the intra-Golgi pH (Hechenberger et al., 1996). Expression of AtClCd fully rescues the gef1 yeast mutant phenotype (Hechenberger et al., 1996; Marmagne et al., 2007; Lv et al., 2009), suggesting that AtClCd may have a function similar to Gef1p. Moreover, AtClCd has been shown to co-localize with the V-type ATPase subunit, VHA-a1, in the TGN (von der Fecht-Bartenbach et al., 2007). Inhibition of VHA-a1 affects Golgi morphology and restricts cell expansion (Dettmer et al., 2005, 2006; Brüx et al., 2008), and this effect is enhanced in the clcd mutant (von der Fecht-Bartenbach et al., 2007), further implying that AtCLCd is involved in adjusting the luminal pH of the TGN. pH homeostasis of the TGN is essential for its functioning (Demaurex et al., 1998; Dettmer et al., 2006). Therefore, AtCLCd most probably regulates the functioning of the TGN by affecting the pH within it.
The TGN is an important platform for sorting cargo proteins to the cell surface or vacuole and lysosome (Viotti et al., 2010; Beck et al., 2012a). A distinctive characteristic of the membrane trafficking system in plants is the convergence of the secretory and endocytic pathways at the TGN (Dettmer et al., 2006; Dhonukshe et al., 2007; Viotti et al., 2010). Endocytic membrane transport has been observed for several plasma membrane receptors in plants, and this seems to be a general regulatory mechanism for perception of extracellular stimuli by plasma membrane receptors (Takano et al., 2002; Robatzek et al., 2006; Beck et al., 2012a). FLS2, the PRR for flg22 (Gómez-Gómez and Boller, 2000, 2002), is localized to the plasma membrane and becomes specifically internalized into highly mobile vesicles upon addition of flg22 (Robatzek et al., 2006). The endocytic transport of FLS2 is critical for its function in PTI (Robatzek et al., 2006). Recently, it was shown that the TGN is an essential compartment for membrane trafficking of FLS2 (Beck et al., 2012b; Choi et al., 2013; Uemura and Nakano, 2013). Interestingly, AtCLCd has been previously suggested to be involved in membrane trafficking, since AtCLCd–green fluorescent protein (GFP) co-localized with endocytosed FM4-64, a dye widely used for tracing endocytic membrane traffic (von der Fecht-Bartenbach et al., 2007). It thus seems likely that AtCLCd regulates PTI via the TGN, probably by affecting FLS2 trafficking. Endocytosis is a feature of most of the PRRs in plants (Beck, et al., 2012a). In agreement with this, it was also found here that different PAMP-induced defence responses were impaired in the AtCLCd-misexpressing plants (Fig. 3). Nevertheless, further studies are needed to reveal whether FLS2 trafficking is affected in the clcd mutant and the overexpressing lines.
In summary, it has been shown here that AtCLCd negatively regulates Arabidopsis PTI, probably by interacting with the PRR signalling pathway. Its sequence indicates that AtCLCd encodes a chloride/proton exchanger (Zifarelli and Pusch, 2010). Future work involving classical and patch–clamp electrophysiology should establish whether the role of AtCLCd in PTI requires a functional channel.
Supplementary data
Supplementary data are available at JXB online.
Table S1. Sequences of primers used in this work
Figure S1. Characterization of Arabidopsis CLC T-DNA insertion lines.
Figure S2. PAMP-triggered immunity is compromised in the AtCLCd-overexpressing lines.
Figure S3. Shoot growth inhibition induced by flg22 in Col-0, clcd mutant and AtCLCd-overexpressing plants.
Figure S4. Morphological phenotypes and FLS2 expression levels in the AtCLCd-misexpressing plants.
Acknowledgements
We thank the Salk Institute and the ABRC for the Arabidopsis T-DNA insertion lines, Dr Jianmin Zhou for the fls2 seeds, Dr Cyril Zipfel for bak1-5, bkk1-1, and bak1-5/bkk1-1 mutant seeds, and Dr Morten Petersen for chitin. This work was supported by the National Basic Research Program of China (973 Program) [2011CB100702], the National Natural Science Foundation of China [31071673], and the Chinese Academy of Sciences [KSCX2-EW-N-06].
References
- Barbier-Brygoo H, De Angeli A, Filleur S, Frachisse JM, Gambale F, Thomine S, Wege S. 2011. Anion channels/transporters in plants: from molecular bases to regulatory networks. Annual Review of Plant Biology 62, 25–51 [DOI] [PubMed] [Google Scholar]
- Bauer Z, Gómez-Gómez L, Boller T, Felix G. 2001. Sensitivity of different ecotypes and mutants of Arabidopsis thaliana toward the bacterial elicitor flagellin correlates with the presence of receptor-binding sites. Journal of Biological Chemistry 276, 45669–45676 [DOI] [PubMed] [Google Scholar]
- Beck M, Heard W, Mbengue M, Robatzek S. 2012a. The INs and OUTs of pattern recognition receptors at the cell surface. Current Opinion in Plant Biology 15, 367–374 [DOI] [PubMed] [Google Scholar]
- Beck M, Zhou J, Faulkner C, MacLean D, Robatzek S. 2012b. Spatio-temporal cellular dynamics of the Arabidopsis flagellin receptor reveal activation status-dependent endosomal sorting. The Plant Cell 24, 4205–4219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T, Felix G. 2009. A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annual Review of Plant Biology 60, 379–406 [DOI] [PubMed] [Google Scholar]
- Boller T, He SY. 2009. Innate immunity in plants: an arms race between pattern recognition receptors in plants and effectors in microbial pathogens. Science 324, 742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüx A, Liu TY, Krebs M, Stierhof YD, Lohmann JU, Miersch O, Wasternack C, Schumacher K. 2008. Reduced V-ATPase activity in the trans-Golgi network causes oxylipin-dependent hypocotyl growth inhibition in Arabidopsis . The Plant Cell 20, 1088–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SW, Tamaki T, Ebine K, Uemura T, Ueda T, Nakano A. 2013. RABA members act in distinct steps of subcellular trafficking of the FLAGELLIN SENSING2 receptor. The Plant Cell 25, 1174–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana . The Plant Journal 16, 735–743 [DOI] [PubMed] [Google Scholar]
- Colcombet J, Mathieu Y, Peyronnet R, Agier N, Lelièvre F, Barbier-Brygoo H, Frachisse JM. 2009. R-type anion channel activation is an essential step for ROS-dependent innate immune response in Arabidopsis suspension cells. Functional Plant Biology 36, 832–843 [DOI] [PubMed] [Google Scholar]
- De Angeli A, Thomine S, Frachisse JM, Ephritikhine G, Gambale F, Barbier-Brygoo H. 2007. Anion channels and transporters in plant cell membranes. FEBS Letters 581, 2367–2374 [DOI] [PubMed] [Google Scholar]
- Demaurex N, Furuya W, D’Souza S, Bonifacino JS, Grinstein S. 1998. Mechanism of acidification of the trans-Golgi network (TGN). In situ measurements of pH using retrieval of TGN38 and furin from the cell surface. Journal of Biological Chemistry 273, 2044–2051 [DOI] [PubMed] [Google Scholar]
- Dettmer J, Hong-Hermesdorf A, Stierhof YD, Schumacher K. 2006. Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis . The Plant Cell 18, 715–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer J, Schubert D, Calvo-Weimar O, Stierhof YD, Schmidt R, Schumacher K. 2005. Essential role of the V-ATPase in male gametophyte development. The Plant Journal 41, 117–124 [DOI] [PubMed] [Google Scholar]
- Dhonukshe P, Aniento F, Hwang I, Robinson DG, Mravec J, Stierhof YD, Friml J. 2007. Clathrin-mediated constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis . Current Biology 17, 520–527 [DOI] [PubMed] [Google Scholar]
- Errakhi R, Meimoun P, Lehner A, Vidal G, Briand J, Corbineau F, Rona JP, Bouteau F. 2008. Anion channel activity is necessary to induce ethylene synthesis and programmed cell death in response to oxalic acid. Journal of Experimental Botany 59, 3121–3129 [DOI] [PubMed] [Google Scholar]
- Felix G, Duran JD, Volko S, Boller T. 1999. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. The Plant Journal 18, 265–276 [DOI] [PubMed] [Google Scholar]
- Gauthier A, Lamotte O, Reboutier D, Bouteau F, Pugin A, Wendehenne D. 2007. Cryptogein-induced anion effluxes: electrophysiological properties and analysis of the mechanisms through which they contribute to the elicitor-triggered cell death. Plant Signaling and Behavior 2, 86–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Gómez L, Boller T. 2000. FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis . Molecular Cell 5, 1003–1011 [DOI] [PubMed] [Google Scholar]
- Gómez-Gómez L, Boller T. 2002. Flagellin perception: a paradigm for innate immunity. Trends in Plant Science 7, 251–256 [DOI] [PubMed] [Google Scholar]
- Hann DR, Rathjen JP. 2007. Early events in the pathogenicity of Pseudomonas syringae on Nicotiana benthamiana . The Plant Journal 49, 607–618 [DOI] [PubMed] [Google Scholar]
- He P, Shan L, Lin NC, Martin GB, Kemmerling B, Nürnberger T, Sheen J. 2006. Specific bacterial suppressors of MAMP signaling upstream of MAPKKK in Arabidopsis innate immunity. Cell 125, 563–575 [DOI] [PubMed] [Google Scholar]
- Hechenberger M, Schwappach B, Fischer WN, Frommer WB, Jentsch TJ, Steinmeyer K. 1996. A family of putative chloride channels from Arabidopsis and functional complementation of a yeast strain with a CLC gene disruption. Journal of Biological Chemistry 271, 33632–33638 [DOI] [PubMed] [Google Scholar]
- Jabs T, Tschöpe M, Colling C, Hahlbrock K, Scheel D. 1997. Elicitor-stimulated ion fluxes and O2− from the oxidative burst are essential components in triggering defense gene activation and phytoalexin synthesis in parsley. Proceedings of the National Academy of Sciences, USA 94, 4800–4805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch TJ. 2008. CLC chloride channels and transporters: from genes to protein structure, pathology and physiology. Critical Reviews in Biochemistry and Molecular Biology 43, 3–36 [DOI] [PubMed] [Google Scholar]
- Jeworutzki E, Roelfsema MRG, Anschütz U, Krol E, Elzenga JTM, Felix G, Boller T, Hedrich R, Becker D. 2010. Early signaling through the Arabidopsis pattern recognition receptors FLS2 and EFR involves Ca2+-associated opening of plasma membrane anion channels. The Plant Journal 62, 367–378 [DOI] [PubMed] [Google Scholar]
- Jones JD, Dangl JL. 2006. The plant immune system. Nature 444, 323–329 [DOI] [PubMed] [Google Scholar]
- Jossier M, Kroniewicz L, Dalmas F, Le Thiec D, Ephritikhine G, Thomine S, Barbier-Brygoo H, Vavasseur A, Filleur S, Leonhardt N. 2010. The Arabidopsis vacuolar anion transporter, AtCLCc, is involved in the regulation of stomatal movements and contributes to salt tolerance. The Plant Journal 64, 563–576 [DOI] [PubMed] [Google Scholar]
- Koers S, Guzel-Deger A, Marten I, Roelfsema MRG. 2011. Barley mildew and its elicitor chitosan promote closed stomata by stimulating guard cell S-type anion channels. The Plant Journal 68, 670–680 [DOI] [PubMed] [Google Scholar]
- Kunze G, Zipfel C, Robatzek S, Niehaus K, Boller T, Felix G. 2004. The N terminus of bacterial elongation factor Tu elicits innate immunity in Arabidopsis plants. The Plant Cell 16, 3496–3507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Q-D, Tang Rj, Liu H, Gao Xs, Li Yz, Zheng Hq, Zhang Hx. 2009. Cloning and molecular analyses of the Arabidopsis thaliana chloride channel gene family. Plant Science 176, 650–661 [Google Scholar]
- Marmagne A, Vinauger-Douard M, Monachello D, de Longevialle AF, Charon C, Allot M, Rappaport F, Wollman FA, Barbier-Brygoo H, Ephritikhine G. 2007. Two members of the Arabidopsis CLC (chloride channel) family, AtCLCe and AtCLCf, are associated with thylakoid and Golgi membranes, respectively. Journal of Experimental Botany 58, 3385–3393 [DOI] [PubMed] [Google Scholar]
- Meyer S, Mumm P, Imes D, Endler A, Weder B, Al-Rasheid KA, Geiger D, Marten I, Martinoia E, Hedrich R. 2010. AtALMT12 represents an R-type anion channel required for stomatal movement in Arabidopsis guard cells. The Plant Journal 63, 1054–1062 [DOI] [PubMed] [Google Scholar]
- Monaghan J, Zipfel C. 2012. Plant pattern recognition receptor complexes at the plasma membrane. Current Opinion in Plant Biology 15, 349–357 [DOI] [PubMed] [Google Scholar]
- Montillet JL, Leonhardt N, Mondy S, Tranchimand S, Rumeau D, Boudsocq M, Garcia AV, Douki T, Bigeard J, Laurière C. 2013. An abscisic acid-independent oxylipin pathway controls stomatal closure and immune defense in Arabidopsis . PLoS Biology 11, e1001513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nürnberger T, Nennstiel D, Jabs T, Sacks WR, Hahlbrock K, Scheel D. 1994. High affinity binding of a fungal oligopeptide elicitor to parsley plasma membranes triggers multiple defense responses. Cell 78, 449–460 [DOI] [PubMed] [Google Scholar]
- Negi J, Matsuda O, Nagasawa T, Oba Y, Takahashi H, Kawai-Yamada M, Uchimiya H, Hashimoto M, Iba K. 2008. CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature 452, 483–486 [DOI] [PubMed] [Google Scholar]
- Pfund C, Tans-Kersten J, Dunning FM, Alonso JM, Ecker JR, Allen C, Bent AF. 2004. Flagellin is not a major defense elicitor in Ralstonia solanacearum cells or extracts applied to Arabidopsis thaliana. Molecular Plant-Microbe Interactions 17, 696–706 [DOI] [PubMed] [Google Scholar]
- Qi Z, Verma R, Gehring C, Yamaguchi Y, Zhao Y, Ryan CA, Berkowitz GA. 2010. Ca2+ signaling by plant Arabidopsis thaliana Pep peptides depends on AtPepR1, a receptor with guanylyl cyclase activity, and cGMP-activated Ca2+ channels. Proceedings of the National Academy of Sciences, USA 107, 21193–21198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robatzek S, Chinchilla D, Boller T. 2006. Ligand-induced endocytosis of the pattern recognition receptor FLS2 in Arabidopsis . Genes and Development 20, 537–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robatzek S, Saijo Y. 2008. Plant immunity from A to Z. Genome Biology 9, 304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux M, Schwessinger B, Albrecht C, Chinchilla D, Jones A, Holton N, Malinovsky FG, Tör M, de Vries S, Zipfel C. 2011. The Arabidopsis leucine-rich repeat receptor-like kinases BAK1/SERK3 and BKK1/SERK4 are required for innate immunity to hemibiotrophic and biotrophic pathogens. The Plant Cell 23, 2440–2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saji S, Bathula S, Kubo A, Tamaoki M, Kanna M, Aono M, Nakajima N, Nakaji T, Takeda T, Asayama M. 2008. Disruption of a gene encoding C4-dicarboxylate transporter-like protein increases ozone sensitivity through deregulation of the stomatal response in Arabidopsis thaliana . Plant and Cell Physiology 49, 2–10 [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Keller BU. 1992. Two types of anion channel currents in guard cells with distinct voltage regulation. Proceedings of the National Academy of Sciences, USA 89, 5025–5029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwessinger B, Roux M, Kadota Y, Ntoukakis V, Sklenar J, Jones A, Zipfel C. 2011. Phosphorylation-dependent differential regulation of plant growth, cell death, and innate immunity by the regulatory receptor-like kinase BAK1. PLoS Genetics 7, e1002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano J, Noguchi K, Yasumori M, Kobayashi M, Gajdos Z, Miwa K, Hayashi H, Yoneyama T, Fujiwara T. 2002. Arabidopsis boron transporter for xylem loading. Nature 420, 337–340 [DOI] [PubMed] [Google Scholar]
- Tsuda K, Katagiri F. 2010. Comparing signaling mechanisms engaged in pattern-triggered and effector-triggered immunity. Current Opinion in Plant Biology 13, 459–465 [DOI] [PubMed] [Google Scholar]
- Uemura T, Nakano A. 2013. Plant TGNs: dynamics and physiological functions. Histochemistry and Cell Biology 140, 341–345 [DOI] [PubMed] [Google Scholar]
- Vahisalu T, Kollist H, Wang YF, Nishimura N, Chan WY, Valerio G, Lamminmäki A, Brosché M, Moldau H, Desikan R. 2008. SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature 452, 487–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viotti C, Bubeck J, Stierhof YD, Krebs M, Langhans M, van den Berg W, van Dongen W, Richter S, Geldner N, Takano J. 2010. Endocytic and secretory traffic in Arabidopsis merge in the trans-Golgi network/early endosome, an independent and highly dynamic organelle. The Plant Cell 22, 1344–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Fecht-Bartenbach J, Bogner M, Dynowski M, Ludewig U. 2010. CLC-b-mediated NO3-/H+ exchange across the tonoplast of Arabidopsis vacuoles. Plant and Cell Physiology 51, 960–968 [DOI] [PubMed] [Google Scholar]
- von der Fecht-Bartenbach J, Bogner M, Krebs M, Stierhof YD, Schumacher K, Ludewig U. 2007. Function of the anion transporter AtCLC-d in the trans-Golgi network. The Plant Journal 50, 466–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendehenne D, Lamotte O, Frachisse JM, Barbier-Brygoo H, Pugin A. 2002. Nitrate efflux is an essential component of the cryptogein signaling pathway leading to defense responses and hypersensitive cell death in tobacco. The Plant Cell 14, 1937–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang C, Han P, Lutziger I, Wang K, Oliver DJ. 1999. A mini binary vector series for plant transformation. Plant Molecular Biology 40, 711–717 [DOI] [PubMed] [Google Scholar]
- Xiang T, Zong N, Zou Y, Wu Y, Zhang J, Xing W, Li Y, Tang X, Zhu L, Chai J, Zhou JM. 2008. Pseudomonas syringae effector AvrPto blocks innate immunity by targeting receptor kinases. Current Biology 18, 74–80 [DOI] [PubMed] [Google Scholar]
- Zifarelli G, Pusch M. 2010. CLC transport proteins in plants. FEBS Letters 584, 2122–2127 [DOI] [PubMed] [Google Scholar]
- Zipfel C, Robatzek S, Navarro L, Oakeley EJ, Jones JD, Felix G, Boller T. 2004. Bacterial disease resistance in Arabidopsis through flagellin perception. Nature 428, 764–767 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.