Abstract
Evidence suggests that glycogen synthase kinase 3β (GSK3β) contributes to AKI; however, its role in post-AKI kidney repair remains uncertain. Here, delayed treatment with a single dose of lithium, a selective inhibitor of GSK3β and a US Food and Drug Administration–approved mood stabilizer, accelerated recovery of renal function, promoted repopulation of renal tubular epithelia, and improved kidney repair in murine models of cisplatin- and ischemia/reperfusion-induced AKI. These effects associated with reduced GSK3β activity and elevated expression of proproliferative molecules, including cyclin D1, c-Myc, and hypoxia-inducible factor 1α (HIF-1α), in renal tubular epithelia. In cultured renal tubular cells, cisplatin exposure led to transient repression of GSK3β activity followed by a prolonged upregulation of activity. Rescue treatment with lithium inhibited GSK3β activity, enhanced nuclear expression of cyclin D1, c-Myc, and HIF-1α, and boosted cellular proliferation. Similarly, ectopic expression of a kinase-dead mutant of GSK3β enhanced the expression of cyclin D1, c-Myc, and HIF-1α and amplified cellular proliferation after cisplatin injury, whereas forced expression of a constitutively active mutant of GSK3β abrogated the effects of lithium. Mechanistically, GSK3β colocalized and physically interacted with cyclin D1, c-Myc, and HIF-1α in tubular cells. In silico analysis revealed that cyclin D1, c-Myc, and HIF-1α harbor putative GSK3β consensus phosphorylation motifs, implying GSK3β-directed phosphorylation and subsequent degradation of these molecules. Notably, cotreatment with lithium enhanced the proapoptotic effects of cisplatin in cultured colon cancer cells. Collectively, our findings suggest that pharmacologic targeting of GSK3β by lithium may be a novel therapeutic strategy to improve renal salvage after AKI.
The current clinical management of AKI is largely limited to symptomatic treatments and general supportive measures, including fluid resuscitation and renal replacement therapy.1 Specific therapeutic interventions that either rescue kidney injury or improve survival are still unavailable in clinical practice. After acute injury, the kidney has a limited potential of self-repair as marked by a spontaneous recovery of kidney function and the repopulated renal tubular epithelia on renal histology in patients and experimental animal models. A burgeoning body of evidence suggests that direct engraftment of hematopoietic mesenchymal stem cells is less likely to be a major mechanism involved in tubular cell regeneration during kidney repair.2 Instead, self-duplication of nonlethally injured, surviving epithelial cells seems to be mainly responsible for nephron repair and renal recovery.3,4 Impaired kidney repair or incomplete renal recovery after AKI has been recognized as an independent risk factor for AKI to CKD transition, which has lately been considered to be an important and underestimated cause of CKD.5
The development, progression, and recovery of AKI is a complex and highly orchestrated pathophysiologic process that is regulated by a myriad of signaling pathways. Of many of these pathways, glycogen synthase kinase 3β (GSK3β) has emerged as the integration point and plays a crucial role in the pathogenesis of AKI. GSK3β is a well conserved, ubiquitously expressed serine/threonine protein kinase originally characterized as one that regulates glucose metabolism.6 Interest in GSK3β expanded greatly with the realization that it is a key regulator of multiple pivotal pathophysiologic processes extending well beyond glycogen metabolism to inflammation, immunomodulation, embryo development, tissue injury, repair, and regeneration.7 More recently, a growing body of evidence suggests that GSK3β plays a detrimental role in AKI.8,9 Nevertheless, the role of GSK3β in renal recovery and kidney repair after AKI remains elusive.
Lithium, a US Food and Drug Administration (FDA)–approved first-line drug commonly used for the past 50 years to treat bipolar affective disorders,10 is a selective inhibitor for GSK3β.11 Lithium has been well known to have a potent promotional effect on tissue repair and regeneration after injury in multiple organ systems, including the central nervous system and hematopoietic system,12–15 whereas the effect of lithium on kidney repair and renal recovery after AKI is unknown. This study examined the effect of delayed administration of a single low dose of lithium on a murine model of cisplatin or ischemia/reperfusion-induced AKI. The potential role and implication of GSK3β, the target of lithium, in kidney repair was delineated.
Results
Rescue Treatment with Lithium Accelerates Renal Recovery in a Murine Model of Cisplatin-Induced AKI
Cisplatin-based chemotherapy for cancer has been greatly limited by multiple adverse effects, including AKI.16 A single intraperitoneal injection of cisplatin (20 mg/kg) in mice elicited severe injury to the kidney. By day 3, cisplatin elicited a typical pattern of acute tubular necrosis, characterized by epithelial simplification, vacuolization of proximal tubular epithelium, luminal ectasia, epithelial necrosis, sloughing of tubular cell into lumen, and loss of brush border (Figure 1A), whereas histology of kidneys from mice treated alone with saline or lithium chloride (80 mg/kg) remained normal. Consistent with the morphologic changes, cisplatin injury resulted in a remarkable elevation in serum creatinine levels that peaked on postinjury day 3 (Figure 1B), congruent with the injury phase of AKI. After day 3, serum creatinine levels in cisplatin-injured mice started to regress gradually but were still significantly higher than those observed in the saline-treated group or the lithium-treated group until postinjury day 7, suggesting a spontaneous but incomplete renal recovery after AKI (Figure 1B). Rescue treatment with a low dose of lithium chloride (40 mg/kg) significantly reduced serum creatinine levels in cisplatin-injured animals by 30% and 47% on days 5 and 7, respectively; treatment with a higher dose of lithium (80 mg/kg) yielded a further reduction of serum creatinine levels by 46% and 66% on days 5 and 7, respectively, signifying an accelerated renal recovery from AKI. The lithium-promoted recovery in renal function was accompanied by enhanced kidney repair, as demonstrated by fewer renal tubular injury lesions and augmented repopulation of tubular epithelia on histology (Figure 1C). Semiquantitative morphometric analysis indicated a markedly accelerated regression of tubular injury scores on day 7 after lithium treatment (Figure 1D). Collectively, these data suggest that lithium possesses a potent proreparative effect that promotes renal function recovery and kidney repair after AKI.
Figure 1.
Delayed lithium therapy accelerates kidney repair and renal recovery in a murine model of cisplatin-induced AKI. On day 0, mice receive cisplatin (20 mg/kg) or an equal volume of saline or LiCl (80 mg/kg) treatment via a single intraperitoneal injection. On day 3, cisplatin-injured mice are treated with saline, low-dose (L) LiCl (40 mg/kg), or high-dose (H) LiCl (80 mg/kg). (A) Representative micrographs of hematoxylin and eosin–stained renal cortex of mice treated with saline (a), lithium (b), or cisplatin (c) on day 3. (B) Serum creatinine levels decrease at a more rapid rate in cisplatin + LiCl–treated mice compared with cisplatin + saline–treated mice (n=5 per group). (C) Representative micrographs of hematoxylin and eosin–stained renal cortex of cisplatin + saline–treated mice (d and g), cisplatin + LiCl (L)–treated mice (e and h), and cisplatin + LiCl (H)–treated mice (f and i) on days 5 and 7. (D) Tubular injury score for mice treated with saline, LiCl (H), cisplatin + saline, cisplatin + LiCl (L), or cisplatin + LiCl (H) on day 7 (n=5 per group). *P<0.05; **P<0.001 versus cisplatin + saline-treated mice. ns, not significant; Scr, serum creatinine. Original magnification, ×200; ×400 in insets.
Lithium Treatment after Cisplatin Injury Inhibits GSK3β Activity and Reinforces Tubular Proliferation and Repair in the Kidney
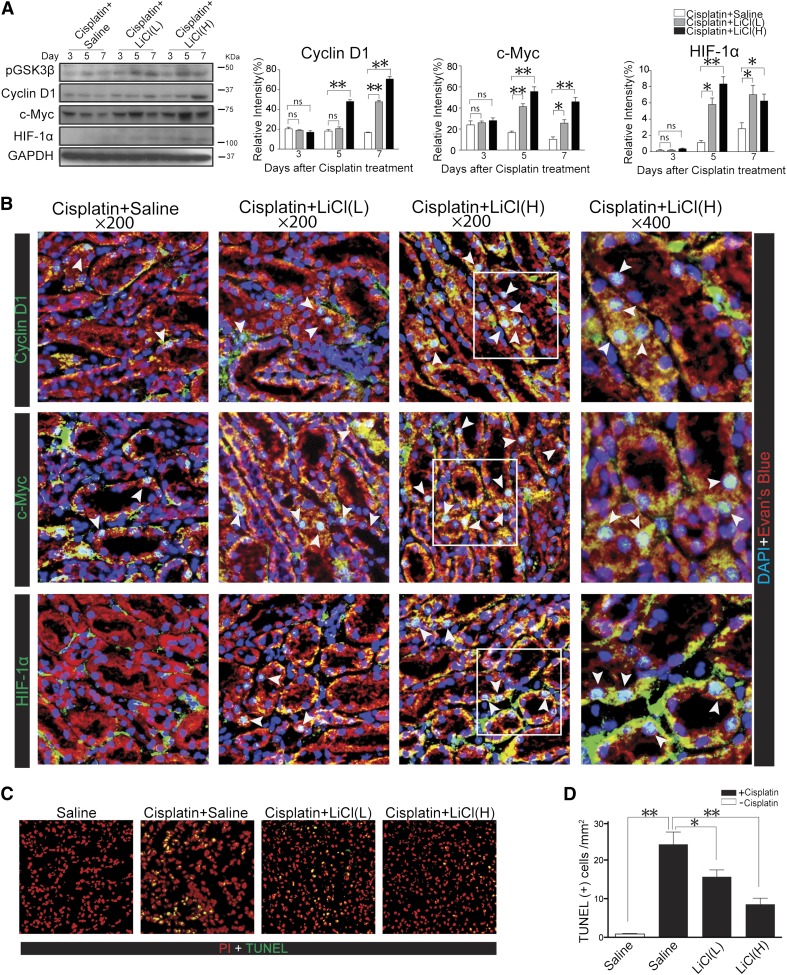
In most organ systems, a number of proproliferative molecules that are active in embryo development are re-expressed after acute injury, resulting in reinforced cell cycle progression. These proproliferative molecules include cell cycle regulators like cyclin D1 and transducers of mitogenic signals like c-Myc, which are proproliferative molecules that govern cellular mitosis and proliferation. Furthermore, renal recovery from AKI is inevitably confounded by ischemia or hypoxia that is secondary to endothelial injury and vascular constriction even if the original etiology is nonischemic.17–19 Recent evidence suggests that hypoxic preconditioning of cisplatin-injured proximal tubular cells increases cell proliferation and that this effect is largely mediated by the induced activation of hypoxia-inducible factor-1α (HIF-1α), which controls survival and proliferation of renal epithelial cells.20 To determine whether lithium inhibited GSK3β activity in vivo in the kidney and to decipher whether cell cycle progression and tubular proliferation was responsible for the lithium-accelerated renal recovery after AKI, kidney homogenates were subjected to immunoblot analysis followed by densitometric analysis (Figure 2A). After cisplatin injury, inhibitory phosphorylation of GSK3β was elevated on days 5 and 7 during the recovery phase, suggesting that GSK3β inactivation is involved in kidney repair. This was associated with mild induction of the expression of cyclin D1, c-Myc, and HIF-1α (Figure 2A). Delayed lithium treatment accentuated the inhibitory phosphorylation of GSK3β in a dose-dependent manner, concomitant with an amplified expression of cyclin D1, c-Myc, and HIF-1α (Figure 2A). To validate the immunoblot data, fluorescence immunohistochemistry staining was carried out on frozen kidney specimens that were obtained on postinjury day 7. As shown in Figure 2B, cisplatin injury elicited very weak and sporadic expression of cyclin D1, c-Myc, and HIF-1α mainly in the injured tubules. Low-dose lithium treatment amplified to a moderate extent, while higher-dose lithium treatment amplified to a greater extent, the expression of cyclin D1, c-Myc, and HIF-1α that was predominantly observed in the renal tubular epithelia. It was noted that the staining of cyclin D1, c-Myc, and HIF-1α in renal tubular cells mainly colocalized with 4′,6-diamidino-2-phenylindole (DAPI) staining and appeared in nuclei in addition to cytoplasm (Figure 2B). During the recovery phase of AKI, even though kidney repair is the dominant histologic event, kidney injury characterized by tubular cell death, especially apoptosis, also occurs continuously for several days after injury. Indeed, apoptotic cells were evidently detected in cisplatin-injured kidneys even on postinjury day 5 and were substantially diminished by lithium in a dose-dependent fashion (Figure 2, C and D), as revealed by the terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) assay, suggesting that delayed lithium therapy improves the late phase kidney injury.
Figure 2.
Lithium rescue treatment promotes renal tubular expression of proproliferative molecules in the recovery phase of cisplatin-induced renal injury. (A) Western immunoblot analysis and densitometric analysis demonstrate higher expression of cyclin D1, c-Myc, and HIF-1α in renal cortical homogenates from cisplatin + LiCl–treated mice (n=5 per group). (B) Immunofluorescence staining shows enhanced expression of cyclin D1, c-Myc, and HIF-1α by proximal tubules in kidney specimens from lithium-treated mice on day 7 after cisplatin injury. Note that the staining of cyclin D1, c-Myc, and HIF-1α in renal tubular cells mainly colocalizes with DAPI staining and appears in nuclei in addition to cytoplasm. (C) GSK3β inhibition protects mice from renal cell apoptosis in cisplatin-injured kidneys. Representative micrographs of TUNEL staining of kidney specimens on day 5. TUNEL-labeled nuclei are revealed as bright green spots in the renal cortex in kidney specimens counterstained with PI. Lithium significantly attenuates apoptosis in cisplatin + LiCl–treated mouse kidneys. (D) Absolute counting of the numbers of TUNEL-positive cells as the means of 20 random high-power fields. *P<0.05; **P<0.001 versus cisplatin + saline–treated mice (n=5 per group). ns, not significant. Original magnification, ×200 in A; ×400 in B.
Lithium Rescue Therapy Accelerates Renal Recovery in a Murine Model of Renal Ischemia Reperfusion Injury
To determine whether this proreparative activity of lithium is also effective in other forms of AKI, another murine model of bilateral kidney ischemia reperfusion injury (IRI) was utilized. Eight hours after IRI, mice were given 40 mg/kg or 80 mg/kg of lithium chloride or saline via intraperitoneal injection. Renal histology was examined 24 and 48 hours after ischemia and exhibited a typical pattern of IRI-induced AKI shown by tubular dilation, swelling, necrosis, sloughing of tubular cells, loss of brush border, luminal congestion, and interstitial hemorrhage. These changes were prominently attenuated in animals treated with low- or high-dose lithium (Figure 3A). Serum creatinine levels were consistently precipitously elevated 24 and 48 hours after renal IRI but were significantly corrected by delayed lithium treatment in a dose-dependent fashion, denoting a promoted renal recovery (Figure 3A). In parallel with renal function and histologic findings, mild expression of cyclin D1, c-Myc, and HIF-1α was induced in the kidney 24 and 48 hours after IRI; this was substantially enhanced after rescue treatment with lithium as revealed by immunoblot analysis of kidney homogenates (Figure 3C).
Figure 3.
Delayed lithium therapy accelerates kidney repair and renal recovery in mice with renal IRI. Mice are subjected to 22 minutes of bilateral renal ischemia and then allowed to recover for 24 or 48 hours. Lithium or saline is given 8 hours after ischemia. (A) Representative micrographs of hematoxylin and eosin–stained renal cortex. (B) Serum creatinine levels are drastically elevated 24 and 48 hours after renal IRI but are significantly corrected by delayed lithium treatment in a dose-dependent fashion (n=5 per group). (C) Western immunoblot analysis of kidney homogenates demonstrates enhanced expression of cyclin D1, c-Myc, and HIF-1α in kidneys with IRI after lithium treatment (n=5 per group). *P<0.05; **P<0.001 versus IRI + saline–treated group. ns, not significant; Scr, serum creatinine. Original magnification, ×200.
Delayed Lithium Treatment Inhibits GSK3β and Promotes Proliferation in Cultured Renal Tubular Cells Injured with Cisplatin
To determine whether lithium promotes renal recovery via a potential proreparative effect directly on renal tubular cells, cultured murine tubular epithelial (TKPT) cells were exposed to cisplatin and then treated with lithium. As shown in Figure 4A, despite a transient induction of inhibitory phosphorylation of GSK3β, cisplatin injury alone resulted in a prolonged repression of the inhibitory phosphorylation of GSK3β for at least 24 hours, denoting upregulated kinase activity of GSK3β. Lithium treatment initiated 12 hours after cisplatin injury markedly enhanced cell proliferation in a dose-dependent fashion at both 24 hours (Figure 4B) and 48 hours (Figure 4G) as assessed by the diphenyltetrazolium bromide (MTT) cell proliferation assay. This was concomitant with dose-dependent suppression of GSK3β activity and induction of the expression of cyclin D1, c-Myc, and HIF-1α (Figure 4, C and H). Densitometric analysis confirmed that the lithium-induced inhibitory phosphorylation of GSK3β positively correlated with the expression of cyclin D1, c-Myc, and HIF-1α, respectively (Figure 4, D–F and I–K), implying that GSK3β inhibition is associated with enhanced expression of proproliferative molecules that drives cell cycle progression and cellular proliferation.
Figure 4.
Inhibition of GSK3β by lithium after cisplatin injury promotes proliferation of cultured renal tubular cells, associated with amplified expression of cyclin D1, c-myc, and HIF-1α. (A) Western immunoblot analysis for phosphorylated GSK3β in renal tubular (TKPT) cells at different time after cisplatin (100 μM) injured; immunoblots from three separate experiments are analyzed by densitometry. (B) MTT assay of TKPT cells injured with cisplatin for 12 hours and then treated with NaCl (10 mM) or different doses of LiCl (1, 5, and 10 mM) for 24 hours (n=6). (C) Western immunoblot analysis for cyclin D1, c-Myc, and HIF-1α in TKPT cells that are subjected to different treatments as described in B. Immunoblots from three separate experiments are subjected to densitometric analysis. (D–F) Densitometric analysis of immunoblot results demonstrates that lithium-induced inhibitory pGSK3β markedly correlates with increased expression of cyclin D1, c-Myc, and HIF-1α at 24 hours (P<0.05). (G) MTT assay of TKPT cells injured with cisplatin for 12 hours and then treated with NaCl (10 mM) or different doses of LiCl (1, 5, and 10 mM) for 48 hours (n=6). (H) Western immunoblot analysis for cyclin D1, c-Myc, and HIF-1α in TKPT cells that are subjected to different treatments as described in G. Immunoblots from three separate experiments were subjected to densitometric analysis. (I–K) Densitometric analysis of immunoblot results demonstrates that lithium-induced inhibitory pGSK3β markedly correlates with increased expression of cyclin D1, c-Myc, and HIF-1α at 48 hours (P<0.05). *P<0.05 versus cisplatin + NaCl–treated group. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ns, not significant.
Lithium Induces Nuclear Accumulation of Proproliferative Molecules in Cisplatin-Injured Renal Tubular Cells
Fluorescence immunocytochemistry was performed to define the subcellular localization of lithium-induced expression of proproliferative molecules, including cyclin D1, c-Myc, and HIF-1α, (Figure 5A). Cells were stimulated with or without cisplatin for 12 hours and then treated with lithium at different concentrations for 24 hours. In exponentially growing renal tubular epithelial cells under basal conditions, cyclin D1 was evidently expressed and mainly localized in the nuclei, whereas c-Myc and HIF-1α were faintly and sporadically expressed in the nuclei of few cells. Cisplatin injury drastically diminished the nuclear expression of cyclin D1 but barely affected the basal weak expression of c-Myc and HIF-1α. Delayed lithium treatment reinstated the nuclear expression of cyclin D1 and considerably induced nuclear accumulation of c-Myc and HIF-1α in a dose-dependent manner. Absolute counting of the nuclei positive for these proproliferative molecules corroborated the morphologic observation (Figure 5, B–D).
Figure 5.
Lithium induces nuclear accumulation of proproliferative molecules in cisplatin-injured renal tubular cells. TKPT cells are treated with cisplatin (100 μM) for 12 hours and then treated with NaCl (10 mM) or different doses of LiCl (1, 5, and 10 mM) for 24 hours. (A) Immunofluorescence staining for cyclin D1, c-Myc, and HIF-1α shows more nuclei positive cells in LiCl-treated TKPT cells after cisplatin injury. (B–D) Absolute counting of cells that are nuclear positive for cyclin D1, c-Myc, or HIF-1α as the means of 20 random high-power fields. *P<0.05; **P<0.001 versus cisplatin + NaCl–treated cells. Original magnification, ×200.
GSK3β Inhibition Is Necessary and Sufficient for Lithium-Promoted Cellular Proliferation and Expression of Proproliferative Molecules
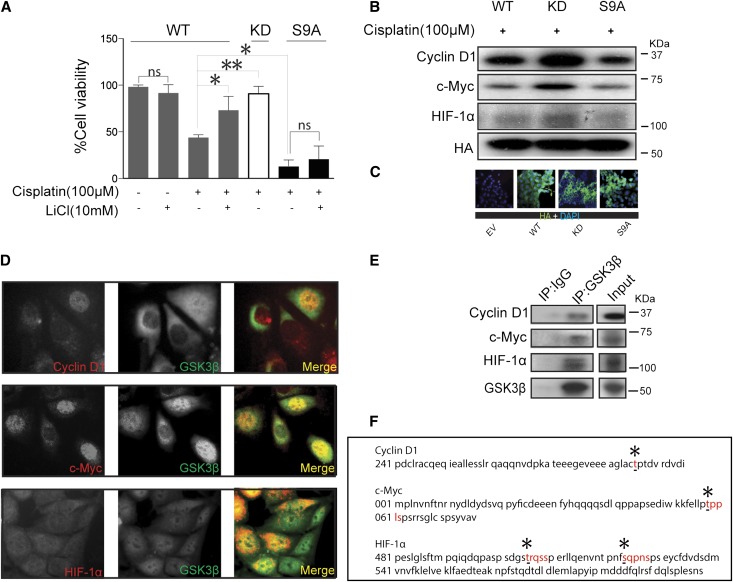
To examine the possible causal relationship between lithium-induced inhibition of GSK3β and lithium-promoted cell proliferation after cisplatin injury, the activity of GSK3β was selectively manipulated by forced expression of vectors encoding the hemagglutinin (HA)–conjugated wild-type (WT), kinase-dead mutant (KD), or constitutively active mutant (S9A) of GSK3β. Cells were transfected with these vectors after cisplatin injury for 12 hours. Immunofluorescence staining for HA revealed a satisfactory transfection efficiency (>75%) (Figure 6C). Ectopic expression of KD after cisplatin injury substantially boosted cell proliferation as measured by the MTT assay, reminiscent of the effect of lithium in WT expressing cells, inferring that GSK3β inhibition is sufficient for lithium-promoted cellular proliferation. In contrast, forced expression of S9A prominently repressed cell proliferation after cisplatin injury and abrogated the promotional effect of lithium, suggesting that GSK3β inhibition is required for lithium-enhanced tubular cell proliferation (Figure 6A). Consistent with the findings in MTT cell proliferation assays, the expressions of the proproliferative molecules, including cyclin D1, c-Myc and HIF-1α, were all markedly upregulated in cells expressing KD, but were evidently abolished in S9A expressing cells as determined by immunoblot analysis (Figure 6B), suggesting that GSK3β inhibition is necessary and sufficient for lithium-enhanced expression of the proproliferative molecules.
Figure 6.
GSK3β inhibition is necessary and sufficient for lithium-promoted cellular proliferation. TKPT cells are injured with cisplatin (100 μM) for 6 hours, and cells are then subjected to liposome-mediated transient transfection with vectors encoding the HA-conjugated WT, KD mutant, or constitutively active (S9A) mutant of GSK3β. Some cells are treated with LiCl (10 mM) 6 hours after the transfection. (A) An MTT assay is carried out 24 hours after cisplatin injury. Forced expression of KD enhances cellular proliferation, whereas S9A shows an opposing effect (n=6). (B) After cisplatin injury and transient transfection as described in A, cells are subjected to different treatments as indicated. Whole cell lysates are harvested and analyzed for cyclin D1, c-Myc, and HIF-1α by Western immunoblot analysis, with three replicates. (C) Fluorescence immunocytochemistry staining of HA demonstrates that the transfection efficiency is >75%. (D) Representative immunofluorescence staining micrographs show colocalization of GSK3β with cyclin D1, c-Myc, or HIF-1α in TKPT cells. (E) Whole cell lysates are immunoprecipitated with anti-GSK3β antibody and immunoprecipitates are probed for cyclin D1, c-Myc, and HIF-1α by immunoblot analysis (n=3). (F) In silico analysis of the amino acid sequences of cyclin D1, c-Myc, and HIF-1α shows consensus motifs for phosphorylation by GSK3β. *P<0.05; **P<0.001 versus cisplatin-treated WT cells. EV, empty vector; ns, not significant. Original magnification, ×400.
Proproliferative Molecules Colocalize and Physically Interact with GSK3β as its Putative Substrates in Renal Tubular Cells
To understand the molecular essence of GSK3β-regulated expression of the proproliferative molecules including cyclin D1, c-Myc, and HIF-1α, we examined the physical relationship between GSK3β and these molecules. Fluorescence immunocytochemistry staining indicated that GSK3β and HIF-1α were expressed in both cytoplasm and nucleus in the cultured tubular epithelial cells, whereas cyclin D1 and c-Myc mainly exhibited a nuclear distribution. Of note, a significant amount of GSK3β staining perfectly colocalized with each of the three proproliferative molecules and this colocalization was predominantly situated in nuclei (Figure 6D). To validate the cytologic findings, immunoprecipitation was performed and revealed that GSK3β evidently coprecipitated with each of the three molecules, indicating that GSK3β physically interacts with cyclin D1, c-Myc, and HIF-1α, respectively (Figure 6E). Evidence suggests that upon phosphorylation, cyclin D1, c-Myc, and HIF-1α will be subjected to nuclear exit and subsequent proteasomal degradation.21–23 To further explore the mechanism by which GSK3β regulates the expression of cyclin D1, c-Myc, and HIF-1α, the amino acid sequences of the three molecules were subjected to computational active site analysis for putative GSK3β consensus motifs (Figure 6F). In silico analysis revealed that residues T286 of cyclin D1, residue T58 of c-Myc, and residues T505, S524, and S598 of HIF-1α reside in consensus motifs for phosphorylation by GSK3β, suggesting that cyclin D1, c-Myc, and HIF-1α are putative cognate substrates for GSK3β.
Lithium Suppresses Growth and Promotes Apoptosis of Colon Cancer Cells
To assess whether lithium treatment affects cancer cell growth or the tumor killing efficacy of cisplatin, cultured SW480 human colon cancer cells were injured with cisplatin in the presence or absence of lithium. Under basal conditions, the addition of lithium to the culture significantly suppressed colon cancer cell growth by >20% after 24 or 48 hours, as assessed by the MTT cell growth assay (Figure 7A). Colon cancer cell growth was much more prominently suppressed by cisplatin. Concomitant treatment with lithium did not blunt the effect of cisplatin, but instead displayed a trend of potentiated anticancer activity, resulting in a further slight suppression of cell growth (Figure 7A). Mechanistically, lithium elicited the activation of proapoptotic caspase-3 in colon cancer cells under basal conditions and slightly promoted its activation in cisplatin-injured cells, as determined by immunoblot analysis (Figure 7B) as well as TUNEL assays (Figure 7, C and D).
Figure 7.
Lithium suppresses cell growth and sensitizes apoptosis in colon cancer cells. SW480 colon cancer cells are treated with cisplatin (20 μM) in the presence or absence of lithium chloride (10 mM) or sodium chloride (10 mM) for 24 or 48 hours. (A) An MTT cell growth assay demonstrates that lithium suppresses growth of SW480 colon cancer cells and slightly potentiates the antitumor activity of cisplatin (n=6). (B) Western immunoblot analysis of cell lysates for cleaved (activated) caspase-3 in SW480 cells that are subjected to different treatments for 24 hours. (C) Representative micrographs of TUNEL staining of SW480 cells after different treatments for 24 hours. (D) Quantitative analysis of the percentages of TUNEL-positive cells after different treatments. *P<0.05 versus cisplatin + NaCl–treated group; **P<0.001 versus vehicle + NaCl–treated group.
Discussion
Emerging evidence suggests that the number of patients with incomplete renal recovery from AKI is underestimated and these individuals are at increased risk for CKD and ESRD.1,24 Renal repair after AKI is influenced by multiple pathobiologic factors, including aging, preexisting kidney diseases, and the nature and the degree of the original injury per se.25,26 It is imperative to develop novel therapeutic modalities to promote kidney repair and maximize the potential of complete renal recovery. This study demonstrated for the first time that rescue therapy with lithium promotes kidney repair and accelerates renal recovery after cisplatin- or IRI-induced AKI in mice.
GSK3β is a key transducer involved in a large number of cellular signaling pathways7 and has been implicated in the regulation of cell proliferation, at least partly through its control of the stability of cell cycle proteins such as cyclin D1, c-Myc, and HIF-1α.27–30 GSK3β is able to render phosphorylation of cyclin D1 and c-Myc and initiate their ubiquitination and proteasomal degradation. In NIH-3T3 cells, phosphorylation of cyclin D1 by GSK3β led to cyclin D1 exclusion from the nucleus to initiate its proteasomal degradation.27 Similarly, mitogen signaling inhibits the GSK3β-mediated degradation of c-Myc, resulting in the activation of its target genes, including cyclin D1 and other cell cycle mediators.28,30 Moreover, Flügel et al.29 demonstrated that both insulin and lithium stabilized HIF-1α in hepatic cells through inhibitory phosphorylation of GSK3β. In our study, inhibition of GSK3β by lithium or forced expression of KD consistently elevated the expression of these proproliferative molecules in renal tubular cells.
GSK3β is also a core element of the canonical Wnt/β-catenin pathway. Recent evidence indicates that activation of β-catenin signaling might be responsible for impaired wound healing and fibrotic kidney repair after AKI.31 For example, in a scratch wound healing study in cultured renal tubular epithelial cells, inhibitors of GSK3β including lithium at high concentrations mildly induced β-catenin accumulation and thereby retarded wound healing.32 A drastic inhibition of GSK3, however, seems to be essential to activate the β-catenin pathway. In support of this, Doble et al. revealed a functional redundancy of GSK3α and GSK3β in Wnt/β-catenin signaling by using compound knockouts of GSK3α and β.33 They found that gene deletion of at least three of the four alleles of both isoforms of GSK3α and β is required to show an appreciable change in β-catenin levels,33 suggesting that there may be a therapeutic window and dose for chemical inhibitors of GSK3β in treating diseases without elevating β-catenin levels33 and thus a risk of β-catenin–mediated adverse effects like renal fibrosis. Regular or low doses of lithium seem to satisfy this concern. Indeed, kidney diseases, like glomerular or interstitial nephropathy, are uncommon for psychiatric patients who received lithium therapy for a long period of time (usually >10 years).34 Of note, both doses of lithium used in our study (40 and 80 mg/kg) are much lower than the standard dose of lithium (120 mg/kg) that has been safely and routinely used for neurobiology research in rodents.35 Consistent with previous work,36 renal expression of β-catenin in our animals was barely affected (data not shown). Nevertheless, more clinical studies are merited to determine the appropriate dose of lithium for humans to inhibit GSK3β without elevating β-catenin levels in the kidney.
Lithium, a first-generation GSK3β inhibitor, has historically been used safely for decades as a mood stabilizer.10 Lithium is well known for its proreparative activities in multiple systems, as well as its beneficial effect on organic neurologic degenerative diseases, including Alzheimer's disease.37 In human patients with psychiatric disorders, lithium treatment was found to induce an increase in brain gray matter.38 In addition, lithium is one of the treatment choices for neutropenia39 and may also assist hematopoietic stem cell mobilization in bone marrow transplant donors.40 Our study consistently demonstrated that rescue therapy with lithium promotes kidney repair after AKI. Thus, lithium possesses a proreparative activity that is exhibited in many organ systems after acute or chronic injury.
Due to the powerful proreparative effect of GSK3β inhibition exhibited in the kidney and other organs, one of the major concerns of clinical application of lithium for cisplatin-induced AKI is its possible effect on cancer cell growth and the tumor killing efficacy of cisplatin, which thus requires heightened awareness of safety considerations. To date, GSK3β has been found to play distinct roles in tumor development and growth in different types of tumors, likely depending on the status of GSK3β overexpression.41 For instance, GSK3β seems to serve as a “tumor suppressor” in skin42 and mammary tumorigenesis.43 By contrast, GSK3β promotes tumorigenesis and development of ovarian, colon, liver, and pancreatic carcinomas.44–46 In support of this, inhibition of GSK3β activity by pharmacologic inhibitors suppressed proliferation of ovarian cancer cells in vitro and prevented the formation of ovarian xenograft tumors in nude mice.44 Similarly, inhibitors of GSK3β significantly inhibited cell growth and proliferation of subcutaneous xenografts of human colon cancer cells in athymic mice.45 Our study consistently demonstrated that lithium suppressed cell growth, induced apoptosis, and slightly potentiated the proapoptotic effect of cisplatin in colon cancer cells, inferring that lithium may actually favor the efficacy of cisplatin-based chemotherapy for certain types of cancers such as colon cancer. Moreover, in patients subjected to systemic chemotherapy for malignant tumors, lithium carbonate has been safely used to accelerate hematopoietic recovery and shorten the period of leukopenia,12,47,48 again suggesting the safety of lithium therapy in chemotherapy patients.
In summary, our study demonstrated that rescue therapy with low-dose lithium promoted renal tubular epithelia repopulation, improved kidney repair, and accelerated renal function recovery after cisplatin or IRI-induced AKI. This proreparative effect of lithium is likely to be attributable to inhibition of GSK3β and subsequent stabilization of proproliferative molecules, including cyclin D1, c-Myc, and HIF-1α. Our findings suggest that therapeutic targeting of GSK3β by using FDA-approved drugs with GSK3β inhibitory activities such as lithium might represent a novel therapeutic strategy for rescue treatment of AKI to promote renal recovery after AKI.
Concise Methods
Animal Experimental Design
Animal studies were approved by the institution’s animal care and use committee and they conformed to US Department of Agriculture regulations and the National Institutes of Health guidelines for humane care and use of laboratory animals.
Murine Models of Cisplatin-Induced AKI
Five FVB/NJ mice aged 8 weeks were randomly assigned to each of the following groups for each observed time point: (1) group control, mice received saline alone; (2) group LiCl, mice were only treated with LiCl (80 mg/kg, single intraperitoneal injection); (3) group cisplatin + saline, mice were subjected to cisplatin (20 mg/kg, single intraperitoneal injection) injury and saline was given on day 3 after cisplatin injury; (4) group cisplatin + LiCl (L), mice were subjected to cisplatin (20 mg/kg, single intraperitoneal injection) injury and low-dose LiCl (40 mg/kg, single intraperitoneal injection) was given on day 3 after cisplatin injury; and (5) group cisplatin + LiCl (H), mice were subjected to cisplatin (20 mg/kg, single intraperitoneal injection) injury and a higher dose of lithium (80 mg/kg, single intraperitoneal injection) was given on day 3 after cisplatin injury. Animals were euthanized on the indicated days and blood and organs were collected for further investigation.
Murine Models of Renal IRI
Male FVB/NJ mice aged 8 weeks were subjected to bilateral renal IRI to induce AKI.49 Briefly, mice were anesthetized with pentobarbital (60 mg/kg, intraperitoneal injection) and placed on a sterile disposable towel over a warming pad. A midline incision was made, and the renal artery and vein were isolated from surrounding tissue and then occluded with a nontraumatic vascular clamp (85 g pressure; Roboz Surgical Instrument Company, Gaithersburg, MD) for 22 minutes. In the sham group, renal pedicles were isolated but no clamps were applied. At the end of the ischemic period, the vascular clamps were removed and the kidneys were observed for 5 minutes to document reflow. Eight hours after ischemia or sham injury, saline or lithium chloride (40 or 80 mg/kg) was administered to mice (single intraperitoneal injection). Five mice were randomized to each of the following groups for each observed time point: (1) sham + saline, mice with sham injury were treated with saline; (2) sham + LiCl, mice with sham injury treated with lithium (80 mg/kg); (3) IRI + saline, mice with IRI treated with saline; (4) IRI + LiCl (L), mice with IRI treated with lithium (40 mg/kg); and (5) IRI + LiCl (H), mice with IRI treated with a higher dose of lithium (80 mg/kg). Blood and organs were collected at 24 and 48 hours after IRI for further investigation.
Renal Pathology
Formalin-fixed kidneys were embedded in paraffin and prepared in 3-mm–thick sections. Sections were stained with hematoxylin and eosin to estimate gross histologic kidney injury. One observer performed semiquantitative morphometric analysis in a blinded manner. Severity of tubulointerstitial injury and tubular dilation/sloughing was semiquantitatively scored on a scale from 0 to 5 and reported as the mean of 20 random high-power (×200) fields per hematoxylin and eosin–stained section as follows: 0, none; 1, <10%; 2, 11%–25%; 3, 26%–45%; 4, 46%–75%; and 5, >76%. Indirect immunofluorescence staining of GSK3β, p-GSK3β, HIF-1α, c-Myc, and cyclin D1 was carried out on methanol/acetone-fixed (1:1) frozen cryostat sections using specific antibodies from Santa Cruz Biotechnology (Santa Cruz, CA), followed by applying Alexa Fluor–conjugated secondary antibodies (Invitrogen, Carlsbad, CA). As a negative control, the primary antibodies were replaced by preimmune IgG from the same species; no staining occurred.
Creatinine Measurements
Serum creatinine levels were measured using a creatinine assay kit (Biovision, Mountain View, CA) according to the manufacturer’s instructions.9,50
Cell Culture and Plasmid Transfection
TKPT cells were grown in DMEM/F12 that contained 5% FBS. The eukaryotic expression vectors encoding EV, WT, S9A, and KD were transfected to TKPT cells was as previously described51 by using Lipofectamine 2000 according to the manufacturer’s instructions (Invitrogen). After transfection with equal amounts of vectors, cells were then subjected to different treatments and assessed by MTT viability assay as previously described.52 SW480 colon cancer cells were cultured in DMEM/F12 that contained 10% FCS. Cells were seeded into a six-well plate at a density of 1×105 cells per well, followed by different treatments. After 24 and 48 hours of treatment, cell growth was assessed by the MTT cell growth assay.52
Western Immunoblot Analyses
Cultured cells were lysed, mice kidneys were homogenized in radioimmunoprecipitation assay buffer supplemented with protease inhibitors, and samples were processed for immunoblot as previously described.51 The antibodies against GSK3β, p-GSK3β, HA, HIF-1α, c-Myc, and cyclin D1 were purchased from Santa Cruz Biotechnology. For detection of the physical interactions between GSK3β and HIF-1α, c-Myc, or cyclin D1, GSK3β antibody (Santa Cruz Biotechnology) was used as the immunoprecipitation antibody and the antibodies against HIF-1α, c-Myc, or cyclin D1 (Santa Cruz Biotechnology) were used to probe the immunoprecipitation products by immunoblot analysis.
Fluorescence Immunocytochemistry
Cultured cells were fixed with 4% paraformaldehyde and then stained with primary antibodies against cyclin D1, c-Myc, HIF-1α, GSK3β, HA, or preimmune IgG and then with the Alexa Fluorophore 488–conjugated secondary antibody (Invitrogen). Finally, cells were counterstained with DAPI or propidium iodide, mounted with Vectashield mounting medium (Vector Laboratories, Burlingame, CA), and visualized using a fluorescence microscope.51
Measurement of Cell Apoptosis
Fixed kidney sections or cell cultures were subjected to TUNEL staining by using a cell apoptosis detection kit (Roche Applied Science, Indianapolis, IN) according to the manufacturer’s instructions, followed by counterstaining with DAPI or propidium iodide.9
Statistical Analyses
For immunoblot analysis, bands were scanned and the integrated pixel density was determined using a densitometer and the National Institutes of Health image analysis program. All data are expressed as the mean ± SD. Statistical analysis of the data from multiple groups was performed by ANOVA followed by the Newman–Keuls test. Data from two groups were compared by the t test. P<0.05 was considered significant.
Disclosures
None.
Acknowledgments
This work was made possible in part by funding from the International Society of Nephrology (ISN) Sister Renal Center Trio Program, the Chinese 973 Fund (2012CB517600), the Natural Science Foundation of China (81270136/H0111 to A.P.), and the US National Institutes of Health (R01DK092485 to R.G.). Z.W. and H.B. are ISN Fellows and recipients of the ISN fellowship.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Lithium in Kidney Diseases: Big Roles for the Smallest Metal,” on pages 421–423.
References
- 1.Murugan R, Kellum JA: Acute kidney injury: What’s the prognosis? Nat Rev Nephrol 7: 209–217, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Humphreys BD, Czerniak S, DiRocco DP, Hasnain W, Cheema R, Bonventre JV: Repair of injured proximal tubule does not involve specialized progenitors. Proc Natl Acad Sci U S A 108: 9226–9231, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV: Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2: 284–291, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Witzgall R, Brown D, Schwarz C, Bonventre JV: Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J Clin Invest 93: 2175–2188, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang L, Humphreys BD, Bonventre JV: Pathophysiology of acute kidney injury to chronic kidney disease: Maladaptive repair. Contrib Nephrol 174: 149–155, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Rayasam GV, Tulasi VK, Sodhi R, Davis JA, Ray A: Glycogen synthase kinase 3: More than a namesake. Br J Pharmacol 156: 885–898, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen P, Frame S: The renaissance of GSK3. Nat Rev Mol Cell Biol 2: 769–776, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Nelson PJ, Cantley L: GSK3beta plays dirty in acute kidney injury. J Am Soc Nephrol 21: 199–200, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Bao H, Ge Y, Zhuang SG, Dworkin LD, Liu ZH, Gong R: Inhibition of glycogen synthase kinase-3β prevents NSAID-induced acute kidney injury. Kidney Int 81: 662–673, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baldessarini RJ, Tondo L, Hennen J: Lithium treatment and suicide risk in major affective disorders: Update and new findings. J Clin Psychiatry 64[Suppl 5]: 44–52, 2003 [PubMed] [Google Scholar]
- 11.Meijer L, Flajolet M, Greengard P: Pharmacological inhibitors of glycogen synthase kinase 3. Trends Pharmacol Sci 25: 471–480, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Vieweg WV, Yank GR, Rowe WT, Hovermale LS, Clayton AH: Increase in white blood cell count and serum sodium level following the addition of lithium to carbamazepine treatment among three chronically psychotic male patients with disturbed affective states. Psychiatr Q 58: 213–217, 1986-1987 [DOI] [PubMed] [Google Scholar]
- 13.Moore GJ, Bebchuk JM, Wilds IB, Chen G, Manji HK: Lithium-induced increase in human brain grey matter. Lancet 356: 1241–1242, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Yan XB, Wang SS, Hou HL, Ji R, Zhou JN: Lithium improves the behavioral disorder in rats subjected to transient global cerebral ischemia. Behav Brain Res 177: 282–289, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Xu J, Culman J, Blume A, Brecht S, Gohlke P: Chronic treatment with a low dose of lithium protects the brain against ischemic injury by reducing apoptotic death. Stroke 34: 1287–1292, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Arany I, Safirstein RL: Cisplatin nephrotoxicity. Semin Nephrol 23: 460–464, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Yasuda H, Yuen PST, Hu X, Zhou H, Star RA: Simvastatin improves sepsis-induced mortality and acute kidney injury via renal vascular effects. Kidney Int 69: 1535–1542, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sutton TA, Fisher CJ, Molitoris BA: Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int 62: 1539–1549, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Mottet D, Dumont V, Deccache Y, Demazy C, Ninane N, Raes M, Michiels C: Regulation of hypoxia-inducible factor-1alpha protein level during hypoxic conditions by the phosphatidylinositol 3-kinase/Akt/glycogen synthase kinase 3beta pathway in HepG2 cells. J Biol Chem 278: 31277–31285, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Weidemann A, Bernhardt WM, Klanke B, Daniel C, Buchholz B, Câmpean V, Amann K, Warnecke C, Wiesener MS, Eckardt KU, Willam C: HIF activation protects from acute kidney injury. J Am Soc Nephrol 19: 486–494, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alao JP: The regulation of cyclin D1 degradation: Roles in cancer development and the potential for therapeutic invention. Mol Cancer 6: 24, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gregory MA, Hann SR: c-Myc proteolysis by the ubiquitin-proteasome pathway: Stabilization of c-Myc in Burkitt’s lymphoma cells. Mol Cell Biol 20: 2423–2435, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yee Koh M, Spivak-Kroizman TR, Powis G: HIF-1 regulation: Not so easy come, easy go. Trends Biochem Sci 33: 526–534, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Wald R, Quinn RR, Luo J, Li P, Scales DC, Mamdani MM, Ray JG, University of Toronto Acute Kidney Injury Research Group : Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA 302: 1179–1185, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Venkatachalam MA, Griffin KA, Lan R, Geng H, Saikumar P, Bidani AK: Acute kidney injury: A springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol 298: F1078–F1094, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmitt R, Coca S, Kanbay M, Tinetti ME, Cantley LG, Parikh CR: Recovery of kidney function after acute kidney injury in the elderly: A systematic review and meta-analysis. Am J Kidney Dis 52: 262–271, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Diehl JA, Cheng MG, Roussel MF, Sherr CJ: Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev 12: 3499–3511, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gregory MA, Qi Y, Hann SR: Phosphorylation by glycogen synthase kinase-3 controls c-myc proteolysis and subnuclear localization. J Biol Chem 278: 51606–51612, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Flügel D, Görlach A, Michiels C, Kietzmann T: Glycogen synthase kinase 3 phosphorylates hypoxia-inducible factor 1alpha and mediates its destabilization in a VHL-independent manner. Mol Cell Biol 27: 3253–3265, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welcker M, Orian A, Jin J, Grim JE, Harper JW, Eisenman RN, Clurman BE: The Fbw7 tumor suppressor regulates glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein degradation. Proc Natl Acad Sci U S A 101: 9085–9090, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng J, Dong Z: Role changes of β-catenin in kidney injury and repair. Kidney Int 82: 509–511, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Peng J, Ramesh G, Sun L, Dong Z: Impaired wound healing in hypoxic renal tubular cells: Roles of hypoxia-inducible factor-1 and glycogen synthase kinase 3β/β-catenin signaling. J Pharmacol Exp Ther 340: 176–184, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doble BW, Patel S, Wood GA, Kockeritz LK, Woodgett JR: Functional redundancy of GSK-3alpha and GSK-3beta in Wnt/beta-catenin signaling shown by using an allelic series of embryonic stem cell lines. Dev Cell 12: 957–971, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bendz H, Schön S, Attman PO, Aurell M: Renal failure occurs in chronic lithium treatment but is uncommon. Kidney Int 77: 219–224, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Min WW, Yuskaitis CJ, Yan Q, Sikorski C, Chen S, Jope RS, Bauchwitz RP: Elevated glycogen synthase kinase-3 activity in Fragile X mice: Key metabolic regulator with evidence for treatment potential. Neuropharmacology 56: 463–472, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Z, Havasi A, Gall J, Bonegio R, Li Z, Mao H, Schwartz JH, Borkan SC: GSK3beta promotes apoptosis after renal ischemic injury. J Am Soc Nephrol 21: 284–294, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phiel CJ, Wilson CA, Lee VM, Klein PS: GSK-3alpha regulates production of Alzheimer’s disease amyloid-beta peptides. Nature 423: 435–439, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Lyoo IK, Dager SR, Kim JE, Yoon SJ, Friedman SD, Dunner DL, Renshaw PF: Lithium-induced gray matter volume increase as a neural correlate of treatment response in bipolar disorder: A longitudinal brain imaging study. Neuropsychopharmacology 35: 1743–1750, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petrini M, Azzarà A: Lithium in the treatment of neutropenia. Curr Opin Hematol 19: 52–57, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Gallicchio VS, Chen MG: Influence of lithium on proliferation of hematopoietic stem cells. Exp Hematol 9: 804–810, 1981 [PubMed] [Google Scholar]
- 41.Luo J: Glycogen synthase kinase 3beta (GSK3beta) in tumorigenesis and cancer chemotherapy. Cancer Lett 273: 194–200, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leis H, Segrelles C, Ruiz S, Santos M, Paramio JM: Expression, localization, and activity of glycogen synthase kinase 3beta during mouse skin tumorigenesis. Mol Carcinog 35: 180–185, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Farago M, Dominguez I, Landesman-Bollag E, Xu X, Rosner A, Cardiff RD, Seldin DC: Kinase-inactive glycogen synthase kinase 3beta promotes Wnt signaling and mammary tumorigenesis. Cancer Res 65: 5792–5801, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Cao Q, Lu X, Feng YJ: Glycogen synthase kinase-3beta positively regulates the proliferation of human ovarian cancer cells. Cell Res 16: 671–677, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Shakoori A, Mai W, Miyashita K, Yasumoto K, Takahashi Y, Ooi A, Kawakami K, Minamoto T: Inhibition of GSK-3 beta activity attenuates proliferation of human colon cancer cells in rodents. Cancer Sci 98: 1388–1393, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ougolkov AV, Fernandez-Zapico ME, Savoy DN, Urrutia RA, Billadeau DD: Glycogen synthase kinase-3beta participates in nuclear factor kappaB-mediated gene transcription and cell survival in pancreatic cancer cells. Cancer Res 65: 2076–2081, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Lyman GH, Williams CC, Preston D, Goldman A, Dinwoodie WR, Saba H, Hartmann R, Jensen R, Shukovsky L: Lithium carbonate in patients with small cell lung cancer receiving combination chemotherapy. Am J Med 70: 1222–1229, 1981 [DOI] [PubMed] [Google Scholar]
- 48.Stein RS, Flexner JM, Graber SE: Lithium and granulocytopenia during induction therapy of acute myelogenous leukemia. Blood 54: 636–641, 1979 [PubMed] [Google Scholar]
- 49.Kelly KJ, Plotkin Z, Dagher PC: Guanosine supplementation reduces apoptosis and protects renal function in the setting of ischemic injury. J Clin Invest 108: 1291–1298, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Keppler A, Gretz N, Schmidt R, Kloetzer HM, Groene HJ, Lelongt B, Meyer M, Sadick M, Pill J: Plasma creatinine determination in mice and rats: An enzymatic method compares favorably with a high-performance liquid chromatography assay. Kidney Int 71: 74–78, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Gong R, Rifai A, Ge Y, Chen S, Dworkin LD: Hepatocyte growth factor suppresses proinflammatory NFkappaB activation through GSK3beta inactivation in renal tubular epithelial cells. J Biol Chem 283: 7401–7410, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gong R, Rifai A, Dworkin LD: Anti-inflammatory effect of hepatocyte growth factor in chronic kidney disease: Targeting the inflamed vascular endothelium. J Am Soc Nephrol 17: 2464–2473, 2006 [DOI] [PubMed] [Google Scholar]