Abstract
Pauci-immune focal necrotizing GN (piFNGN) is usually associated with ANCAs that are thought to be pathogenic. However, 10%–15% of patients are ANCA negative and the cause of their injury is unknown. We previously reported a high frequency of autoantibodies to human lysosome-associated membrane protein-2 (hLAMP-2) in ANCA-associated piFNGN, and have now investigated whether the same is true in ANCA-negative patients. Of 11 patients, 8 (73%) had anti–hLAMP-2 antibodies detected by ELISA and confirmed by immunoblotting and indirect immunofluorescence. The autoantibodies from all 8 patients bound to native LAMP-2 purified from human glomeruli and recombinant hLAMP-2 expressed in ldlD cells, both with molecular masses of 110 kD. However, in contrast to anti–LAMP-2 antibodies from ANCA-positive patients, these antibodies from ANCA-negative patients failed to bind the more complexly glycosylated native neutrophil hLAMP-2 (190 kD). Treatment with the deglycosylating enzyme, endo-β-galactosidase, reduced the mass of neutrophil hLAMP-2 to 110 kD and enabled autoantibody binding. Similarly, pretreating neutrophils with endo-β-galactosidase or neuraminidase converted ANCA assay results from negative to positive. Finally, IgG from LAMP-2-positive ANCA-negative patients bound specifically to normal human kidney sections and to human glomerular endothelial cells in culture. In conclusion, in patients with ANCA-negative piFNGN, we have identified autoantibodies to hLAMP-2 that bind native glomerular but not neutrophil hLAMP-2, suggesting a role in pathogenesis.
Pauci-immune focal necrotizing GN (piFNGN) is a severe inflammatory disease that commonly causes renal failure and typically occurs as part of a systemic small vessel vasculitis.1,2 Between 85% and 90% of affected individuals have ANCA with specificity for myeloperoxidase (MPO) or proteinase-3 (PR3), leading to the term ANCA-associated vasculitis (AAV).3,4 The high frequency of ANCA, combined with evidence from in vitro studies and experimental models, provides compelling evidence that antibodies to MPO and PR3 can be pathogenic.5,6 This raises the question of what causes injury in the 10%–15% of patients in whom ANCA cannot be detected.
Injury in ANCA-negative piFNGN is morphologically very similar to ANCA-positive disease, and there is little to suggest that they are separate entities, although the available evidence on ANCA-negative patients is limited to three small series and isolated case descriptions.7–10 In Europeans, the clinical expression appears identical with no discernible difference in the nature and severity of the renal injury or in the extent of systemic involvement.7 However, in Chinese individuals, ANCA-negative disease has been reported to be more protracted and to have less extensive extrarenal involvement.9 Glomerular neutrophil infiltration may be less intense11 although glomerular deposition of Ig and complement are similar and systemic complement activation occurs in active disease regardless of ANCA status.12 The cause of injury in ANCA-negative patients is uncertain but the following possibilities have been suggested: low titers of anti-PR3 antibodies that can only be detected using extrasensitive immunoassays,13,14 inhibition of assays for anti-MPO antibodies by ceruloplasmin fragments,15 podocyte-specific nonimmune triggers to crescent formation that have been identified in murine models,16,17 and autoantibodies to lysosome-associated membrane protein-2 (LAMP-2) similar to those in ANCA-positive disease.18,19
LAMP-2 is a heavily glycosylated membrane protein that traffics from the cell surface to lysosomes, where it is most abundant and is critical for cellular homeostasis and responses to stress.20 We originally discovered autoantibodies to human LAMP-2 (hLAMP-2) as part of a systematic search for autoantibodies to glomerular membrane proteins in piFNGN21 and have reported their high prevalence in piFNGN. We consistently find that >80% of patients presenting with piFNGN in European cohorts have circulating autoantibodies to hLAMP-2 that rapidly became undetectable after immunosuppressive treatment.18,19,21 Although another group reported a lower overall incidence, the frequency of anti-hLAMP-2 antibodies at presentation in their cohort was still highly significantly increased, with a frequency 10-fold higher than healthy controls.22
Results
There were a few individuals with ANCA-negative piFNGN in our previous cohorts who had autoantibodies to hLAMP-2 detected by ELISA and confirmed by Western blotting and indirect immunofluorescence assays.18,19 This was unexpected because LAMP-2 is expressed in neutrophils (Figure 1A) and patients’ autoantibodies almost invariably recognize peptide epitopes that remain accessible after glycosylation.18,19,21 Accordingly, anti–hLAMP-2 antibodies would be expected to have positive fluorescence ANCA assays even when antibodies to MPO and PR3 are absent. In an attempt to explain the apparent paradox, we identified all of the ANCA-negative patients with piFNGN treated by us and re-analyzed sera taken at the time they first presented with biopsy-proven active disease.
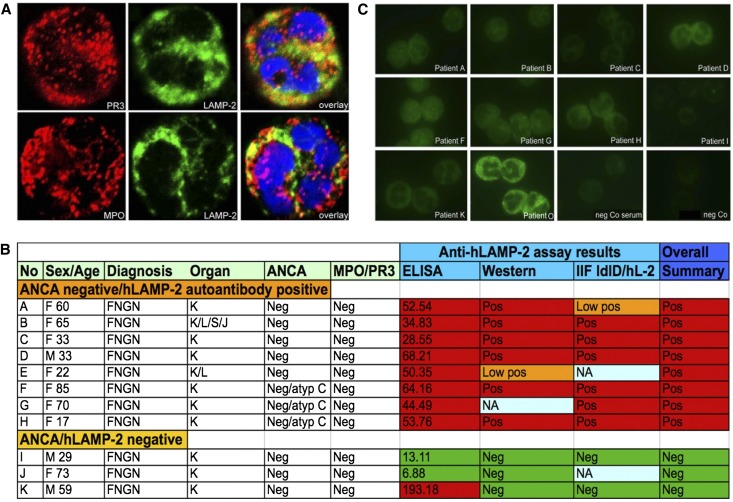
Figure 1.
LAMP-2 in human neutrophils and characteristics of ANCA-negative patients. (A) LAMP-2 is found in compartments that partially overlap with PR3 and MPO in human PMNs. (B) Clinical characteristics and results of ANCA, anti-MPO, anti-PR3, and anti-hLAMP-2 assays of 11 ANCA-negative patients with (n=8) or without (n=3) evidence of autoantibodies to hLAMP-2 with piFNGN with either isolated renal disease (K) or systemic disease (L, lung; S, skin; J, joint). Sera from ANCA-negative patients react with hLAMP-2. (C) IgG in sera from LAMP-2–positive/ANCA-negative patients does not give positive ANCA reactivity using commercially available PMN cytospin preparations by indirect immunofluorescence. Original magnification, ×630 in A; ×400 in C. IIF, indirect immunofluorescence.
We identified 11 patients who had ANCA-negative results at presentation with piFNGN and without detectable antibodies to MPO or PR3 whose subsequent assays remained consistently negative (Figure 1B): two of these patients were included in our previously reported cohorts and nine were not (Table 1). All of the patients presented with deteriorating renal function and typical features of piFNGN. Renal biopsies confirmed the diagnosis and presence of active injury with focal necrosis and crescents affecting at least 50% of glomeruli. The morphologic appearances were indistinguishable from ANCA-positive patients in our cohorts but injury was apparently confined to the kidney in 9 of 11 patients (82%), suggesting more limited disease. On retesting, all 11 patients had negative indirect immunofluorescence ANCA assays (Figure 1C) as well as ELISA results for antibodies to MPO and PR3. This confirms they were true ANCA-negative patients.
Table 1.
Effect of removal of sialic acid residues and/or removal of polylactosamines on ANCA staining pattern
| Patienta | Sex | Age (yr) | IIF | MPO/PR3 (U/ml) | hLAMP-2 | ANCA | |||
|---|---|---|---|---|---|---|---|---|---|
| ANCA | GEnC | Neuraminidase | PNGaseF | Endo-β-Galactosidase | |||||
| ANCA-negative/hLAMP-2 autoantibody positive | |||||||||
| A | F | 60 | − | + | − | + | Membrane ++ | No difference | C+ |
| B | F | 65 | − | NA | − | + | Atypical C/ membrane ++ | No difference | C+ |
| C | F | 33 | − | + | − | + | Atypical C+ | No difference | C++ |
| D (39) | M | 33 | − | + | − | + | Atypical C+ | No difference | Atypical C++ |
| E | F | 22 | − | + | − | + | Atypical C++ | C++ | Negative |
| F | F | 85 | − | + | − | + | C | No difference | C± |
| G | F | 70 | − | NA | − | + | C | No difference | C |
| H (70) | F | 17 | − | + | − | + | C++ | No difference | C++ |
| ANCA-negative/hLAMP-2 autoantibody negative | |||||||||
| I | M | 29 | − | NA | − | − | No difference | NA | NA |
| J | F | 73 | − | + | − | − | No difference | NA | NA |
| K | M | 59 | − | + | − | − | No difference | No difference | No difference |
| ANCA-positive/hLAMP-2 autoantibody negative controls | |||||||||
| L | M | 78 | P | NA | MPO 22 | − | No difference | NA | NA |
| M (57) | F | 57 | C | NA | PR3 20 | − | No difference | No difference | No difference |
| N (51) | M | 58 | C | − | PR3 50.55 | − | No difference | NA | NA |
| O (9) | F | 76 | C | − | PR3 360 | − | No difference | No difference | No difference |
| P | M | 45 | C | − | PR3 39.8 | − | No difference | NA | NA |
All 11 ANCA-negative patients (patients A–K) had negative results in standard, commercially available indirect immunofluorescence assays; however, patient sera with antibodies to hLAMP-2 exhibited binding to GEnC by IIF using either frozen sections of human kidney or immortalized glomerular endothelial cells (n=8, A–H). Using sera from those eight patients for IIF, removal of either N-glycans (n=1, E) or both, removal of sialic acid and polylactosamines (n=7, A–D, F–H) from normal human granulocytes, enhanced fluorescence intensity and revealed either a membrane bound staining (n=2) and/or an (atypical) cANCA pattern (n=7). The remaining three patients did not exhibit hLAMP-2 antibodies by ELISA and treatment of granulocytes did not alter their ANCA negativity by IIF assays (patients I–K). The ANCA staining pattern of five patients with either cANCA/anti-PR3 antibodies (n=4) or pANCA/anti-MPO antibodies (n=1) was not altered by carbohydrate removal (patients L–P), nor did treatment with the enzymes change negative results from sera of healthy controls (n=3). IIF, indirect immunofluorescence; F, female; −, negative; +, positive; membrane ++, membrane accentuated staining; C, cytoplasmic ANCA; NA, not available; M, male; atypical, atypical staining pattern; no difference, no difference in staining pattern to untreated cells; P, perinuclear ANCA.
Numbers in parentheses next to the patients’ assignation (A–P) refer to patients in Supplemental Table 3 published previously by Kain et al. (18).
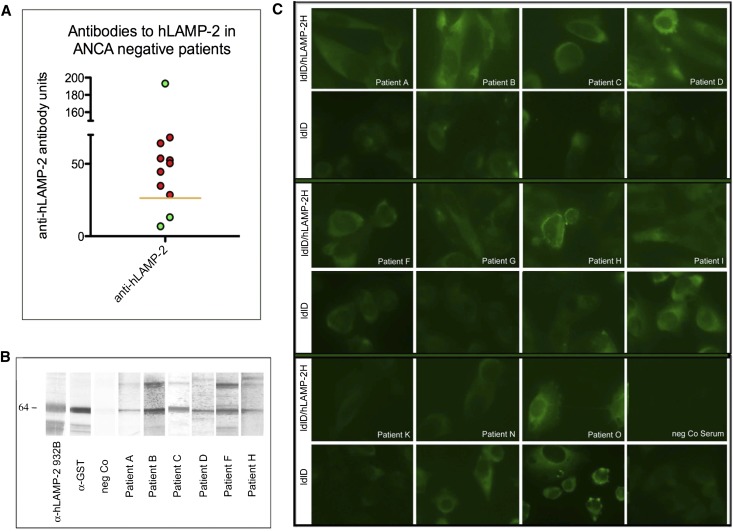
When tested for antibodies to hLAMP-2, 9 of 11 patients (82%) had positive results in our standard ELISA with bacterially expressed unglycosylated recombinant human LAMP-2 as a substrate, whereas two were negative (Figure 2A). The positive ELISA results were confirmed in eight of these by Western blots (Figure 2B) and indirect immunofluorescence on ldlD cells stably transfected to express glycosylated human LAMP-2 targeted to the plasma membrane (ldlD/hLAMP-2H; Figure 2C). Thus, positive results in three independent validated assays unequivocally established that 8 of 11 (73%) ANCA-negative patients had autoantibodies to hLAMP-2 with titers indistinguishable from those of ANCA-positive patients.18,19 Anti–LAMP-2 antibodies were not detected in the remaining three patients.
Figure 2.
Assays for autoantibodies specific for LAMP-2. Sera from ANCA-negative patients bind to hLAMP-2 using recombinant, E. coli expressed GST fusion protein by ELISA (A) and Western blot (B). (C) Reactivity is confirmed by indirect immunofluorescence on ldlD parental cells (ldlD) or cells expressing hLAMP-2 on the cell surface (ldlD/hLAMP-2H).
ELISA and Western blotting showed that the autoantibodies from ANCA-negative patients bind to the protein backbone of hLAMP-2, whereas indirect immunofluorescence confirmed that the epitopes recognized remained accessible in glycosylated recombinant hLAMP-2 expressed in ldlD cells but were cryptic in native neutrophil hLAMP-2 (Figure 1C). hLAMP-2 is heavily glycosylated with 19 potential N-linked glycosylation sites and a cluster of O-linked glycosylation sites at the hinge region between the two homologous halves of the extracellular domain.23 Carbohydrate contributes between a half and three quarters of the molecular mass of hLAMP-2, depending on the complexity of glycosylation that varies with cell type and activation status. Neutrophil hLAMP-2 is particularly heavily glycosylated and carries complex polylactosamine carbohydrate moieties, presumptively to protect it from degradation by neutrophil proteases.24 By contrast, native hLAMP-2 from glomeruli is less complexly glycosylated and has a molecular mass of 110 kD, which is identical to recombinant hLAMP-2 expressed in Chinese hamster ovary cells or the mutant ldlD cell line we use for indirect immunofluorescence assays.18,19
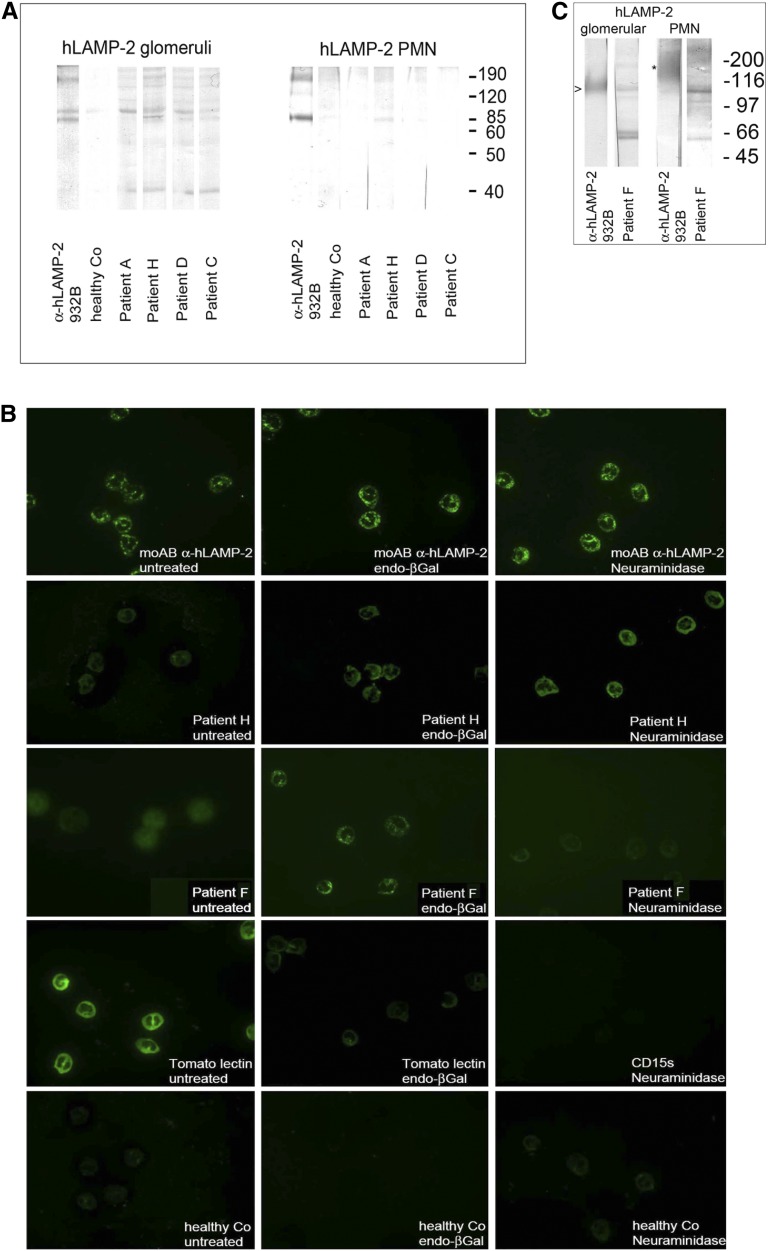
To test the influence of glycosylation on autoantibody binding, we purified LAMP-2 separately from membrane-enriched fractions of lysates of human glomeruli and neutrophils by two-stage affinity purification using a rabbit polyclonal anti–hLAMP-2 and a mouse mAb (H4B4), respectively, coupled to protein A Sepharose columns. The affinity-purified glomerular and neutrophil hLAMP-2 had the expected molecular masses of 110 and 190 kD, respectively, reflecting the complexity of their glycosylation.18,19 Autoantibodies from the eight LAMP-2 positive patients bound glomerular hLAMP-2 but failed to bind to 190-kD neutrophil hLAMP-2 (Figure 3A). However, enzymatic treatment with endo-β-galactosidase, which removes polylactosamines, reduced the molecular mass of the neutrophil hLAMP-2 to 110 kD, similar to that of glomerular hLAMP-2,23 and facilitated autoantibody binding. By contrast, anti–hLAMP-2 antibodies from ANCA-positive patients usually bind fully glycosylated native neutrophil hLAMP-2.21 Interestingly, the specific polyclonal anti–LAMP-2 antibody recognized a second weak band of 110 kD in some neutrophil LAMP-2 preparations (Figure 3, A and C). This probably represents incompletely glycosylated hLAMP-2 isolated from immature neutrophils as has previously been described.21 This band was present in LAMP-2 preparations used to test sera from two patients (H and F): IgG from their sera reacted with it but not with the dominant 190 kD species (Figure 3, A and C). IgG from the three ANCA-negative patients without detectable anti–LAMP-2 antibodies failed to bind any of the LAMP-2 preparations (Figure 2C, Supplemental Figures 1A and 2).
Figure 3.
Autoantibodies in ANCA-negative patients bind to native human glomerular but not fully glycosylated neutrophil LAMP-2. (A and C) Patients’ autoantibodies bind to affinity-purified native human glomerular hLAMP-2, whereas immunopurified, fully glycosylated 109 kD hLAMP-2 from neutrophils is not recognized. However, in some patients (F and H), reactivity with an incompletely glycosylated form of hLAMP-2 at 110 kD is observed (A and C, right). (B) Enzymatic deglycosylation of PMN preparations with neuraminidase and endo-β-galactosidase (endo-βGal) treatment before indirect immunofluorescence abolishes staining with CD15s (SialylLex) and Tomato lectin staining, respectively. IgG from patients’ sera containing anti–LAMP-2 antibodies binds only to neutrophils after enzymatic deglycosylation, whereas binding of a mAb to hLAMP-2 is not influenced by enzymatic treatment. The control serum from a healthy control remains negative in all conditions. Original magnification, ×400.
The Western blots suggest that the anti–LAMP-2 antibodies in ANCA-negative patients fail to bind to fixed neutrophils because the epitopes they recognized are occluded by carbohydrate moieties. We tested this by pretreating the human neutrophils with deglycosylating enzymes before performing the ANCA assay. Pretreatment with neuraminidase to remove sialic acid residues23 enabled the patients’ autoantibodies to bind neutrophil LAMP-2 (Supplemental Figure 1B) and resulted in positive indirect immunofluorescence for ANCA in eight relevant ANCA-negative patients, whereas pretreatment with endo-β-galactosidase to remove polylactosamine side chains23,24 rescued binding in 7 patients (Figure 3B, Table 1). Again, the sera from the three ANCA-negative patients without anti–hLAMP-2 antibodies failed to bind neutrophils even after treatment with deglycosylating enzymes (Supplemental Figure 1C). Binding of antibodies to MPO and PR3 in neutrophils was unaffected by treatment with either deglycosylating enzyme (Supplemental Figure 1C) using sera from ANCA-positive patients without detectable autoantibodies to hLAMP-2 (Supplemental Figure 2, A and B). Thus, anti–LAMP-2 antibodies in ANCA-negative patients do not bind fully glycosylated LAMP-2 in mature human neutrophils.
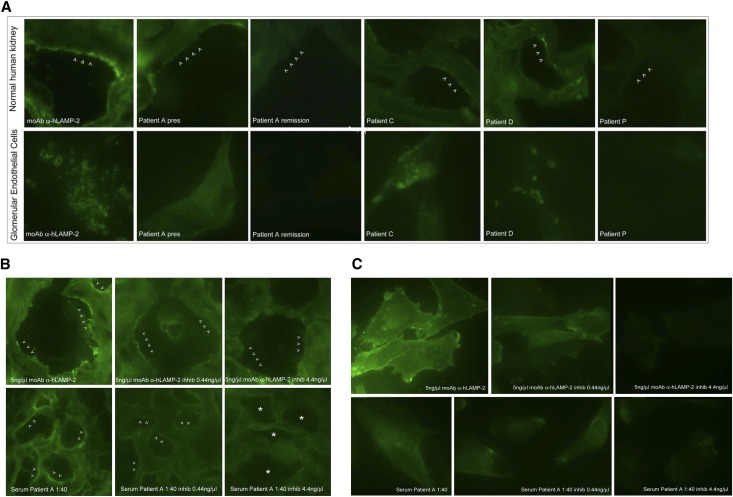
The autoantibodies in sera from the ANCA-negative patients bound native glomerular hLAMP-2 in Western blots; thus, we used indirect immunofluorescence to test whether they also bind LAMP-2 in glomeruli of tissue sections of normal human kidney. Anti–LAMP-2–positive and anti–LAMP-2–negative sera were incubated with sections of unfixed frozen normal human tissue that had been preincubated with high salt (1.8% NaCl) citrate buffer to remove nonspecifically bound IgG and binding visualized with an affinity-purified sheep–anti-human IgG antibody. Sera were available from 8 of 11 ANCA-negative patients (6 positive for antibodies to LAMP-2 and 2 negative—there was insufficient serum to test patients B, G, and I). IgG from all six LAMP-2–positive patients tested bound specifically to glomeruli with a predominantly endothelial pattern (Figure 4A, Supplemental Figure 2C) similar to what we previously described for ANCA-positive patients with coexisting anti–LAMP-2 antibodies.21 We confirmed that autoantibodies in all six sera bound the glomerular endothelium using conditionally immortalized human glomerular endothelial cells (GEnCs),25 kindly provided by Dr. Simon Satchell and Professor Peter Mathieson (Bristol, UK) (Figure 4A). By contrast, IgG from the two ANCA-negative LAMP-2–negative patients did not bind to glomeruli, nor did IgG from two LAMP-2–negative/ANCA-positive patients used as controls (Supplemental Figure 2C).
Figure 4.
Anti–LAMP-2 antibodies in ANCA-negative patients bind specifically to human glomerular endothelium. (A) Sera from ANCA-negative/hLAMP-2–positive (patients A, C, and D) patients, but not ANCA-positive/hLAMP-2–negative (patient P) patients, bind specifically to glomeruli with a predominantly endothelial pattern in 4-μm unfixed frozen sections of normal human kidney (upper) and to conditionally immortalized human GEnC, which results in a mixed lysosomal and surface staining pattern (lower). The slightly granular linear IgG staining of glomeruli in tissue sections with patient A’s serum is identical to that obtained with the mAb to hLAMP-2 (H4B4) (B) and both patient sera and H4B4 give similar surface staining on ldlD cells stably expressing hLAMP-2 in the plasma membrane (C). Preincubating the antibodies with increasing concentrations of immune-purified native glomerular hLAMP-2 results in complete inhibition of antibody binding at 4.4 ng/μl (B and C inhibited).
Finally, we used inhibition with purified soluble human glomerular LAMP-2 to confirm the specificity of autoantibody binding to human glomeruli and GEnCs. First we defined the optimal concentration of soluble LAMP-2 in a dose response curve to inhibit IgG binding from the serum of one patient with high anti–LAMP-2 antibody concentration (patient A). Soluble glomerular hLAMP-2 inhibited binding in a dose-dependent fashion both of the patient’s autoantibodies and of the monoclonal anti–LAMP-2 antibody. The results were similar using either normal human kidney sections or ldlD cells stably expressing hLAMP-2 (Figure 4, B and C). Complete inhibition of binding was achieved with a hLAMP-2 concentration of 4.4 ng/μl. Accordingly, double this concentration was used for inhibition experiments with the remaining five sera samples and this abrogated IgG binding in every case (Supplemental Figure 2C).
Discussion
The results, summarized in Table 1, clearly identify anti–hLAMP-2 antibodies in patients with biopsy-proven active piFNGN in a situation in which canonical ANCA cannot be detected and thus are highly unlikely to be the cause of injury. This mirrors the situation in two experimental models in rats: (1) WKY rats immunized with the hLAMP-2 molecular mimic, FimH,18 who develop autoantibodies to rat LAMP-2 associated with piFNGN without anti-MPO or PR3 antibodies; and (2) env-pX rats that spontaneously develop small vessel vasculitis together with high titers of autoantibodies to LAMP-2 antibodies again without antibodies specific for MPO or PR3.26 Thus, clinically and in rodent models, the presence of autoantibodies to hLAMP-2 is correlated with active vasculitis even in the absence of antibodies to MPO and PR3. Notably, the converse occurs in patients with AAV in remission when antibodies to LAMP-2 are rare despite the continued presence of autoantibodies to MPO or PR3,4,27,28 further strengthening the case that autoantibodies to LAMP-2 are pathogenic.
In conclusion, we have identified injury-associated autoantibodies to hLAMP-2 that bind glomerular but not neutrophil LAMP-2 in a group of ANCA-negative patients. This suggests that autoantibody binding to hLAMP-2 in the glomerulus is sufficient to cause injury in these patients and that binding to neutrophils is dispensable. This has implications for understanding the pathogenesis of AAV more generally. It also has important implications for the development of anti–LAMP-2 assays. There are currently no clinical-grade assays for measuring hLAMP-2, necessitating our use of three independent assays. We and others are working intensively to develop assays suitable for general use; however, the results reported here caution against the use of heavily glycosylated hLAMP-2 preparations as assay substrates,22 such as those expressed in human embryonic kidney 293 cells, because of the risk that pathogenetically relevant epitopes will be occluded and that autoantibodies to them will go undetected.
Concise Methods
Patients and Controls
Sera from 11 ANCA-negative patients (8 hLAMP-2 positive and 3 hLAMP-2 negative) and 5 ANCA-positive (hLAMP-2 negative) control sera were identified from two independent series of patients presenting with FNGN in the context of small vessel vasculitis from Vienna and Aberdeen. The patients’ details are summarized in Figure 1A and Supplemental Figure 1. Two of these patients were included in our previous study and nine were not.18 Sera from three healthy volunteers served as controls in all experiments.
Permission to use patients’ sera for autoantibody testing was granted by the relevant ethics committees of the Medical University of Vienna and the University of Aberdeen.
Standard ANCA and Autoantibody Assays
Serial dilutions of all sera were routinely tested for ANCA (The Binding Site), as previously described.18,19,21 One or more serum samples from each patient was tested for ANCA specificity using commercially available ELISA systems (PR3/MPO Combikit, ORGenTec, or VARELISA, Phadia).
Generation of Recombinant and Purification Mutant hLAMP-2 for Western Blotting and ELISA and Indirect Immunofluorescence Assay for Antibodies to hLAMP-2
Methods and assays to test for the presence of autoantibodies to hLAMP-2 in patients’ sera using Escherichia coli expressed hLAMP-2 extracellular domain as GST fusion protein for ELISA and Western blot and ldlD cells stably expressing hLAMP-2 on the cell surface for indirect immunofluorescence were previously described in detail.18,19
Indirect Immunofluorescence on Sections of Human Kidney, Immortalized GEnCs, and Polymorphonuclear Neutrophilic Granulocytes
Reactivity with structures of human kidney was tested on 4-μm unfixed frozen sections of a normal human kidney that had proved unsuitable for transplantation. Kidney sections were treated with 100 mM sodium citrate buffer (pH 6.6) with 1.8% NaCl to remove nonspecifically bound IgG before indirect immunofluorescence with patient sera and antibodies specific for hLAMP-2. Reactivity of patients’ IgG with GEnCs, maintained in the presence of and without the addition of TNF-α, and preparations of normal human granulocytes was assayed by indirect immunofluorescence using commercially available cytospin preparations of polymorphonuclear neutrophilic granulocytes (PMNs) (Inova). To test the influence of glycosylation, neutrophils were treated with PNGaseF (P0704S; BioLabs), neuraminidase (sialidase, P07205; BioLabs), and endo-β-galactosidase (Escherichia freundii, 100455; Seikagaku Corporation) in their appropriate buffers and used for indirect immunofluorescence with hLAMP-2–positive and hLAMP-2–negative sera from 11 ANCA/MPO/PR3-negative patients. As controls, we used hLAMP-2–negative sera from five patients with ANCA-associated FNGN (one with pANCA/MPO and four with cANCA/PR3 antibodies) and sera from three healthy volunteers. Pictures were taken in each experiment at fixed exposure times determined by a positive control antibody to hLAMP-2 (H4B4) and ranged between 400–600 milliseconds.
Purification of Native hLAMP-2, Western Blotting, and Inhibition Experiments
Native hLAMP-2 was purified from human glomeruli and PMNs. In a two-stage procedure, membrane protein-enriched fractions (TX 114 fraction)21 of glomerular and PMN cell lysates were passed sequentially through two protein A Sepharose columns, respectively, containing immobilized polyclonal and monoclonal (H4B4) antibodies specific for hLAMP-2. Bound hLAMP-2 protein was recovered by acidic elution and separated by SDS-PAGE with subsequent transfer onto polyvinylidene fluoride or nitrocellulose membranes and used for Western blot analysis to determine the capacity of patients’ autoantibodies to bind to unglycosylated and glycosylated forms of hLAMP-2. Increasing concentrations of immunopurified glomerular hLAMP-2 (0.044, 0.44, 4.4, and 8.4 ng/μl) were used to inhibit binding of patients’ sera or H4B4, a mAb to hLAMP-2, to hLAMP-2 in sections of normal human kidney, immortalized GEnCs, or ldlD cells stably expressing hLAMP-2 on the cells surface.
Antibodies and Reagents
The following primary antibodies were used: monoclonal mouse anti–HLAMP-2, clone H4B4 (Developmental Studies Hybridoma Bank, University of Iowa), and monoclonal mouse anti–hLAMP-2 (clone CD3) and rabbit anti–hLAMP-2 (932B) (kindly provided by Prof. Minoru Fukuda of Burnham Institute, La Jolla, CA). Secondary antibodies used in indirect immunofluorescence were Alexa Fluor 488–conjugated F(ab′)2 fragment of goat anti-mouse IgG (H+L) and goat anti-rabbit IgG (H+L) (Invitrogen) and FITC-conjugated sheep Ig to human IgG (INOVA Diagnostics).
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank A. Röthel for technical assistance.
The research leading to these results received funding from the European Union Seventh Framework Programme (FP7/2007-2013 Grant 261382) and was supported by the Vienna Science and Technology Fund (Project LS09-075).
Parts of this work were presented at the 2011 Annual Meeting of the American Society of Nephrology, held November 8–13, 2011, in Philadelphia, Pennsylvania.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013030320/-/DCSupplemental.
References
- 1.Jennette JC, Falk RJ: Small-vessel vasculitis. N Engl J Med 337: 1512–1523, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Morgan MD, Harper L, Williams J, Savage C: Anti-neutrophil cytoplasm-associated glomerulonephritis. J Am Soc Nephrol 17: 1224–1234, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, Flores-Suarez LF, Gross WL, Guillevin L, Hagen EC, Hoffman GS, Jayne DR, Kallenberg CGM, Lamprecht P, Langford CA, Luqmani RA, Mahr AD, Matteson EL, Merkel PA, Ozen S, Pusey CD, Rasmussen N, Rees AJ, Scott DGI, Specks U, Stone JH, Takahashi K, Watts RA: 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 65: 1–11, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Jennette JC, Falk RJ, Hu P, Xiao H: Pathogenesis of antineutrophil cytoplasmic autoantibody-associated small-vessel vasculitis. Annu Rev Pathol 8: 139–160, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heeringa P, Little MA: In vivo approaches to investigate ANCA-associated vasculitis: Lessons and limitations. Arthritis Res Ther 13: 204, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schreiber A, Kettritz R: The neutrophil in antineutrophil cytoplasmic autoantibody-associated vasculitis. J Leukoc Biol 94: 623–631, 2013 [DOI] [PubMed]
- 7.Hedger N, Stevens J, Drey N, Walker S, Roderick P: Incidence and outcome of pauci-immune rapidly progressive glomerulonephritis in Wessex, UK: A 10-year retrospective study. Nephrol Dial Transplant 15: 1593–1599, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Eisenberger U, Fakhouri F, Vanhille P, Beaufils H, Mahr A, Guillevin L, Lesavre P, Noël LH: ANCA-negative pauci-immune renal vasculitis: Histology and outcome. Nephrol Dial Transplant 20: 1392–1399, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Chen M, Yu F, Wang SX, Zou WZ, Zhao MH, Wang HY: Antineutrophil cytoplasmic autoantibody-negative pauci-immune crescentic glomerulonephritis. J Am Soc Nephrol 18: 599–605, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Chen M, Kallenberg CGM, Zhao MH: ANCA-negative pauci-immune crescentic glomerulonephritis. Nat Rev Nephrol 5: 313–318, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Xing GQ, Chen M, Liu G, Wang SX, Zhao MH: Renal neutrophils infiltration in antineutrophil cytoplasmic antibodies-negative pauci-immune crescentic glomerulonephritis. Am J Med Sci 340: 474–480, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Gou S-J, Yuan J, Chen M, Yu F, Zhao M-H: Circulating complement activation in patients with anti-neutrophil cytoplasmic antibody-associated vasculitis. Kidney Int 83: 129–137, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Silva F, Hummel AM, Jenne DE, Specks U: Discrimination and variable impact of ANCA binding to different surface epitopes on proteinase 3, the Wegener’s autoantigen. J Autoimmun 35: 299–308, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Selga D, Segelmark M, Gunnarsson L, Hellmark T: Epitope shift of proteinase-3 anti-neutrophil cytoplasmic antibodies in patients with small vessel vasculitis. Clin Exp Immunol 160: 318–324, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roth AJ, Ooi JD, Hess JJ, van Timmeren MM, Berg EA, Poulton CE, McGregor J, Burkart M, Hogan SL, Hu Y, Winnik W, Nachman PH, Stegeman CA, Niles J, Heeringa P, Kitching AR, Holdsworth S, Jennette JC, Preston GA, Falk RJ: Epitope specificity determines pathogenicity and detectability in ANCA-associated vasculitis. J Clin Invest 123: 1773–1783, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding M, Cui S, Li C, Jothy S, Haase V, Steer BM, Marsden PA, Pippin J, Shankland S, Rastaldi MP, Cohen CD, Kretzler M, Quaggin SE: Loss of the tumor suppressor Vhlh leads to upregulation of Cxcr4 and rapidly progressive glomerulonephritis in mice. Nat Med 12: 1081–1087, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Bollee G, Flamant M, Schordan S, Fligny C, Rumpel E, Milon M, Schordan E, Sabaa N, Vandermeersch S, Galaup A, Rodenas A, Casa I, Sunnarborg SW, Salant DJ, Kopp JB, Threadgill DW, Quaggin SE, Dussaule JC, Germain S, Mesnard L, Endlich K, Boucheix C, Belenfant X, Callard P, Endlich N, Tharaux PL: Epidermal growth factor receptor promotes glomerular injury and renal failure in rapidly progressive crescentic glomerulonephritis. Nat Med 17: 1242–1250, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kain R, Exner M, Brandes R, Ziebermayr R, Cunningham D, Alderson CA, Davidovits A, Raab I, Jahn R, Ashour O, Spitzauer S, Sunder-Plassmann G, Fukuda M, Klemm P, Rees AJ, Kerjaschki D: Molecular mimicry in pauci-immune focal necrotizing glomerulonephritis. Nat Med 14: 1088–1096, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kain R, Tadema H, McKinney EF, Benharkou A, Brandes R, Peschel A, Hubert V, Feenstra T, Sengölge G, Stegeman C, Heeringa P, Lyons PA, Smith KG, Kallenberg C, Rees AJ: High prevalence of autoantibodies to hLAMP-2 in anti-neutrophil cytoplasmic antibody-associated vasculitis. J Am Soc Nephrol 23: 556–566, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saftig P, Klumperman J: Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat Rev Mol Cell Biol 10: 623–635, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Kain R, Matsui K, Exner M, Binder S, Schaffner G, Sommer EM, Kerjaschki D: A novel class of autoantigens of anti-neutrophil cytoplasmic antibodies in necrotizing and crescentic glomerulonephritis: The lysosomal membrane glycoprotein h-lamp-2 in neutrophil granulocytes and a related membrane protein in glomerular endothelial cells. J Exp Med 181: 585–597, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roth AJ, Brown MC, Smith RN, Badhwar AK, Parente O, Chung H, Bunch DO, McGregor JG, Hogan SL, Hu Y, Yang JJ, Berg EA, Niles J, Jennette JC, Preston GA, Falk RJ: Anti-LAMP-2 antibodies are not prevalent in patients with antineutrophil cytoplasmic autoantibody glomerulonephritis. J Am Soc Nephrol 23: 545–555, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carlsson SR, Roth J, Piller F, Fukuda M: Isolation and characterization of human lysosomal membrane glycoproteins, h-lamp-1 and h-lamp-2. Major sialoglycoproteins carrying polylactosaminoglycan. J Biol Chem 263: 18911–18919, 1988 [PubMed] [Google Scholar]
- 24.Lee N, Wang WC, Fukuda M: Granulocytic differentiation of HL-60 cells is associated with increase of poly-N-acetyllactosamine in Asn-linked oligosaccharides attached to human lysosomal membrane glycoproteins. J Biol Chem 265: 20476–20487, 1990 [PubMed] [Google Scholar]
- 25.Satchell SC, Tasman CH, Singh A, Ni L, Geelen J, von Ruhland CJ, O’Hare MJ, Saleem MA, van den Heuvel LP, Mathieson PW: Conditionally immortalized human glomerular endothelial cells expressing fenestrations in response to VEGF. Kidney Int 69: 1633–1640, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Takeuchi S, Kimura S, Soma Y, Waki M, Yamaguchi M, Nakazawa D, Tomaru U, Ishizu A, Kawakami T: Lysosomal-associated membrane protein-2 plays an important role in the pathogenesis of primary cutaneous vasculitis. Rheumatology (Oxford) 52: 1592–1598, 2013 [DOI] [PubMed]
- 27.Birck R, Schmitt WH, Kaelsch IA, van der Woude FJ: Serial ANCA determinations for monitoring disease activity in patients with ANCA-associated vasculitis: Systematic review. Am J Kidney Dis 47: 15–23, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Tomasson G, Grayson PC, Mahr AD, Lavalley M, Merkel PA: Value of ANCA measurements during remission to predict a relapse of ANCA-associated vasculitis—a meta-analysis. Rheumatology (Oxford) 51: 100–109, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.