Abstract
Nucleotide phosphohydrolysis by the ecto-5′-nucleotidase (CD73) is the main source for extracellular generation of adenosine. Extracellular adenosine subsequently signals through four distinct adenosine A receptors (Adora1, Adora2a, Adora2b, or Adora3). Here, we hypothesized a functional role for CD73-dependent generation and concomitant signaling of extracellular adenosine during diabetic nephropathy. CD73 transcript and protein levels were elevated in the kidneys of diabetic mice. Genetic deletion of CD73 was associated with more severe diabetic nephropathy, whereas treatment with soluble nucleotidase was therapeutic. Transcript levels of renal adenosine receptors showed a selective induction of Adora2b during diabetic nephropathy. In a transgenic reporter mouse, Adora2b expression localized to the vasculature and increased after treatment with streptozotocin. Adora2b−/− mice experienced more severe diabetic nephropathy, and studies in mice with tissue-specific deletion of Adora2b in tubular epithelia or vascular endothelia implicated endothelial Adora2b signaling in protection from diabetic nephropathy. Finally, treatment with a selective Adora2b agonist (BAY 60–6583) conveyed potent protection from diabetes-associated kidney disease. Taken together, these findings implicate CD73-dependent production of extracellular adenosine and endothelial Adora2b signaling in kidney protection during diabetic nephropathy.
During conditions of inflammation and tissue injury, nucleotides in the form of ATP or ADP are released from multiple cell types.1–4 Nucleotide release pathways include the spillover of ATP from necrotic cells,5 its controlled release from apoptotic cells via pannexin hemichannels6 or from inflammatory or endothelial cells via connexin hemichannels,7,8 and vesicular nucleotide release from platelets.3,9–12 Once released into the extracellular compartment, ATP and ADP are rapidly converted to adenosine via enzymatically-controlled pathways.13–19 The pacemaker reaction for the extracellular generation of adenosine is catalyzed by the ecto-5′-nucleotidase CD73, a glycosylphosphatidylinositol-anchored ecto-enzyme that converts AMP into adenosine.9,18,20 Once generated into the extracellular compartment, adenosine can signal through four distinct adenosine receptors (Adora1, Adora2a, Adora2b, or Adora3). Previous studies had implicated CD73-dependent generation of extracellular adenosine and concomitant adenosine signaling in tissue protection during conditions of acute tissue injury, such as AKI,21–26 hepatic10,20,27 or intestinal17,18,28 ischemia, or myocardial infarction.10,29–32 In contrast, the functional role of extracellular adenosine generation and signaling during chronic disease states is less clear. For example, extracellular adenosine generation and signaling appears to be protective during the acute phase of lung injury, but has been implicated in promoting a fibrotic response during chronic forms of lung disease.33–37
Diabetic nephropathy is among the leading causes of morbidity and mortality in patients with diabetes mellitus. It belongs to the group of CKDs and is characterized by its progressive nature, eventually leading to nephrotic syndrome and diffuse glomerulosclerosis. It typically occurs in the context of longstanding diabetes mellitus and is among the primary reasons patients require dialysis or kidney transplantation. For example, patients with type 1 diabetes carry a 20%–50% risk of developing ESRD requiring dialysis or renal transplantation.38–40 Although effective interventions to slow the progression of diabetes-induced CKD have been described,41 enhanced therapeutic approaches are urgently needed. Therefore, studies to identify novel therapeutic interventions to prevent diabetic nephropathy or slow its progression are an area of intense investigation and would significantly affect the disease course of diabetes mellitus.
In the present study, we hypothesized a functional role for extracellular adenosine generation and signaling during diabetic nephropathy. Because adenosine responses can differ during acute or chronic disease stages,42 we pursued these studies without anticipating what the outcome would be. However, we were surprised that combinations of pharmacologic and genetic studies provided a very clear read-out. Indeed, we found that CD73-dependent adenosine generation and signaling through endothelial Adora2b receptors represent an endogenous pathway that protects during diabetic nephropathy and can be targeted for the treatment of kidney diseases caused by chronic elevation of serum glucose levels.
Results
CD73 Transcript and Protein Levels Are Elevated during Diabetic Nephropathy
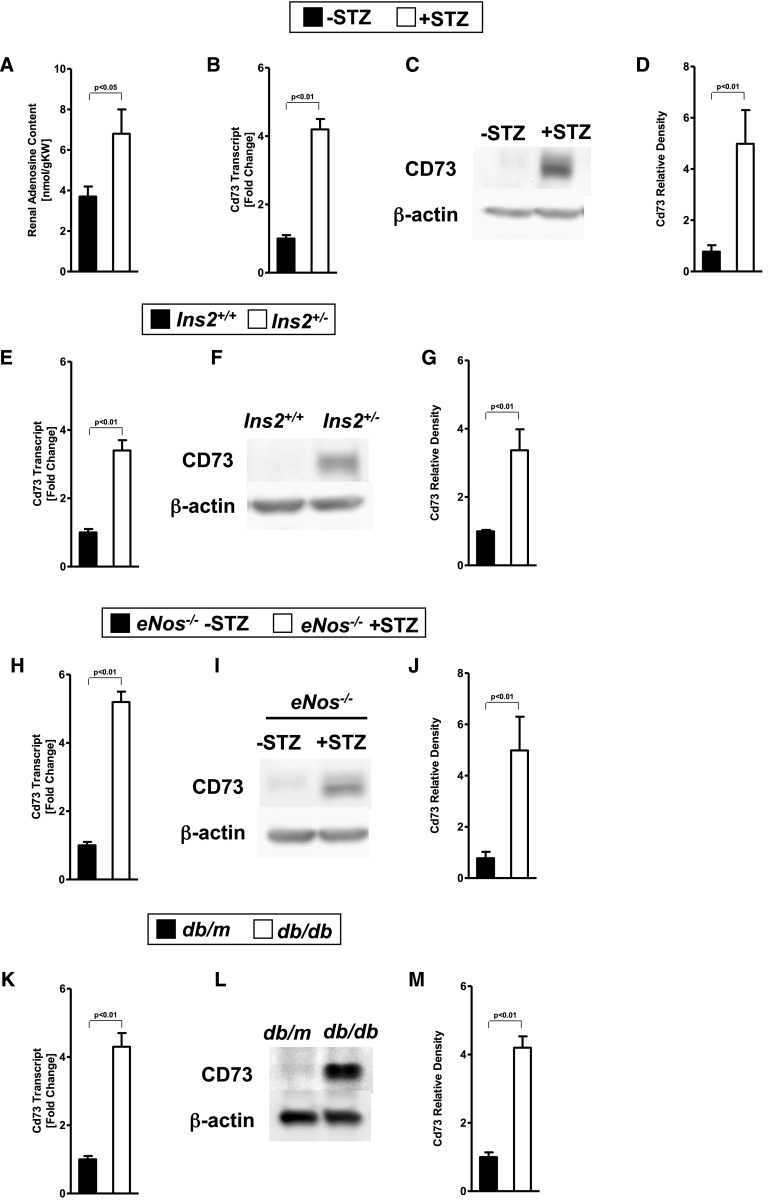
To study the role of extracellular adenosine generation and signaling during diabetic nephropathy, we induced diabetes in mice at 8 weeks of age by streptozotocin (STZ) treatment. Sixteen weeks after STZ treatment, we performed studies to address the role of extracellular adenosine generation and signaling during diabetic nephropathy. Initial studies in C57BL6 mice showed that renal adenosine levels were significantly elevated 16 weeks after STZ treatment compared with vehicle-treated controls matched by age, sex, and weight (Figure 1A). Because adenosine is generated in the extracellular compartment by enzymatic conversion of AMP to adenosine catalyzed by CD73, we subsequently measured renal CD73 transcript and protein levels. Indeed, we observed that renal CD73 transcript (Figure 1B) and protein levels (Figure 1, C and D) were significantly elevated in mice with STZ-induced diabetic nephropathy. We could confirm an upregulation of CD73 on a transcript and protein level also in the genetic Akita (Ins2+/−) model (Figure 1, E–G), the STZ-induced eNOS−/− model (Figure 1, H–J), and the db/db model (Figure 1, K–M). These findings demonstrate elevations of renal adenosine levels and concomitant induction of CD73 transcript and protein levels in different models of diabetic nephropathy.
Figure 1.
CD73 is induced by diabetic nephropathy. Age-, sex-, and weight-matched mice were subjected to STZ-induced diabetes for 16 weeks until organs were removed for analysis. (A) Wild-type mice show increased renal adenosine concentrations after 16 weeks of diabetes compared with wild-type controls without diabetes (n=3–4). Cd73 mRNA and CD73 protein renal tissue content (quantification by densitometry [n=3] was induced during diabetic nephropathy in (B–D) the STZ-induced diabetic model in wild-type mice, (E–G) Akita (Ins2+/−) mice, (H–J) STZ-induced eNOS−/− mice, and (K–M) db/db mice. Kidneys were excised, total RNA isolated, and Cd73 mRNA levels determined by real-time RT-PCR. Data were calculated relative to β-actin and are expressed as fold change compared with control (no diabetes)±SD (n=4). Renal CD73 protein levels were assessed by Western blot (β-actin to control loading conditions; one representative blot among 3 is shown) and quantification by densitometry (n=3). All data are shown as mean±SD.
Genetic and Pharmacologic Studies Implicate CD73-Dependent Adenosine Generation in Renal Protection from Diabetic Nephropathy
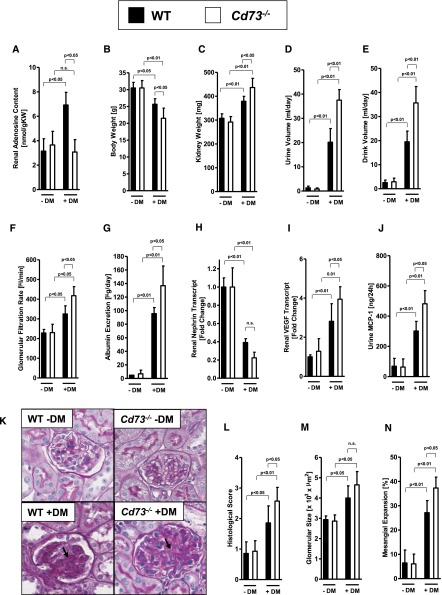
To demonstrate a functional role for CD73 during diabetic nephropathy, we subsequently performed studies in previously described Cd73−/− mice13,18,20,25,31,43 by inducing diabetes mellitus with STZ treatment and performed measurements 16 weeks afterward. We observed that systolic BP and blood glucose levels were similar in all treatment groups (Supplemental Figure 1), indicating that STZ-diabetes is similar in both groups. Next, we measured renal adenosine levels. We found that baseline levels of renal adenosine between wild-type and Cd73−/− mice were similar (Figure 2A), which is consistent with the notion that CD73-dependent production of extracellular adenosine predominantly occurs during injurious conditions wherein increased levels of precursor nucleotides are being liberated.4 Importantly, we found that elevations of renal adenosine levels during diabetic nephropathy were almost completely abolished. Moreover, Cd73−/− mice showed a more profound loss of body weight (Figure 2B), whereas their kidney weights were elevated (Figure 2C) in conjunction with elevated urine and drinking volumes (Figure 2, D and E). Moreover, Cd73−/− mice exhibited a more severe degree of renal dysfunction as assessed by measurements of GFR (Figure 2F) and albuminuria (Figure 2G). Similarly, transcript levels of renal nephrin—a protein necessary for the proper functioning of the renal filtration barrier, previously implicated in kidney protection during diabetic nephropathy44—was more repressed in Cd73−/− mice, indicating a more severe degree of renal dysfunction. Furthermore, transcript levels of vascular endothelial growth factor (VEGF) and urinary monocyte chemoattractant protein-13 (MCP-13)—both implicated in the pathogenesis of diabetic nephropathy45,46— were more profoundly elevated in Cd73−/− mice than in controls (Figure 2, I and J). In addition, elevations of inflammatory markers (TNF-α and MCP-1) in Cd73−/− mice were specific for the kidneys because there were no differences in the liver or the lungs of diabetic Cd73−/− mice or wild-type littermate controls (Supplemental Figure 2). Finally, histologic tissue injury in Cd73−/− mice was more severe during diabetic nephropathy, including more profound increases in glomerular size and a higher percentage of glomeruli with mesangial expansion (Figure 2, K–N). Taken together, these findings indicate a protective role for extracellular adenosine production during diabetic nephropathy.
Figure 2.
Diabetic nephropathy is increased in Cd73−/− mice. Cd73−/− mice and age-, weight-, and sex-matched wild-type mice were subjected to STZ-induced diabetes for 16 weeks until measurement of (A) renal adenosine content, (B) body weight, (C) kidney weight, (D) urine volume, (E) drink volume, (F) GFR, (G) albumin excretion, (H) renal nephrin transcript, (I) renal VEGF transcript, (J) and urine MCP-1 (n=6–8 in each group). (K) Extracellular matrix deposition as determined by PAS staining. PAS staining was increased in diabetic wild-type (WT) mice and in particular in Cd73−/− mice (arrows; original magnification, ×400). (L) Histologic score of PAS staining sections. (M) Glomerular size in all groups. (N) Mesangial expansion. M and N show the result of 30 glomeruli in sections of six mice per group. DM, diabetes mellitus; n.s., not significant. All data are shown as mean±SD.
Pharmacologic Studies Implicate CD73 Function in Renal Protection from Diabetic Nephropathy
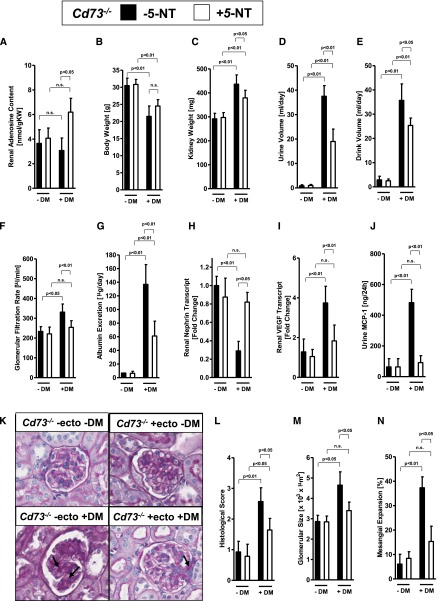
As proof of principle for the assertion that CD73 plays an important role in diabetes, we subsequently reconstituted Cd73−/− mice with soluble 5′-nucleotidase (5-NT). For this purpose, we implanted osmotic pumps (Alzet pump 2006) for continuous subcutaneous 5-NT treatment over 12 weeks. Therefore, the Alzet pumps were implanted at weeks 4 and 10 after the induction of diabetes (STZ, 50 mg/kg on 5 successive days), and kidney function was examined 16 weeks after STZ injection. Systolic BP and blood glucose levels were similar in Cd73 gene-targeted mice with nucleotidase or without nucleotidase treatment (Supplemental Figure 3), indicating that STZ-induced diabetes is similar in both groups. Interestingly, renal adenosine levels in Cd73−/− mice treated with 5-NT returned to higher renal adenosine levels during diabetes, as observed in diabetic wild-type mice (Figures 2A and 3A). Indeed, 5-NT treatment (4 U per mouse per day) of Cd73−/− mice was associated with the reconstitution of a normal phenotype, as shown for their body weight, kidney weight, urine volume, drinking volume, GFR, and albuminuria (Figure 3, B–G). Furthermore, the decrease of renal nephrin transcript in Cd73−/− mice was attenuated and VEGF expression and urinary MCP-1 excretion were decreased (Figure 3, I and J). These findings could also be confirmed on a histologic level (Figure 3, K–N). Taken together, these pharmacologic studies confirm our findings in Cd73−/− mice and implicate CD73-dependent production of extracellular adenosine in kidney protection during diabetic nephropathy.
Figure 3.
Treatment with soluble 5′-NT attenuates diabetic nephropathy in Cd73−/− mice. Cd73−/− mice with or without 5′-NT treatment (from Crotalus atrox venom via Alzet pump, 4 U per mouse per day) and age-, weight-, and sex-matched Cd73−/− mice without 5′-NT treatment were subjected to STZ-induced diabetes for 16 weeks until measurement of (A) renal adenosine content, (B) body weight, (C) kidney weight, (D) urine volume, (E) drink volume, (F) GFR, (G) albumin excretion, (H) renal nephrin transcript, (I) renal VEGF transcript, and (J) urine MCP-1 (n=6–8 in each group). (K) Extracellular matrix deposition as determined by PAS staining. PAS staining was attenuated in Cd73−/− mice with 5′-NT treatment compared with untreated mice (arrows; original magnification, ×400). (L) Histologic score of PAS staining sections. (M) Glomerular size in all groups. (N) Mesangial expansion. M and N show the result of 30 glomeruli in sections of six mice per group. DM, diabetes mellitus; n.s., not significant. All data are shown as mean±SD.
Selective Induction of the Adora2b Adenosine Receptor
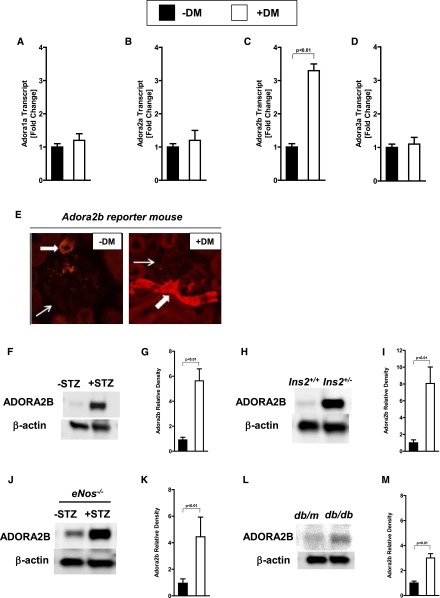
On the basis of the above genetic and pharmacologic studies implicating extracellular adenosine generation in kidney protection from diabetic nephropathy, we subsequently examined transcriptional responses of extracellular adenosine receptors during diabetic nephropathy. Indeed, we observed a selective and robust induction of the Adora2b (Figure 4, A–D) in mice with diabetic nephropathy. According to these findings, we next examined Adora2b protein expression using a previously described Adora2b gene reporter mouse.47 Consistent with previous studies,23,24 we observed that Adora2b protein expression at baseline was predominantly associated with vascular structures (Figure 4E). Moreover, Adora2b induction at 16 weeks after STZ treatment was dramatically increased at vascular sites (Figure 4E). Furthermore, we could show an upregulation of Adora2b protein by Western blot analysis in the STZ-induced diabetic wild-type and eNOS−/− model and in Ins2+/− and db/db mice (Figure 4, F–M). Taken together, these findings indicate vascular induction of the Adora2b during diabetic nephropathy.
Figure 4.
Adora2b expression is induced during diabetic nephropathy. Age-, sex-, and weight-matched mice were subjected to STZ-induced diabetes for 16 weeks until kidneys were removed for analysis. (A–D) Adenosine receptors (Adora1, Adora2a, Adora2b, and Adora3) transcript levels in kidneys with or without diabetic nephropathy assessed by real-time RT-PCR relative to housekeeping gene β-actin (n=4). (E) Renal tissue of Adora2b reporter mice (Adora2b-KO/β-gal–knockin mice) with or without diabetes were stained for β-galactosidase as an indicator of the Adora2b gene promoter, which drives the expression of the reporter gene (original magnification, ×400; one representative slide of three is displayed). Small arrows indicate the glomeruli. Bold arrows indicate the Adora2b staining. ADORA2B protein levels in kidneys (quantification by densitometry [n=3]) of (F and G) STZ-induced wild-type mice, (H and I) Akita (Ins2+/−) mice, (J and K) STZ-induced eNOS−/− mice, and (L and M) db/db mice. Renal ADORA2B protein levels were assessed by Western blot (β-actin to control loading conditions; one representative blot of three is shown). DM, diabetes mellitus. All data are shown as mean±SD.
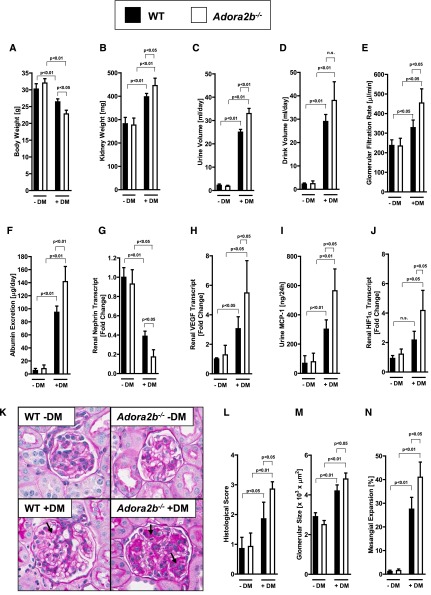
Diabetic Nephropathy Is More Severe in Adora2b−/− Mice
Based on the above studies showing a selective induction of the renal Adora2b adenosine receptor in mice with diabetes, we subsequently pursued studies in genetic models to address the functional role of the Adora2b. As first step, we induced diabetic nephropathy in previously described Adora2b−/− mice.23,28–31,48–51Similar to the preceding studies in Cd73−/− mice, we observed a more severe degree of diabetic nephropathy in Adora2b−/− mice without differences regarding systolic BP and blood glucose levels (Supplemental Figure 4, A and B), but a more severe degree of body weight loss (Figure 5A), increased kidney weight (Figure 5B), and elevated urine (Figure 5C) and drinking (Figure 5D) volumes. Determination of the GFR showed a more severe hyperfiltration in Adora2b−/− mice compared with diabetic control mice (Figure 5E). In addition, urinary albumin excretion was significantly increased in diabetic Adora2b−/− mice compared with the respective diabetic control mice (Figure 5F). Renal nephrin expression was significantly reduced in diabetic Adora2b−/− mice compared with the diabetic control group, whereas renal VEGF expression and urinary MCP-1 excretion were significantly increased (Figure 5, G–I). Consistent with previous studies implicating transcriptional mechanisms in the control of hypoxia-inducible factor-α (HIF1-α),52 we found that HIF1-α mRNA levels were elevated in the kidneys of diabetic Adora2b−/− mice. Because HIF1-α is a key transcriptional regulator of VEGF,1 these findings explain elevated VEGF levels in diabetic Adora2b−/− mice (Figure 5J).
Figure 5.
Diabetic nephropathy is increased in Adora2b−/− mice. Adora2b−/− mice and age-, weight-, and sex-matched wild-type (WT) mice were subjected to STZ-induced diabetes for 16 weeks before blood and organs were taken for (A) body weight, (B) kidney weight, (C) urine volume, (D) drink volume, (E) GFR, (F) albumin excretion, (G) renal nephrin transcript, (H) renal VEGF transcript, (I) urine MCP-1, and (J) renal HIF1-α transcript (n=6 in each group). (K) Extracellular matrix deposition as determined by PAS staining. PAS staining was increased in diabetic wild-type mice and in particular in Adora2b−/− mice (arrows; original magnification, ×400). (L) Histologic score of PAS staining sections. (M) Glomerular size in all groups. (N) Mesangial expansion. M and N show the result of 30 glomeruli in sections of six mice per group. DM, diabetes mellitus; n.s., not significant. All data are shown as mean±SD.
Accordingly, histologic characterization of diabetic nephropathy was more severe in Adora2b−/− mice (Figure 5, K–N). Furthermore, we could confirm the increase of VEGF transcript in diabetic Adora2−/− mice on a protein level showing increased VEGF protein in glomerular epithelia and tubular cells of diabetic Adora2−/− mice compared with diabetic wild-type mice (Supplemental Figure 4C). To exclude potential nonspecific tissue inflammatory effects that might occur in the STZ model of type 1 diabetes, we used genetic insulin-deficient Akita mice. Therefore, we crossed male Akita mice with female Adora2b−/− mice. Ins2+/− Adora2b−/− mice had a severe decrease in body weight and showed an increase in kidney weight, and drink and urine volumes and albuminuria compared with Ins2+/− mice (Supplemental Figure 5, A–G). Taken together, these studies implicate Adora2b signaling in kidney protection during diabetic nephropathy in a drug-induced and genetic model.
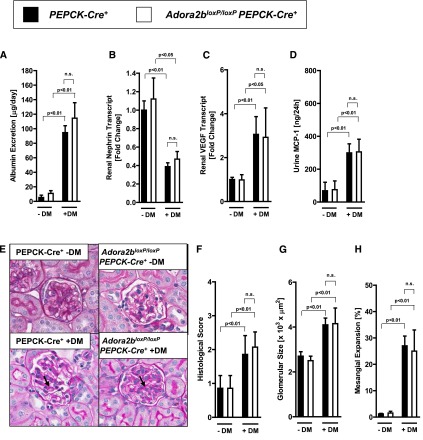
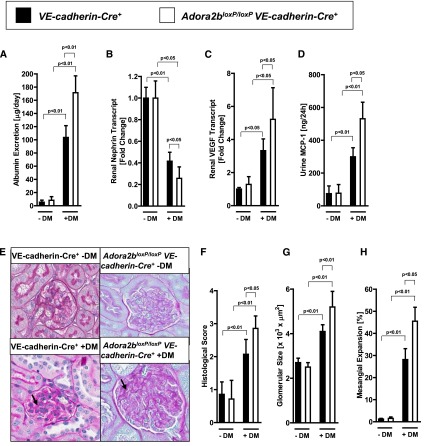
Adora2b-Mediated Kidney Protection during Diabetic Nephropathy Involves Endothelial Adora2b Signaling
To address the tissue-specific role for Adora2b signaling during diabetic nephropathy, we subsequently examined previously described mice with deletion of the Adora2b in tubular epithelia or vascular endothelial cells.23 Consistent with the above studies localizing Adora2b induction during diabetic nephropathy to the vasculature, we failed to observe a phenotype in mice with tubular epithelial Adora2b deletion (Adora2bloxP/loxPPEPCK-Cre+) during diabetic nephropathy (Figure 6, A–H). In contrast, mice with deletion of the Adora2b on vascular endothelia (Adora2bloxP/loxPVE-cadherin-Cre+) showed a more severe degree of diabetic nephropathy. Their renal phenotype was characterized by a more severe degree of albuminuria (Figure 7A), a more severe degree of vascular and inflammatory parameters (Figure 7, B–D), and a more severe degree of histologic signs for diabetic nephropathy (Figure 7, E–H). The VE-cadherin mouse line has been widely used to investigate endothelial-specific gene deletion in different experimental settings, although Cre expression in this mouse line exists to a lesser extent in hematopoietic cells.53–55 In conjunction with our studies using Adora2b reporter mice showing almost exclusive expression of the Adora2b in the vasculature of diabetic kidneys, we believe that the most likely explanation of our findings is vascular Adora2b signaling as a mediator of kidney protection during diabetic nephropathy.
Figure 6.
Renal epithelial-specific deletion of the Adora2b has no impact on the severity of diabetic nephropathy. Mice with deletion of the Adora2b in tubular epithelia of the kidneys were generated using the Cre-flox system (Adora2bloxP/loxP PEPCK-Cre+). Adora2bloxP/loxP PEPCK-Cre+ mice and age-, weight-, and sex-matched PEPCK-Cre+ mice were subjected to STZ-induced diabetes for 16 weeks before blood, urine, and organs were taken for measurement of (A) albumin excretion, (B) renal nephrin transcript, (C) renal VEGF transcript, and (D) urine MCP-1 (n=6–8 in each group). (E) Extracellular matrix deposition as determined by PAS staining. PAS staining was increased in diabetic PEPCK-Cre+ mice and in Adora2bloxP/loxP PEPCK-Cre+ mice (arrows; original magnification, ×400). (F) Histologic score of PAS staining sections. (G) Glomerular size in all groups. (H) Mesangial expansion. G and H show the result of 30 glomeruli in sections of six mice per group. DM, diabetes mellitus; n.s., not significant. All data are shown as mean±SD.
Figure 7.
Renal epithelial-specific deletion of the Adora2b increases the severity of diabetic nephropathy. Mice with deletion of the Adora2b in vascular endothelia of the kidneys were generated using the Cre-flox system (Adora2bloxP/loxP VE-cadherin-Cre+). Adora2bloxP/loxP VE-cadherin-Cre+ mice and age-, weight-, and sex-matched VE-cadherin-Cre+ mice were subjected to STZ-induced diabetes for 16 weeks before blood, urine, and organs were taken for measurement of (A) albumin excretion, (B) renal nephrin transcript, (C) renal VEGF transcript, and (D) urine MCP-1 (n=6–8 in each group). (E) Extracellular matrix deposition as determined by PAS staining. PAS staining was increased in diabetic PEPCK-Cre+ mice and in Adora2bloxP/loxP VE-cadherin-Cre+ mice (arrows; original magnification, ×400). (F) Histologic score of PAS staining sections. (G) Glomerular size in all groups. (H) Mesangial expansion. G and H show the result of 30 glomeruli in sections of six mice per group. DM, diabetes mellitus. All data are shown as mean±SD.
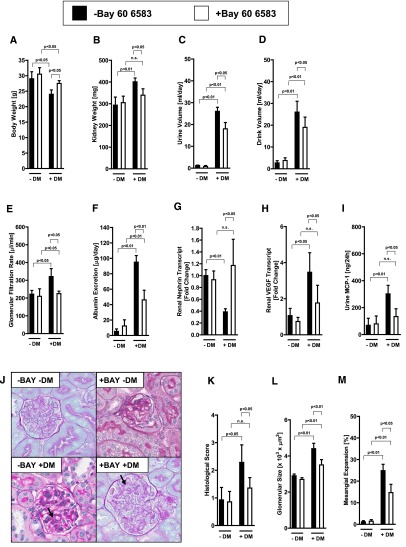
Adora2b Agonist Treatment (BAY 60–6583) Provides Potent Kidney Protection during Diabetic Nephropathy
On the basis of these genetic findings implicating endothelial Adora2b signaling in kidney protection during diabetic nephropathy, we subsequently pursued pharmacologic studies with the selective Adora2b agonist BAY 60–6583. We had shown in several previous studies that this compound has pharmacologic effects in wild-type or Adora2a−/− mice, but not in Adora2b−/− mice.24,31,50,51 For the purpose of our studies, we administered BAY 60–6583 via osmotic pump (0.3 μg per mouse per hour). Indeed, we observed that continuous BAY 60–6583 treatment had no effect on systolic BP or blood glucose levels (Supplemental Figure 6, A and B) but was associated with an attenuated wasting syndrome after STZ treatment (Figure 8, A–D). Hyperfiltration was attenuated in diabetic mice with BAY 60–6583 treatment (Figure 7E). Moreover, changes in albuminuria, renal nephrin and VEGF expression, and urinary MCP-1 excretion, as well as histologic signs of diabetic nephropathy, were attenuated in the mice treated with BAY 60–6583 (Figure 7, G–M). Furthermore, we tested the Adora2b agonist treatment in the genetic diabetic Akita model (Ins2+/−). Akita mice treated with the Adora2b agonist BAY 60 6583 showed a less severe phenotype of diabetic nephropathy compared with untreated mice at age 6 months (Supplemental Figure 7, A–G). Systolic BP and blood glucose levels were similar in all groups (Supplemental Figure 7, A and B). Taken together, these data indicate that treatment with a selective Adora2b agonist is associated with significant kidney protection from diabetic nephropathy in the STZ-induced diabetic and the Akita model.
Figure 8.
Adora2b agonist treatment (BAY 60–6583) attenuates diabetic nephropathy. Wild-type mice were subjected to STZ-induced diabetes for 16 weeks. Four weeks after STZ injection and confirmation of diabetes (glucose levels>400 mg/dl), mice were treated with BAY 60–6583 or vehicle via Alzet pump (0.3 μg per mouse per hour). After 16 weeks of diabetes with or without BAY 60–6583 treatment, blood, urine, and organs were taken for measurement of (A) body weight, (B) kidney weight, (C) urine volume, (D) drink volume, (E) GFR, (F) albumin excretion, (G) renal nephrin transcript, (H) renal VEGF transcript, and (I) urine MCP-1 (n=6–8 in each group). (J) Extracellular matrix deposition as determined by PAS staining. PAS staining was increased in diabetic wild-type mice and attenuated in BAY 60–6583 treated mice (arrows; original magnification, ×400). (K) Histologic score of PAS staining sections. (L) Glomerular size in all groups. (M) Mesangial expansion. L and M show the result of 30 glomeruli in sections of six mice per group. DM, diabetes mellitus; n.s., not significant. All data are shown as mean±SD.
Discussion
In the present studies we combined genetic and pharmacologic approaches to investigate the functional roles of extracellular adenosine production and signaling during diabetic nephropathy. Initial findings suggested that renal CD73 transcript and protein levels are induced during diabetic nephropathy. These findings coincided with elevations of renal adenosine levels that were abolished in gene-targeted mice for CD73, thereby implicating CD73 to function as an endogenous regulator for extracellular adenosine levels during diabetic nephropathy. Indeed, functional studies using Cd73−/− mice or treatment with soluble nucleotidase pointed to CD73-dendent adenosine production as an endogenous protective pathway during diabetic nephropathy. Subsequent studies demonstrated a selective induction of Adora2b during diabetic nephropathy, and studies in mice with global or tissue-specific Adora2b deletion identified a protective role for endothelial Adora2b signaling during diabetic nephropathy. Importantly, continuous treatment via an osmotic pump delivery system of a selective Adora2b agonist (BAY 60–6583) provided potent protection from diabetic nephropathy, thereby implicating the Adorad2b as a therapeutic target.
The results from the present study are consistent with previous findings implicating extracellular adenosine signaling in kidney protection from diabetic nephropathy. For example, a previous study provided compelling evidence that signaling through Adora2a mediates protection from kidney dysfunction during STZ-induced diabetes. These studies were carried out in Sprague-Dawley rats that were treated with the selective Adora2a agonist ATL146e.56 Indeed, a serious of previous investigations from the laboratory of Dr. Okusa had shown that activation of Adora2a receptors on T cells provides protection during acute ischemic kidney disease.2,3,21,22,57,58 As such, it is conceivable that Adora2a receptors expressed on inflammatory cells and vascular Adora2b receptors function together to protect the kidneys during diabetic nephropathy.
The Adora2b adenosine receptor is the most adenosine “insensitive” adenosine receptor, typically requiring at least micromolar adenosine concentrations for signaling to occur. However, in the context of a chronic inflammatory disease, such increases of extracellular adenosine levels may occur—particularly in a specific microenvironment. Our findings of elevated renal adenosine levels and transcriptional induction of the Adora2b further support this notion. Indeed, previous studies had shown that the Adora2b is highly regulated on a transcriptional level. For example, conditions of inflammation and tissue hypoxia can result in the stabilization of the transcription factor HIF.1 Previous studies had shown that the Adora2b is a classic HIF target gene, and conditions of ambient hypoxia, ischemia, or inflammation are associated with HIF-dependent induction of the Adora2b.17,30,59,60
The downstream mechanisms of adenosine signaling in the development of chronic forms or renal disease are somewhat controversial. VEGF has been discussed as key factor in the progression of diabetic nephropathy. Abnormal angiogenesis has been shown in human diabetic nephropathy and has been linked to increased VEGF expression.46 VEGF also mediates renal hypertrophy, increases in GFR, and urinary protein excretion in early diabetic nephropathy.61 The functional role of Adora2b signaling on VEGF release has been controversial, with some studies showing VEGF promotion and other studies showing VEGF reduction.62,63 To our knowledge, the present studies provide the first genetic evidence for a functional role of Adora2b signaling in attenuating renal VEGF levels. Indeed, we found that renal VEGF expression was markedly increased in Adora2b gene-targeted mice after 16 weeks of diabetes compared with wild-type mice. VEGF is mainly produced by podocytes and has a paracrine role to mediate angiogenesis beyond development via VEGF receptors on endothelial cells.64 These findings are consistent with our studies to address the tissue-specific functions of Adora2b signaling. Indeed, we found in an Adora2b reporter mouse model that Adora2b expression is predominantly on the vasculature. Moreover, we found that tissue-specific deletion of Adora2b signaling on vascular endothelial cells conveys increased disease susceptibility during diabetic nephropathy. In fact, Adora2bloxP/loxP VE-cadherin-Cre+ mice show dramatically increased levels of VEGF transcript levels during diabetic nephropathy. Together, these findings suggest a potential role for endothelial Adora2b signaling in attenuating VEGF production during diabetic nephropathy as a potential mechanism for adenosine-mediated kidney protection during diabetic nephropathy.
Another possible downstream mechanism could be the known potency of adenosine in mediating the resolution of inflammation and wound healing via promoting regulatory T cells. For example, a recent study demonstrated that Cd73−/− mice experience a dramatic failure to resolve lipopolysaccharide-induced inflammation due to a lack of regulatory T cells.65 Similarly, Adora2b signaling has been implicated in promoting regulatory T cells and inflammation resolution.66 Because T cell activation plays an important role in the pathophysiology of diabetic organ injury,67 future studies toward a possible role of adenosine in regulating T-cell activation could be of great clinical relevance.
Taken together, the present studies implicate the CD73-dependent generation of extracellular adenosine and signaling through endothelial-expressed Adora2b receptors in kidney protection during diabetic nephropathy. Future challenges will include the translation of the present findings from bench to bedside. Particularly, it will be critical to move forward with Adora2b agonist treatment in human disease conditions. At present, an Adora2b agonist has never been used in a clinical setting to treat patients.
Concise Methods
Gene-Targeted Mouse Strains
The animal protocol was approved by the Institutional Animal Care and Use Committee of the University of Colorado Denver and is in accordance with the National Institutes of Health guidelines for use of live animals. Previously described Cd73−/–,13 Adora2b−/−, Adora2bloxP/loxPPEPCK-Cre+, Adora2bloxP/loxPVE-cadherin-Cre+,31,23 and Adora2b reporter mice (Adora2b-KO/β-gal–knock-in mice) mice47 on the C57BL/6 strain or the respective littermate controls, matched for age, sex, and weight, were used.
Mouse Models of Diabetes
For the purpose of studying the role of adenosine generation and signaling during diabetic nephropathy, we used four murine models of diabetes (type 1 and 2). In most of our functional studies we used the low-dose STZ model.68 For this purpose, mice at 8 weeks of age received daily STZ injections intraperitoneally (50 mg/kg, made fresh in 0.1 M citrate buffer, pH 4.5) for 5 consecutive days. Vehicle-injected mice served as controls. Development of diabetes (defined by blood glucose>400 mg/dl) was verified 2 weeks after the first STZ injection.
Another type 1 diabetes mellitus murine model with more progressive renal vascular injury is the STZ-induced diabetes in eNOS−/− mice (The Jackson Laboratory, Bar Harbor, ME).69 C57BL/6J mice (C57BL/6) and C57BL/6J-Nos3tm1Unc (eNOS−/− mice; The Jackson Laboratory) that were 8 weeks of age were rendered diabetic by using an established protocol (STZ, 100 mg/kg per day for 2 consecutive days; freshly dissolved in 0.01 M citrate buffer, pH 4.5).70 Development of diabetes (defined by blood glucose>400 mg/dl) was verified 2 weeks after the first STZ injection. We had an approximately 10% loss of diabetic mice in all STZ-induced diabetes groups during the 16 weeks of investigation.
To exclude potential nonspecific tissue inflammatory effects that might occur in the STZ model of type 1 diabetes, we used genetic insulin-deficient Akita mice. Insulin deficiency is the consequence of a severe pancreatic cell decline resulting from a mutation of the insulin 2 gene and the proteotoxic effect of misfolded insulin.71,72 Male Akita mice (Ins2+/− C57BL/6 background) from The Jackson Laboratories were crossed with female C57BL/6 WT mice. For generation of Ins2+/−/Adora2b−/− double mutants, female Adora2b−/− mice (C57BL/6 background) were crossed with male mice that were heterozygous for both the Ins2 (Akita) and Adora2b mutations. All experiments were performed in male mice at 6 months of age.
To investigate CD73 and Adora receptor regulation also in a type 2 diabetes mouse model, we used the db/db mutation on the C57BLKS background, which has been investigated intensively and exhibits many features similar to human diabetic nephropathy.73 Db/db mice and their respective controls (db/m) were purchased from The Jackson Laboratories.
Measurement of Blood Glucose Levels
Fasting blood glucose levels were examined weekly with a One Touch Ultra Smart blood glucose meter (detection range, 20–600 mg/dl) (LifeScan, Inc.). For glucose measurements in Akita mice, we used a glucose kit from Sigma-Aldrich, GAGO20, to detect values above 600 mg/dl (detection range unlimited depending on prior dilution). To validate the phenotype in our diabetic STZ model, glycosylated hemoglobin levels were determined in diabetic C57BL/6 mice 16 weeks after STZ treatment. These studies showed hemoglobin A1c concentrations similar to those in previous studies in the STZ model (Supplemental Figure 8).74
Alzet Pump Implantation
For continuous application of soluble 5-NT (4 U per mouse per day) or the Adora2b agonist BAY 60–6583 (0.3 μg per mouse per hour), Alzet pumps (2006, delivery rate: 0.15 μl/h for 6 weeks) were subcutaneously implanted under isoflurane anesthesia. Implantation was performed at week 4 after STZ injections, and Alzet pumps were replaced at week 10 to ensure continuous compound delivery. For BAY 60–6583 (0.3 μg per mouse per hour) treatment in Akita mice, Alzet pumps were implanted at 6 weeks of age and were replaced three times to ensure a continuous treatment for the duration of 18 weeks until mice were euthanized after 6 months of age.
Determination of GFR
Inulin clearance was measured 16 weeks after STZ-induced diabetes as described previously.23 Briefly, mice were anesthetized using pentobarbital, 50 mg/kg intraperitoneally. Animals were then placed on a temperature-controlled operating table to keep rectal temperature at 37°C. The right jugular vein was cannulated for continuous infusion. Blood samples were taken via retroorbital vein plexus puncture. A catheter was placed in the urinary bladder for timed urine collection after removal of the right kidney. After surgery, mice received a bolus of 0.45% sodium chloride solution in an amount equal to 20% body weight. Continuous infusion was maintained at a rate of 800 μl/h per 25 g body weight, and FITC-labeled inulin (0.75 g/100 ml; Sigma-Aldrich) was added to the infusion for evaluation of whole kidney GFR. After stabilization of the animals for 20 minutes, 20-minute timed urine collections were performed. Blood was obtained in the middle of every urine collection period for measurement of FITC-inulin. Concentration of inulin in plasma and urine were determined by measurement of wavelength using a spectrophotometer (Biotek Synergy 2), and GFR was calculated by standard formulas.
Metabolic Cage Investigation
Experimental mice were placed in metabolic cages (Tecniplast, Italy) for urine collection after 16 weeks of STZ-induced diabetes or at 6 months of age (Ins+/−), respectively. Urine was collected and drinking volume measured. The urinary excretion amount per day and drinking volume per day were calculated. Urinary albumin and MCP-1 excretion were determined. Blood and organs were harvested after metabolic cage investigations and were stored at −80°C until further analysis.
Determination of Albuminuria
Urine was collected over 24 hours via metabolic cage investigations, and mouse albumin ELISA was used by following the manufacturer’s instructions (Exocell).
ELISA Measurements
Blood hemoglobin A1c was measured using an ELISA (A1Cnow) from Bayer AG (Germany). Urine MCP-1 concentrations were determined by ELISA (Pharmingen, San Diego, CA).
Adenosine Measurement via HPLC-Ultraviolet
Whole kidneys from mice with and without diabetes were removed and immediately snap-frozen, and tissue adenosine levels were determined as previously described.23
Transcriptional Studies
We used real-time RT-PCR (iCycler; Bio-Rad Laboratories, Inc.) to examine cd73 and Adora1, Adora2a, Adora2b, and Adora3 expression in renal tissue as previously described.23 Furthermore, we determined renal transcript of MCP-1, VEGF, nephrin (Applied Biosystems), and HIF1-α (Qiagen; catalog number QT01039542).45,75,76
Immunoblotting Experiments
Renal tissues from different diabetic mouse strains with or without diabetes were blotted using polyclonal goat anti-CD73 or anti-Adora2b (Santa Cruz Biotechnology).24,25 Tissues were homogenized and lysed for 10 minutes in ice-cold lysis buffer (150 mM NaCl, 25 mM Tris [pH 8.0], 5 mM EDTA, 2% Triton X-100, and 10% mammalian tissue protease inhibitor cocktail; Sigma-Aldrich), and further processed as previously described.23 To control for protein loading, blots were stripped in stripping buffer for 30 minutes and washed for 10 minutes with Tris-buffered saline and Tween 20, and membranes were blocked for 1 hour at room temperature in PBS-Tween and 4% BSA. Thereafter, the membrane was incubated with β-actin (Abcam, Inc.).
Renal Histology and Quantification of Morphology
Kidneys were excised and harvested as previously described.24 Extracellular matrix deposition in glomeruli was assessed by periodic acid-Schiff (PAS) staining. An investigator scored sections in a blinded fashion, according to an established scoring system (range, 0–4: 0, no extracellular matrix deposition; 4, extracellular matrix deposition in all sections of the glomeruli).77 All quantifications were performed in a masked manner. With use of coronal sections of the kidney, 30 consecutive glomeruli per mouse, six mice per group were examined for histologic evaluation. The glomerular area was traced along the outline of the capillary loop using AxioVision image analyzer (Carl Zeiss, Thornwood, NY). The extent of the mesangial expansion was determined by assessing the PAS-positive and nuclei-free area in the mesangium on 30 glomeruli of six mice in each group using ScanScope image analyzer (Aperio Technologies, Vista, CA).78
Immunohistochemistry
Formalin-fixed tissues were deparaffinized, rehydrated, and processed for antigen retrieval using 10 mM citrate buffer (pH 6.0) for VEGF (Millipore, Billerica, MA). The sections were incubated with primary antibody overnight at 4°C, followed by treatment with peroxidase-coupled secondary antibody. Color development was achieved using diaminobenzidine.
Analysis of β-Gal Expression
To localize the Adora2b in renal tissues, we analyzed β-gal expression in renal sections in Adora2b-KO/β-gal–knockin mice. Kidneys were harvested and fixed as previously described.23
Statistical Analyses
Data are presented as mean±SD from six to eight animals per condition. One-way ANOVA followed by Bonferroni correction was used to compare more than two groups. For comparison between two groups, the unpaired two-tailed t test was performed. All tests were performed using the software program GraphPad Prism (GraphPad Software, San Diego, CA). P<0.05 was considered to represent a statistically significant difference.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Linda Thompson for providing us with her Cd73−/− mice and Thomas Krahn from BayerAG for providing us with BAY 60-6583.
The present research work was supported by the National Institutes of Health grant 1KO8HL103900-01 to M.A.Z., a grant by the Juvenile Diabetes Research Foundation and an American Heart Association grant to A.G., the National Institutes of Health grants R01-DK097075, R01-HL0921, R01-DK083385, R01- HL098294, and POIHL114457-01, and a grant by the Crohn’s and Colitis Foundation of America to H.K.E.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012101014/-/DCSupplemental.
References
- 1.Eltzschig HK, Carmeliet P: Hypoxia and inflammation. N Engl J Med 364: 656–665, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eltzschig HK, Eckle T: Ischemia and reperfusion—from mechanism to translation. Nat Med 17: 1391–1401, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eltzschig HK: Adenosine: An old drug newly discovered. Anesthesiology 111: 904–915, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen JF, Eltzschig HK, Fredholm BB: Adenosine receptors as drug targets—what are the challenges? Nat Rev Drug Discov 12: 265–286, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hotchkiss RS, Strasser A, McDunn JE, Swanson PE: Cell death. N Engl J Med 361: 1570–1583, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, Penuela S, Laird DW, Salvesen GS, Isakson BE, Bayliss DA, Ravichandran KS: Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature 467: 863–867, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faigle M, Seessle J, Zug S, El Kasmi KC, Eltzschig HK: ATP release from vascular endothelia occurs across Cx43 hemichannels and is attenuated during hypoxia. PLoS ONE 3: e2801, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eltzschig HK, Eckle T, Mager A, Küper N, Karcher C, Weissmüller T, Boengler K, Schulz R, Robson SC, Colgan SP: ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res 99: 1100–1108, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Eckle T, Koeppen M, Eltzschig HK: Role of extracellular adenosine in acute lung injury. Physiology (Bethesda) 24: 298–306, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Hart ML, Gorzolla IC, Schittenhelm J, Robson SC, Eltzschig HK: SP1-dependent induction of CD39 facilitates hepatic ischemic preconditioning. J Immunol 184: 4017–4024, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eltzschig HK, Köhler D, Eckle T, Kong T, Robson SC, Colgan SP: Central role of Sp1-regulated CD39 in hypoxia/ischemia protection. Blood 113: 224–232, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eltzschig HK, Macmanus CF, Colgan SP: Neutrophils as sources of extracellular nucleotides: functional consequences at the vascular interface. Trends Cardiovasc Med 18: 103–107, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson LF, Eltzschig HK, Ibla JC, Van De Wiele CJ, Resta R, Morote-Garcia JC, Colgan SP: Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. J Exp Med 200: 1395–1405, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eltzschig HK, Thompson LF, Karhausen J, Cotta RJ, Ibla JC, Robson SC, Colgan SP: Endogenous adenosine produced during hypoxia attenuates neutrophil accumulation: Coordination by extracellular nucleotide metabolism. Blood 104: 3986–3992, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Eltzschig HK, Ibla JC, Furuta GT, Leonard MO, Jacobson KA, Enjyoji K, Robson SC, Colgan SP: Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: Role of ectonucleotidases and adenosine A2B receptors. J Exp Med 198: 783–796, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Synnestvedt K, Furuta GT, Comerford KM, Louis N, Karhausen J, Eltzschig HK, Hansen KR, Thompson LF, Colgan SP: Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest 110: 993–1002, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hart ML, Grenz A, Gorzolla IC, Schittenhelm J, Dalton JH, Eltzschig HK: Hypoxia-inducible factor-1α-dependent protection from intestinal ischemia/reperfusion injury involves ecto-5′-nucleotidase (CD73) and the A2B adenosine receptor. J Immunol 186: 4367–4374, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Hart ML, Henn M, Köhler D, Kloor D, Mittelbronn M, Gorzolla IC, Stahl GL, Eltzschig HK: Role of extracellular nucleotide phosphohydrolysis in intestinal ischemia-reperfusion injury. FASEB J 22: 2784–2797, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hart ML, Köhler D, Eckle T, Kloor D, Stahl GL, Eltzschig HK: Direct treatment of mouse or human blood with soluble 5′-nucleotidase inhibits platelet aggregation. Arterioscler Thromb Vasc Biol 28: 1477–1483, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Hart ML, Much C, Gorzolla IC, Schittenhelm J, Kloor D, Stahl GL, Eltzschig HK. Extracellular adenosine production by ecto-5′-nucleotidase protects during murine hepatic ischemic preconditioning. Gastroenterology 135:1739–1750 e1733, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Day YJ, Huang L, Ye H, Li L, Linden J, Okusa MD: Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: the role of CD4+ T cells and IFN-gamma. J Immunol 176: 3108–3114, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Day YJ, Huang L, McDuffie MJ, Rosin DL, Ye H, Chen JF, Schwarzschild MA, Fink JS, Linden J, Okusa MD: Renal protection from ischemia mediated by A2A adenosine receptors on bone marrow-derived cells. J Clin Invest 112: 883–891, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grenz A, Bauerle JD, Dalton JH, Ridyard D, Badulak A, Tak E, McNamee EN, Clambey E, Moldovan R, Reyes G, Klawitter J, Ambler K, Magee K, Christians U, Brodsky KS, Ravid K, Choi DS, Wen J, Lukashev D, Blackburn MR, Osswald H, Coe IR, Nürnberg B, Haase VH, Xia Y, Sitkovsky M, Eltzschig HK: Equilibrative nucleoside transporter 1 (ENT1) regulates postischemic blood flow during acute kidney injury in mice. J Clin Invest 122: 693–710, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Grenz A, Osswald H, Eckle T, Yang D, Zhang H, Tran ZV, Klingel K, Ravid K, Eltzschig HK: The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Med 5: e137, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grenz A, Zhang H, Eckle T, Mittelbronn M, Wehrmann M, Köhle C, Kloor D, Thompson LF, Osswald H, Eltzschig HK: Protective role of ecto-5′-nucleotidase (CD73) in renal ischemia. J Am Soc Nephrol 18: 833–845, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Grenz A, Zhang H, Hermes M, Eckle T, Klingel K, Huang DY, Müller CE, Robson SC, Osswald H, Eltzschig HK: Contribution of E-NTPDase1 (CD39) to renal protection from ischemia-reperfusion injury. FASEB J 21: 2863–2873, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Lappas CM, Day YJ, Marshall MA, Engelhard VH, Linden J: Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med 203: 2639–2648, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hart ML, Jacobi B, Schittenhelm J, Henn M, Eltzschig HK: Cutting Edge: A2B Adenosine receptor signaling provides potent protection during intestinal ischemia/reperfusion injury. J Immunol 182: 3965–3968, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Eckle T, Hartmann K, Bonney S, Reithel S, Mittelbronn M, Walker LA, Lowes BD, Han J, Borchers CH, Buttrick PM, Kominsky DJ, Colgan SP, Eltzschig HK: Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat Med 18: 774–782, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eckle T, Köhler D, Lehmann R, El Kasmi KC, Eltzschig HK: Hypoxia-inducible factor-1 is central to cardioprotection: A new paradigm for ischemic preconditioning. Circulation 118: 166–175, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Eckle T, Krahn T, Grenz A, Köhler D, Mittelbronn M, Ledent C, Jacobson MA, Osswald H, Thompson LF, Unertl K, Eltzschig HK: Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation 115: 1581–1590, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Eltzschig HK, Bonney SK, Eckle T: Attenuating myocardial ischemia by targeting A2B adenosine receptors. Trends Mol Med 19: 345–354, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou Y, Schneider DJ, Morschl E, Song L, Pedroza M, Karmouty-Quintana H, Le T, Sun CX, Blackburn MR: Distinct roles for the A2B adenosine receptor in acute and chronic stages of bleomycin-induced lung injury. J Immunol 186: 1097–1106, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schingnitz U, Hartmann K, Macmanus CF, Eckle T, Zug S, Colgan SP, Eltzschig HK: Signaling through the A2B adenosine receptor dampens endotoxin-induced acute lung injury. J Immunol 184: 5271–5279, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reutershan J, Vollmer I, Stark S, Wagner R, Ngamsri KC, Eltzschig HK: Adenosine and inflammation: Cd39 and cd73 are critical mediators in lps-induced pmn trafficking into the lungs. FASEB J 23: 473–482, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Eckle T, Füllbier L, Grenz A, Eltzschig HK: Usefulness of pressure-controlled ventilation at high inspiratory pressures to induce acute lung injury in mice. Am J Physiol Lung Cell Mol Physiol 295: L718–L724, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Eckle T, Füllbier L, Wehrmann M, Khoury J, Mittelbronn M, Ibla J, Rosenberger P, Eltzschig HK: Identification of ectonucleotidases CD39 and CD73 in innate protection during acute lung injury. J Immunol 178: 8127–8137, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS: Regression of microalbuminuria in type 1 diabetes. N Engl J Med 348: 2285–2293, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Schernthaner G: Kidney disease in diabetology: lessons from 2008. Nephrol Dial Transplant 24: 396–399, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Amin R, Widmer B, Prevost AT, Schwarze P, Cooper J, Edge J, Marcovecchio L, Neil A, Dalton RN, Dunger DB: Risk of microalbuminuria and progression to macroalbuminuria in a cohort with childhood onset type 1 diabetes: Prospective observational study. BMJ 336: 697–701, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mauer M, Zinman B, Gardiner R, Suissa S, Sinaiko A, Strand T, Drummond K, Donnelly S, Goodyer P, Gubler MC, Klein R: Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med 361: 40–51, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blackburn MR: Too much of a good thing: adenosine overload in adenosine-deaminase-deficient mice. Trends Pharmacol Sci 24: 66–70, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Grenz A, Zhang H, Weingart J, von Wietersheim S, Eckle T, Schnermann JB, Kohle C, Kloor D, Gleiter CH, Vallon V, Eltzschig HK, Osswald H. Lack of effect of extracellular adenosine generation and signalling on renal erythropoietin secretion during hypoxia. Am J Physiol Renal Physiol 293: F1501–F1511, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Nadarajah R, Milagres R, Dilauro M, Gutsol A, Xiao F, Zimpelmann J, Kennedy C, Wysocki J, Batlle D, Burns KD: Podocyte-specific overexpression of human angiotensin-converting enzyme 2 attenuates diabetic nephropathy in mice. Kidney Int 82: 292–303, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Friedman DJ, Rennke HG, Csizmadia E, Enjyoji K, Robson SC: The vascular ectonucleotidase ENTPD1 is a novel renoprotective factor in diabetic nephropathy. Diabetes 56: 2371–2379, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Kanesaki Y, Suzuki D, Uehara G, Toyoda M, Katoh T, Sakai H, Watanabe T: Vascular endothelial growth factor gene expression is correlated with glomerular neovascularization in human diabetic nephropathy. Am J Kidney Dis 45: 288–294, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Yang D, Zhang Y, Nguyen HG, Koupenova M, Chauhan AK, Makitalo M, Jones MR, St Hilaire C, Seldin DC, Toselli P, Lamperti E, Schreiber BM, Gavras H, Wagner DD, Ravid K: The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. J Clin Invest 116: 1913–1923, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aherne CM, Kewley EM, Eltzschig HK. The resurgence of a2b adenosine receptor signaling. Biochim Biophys Acta 1808:1329-1339, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frick JS, MacManus CF, Scully M, Glover LE, Eltzschig HK, Colgan SP: Contribution of adenosine A2B receptors to inflammatory parameters of experimental colitis. J Immunol 182: 4957–4964, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eckle T, Faigle M, Grenz A, Laucher S, Thompson LF, Eltzschig HK: A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood 111: 2024–2035, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eckle T, Grenz A, Laucher S, Eltzschig HK: A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J Clin Invest 118: 3301–3315, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG, Karin M: NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature 453: 807–811, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alva JA, Zovein AC, Monvoisin A, Murphy T, Salazar A, Harvey NL, Carmeliet P, Iruela-Arispe ML: Ve-cadherin-cre-recombinase transgenic mouse: A tool for lineage analysis and gene deletion in endothelial cells. Development Dynam 235: 759–767, 2006 [DOI] [PubMed] [Google Scholar]
- 54.Skuli N, Liu L, Runge A, Wang T, Yuan L, Patel S, Iruela-Arispe L, Simon MC, Keith B: Endothelial deletion of hypoxia-inducible factor-2alpha (HIF-2alpha) alters vascular function and tumor angiogenesis. Blood 114: 469–477, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Skuli N, Majmundar AJ, Krock BL, Mesquita RC, Mathew LK, Quinn ZL, Runge A, Liu L, Kim MN, Liang J, Schenkel S, Yodh AG, Keith B, Simon MC: Endothelial HIF-2α regulates murine pathological angiogenesis and revascularization processes. J Clin Invest 122: 1427–1443, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Awad AS, Huang L, Ye H, Duong ET, Bolton WK, Linden J, Okusa MD: Adenosine A2A receptor activation attenuates inflammation and injury in diabetic nephropathy. Am J Physiol Renal Physiol 290: F828–F837, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Wallace KL, Linden J: Adenosine A2A receptors induced on iNKT and NK cells reduce pulmonary inflammation and injury in mice with sickle cell disease. Blood 116: 5010–5020, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Day YJ, Marshall MA, Huang L, McDuffie MJ, Okusa MD, Linden J: Protection from ischemic liver injury by activation of A2A adenosine receptors during reperfusion: inhibition of chemokine induction. Am J Physiol Gastrointest Liver Physiol 286: G285–G293, 2004 [DOI] [PubMed] [Google Scholar]
- 59.Grenz A, Clambey E, Eltzschig HK: Hypoxia signaling during intestinal ischemia and inflammation. Curr Opin Crit Care 18: 178–185, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kong T, Westerman KA, Faigle M, Eltzschig HK, Colgan SP: HIF-dependent induction of adenosine A2B receptor in hypoxia. FASEB J 20: 2242–2250, 2006 [DOI] [PubMed] [Google Scholar]
- 61.Liu E, Morimoto M, Kitajima S, Koike T, Yu Y, Shiiki H, Nagata M, Watanabe T, Fan J: Increased expression of vascular endothelial growth factor in kidney leads to progressive impairment of glomerular functions. J Am Soc Nephrol 18: 2094–2104, 2007 [DOI] [PubMed] [Google Scholar]
- 62.Cardenas A, Toledo C, Oyarzun C, Sepulveda A, Quezada C, Guillen-Gomez E, Diaz-Encarnacion MM, Pastor-Anglada M, San Martin R: Adenosine a(2b) receptor-mediated vegf induction promotes diabetic glomerulopathy. Lab Invest 93: 135–144, 2013 [DOI] [PubMed] [Google Scholar]
- 63.Valladares D, Quezada C, Montecinos P, Concha II, Yañez AJ, Sobrevia L, San Martín R: Adenosine A(2B) receptor mediates an increase on VEGF-A production in rat kidney glomeruli. Biochem Biophys Res Commun 366: 180–185, 2008 [DOI] [PubMed] [Google Scholar]
- 64.Foster RR: The importance of cellular VEGF bioactivity in the development of glomerular disease. Nephron, Exp Nephrol 113: e8–e15, 2009 [DOI] [PubMed] [Google Scholar]
- 65.Ehrentraut H, Clambey ET, McNamee EN, Brodsky KS, Ehrentraut SF, Poth JM, Riegel AK, Westrich JA, Colgan SP, Eltzschig HK: Cd73+ regulatory T cells contribute to adenosine-mediated resolution of acute lung injury. FASEB J 27: 2207–2019, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ehrentraut H, Westrich JA, Eltzschig HK, Clambey ET: Adora2b adenosine receptor engagement enhances regulatory T cell abundance during endotoxin-induced pulmonary inflammation. PLoS ONE 7: e32416, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Homann D, von Herrath M: Regulatory T cells and type 1 diabetes. Clin Immunol 112: 202–209, 2004 [DOI] [PubMed] [Google Scholar]
- 68.Breyer MD, Böttinger E, Brosius FC, 3rd, Coffman TM, Harris RC, Heilig CW, Sharma K, AMDCC : Mouse models of diabetic nephropathy. J Am Soc Nephrol 16: 27–45, 2005 [DOI] [PubMed] [Google Scholar]
- 69.Quaggin SE, Coffman TM: Toward a mouse model of diabetic nephropathy: is endothelial nitric oxide synthase the missing link? J Am Soc Nephrol 18: 364–366, 2007 [DOI] [PubMed] [Google Scholar]
- 70.Beitner-Johnson D, Shull GE, Dedman JR, Millhorn DE: Regulation of gene expression by hypoxia: A molecular approach. Respir Physiol 110: 87–97, 1997 [DOI] [PubMed] [Google Scholar]
- 71.Wang J, Takeuchi T, Tanaka S, Kubo SK, Kayo T, Lu D, Takata K, Koizumi A, Izumi T: A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse. J Clin Invest 103: 27–37, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oyadomari S, Koizumi A, Takeda K, Gotoh T, Akira S, Araki E, Mori M: Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest 109: 525–532, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sharma K, McCue P, Dunn SR: Diabetic kidney disease in the db/db mouse. Am J Physiol Renal Physiol 284: F1138–F1144, 2003 [DOI] [PubMed] [Google Scholar]
- 74.Chow FY, Nikolic-Paterson DJ, Atkins RC, Tesch GH. Macrophages in streptozotocin-induced diabetic nephropathy: Potential role in renal fibrosis. Nephrol Dial Transplant 19: 2987–2996, 2004 [DOI] [PubMed] [Google Scholar]
- 75.Nakagawa T, Sato W, Glushakova O, Heinig M, Clarke T, Campbell-Thompson M, Yuzawa Y, Atkinson MA, Johnson RJ, Croker B: Diabetic endothelial nitric oxide synthase knockout mice develop advanced diabetic nephropathy. J Am Soc Nephrol 18: 539–550, 2007 [DOI] [PubMed] [Google Scholar]
- 76.Dai HY, Zheng M, Lv LL, Tang RN, Ma KL, Liu D, Wu M, Liu BC: The roles of connective tissue growth factor and integrin-linked kinase in high glucose-induced phenotypic alterations of podocytes. J Cell Biochem 113: 293–301, 2012 [DOI] [PubMed] [Google Scholar]
- 77.Calkin AC, Giunti S, Jandeleit-Dahm KA, Allen TJ, Cooper ME, Thomas MC: Ppar-alpha and -gamma agonists attenuate diabetic kidney disease in the apolipoprotein e knockout mouse. Nephrol Dial Transplant 21: 2399–2405, 2006 [DOI] [PubMed] [Google Scholar]
- 78.Kosugi T, Nakayama T, Heinig M, Zhang L, Yuzawa Y, Sanchez-Lozada LG, Roncal C, Johnson RJ, Nakagawa T: Effect of lowering uric acid on renal disease in the type 2 diabetic db/db mice. Am J Physiol Renal Physiol 297: F481–F488, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.