Abstract
Recent data suggest that nonlinear GFR trajectories are common among patients with CKD, but the modifiable risk factors underlying these changes in CKD progression rate are unknown. Analyses relating baseline risk factors to subsequent GFR decline are suboptimal because these relationships often attenuate as follow-up time increases and these analyses do not account for temporal changes in risk factors. We identified 74 participants in the African American Study of Kidney Disease and Hypertension who had both a period of rapid GFR decline and an extended period of stability during a follow-up period of ≥12 years. We performed a within-patient comparison of time-varying risk factors measured during the periods of GFR decline and stability and identified several risk factors associated with faster GFR decline: more hospitalization episodes and hospitalization days per year; higher BP, serum phosphorus, and urine protein-to-creatinine ratio; lower serum albumin and urine sodium-to-potassium ratio; slower rate of decline of serum urea nitrogen, serum creatinine, serum uric acid, and serum phosphorus; and faster rate of decline of serum hematocrit and serum bicarbonate. By allowing each patient to serve as his or her own control, this novel, within-patient analytic approach holds considerable promise as a means to identify time-varying risk factors associated with stabilization of GFR or acceleration of GFR decline.
Anecdotal observations of nephrologists have long suggested that the rate of GFR decline can be punctuated by episodes of rapid decline as well as periods of stability. However, until recently, most statistical analyses of GFR decline have relied on the assumption of linear rates of decline.1,2 This analytic approach is used in part because of its simplicity and in part because the relatively brief follow-up times of most longitudinal studies of patients with CKD limited the ability to detect deviations from linearity. Recent analyses of CKD cohorts with extended duration of follow-up have provided rigorous statistical confirmation that nonlinear trajectories are in fact commonplace.1,2 Using Bayesian analysis, we established that over a median follow-up of 9 years, 42% of the participants from the African American Study of Kidney Disease (AASK) had a ≥0.9 probability of having a nonlinear trajectory or a prolonged period of nonprogression.1
The development of rigorous methods for identifying nonlinear trajectories provides intriguing new possibilities for epidemiologic investigation of the relationships between CKD progression and potential risk factors. In particular, if specific periods of stable GFR and rapidly declining GFR can be identified, then it would be possible to investigate which factors changed over time that may have led to changes in the rate of CKD progression. In this approach, risk factors would be compared between periods of rapidly declining GFR and periods of stable GFR in the same patients. Although observational in nature, such a within-patient approach would overcome three fundamental problems that hamper the current standard epidemiologic approaches in CKD cohort studies in which baseline (i.e., time-invariant) risk factors are related to subsequent CKD progression. First, by relating the rate of progression in specific periods to measurements of risk factors in those same periods, the within-patient approach avoids the drawback seen with use of baseline risk factors: that the relationship between GFR decline and the baseline risk factors often attenuates as follow-up time increases. Second, by limiting assessment of the risk factor to a single measurement at baseline, the standard approach is unable to account for changes in risk factors occurring during the study that may lead to changes in rates of progression. Third, by comparing risk factors between periods of rapid progression and stable GFR in the same patients, each patient is used as his or her own control; thus, we can eliminate the effects of patient-specific confounders, both measured and unmeasured, that are often present in the standard cohort design when progression rates are related to risk factors across different patients with different characteristics.
In this study we use a novel within-patient crossover design and analytic approach to study time-varying risk factors of CKD progression. We previously identified 74 AASK participants whose estimated GFR (eGFR) trajectories had both a period of rapid decline and an extended period of stability, according to conservative prespecified criteria.1 Using data from these participants, we compared time-varying risk factors measured during the decline and stable periods within each participant to identify potentially-modifiable risk factors associated with GFR decline.
Results
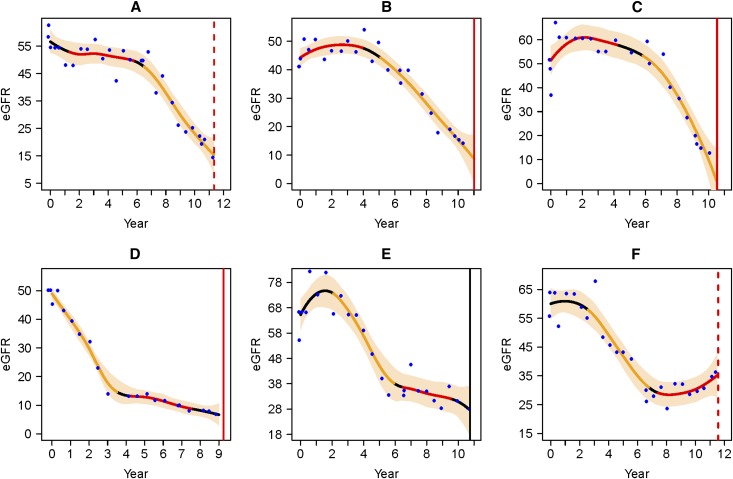
Table 1 summarizes the baseline characteristics of the 74 AASK participants in this analysis, which includes 45 participants who had the stable period first (stable/decline) and 29 who had the decline period first (decline/stable). For the stable/decline group, the median eGFRs (interquartile range) for the stable and the decline periods were 53.6 (46.3–61.6) ml/min per 1.73 m2 and 34.7 (29.5–52.8) ml/min per 1.73 m2, respectively. For the decline/stable group, median eGFRs (interquartile range) for the decline and the stable periods were 52.6 (44.0–59.8) ml/min per 1.73 m2 and 39.1 (28.4–46.2) ml/min per 1.73 m2, respectively. Median duration, including all 74 participants, was 32 months for the decline periods and 50 months for the stable periods. Of note, the earlier period always had a higher mean eGFR, regardless of whether it was a stable or decline period, because the overall trend in this cohort was that the eGFR declined over time. Figure 1 illustrates the nonlinear eGFR trajectories for six individuals in our analysis.
Table 1.
Summary statistics of the study sample
| Baseline Characteristics | Summary Statistics | ||
|---|---|---|---|
| Stable/Decline (n=45) | Decline/Stable (n=29) | All 74 Patients | |
| Age at randomization (yr) | 53.1 (45.6, 57.8) | 54.9 (44.5, 62.1) | 53.7 (45.5, 58.9) |
| Women | 13 (28.9) | 12 (41.4) | 25 (33) |
| Baseline eGFR (ml/min per 1.73 m2)a | 49.7 (41.1, 57.3) | 60.6 (55.4, 68.0) | 55.8 (44.1, 61.8) |
| Baseline urine protein-to-creatinine ratio (g/g) | 0.05 (0.02, 0.12) | 0.05 (0.03, 0.11) | 0.05 (0.02, 0.11) |
| Duration of stable periods (mo) | 51 (41, 57) | 49 (41, 58) | 50 (41, 58) |
| Duration of decline periods (mo) | 34 (24, 50) | 28 (24, 41) | 32 (24, 42) |
| AASK randomized intervention: mean arterial pressure goal | |||
| ≤92 mmHg | 18 (40) | 14 (48.3) | 32 (43) |
| 102–107 mmHg | 27 (60) | 15 (51.7) | 42 (57) |
| AASK randomized intervention: antihypertensive drugsb | |||
| Ramipril | 19 (42.2) | 10 (34.5) | 29 (39) |
| Metoprolol | 18 (40) | 11 (37.9) | 29 (39) |
| Amlodipine | 8 (17.8) | 8 (27.6) | 16 (22) |
| Average eGFR on segments of estimated trajectory (ml/min per 1.73 m2) | |||
| On stable periods | 53.6 (46.3, 61.6) | 39.1 (28.4, 46.2) | 48.5 (37.2, 58.0) |
| On decline periods | 34.7 (29.5, 52.8) | 52.6 (44.0, 59.8) | 43.1 (31.8, 54.8) |
Continuous variables were summarized by median (25th and 75th percentiles). Categorical variables were summarized by number (percentage).
Averaged over two baseline values, <3 months apart.
The randomized antihypertensive drugs were allocated in 2:2:1 ratio. Amlodipine was stopped 3 years after randomization.
Figure 1.
It can be seen that a patient's trajectory can have both a period of stability and a period of decline. The eGFR trajectories of six AASK patients in the analysis. On each plot, the horizontal axis is year since randomization, and the vertical axis is eGFR (ml/min per 1.73 m2). The blue dots are eGFR data, and the black smooth curve is the estimated trajectory; the yellow segment represents the declining eGFR period, and the red segment represents the stable or increasing eGFR period. The bisque-colored band is the pointwise 95% Bayesian confidence intervals. The red vertical line represents time of censoring (dashed) or dialysis (solid). The black vertical line represents time of death. Plots a–c represent individuals who had the stable period first, and d–f represent individuals who had the decline period first.
Hospitalizations
Participants experienced more hospitalization episodes per year (0.25 versus 0.12; P=0.01) and higher total days of hospitalization per year (1.8 versus 0.6; P=0.03) during the decline period than the stable period; results remained similar after adjustment for early/late period and mean eGFR during these periods (Table 2). The adjusted odds ratio of having a hospitalization was 0.7 (95% confidence interval, 0.3 to 1.5; P=0.3) for the stable versus decline comparison (Table 2). This nonsignificant result may be partly due to lower statistical power with the binary outcome variable.
Table 2.
Comparison of three hospitalization metrics between the stable and decline periods
| Hospitalization Metrics | Unadjusted Comparison | Adjusted for Early/Late Periods and Mean eGFR | ||||
|---|---|---|---|---|---|---|
| Stable Periods | Decline Periods | Unadjusted Difference (Stable–Decline) | P Value | Adjusted Difference (Stable–Decline) | P Value | |
| Periods with hospitalization (%) | 31 | 41 | OR, 0.63 (0.31 to 1.3) | 0.21 | OR, 0.66 (0.29 to 1.5) | 0.31 |
| Hospitalization episodes per year (n) | 0.12 | 0.25 | −0.13 (0.05) | 0.013 | −0.13 (0.06) | 0.02 |
| Total duration of hospitalizations per year (d) | 0.61 | 1.8 | −1.2 (0.55) | 0.03 | −1.1 (0.57) | 0.05 |
For the first binary metric, the difference is expressed as odds ratio (OR) with 95% confidence intervals. For the other two continuous metrics, the differences are expressed as the mean differences with estimated SEMs.
Because the sample size was small, we did not have enough statistical power for formal comparisons of specific hospitalization diagnoses. However, Supplemental Table A1 shows a trend toward more hospitalizations for cardiovascular (19 compared with 12), surgery (16 compared with 2), and cancer (6 compared with 2) diagnoses during the decline periods compared with the stable periods.
Medication
Self-reported medication use was generally similar between the stable and decline periods, with no significant differences in use of angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers, lipid-lowering medications, nonsteroid anti-inflammatory drugs, uric acid–lowering medications, or other BP medications (Supplemental Table A2).
BP
After adjustment for early/late period and mean eGFR of each period, systolic BP was on average 3.8 mmHg higher during the decline period than during the stable period (P=0.02). Likewise, diastolic BP was 1.6 mmHg (P=0.06) higher, and mean arterial BP was 2.3 mmHg (P=0.03) higher during the decline period compared with the stable period (Table 3).
Table 3.
Comparison of the average BP between the stable and decline periods
| Blood Pressure | Unadjusted Comparison | Adjusted for Early/Late and Mean eGFR | ||||
|---|---|---|---|---|---|---|
| Mean of Stable Periods | Mean of Decline Periods | Unadjusted Mean Difference (Stable–Decline) | P Value | Adjusted Mean Difference (Stable–Decline) | P Value | |
| Systolic (mmHg) | 131.1 (107.1, 161.7) | 134.0 (100.5, 169.8) | −2.8 (1.6) | 0.08 | −3.8 (1.6) | 0.02 |
| Diastolic (mmHg) | 81.2 (54.6, 96.1) | 81.2 (56.6, 111.3) | 0 (1.1) | 1.0 | −1.6 (0.9) | 0.06 |
| Mean arterial pressure (mmHg) | 98.0 (75.6, 114.2) | 98.9 (72.6, 128.2) | −0.9 (1.3) | 0.47 | −2.3 (1.1) | 0.03 |
The numbers in the parentheses are the range of BP or the SEM of the unadjusted or adjusted mean differences.
Biomarkers
Mean levels of serum phosphorus, urine protein, and urine protein-to-creatinine ratio were higher, and mean levels of albumin and urine sodium-to-potassium ratio were lower in the decline periods than the stable periods, after adjustment for early/late period and mean eGFR of each period (Table 4).
Table 4.
Comparison of the average biomarker levels between the stable and decline periods
| Biomarker | Unadjusted Comparison | Adjusted for Early/Late Periods and Mean eGFR | ||||
|---|---|---|---|---|---|---|
| Mean of Stable Periods | Mean of Decline Periods | Unadjusted Mean Difference (Stable–Decline) | P Value | Adjusted Mean Difference (Stable–Decline) | P Value | |
| Serum | ||||||
| Albumin (g/dl) | 4.16 | 4.03 | 0.13 (0.03) | <0.001 | 0.12 (0.03) | <0.001 |
| Alkaline phosphatase (U/L) | 93.2 | 97.4 | −4.27 (2.45) | 0.08 | −1.93 (2.31) | 0.38 |
| Calcium (mg/dl) | 9.34 | 9.44 | −0.1 (0.07) | 0.14 | −0.01 (0.05) | 0.87 |
| Glucose (mg/dl) | 100.9 | 100.6 | 0.32 (2.06) | 0.88 | 1.74 (1.95) | 0.35 |
| Serum urea nitrogen (mg/dl) | 26.5 | 31.4 | −4.87 (2.26) | 0.03 | −0.89 (1.45) | 0.50 |
| Serum creatinine (mg/dl) | 2.05 | 2.43 | −0.38 (0.14) | 0.005 | −0.15 (0.09) | 0.12 |
| Serum urea nitrogen-to-creatinine ratio | 13.09 | 13.06 | 0.03 (0.52) | 0.95 | 0.5 (0.49) | 0.30 |
| eGFR (ml/min per 1.73 m2) | 47.5 | 43.2 | 4.36 (1.71) | 0.010 | 0.67 (0.27) | 0.02 |
| Serum uric acid (mg/dl) | 8.55 | 8.54 | 0.01 (0.23) | 0.95 | 0.13 (0.24) | 0.62 |
| Serum phosphorus (mg/dl) | 3.42 | 3.71 | −0.29 (0.08) | <0.001 | −0.18 (0.07) | 0.005 |
| Serum sodium (mmol/L) | 138.4 | 139.0 | −0.63 (0.35) | 0.07 | −0.27 (0.33) | 0.45 |
| Serum hematocrit (%) | 39.3 | 38.3 | 1.05 (0.43) | 0.01 | 0.55 (0.38) | 0.13 |
| Serum bicarbonate (mmol/L) | 26.0 | 25.9 | 0.08 (0.35) | 0.82 | −0.16 (0.35) | 0.61 |
| Urine | ||||||
| Urine urea nitrogen (g/d) | 8.37 | 8.10 | 0.27 (0.28) | 0.33 | 0.07 (0.28) | 0.81 |
| Urine protein (g/d) | 0.36 | 1.01 | −0.65 (0.15) | <0.001 | −0.49 (0.14) | <0.001 |
| Urine creatinine (g/d) | 1.60 | 1.57 | 0.04 (0.05) | 0.47 | 0 (0.05) | 0.95 |
| Urine protein/creatinine ratio (g/g) | 0.21 | 0.69 | −0.48 (0.12) | <0.001 | −0.36 (0.11) | <0.001 |
| Urine sodium (g/d) | 4.02 | 3.72 | 0.3 (0.19) | 0.11 | 0.27 (0.19) | 0.12 |
| Urine potassium (g/d) | 2.06 | 2.05 | 0.01 (0.09) | 0.94 | −0.07 (0.09) | 0.43 |
| Urine sodium-to-potassium ratio (g/g) | 2.29 | 2.10 | 0.19 (0.11) | 0.07 | 0.26 (0.11) | 0.02 |
The unadjusted or adjusted mean differences are expressed as the estimator (SEM).
In addition to studying the relationship of GFR decline rate with the mean level of a biomarker, we also considered its relationship with the rate of change of the biomarker, which was quantified by the least-squares slope of the biomarker within each stable or decline period. Mean slopes of serum urea nitrogen, serum creatinine, serum uric acid, and serum phosphorus were higher, and mean slopes of serum hematocrit and bicarbonate were lower in the decline periods than the stable periods, after adjustment for early/late period and mean eGFR of each period (Table 5). Note that a higher slope (more positive or less negative) generally indicates faster rate of increase or slower rate of decline of a biomarker, while a lower slope (more negative or less positive) generally indicates slower rate of increase or faster rate of decline.
Table 5.
Comparison of the rate of change (per year) of biomarkers between the stable and decline periods
| Biomarker | Unadjusted Comparison | Adjusted for Early/Late Periods and Mean eGFR | ||||
|---|---|---|---|---|---|---|
| Mean Slope of Stable Periods | Mean Slope of Decline Periods | Unadjusted Mean Slope Difference (Stable–Decline) | P Value | Adjusted Mean Slope Difference (Stable–Decline) | P Value | |
| Serum | ||||||
| Albumin (g/dl) | −0.01 | 0.034 | −0.04 (0.05) | 0.35 | −0.01 (0.05) | 0.74 |
| Alkaline phosphatase (U/L) | 1.38 | 1.00 | 0.38 (2.7) | 0.89 | −0.45 (2.9) | 0.87 |
| Calcium (mg/dl) | 0.043 | 0.068 | −0.03 (0.06) | 0.69 | −0.06 (0.07) | 0.25 |
| Glucose (mg/dl) | 0.11 | −1.30 | 1.4 (4.23) | 0.74 | 1.6 (4.49) | 0.71 |
| Serum urea nitrogen (mg/dl) | −0.72 | 9.34 | −10.1 (1.8) | <0.001 | −9.44 (1.95) | <0.001 |
| Serum creatinine (mg/dl) | −0.042 | 0.77 | −0.81 (0.18) | <0.001 | −0.71 (0.19) | <0.001 |
| Serum urea nitrogen-to-creatinine ratio | 0.044 | 0.44 | −0.39 (0.46) | 0.39 | −0.45 (0.49) | 0.37 |
| eGFR (ml/min per 1.73 m2) | 1.49 | −8.04 | 9.53 (1.74) | <0.001 | 9.21 (1.78) | <0.001 |
| Serum uric acid (mg/dl) | −0.32 | 0.63 | −0.95 (0.21) | <0.001 | −1.12 (0.21) | <0.001 |
| Serum phosphorus (mg/dl) | −0.012 | 0.24 | −0.25 (0.08) | 0.002 | −0.2 (0.09) | 0.004 |
| Serum sodium (mmol/L) | −0.21 | 0.47 | −0.68 (0.35) | 0.050 | −0.74 (0.37) | 0.056 |
| Serum hematocrit (%) | 0.41 | −1.48 | 1.88 (0.42) | <0.001 | 1.87 (0.44) | <0.001 |
| Serum bicarbonate (mmol/L) | 0.57 | −0.53 | 1.1 (0.36) | 0.002 | 0.87 (0.37) | 0.007 |
| Urine | ||||||
| Urine urea nitrogen (g/d) | 0.11 | −0.20 | 0.31 (0.34) | 0.36 | 0.31 (0.34) | 0.32 |
| Urine protein (g/d) | 0.087 | 0.22 | −0.13 (0.12) | 0.25 | −0.15 (0.12) | 0.24 |
| Urine creatinine (g/d) | 0.016 | −0.047 | 0.06 (0.04) | 0.14 | 0.06 (0.04) | 0.18 |
| Urine protein/creatinine ratio (g/g) | 0.049 | 0.12 | −0.07 (0.07) | 0.30 | −0.06 (0.07) | 0.34 |
| Urine sodium (g/d) | 0.23 | 0.026 | 0.2 (0.21) | 0.33 | 0.2 (0.21) | 0.31 |
| Urine potassium (g/d) | 0.046 | −0.013 | 0.06 (0.11) | 0.60 | 0.01 (0.12) | 0.95 |
| Urine sodium-to-potassium ratio (g/g) | 0.12 | 0.018 | 0.1 (0.11) | 0.35 | 0.16 (0.11) | 0.17 |
The unadjusted or adjusted mean slope differences are expressed as the estimator (SEM).
Discussion
In this study comparing periods of stable GFR with periods of declining GFR in 74 individuals with hypertensive kidney disease, we illustrated how a novel, within-patient crossover design can be used to identify time-varying risk factors for GFR decline. This approach has many advantages. First, the design is conceptually simple and the analytic approach is intuitive and easy to interpret. Second, the selected subset of AASK participants, with clear-cut stable and decline periods, was most informative for the investigation of the association of contemporaneously measured time-varying risk factors with changes in the rate of GFR decline. Third, this design removes the confounding effects of all time-invariant (e.g., baseline) risk factors, regardless of whether they are measured or unmeasured, because the stable and decline periods of each patient share the same time-invariant risk factors. Fourth, because risk factors are measured concurrently with eGFR in this design, there may be increased power to detect time-varying risk factors associated with periods of GFR decline compared with studies that use baseline risk factors, where the relationships between risk factors and the outcome attenuate over time. Fifth, our analytic approach can be used to study the association between CKD progression and both the level (Table 4) and rate (Table 5) of change in each time-varying risk factor.
This latter approach using data on rates of changes in biomarkers and other risk factors could help improve our understanding of CKD progression because risk factors often change over time as GFR declines. Moreover, this type of dynamic data may become increasingly available for clinicians and researchers alike with the widespread adoption of electronic medical records. Using this method, we identified serum phosphorus, bicarbonate, and uric acid as potentially modifiable risk factors, although we cannot prove causality. Most,3–5 but not all,6 traditional observational studies examining baseline risk factors have found that higher serum phosphorus levels are associated with rapid GFR decline and ESRD. Other researchers have also found an association between lower serum bicarbonate and CKD progression,7,8 and small, randomized trials have found that bicarbonate supplementation slows the rate of CKD progression.9,10 Some have suggested a pathogenic role of hyperuricemia in hypertension and renal injury,11,12 although hyperuricemia has been associated with cardiovascular events and mortality, but not rapid renal progression or ESRD.13,14
Several studies have found an association between episodes of AKI and risk of rapid GFR decline and ESRD.2,15,16 In our study, we found that the number of hospital episodes and hospitalization days per year were significantly greater in the decline periods than the stable periods. We did not have information beyond International Classification of Diseases, Ninth Revision, codes for primary and secondary diagnoses, so we cannot ascertain whether episodes of AKI occurred during these hospitalizations. Because AKI often occurs in the setting of cardiovascular, cancer, and surgery hospitalizations,17,18 we speculate that episodes of AKI may have occurred, explaining the trend toward more hospitalizations for these diagnoses during periods of decline. However, it is also possible that the decline periods may simply represent worsening overall health, resulting in more hospitalizations.
Although this study design has many advantages, we still are unable to prove that these associations are causal in nature. For instance, mean slope of hematocrit was lower in the decline period than the stable period, a finding consistent with those of other showing that lower hemoglobin levels are associated with progression to ESRD.19,20 However, randomized controlled trials treating anemia with erythropoietin-stimulating agents have resulted in no difference in CKD progression, while one small study found no effect of iron supplementation on CKD progression.21,22 Other studies have suggested potential renal risks with targeting higher hemoglobin goals with erythropoietin-stimulating agents.23,24 Biomarkers, BP, and overall health may simply worsen as kidney function declines, so that difference in the rates of eGFR decline between the decline and stable periods may have influenced the studied time-varying risk factors rather than the converse. Creatinine-based eGFR does not account for some of the observed differences in biomarkers may reflect differences in kidney function.
Our design and analytic approach have some limitations. First, the design restricts the analysis to a subset of the AASK participants who had both a period of rapidly declining GFR and a period of stable GFR according to conservative prespecified criteria. The use of a focused subset of the full cohort is common to a variety of designs that are widely used in epidemiologic research, including matched case-control and case-crossover designs. The rationale for these designs is to facilitate robust analyses that minimize confounding and other forms of bias while focusing on the topics that are most informative for the research question under consideration. Although used in other lines of epidemiologic research, the case-crossover study design has not previously been used to examine risk factors for accelerated CKD progression. Second, we have considered time-varying risk factors one at a time and have not investigated the joint dependence of GFR decline on multiple risk factors. Future work is warranted to develop multivariable time-varying risk factor models that use multiple time-varying exposures. Third, although our design eliminates confounding from all time-invariant risk factors, both measured and unmeasured, there could still be residual confounding from other time-varying risk factors. However, because we incorporated covariate adjustment for the temporal ordering of the stable and decline periods, as well as the mean eGFR levels during the respective periods, our adjusted comparisons are unlikely to have been affected by confounding associated with time or the level of eGFR itself.
In summary, this novel, within-patient analytic approach holds considerable promise as a means to identify time-varying risk factors associated with stabilization of GFR or acceleration of GFR decline. Our analyses identified several modifiable factors (serum uric acid, serum phosphorus, and serum bicarbonate) that could be therapeutic targets in clinical trials.
Concise Methods
Study Population
AASK was a multicenter, randomized clinical trial of 1094 African American patients aged 18–70 years with a baseline GFR between 20 and 65 ml/min per 1.73 m2.25 The participants were randomly assigned in a 3×2 factorial design to one of three antihypertensive drug regimens (ramipril, amlodipine, or metoprolol) and two levels of BP control (mean arterial pressure≤92 mmHg or 102–107 mmHg). At the completion of the trial, 787 participants were alive and not undergoing dialysis; of these, 691 were enrolled in the subsequent AASK Cohort Study.25 The maximum follow-up of both trial and cohort phases was 12 years. Serum creatinine was measured twice at baseline, less than 3 months apart, and at follow-up months 3 and 6, and then every 6 months for the rest of the follow-up.
Previously,1 we analyzed the combined trial and cohort phase data from 846 AASK participants with at least 3 years of follow-up and at least eight visits in which GFR could be estimated from serum creatinine measurements using the AASK equation26:
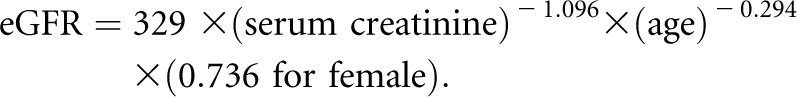 |
Bayesian penalized splines were used to estimate the eGFR trajectory of each patient as a smooth curve, removing much of the noise, short-term variation, and measurement error in the eGFR and revealing the overall trend over time.
Definitions of Stable and Decline Periods
Using the eGFR trajectories, we defined a period of stable GFR to be a period of time that satisfied the following three conditions: (1) the period was at least 3 years; (2) the trajectory increased or declined slowly (i.e., at a rate of <2 ml/min per 1.73 m2 per year throughout); and (3) the total decrease in eGFR, if any, was no more than 4.5 ml/min per 1.73 m2. We defined a period of rapidly declining GFR to be a period of time that satisfied the following two conditions: (1) the trajectory decreased at a rate of at least 4 ml/min per 1.73 m2 per year during the entire period; and (2) the total decline was at least 8 ml/min per 1.73 m2. It was found that 74 AASK participants had both a stable period and a decline period (1). Of the 74 patients, the stable period preceded the decline period in 45 participants, and the decline period preceded the stable period in 29 participants. The mean (minimum, maximum) length of the stable periods was 52 (36, 93) months, and that of the decline periods was 36 (13, 79) months.
The Within-Patient Crossover Design
We used data from these 74 participants with both stable and decline periods to determine whether time-varying risk factors were associated with eGFR decline. Essentially, a difference in a time-varying risk factor between the stable and decline periods implies that this time-varying risk factor may be associated with GFR decline. This approach allows us to study a large number of time-varying risk factors that are associated with GFR decline or stabilization without complicated modeling assumptions.
Statistical Analyses
We considered four classes of time-dependent risk factors for their association with the eGFR decline: hospitalizations, medications, BP levels, and biomarkers. Below we define clinically relevant metrics for each class.
Hospitalization
We used three metrics to quantify hospitalizations: (1) the percentage of patients with at least one hospitalization episode; (2) the number of hospitalization episodes per year; and (3) the total days of hospitalization per year. The primary and secondary International Classification of Diseases, Ninth Revision, codes of hospitalization were summarized and compared between the stable and decline periods by frequency tables.
Medication
The current medications taken by the patient were recorded at each follow-up visit. We made the simplifying assumption that if a patient was receiving a certain medication at a particular visit, this patient had been receiving that medication between that visit and the preceding visit. The proportion of time that a patient was receiving a certain medication was calculated for each patient’s stable and decline periods and was used as a metric to quantify medication use.
BP
We calculated the mean BP (arterial, systolic, diastolic) within each patient’s stable and decline periods.
Biomarkers
We used two different metrics to characterize the levels and the average rates of change of biomarkers during the stable and decline periods. First, we calculated the mean biomarker value for each patient’s stable and decline periods. Second, we computed the least-squares slopes of separate linear regressions of the biomarker values versus time within each patient’s stable and decline periods.
The general analytic strategy was to calculate the change in the aforementioned metrics of the time-varying risk factors between the stable and decline periods within the same patient and average the results across all 74 patients. The later period generally had lower eGFR than the earlier period, and on average the decline periods also had lower mean eGFR than the stable periods. Hence, we provide results both with and without adjusting for temporal confounding and for confounding by the level of GFR by including as covariates an indicator of early/late period and the mean eGFR level of each period. Time-varying risk factors exhibiting statistically significant differences between the stable and decline periods in the adjusted analysis were identified as being associated with GFR decline or stabilization, independent of the temporal ordering of the periods or the level of eGFR. We interpreted our analyses as representing the discovery phase of risk factor identification, and thus performed all hypothesis tests on a comparison-wise basis using a two-sided α=0.05, without adjustment for multiple comparisons. Details on the statistical models can be found in the Supplemental Materials.
Disclosures
None.
Supplementary Material
Acknowledgments
This research was sponsored by grants 5U01DK048648 and 1R01DK090046 from the National Institutes of Health.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013050464/-/DCSupplemental.
References
- 1.Li L, Astor BC, Lewis J, Hu B, Appel LJ, Lipkowitz MS, Toto RD, Wang X, Wright JT, Jr, Greene TH: Longitudinal progression trajectory of GFR among patients with CKD. Am J Kidney Dis 59: 504–512, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Hare AM, Batten A, Burrows NR, Pavkov ME, Taylor L, Gupta I, Todd-Stenberg J, Maynard C, Rodriguez RA, Murtagh FE, Larson EB, Williams DE: Trajectories of kidney function decline in the 2 years before initiation of long-term dialysis. Am J Kidney Dis 59: 513–522, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zoccali C, Ruggenenti P, Perna A, Leonardis D, Tripepi R, Tripepi G, Mallamaci F, Remuzzi G, REIN Study Group : Phosphate may promote CKD progression and attenuate renoprotective effect of ACE inhibition. J Am Soc Nephrol 22: 1923–1930, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scialla JJ, Astor BC, Isakova T, Xie H, Appel LJ, Wolf M: Mineral metabolites and CKD progression in African Americans. J Am Soc Nephrol 24: 125–135, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chue CD, Edwards NC, Davis LJ, Steeds RP, Townend JN, Ferro CJ: Serum phosphate but not pulse wave velocity predicts decline in renal function in patients with early chronic kidney disease. Nephrol Dial Transplant 26: 2576–2582, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Mehrotra R, Peralta CA, Chen SC, Li S, Sachs M, Shah A, Norris K, Saab G, Whaley-Connell A, Kestenbaum B, McCullough PA: No independent association of serum phosphorus with risk for death or progression to end-stage renal disease in a large screen for chronic kidney disease [published online ahead of print April 24, 2013]. Kidney Int 10.1038/ki.2013.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah SN, Abramowitz M, Hostetter TH, Melamed ML: Serum bicarbonate levels and the progression of kidney disease: a cohort study. Am J Kidney Dis 54: 270–277, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dobre M, Yang W, Chen J, Drawz P, Hamm LL, Horwitz E, Hostetter T, Jaar B, Lora CM, Nessel L, Ojo A, Scialla J, Steigerwalt S, Teal V, Wolf M, Rahman M, CRIC Investigators : Association of serum bicarbonate with risk of renal and cardiovascular outcomes in CKD: A report from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis 62: 670–678, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahajan A, Simoni J, Sheather SJ, Broglio KR, Rajab MH, Wesson DE: Daily oral sodium bicarbonate preserves glomerular filtration rate by slowing its decline in early hypertensive nephropathy. Kidney Int 78: 303–309, 2010 [DOI] [PubMed] [Google Scholar]
- 10.de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM: Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol 20: 2075–2084, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang DH, Nakagawa T, Feng L, Watanabe S, Han L, Mazzali M, Truong L, Harris R, Johnson RJ: A role for uric acid in the progression of renal disease. J Am Soc Nephrol 13: 2888–2897, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Johnson RJ, Nakagawa T, Jalal D, Sánchez-Lozada LG, Kang DH, Ritz E: Uric acid and chronic kidney disease: Which is chasing which? Nephrol Dial Transplant 28: 2221–2228, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu WC, Hung CC, Chen SC, Yeh SM, Lin MY, Chiu YW, Kuo MC, Chang JM, Hwang SJ, Chen HC: Association of hyperuricemia with renal outcomes, cardiovascular disease, and mortality. Clin J Am Soc Nephrol 7: 541–548, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Madero M, Sarnak MJ, Wang X, Greene T, Beck GJ, Kusek JW, Collins AJ, Levey AS, Menon V: Uric acid and long-term outcomes in CKD. Am J Kidney Dis 53: 796–803, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ: Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol 20: 223–228, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee P, Johansen K, Hsu CY: End-stage renal disease preceded by rapid declines in kidney function: a case series. BMC Nephrol 12:5-2369-12-5, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salahudeen AK, Doshi SM, Pawar T, Nowshad G, Lahoti A, Shah P: Incidence rate, clinical correlates, and outcomes of AKI in patients admitted to a comprehensive cancer center. Clin J Am Soc Nephrol 8: 347–354, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thakar CV: Perioperative acute kidney injury. Adv Chronic Kidney Dis 20: 67–75, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Keane WF, Zhang Z, Lyle PA, Cooper ME, de Zeeuw D, Grunfeld JP, Lash JP, McGill JB, Mitch WE, Remuzzi G, Shahinfar S, Snapinn SM, Toto R, Brenner BM, RENAAL Study Investigators : Risk scores for predicting outcomes in patients with type 2 diabetes and nephropathy: the RENAAL study. Clin J Am Soc Nephrol 1: 761–767, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Hsu CY, Iribarren C, McCulloch CE, Darbinian J, Go AS: Risk factors for end-stage renal disease: 25-year follow-up. Arch Intern Med 169: 342–350, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJ, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto R, TREAT Investigators : A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 361: 2019–2032, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Kim SM, Lee CH, Oh YK, Joo KW, Kim YS, Kim S, Lim CS: The effects of oral iron supplementation on the progression of anemia and renal dysfunction in patients with chronic kidney disease. Clin Nephrol 75: 472–479, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Inrig JK, Barnhart HX, Reddan D, Patel UD, Sapp S, Califf RM, Singh AK, Szczech LA: Effect of hemoglobin target on progression of kidney disease: A secondary analysis of the CHOIR (Correction of Hemoglobin and Outcomes in Renal Insufficiency) trial. Am J Kidney Dis 60: 390–401, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drüeke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, Burger HU, Scherhag A, CREATE Investigators : Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 355: 2071–2084, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Appel LJ, Middleton J, Miller ER, 3rd, Lipkowitz M, Norris K, Agodoa LY, Bakris G, Douglas JG, Charleston J, Gassman J, Greene T, Jamerson K, Kusek JW, Lewis JA, Phillips RA, Rostand SG, Wright JT: The rationale and design of the AASK cohort study. J Am Soc Nephrol 14[Suppl 2]: S166–S172, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Lewis J, Agodoa L, Cheek D, Greene T, Middleton J, O’Connor D, Ojo A, Phillips R, Sika M, Wright J, Jr, African-American Study of Hypertension and Kidney Disease : Comparison of cross-sectional renal function measurements in African Americans with hypertensive nephrosclerosis and of primary formulas to estimate glomerular filtration rate. Am J Kidney Dis 38: 744–753, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.