Abstract
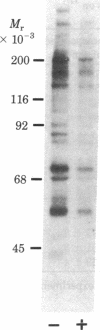
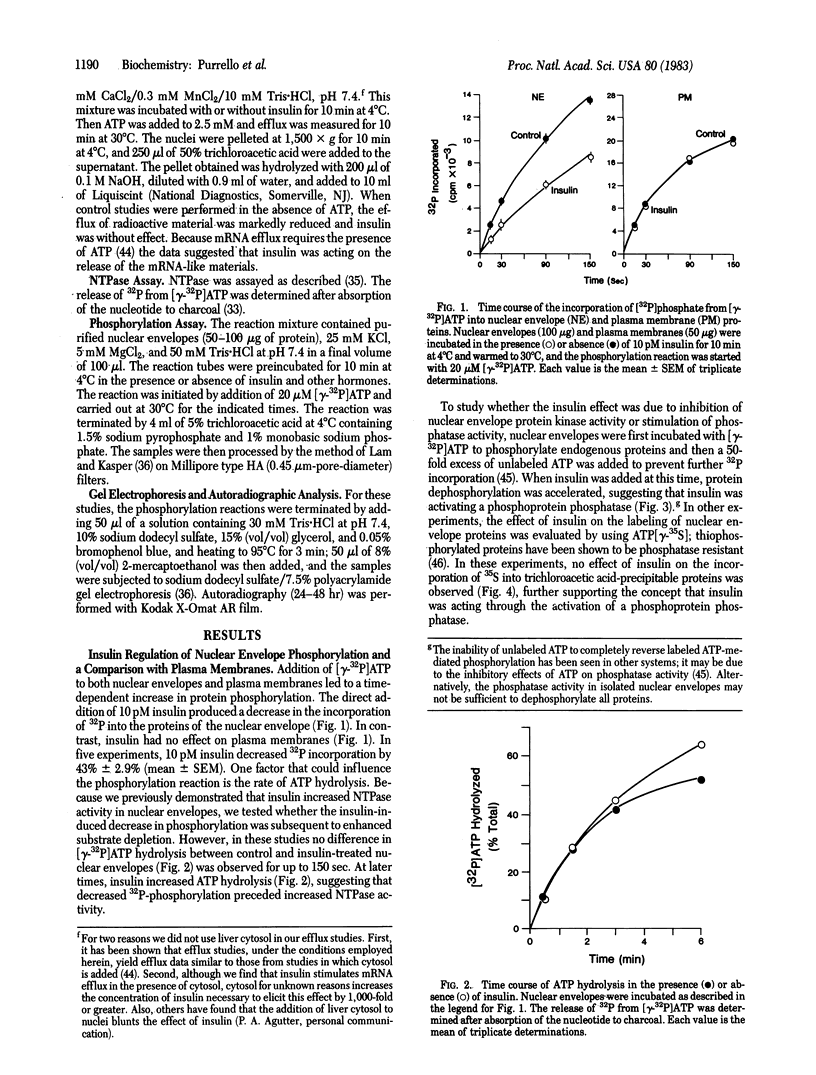
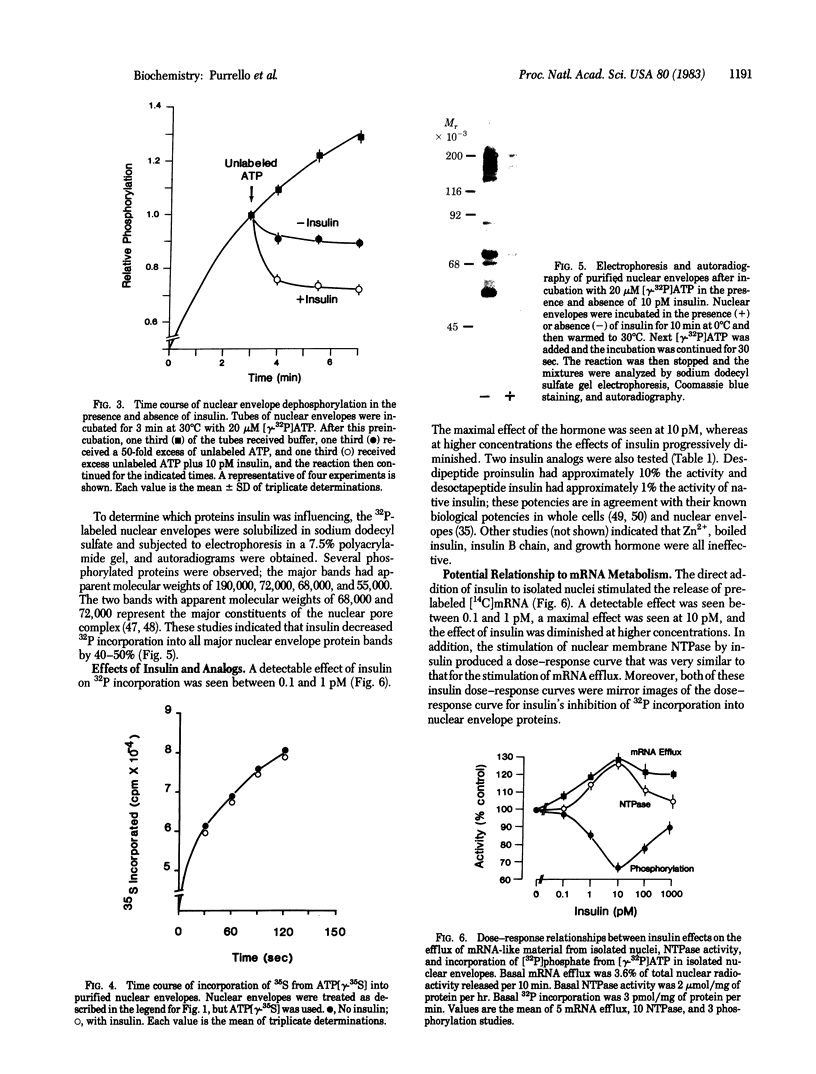
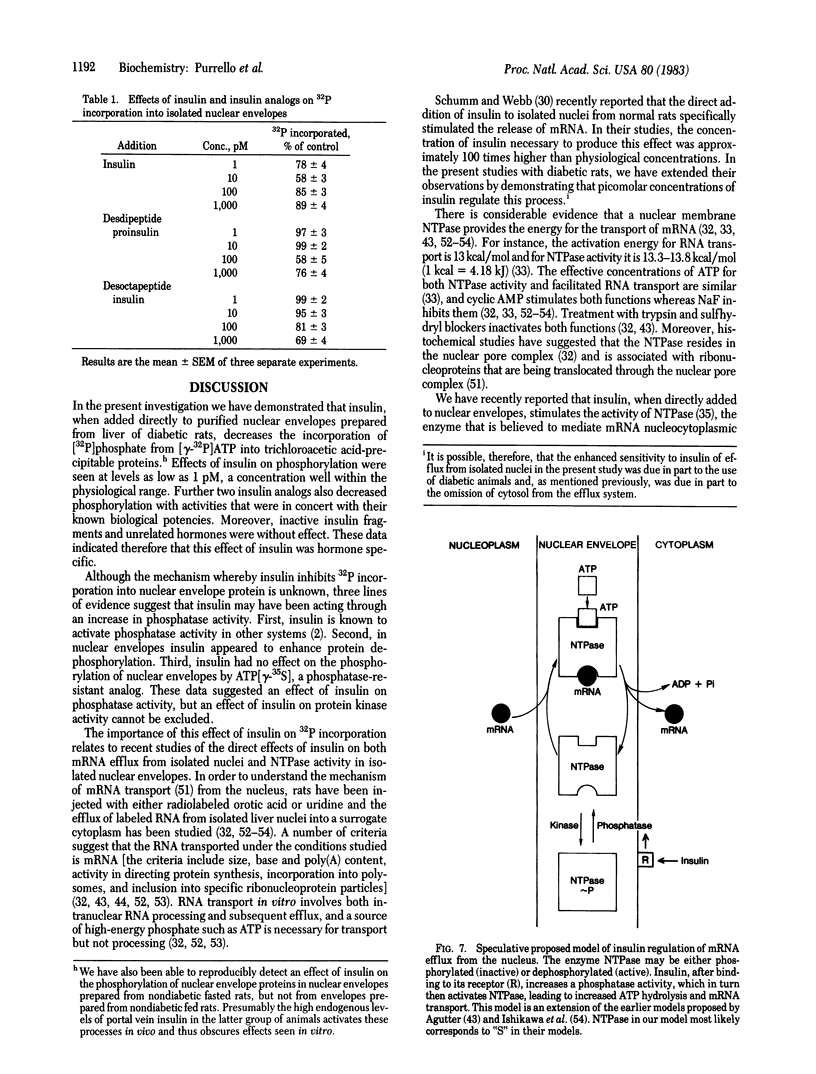
The direct addition of insulin to highly purified nuclear envelopes prepared from the livers of diabetic rats resulted in a decrease in the incorporation of 32P into trichloroacetic acid-precipitable proteins. Autoradiography of 32P-labeled envelopes, solubilized in sodium dodecyl sulfate and subjected to electrophoresis, revealed that insulin decreased the phosphorylation of all major protein bands. Insulin produced detectable effects at concentrations between 0.1 and 1 pM, maximal effects at 10 pM, and progressively diminished effects at higher concentrations. Two insulin analogs, desdipeptide proinsulin and desoctapeptide insulin, had approximately 10% and 1%, respectively, the activity of native insulin. When nuclear envelopes were first phosphorylated with [gamma-32P]ATP and insulin was then added with an excess of unlabeled ATP, dephosphorylation was enhanced, suggesting that insulin was regulating nuclear envelope phosphatase activity. The direct addition of insulin to isolated rat liver nuclei in the presence of ATP stimulated the release of previously 14C-labeled trichloroacetic acid-precipitable mRNA-like material, and the direct addition of insulin to nuclear envelopes stimulated the activity of nucleoside triphosphatase, the enzyme that participates in mRNA nucleocytoplasmic transport. Moreover, the dose-response curves for these functions mirrored insulin's inhibition of nuclear envelope phosphorylation. These data suggest, therefore, a mechanism whereby insulin directly inhibits the phosphorylation of the nuclear envelope, leading in turn to the regulation of mRNA metabolism.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson R. P., Blobel G. Isolation of nuclear pore complexes in association with a lamina. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1007–1011. doi: 10.1073/pnas.72.3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agutter P. S., Cockrill J. B., Lavine J. E., McCaldin B., Sim R. B. Properties of mammalian nuclear-envelope nucleoside triphosphatase. Biochem J. 1979 Sep 1;181(3):647–658. doi: 10.1042/bj1810647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agutter P. S. Influence of nucleotides, cations and nucleoside triphosphatase inhibitors on the release of ribonucleic acid from isolated rat liver nuclei. Biochem J. 1980 Apr 15;188(1):91–97. doi: 10.1042/bj1880091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agutter P. S., McArdle H. J., McCaldin B. Evidence for involvement of nuclear envelope nucleoside triphosphatase in nucleocytoplasmic translocation of ribonucleoprotein. Nature. 1976 Sep 9;263(5573):165–167. doi: 10.1038/263165a0. [DOI] [PubMed] [Google Scholar]
- Agutter P. S., McCaldin B., McArdle H. J. Importance of mammalian nuclear-envelope nucleoside triphosphatase in nucleo-cytoplasmic transport of ribonucleoproteins. Biochem J. 1979 Sep 15;182(3):811–819. doi: 10.1042/bj1820811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Nuclei from rat liver: isolation method that combines purity with high yield. Science. 1966 Dec 30;154(3757):1662–1665. doi: 10.1126/science.154.3757.1662. [DOI] [PubMed] [Google Scholar]
- Bolander F. F., Jr, Nicholas K. R., Van Wyk J. J., Topper Y. J. Insulin is essential for accumulation of casein mRNA in mouse mammary epithelial cells. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5682–5684. doi: 10.1073/pnas.78.9.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson-Lougarre A., Blum C. J. Spécificité et réversibilité de la liaison de l'insuline aux noyaux isolés thyroïdiens. C R Seances Acad Sci D. 1980 Mar 31;290(13):889–892. [PubMed] [Google Scholar]
- Caroni P., Carafoli E. Regulation of Ca2+-pumping ATPase of heart sarcolemma by a phosphorylation-dephosphorylation Process. J Biol Chem. 1981 Sep 25;256(18):9371–9373. [PubMed] [Google Scholar]
- Carpenter G., King L., Jr, Cohen S. Rapid enhancement of protein phosphorylation in A-431 cell membrane preparations by epidermal growth factor. J Biol Chem. 1979 Jun 10;254(11):4884–4891. [PubMed] [Google Scholar]
- Cassel D., Glaser L. Resistance to phosphatase of thiophosphorylated epidermal growth factor receptor in A431 membranes. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2231–2235. doi: 10.1073/pnas.79.7.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clawson G. A., James J., Woo C. H., Friend D. S., Moody D., Smuckler E. A. Pertinence of nuclear envelope nucleoside triphosphatase activity to ribonucleic acid transport. Biochemistry. 1980 Jun 10;19(12):2748–2756. doi: 10.1021/bi00553a033. [DOI] [PubMed] [Google Scholar]
- Clawson G. A., James J., Woo C. H., Friend D. S., Moody D., Smuckler E. A. Pertinence of nuclear envelope nucleoside triphosphatase activity to ribonucleic acid transport. Biochemistry. 1980 Jun 10;19(12):2748–2756. doi: 10.1021/bi00553a033. [DOI] [PubMed] [Google Scholar]
- Czech M. P. Molecular basis of insulin action. Annu Rev Biochem. 1977;46:359–384. doi: 10.1146/annurev.bi.46.070177.002043. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Brownsey R. W., Belsham G. J. A partial view of the mechanism of insulin action. Diabetologia. 1981 Oct;21(4):347–362. doi: 10.1007/BF00252681. [DOI] [PubMed] [Google Scholar]
- Franke W. W. Structure, biochemistry, and functions of the nuclear envelope. Int Rev Cytol. 1974;Suppl 4:71–236. [PubMed] [Google Scholar]
- Goidl J. A. Insulin binding to isolated liver nuclei from obese and lean mice. Biochemistry. 1979 Aug 21;18(17):3674–3679. doi: 10.1021/bi00584a006. [DOI] [PubMed] [Google Scholar]
- Goldfine I. D., Jones A. L., Hradek G. T., Wong K. Y. Electron microscope autoradiographic analysis of [125I]iodoinsulin entry into adult rat hepatocytes in vivo: evidence for multiple sites of hormone localization. Endocrinology. 1981 May;108(5):1821–1828. doi: 10.1210/endo-108-5-1821. [DOI] [PubMed] [Google Scholar]
- Goldfine I. D., Jones A. L., Hradek G. T., Wong K. Y., Mooney J. S. Entry of insulin into human cultured lymphocytes: electron microscope autoradiographic analysis. Science. 1978 Nov 17;202(4369):760–763. doi: 10.1126/science.715440. [DOI] [PubMed] [Google Scholar]
- Goldfine I. D., Smith G. J. Binding of insulin to isolated nuclei. Proc Natl Acad Sci U S A. 1976 May;73(5):1427–1431. doi: 10.1073/pnas.73.5.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfine I. D., Vigneri R., Cohen D., Pliam N. B., Kahn C. R. Intracellular binding sites for insulin are immunologically distinct from those on the plasma membrane. Nature. 1977 Oct 20;269(5630):698–700. doi: 10.1038/269698a0. [DOI] [PubMed] [Google Scholar]
- Greengard P. Phosphorylated proteins as physiological effectors. Science. 1978 Jan 13;199(4325):146–152. doi: 10.1126/science.22932. [DOI] [PubMed] [Google Scholar]
- Hill R. E., Lee K. L., Kenney F. T. Effects of insulin on messenger RNA activities in rat liver. J Biol Chem. 1981 Feb 25;256(4):1510–1513. [PubMed] [Google Scholar]
- Horvat A., Li E., Katsoyannis P. G. Cellular binding sites for insulin in rat liver. Biochim Biophys Acta. 1975 Apr 8;382(4):609–620. doi: 10.1016/0005-2736(75)90226-6. [DOI] [PubMed] [Google Scholar]
- Ishikawa K., Sato-Odani S., Ogata K. The role of ATP in the transport of rapidly-labeled RNA from isolated nuclei of rat liver in vitro. Biochim Biophys Acta. 1978 Dec 21;521(2):650–661. doi: 10.1016/0005-2787(78)90306-4. [DOI] [PubMed] [Google Scholar]
- Kiechle F. L., Jarett L., Kotagal N., Popp D. A. Partial purification from rat adipocyte plasma membranes of a chemical mediator which simulates the action of insulin on pyruvate dehydrogenase. J Biol Chem. 1981 Mar 25;256(6):2945–2951. [PubMed] [Google Scholar]
- Korc M., Iwamoto Y., Sankaran H., Williams J. A., Goldfine I. D. Insulin action in pancreatic acini from streptozotocin-treated rats. I. Stimulation of protein synthesis. Am J Physiol. 1981 Jan;240(1):G56–G62. doi: 10.1152/ajpgi.1981.240.1.G56. [DOI] [PubMed] [Google Scholar]
- Korc M., Owerbach D., Quinto C., Rutter W. J. Pancreatic islet-acinar cell interaction: amylase messenger RNA levels ar determined by insulin. Science. 1981 Jul 17;213(4505):351–353. doi: 10.1126/science.6166044. [DOI] [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- Krohne G., Franke W. W., Scheer U. The major polypeptides of the nuclear pore complex. Exp Cell Res. 1978 Oct 1;116(1):85–102. doi: 10.1016/0014-4827(78)90067-8. [DOI] [PubMed] [Google Scholar]
- Lam K. S., Kasper C. B. Selective phosphorylation of a nuclear envelope polypeptide by an endogenous protein kinase. Biochemistry. 1979 Jan 23;18(2):307–311. doi: 10.1021/bi00569a012. [DOI] [PubMed] [Google Scholar]
- Larner J., Galasko G., Cheng K., DePaoli-Roach A. A., Huang L., Daggy P., Kellogg J. Generation by insulin of a chemical mediator that controls protein phosphorylation and dephosphorylation. Science. 1979 Dec 21;206(4425):1408–1410. doi: 10.1126/science.228395. [DOI] [PubMed] [Google Scholar]
- Lolait S. J., Toh B. H. Binding sites of fluorescent derivatives of insulin in nuclei, rough endoplasmic reticulum, and mitochondria. Cell Tissue Res. 1980;210(1):145–153. doi: 10.1007/BF00232150. [DOI] [PubMed] [Google Scholar]
- Monneron A., Blobel G., Palade G. E. Fractionation of the nucleus by divalent cations. Isolation of nuclear membranes. J Cell Biol. 1972 Oct;55(1):104–125. doi: 10.1083/jcb.55.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peavy D. E., Taylor J. M., Jefferson L. S. Correlation of albumin production rates and albumin mRNA levels in livers of normal, diabetic, and insulin-treated diabetic rats. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5879–5883. doi: 10.1073/pnas.75.12.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pry T. A., Porter J. W. Control of fatty acid synthetase mRNA levels in rat liver by insulin, glucagon, and dibutyl cyclic AMP. Biochem Biophys Res Commun. 1981 Jun 16;100(3):1002–1009. doi: 10.1016/0006-291x(81)91923-9. [DOI] [PubMed] [Google Scholar]
- Purrello F., Vigneri R., Clawson G. A., Goldfine I. D. Insulin stimulation of nucleoside triphosphatase activity in isolated nuclear envelopes. Science. 1982 May 28;216(4549):1005–1007. doi: 10.1126/science.6281885. [DOI] [PubMed] [Google Scholar]
- Ray T. K. A modified method for the isolation of the plasma membrane from rat liver. Biochim Biophys Acta. 1970 Jan 6;196(1):1–9. doi: 10.1016/0005-2736(70)90159-8. [DOI] [PubMed] [Google Scholar]
- Rosen O. M., Rubin C. S., Cobb M. H., Smith C. J. Insulin stimulates the phosphorylation of ribosomal protein S6 in a cell-free system derived from 3T3-L1 adipocytes. J Biol Chem. 1981 Apr 25;256(8):3630–3633. [PubMed] [Google Scholar]
- Roy A. K., Chatterjee B., Prasad M. S., Unakar N. J. Role of insulin in the regulation of the hepatic messenger RNA for alpha 2u-globulin in diabetic rats. J Biol Chem. 1980 Dec 10;255(23):11614–11618. [PubMed] [Google Scholar]
- Sankaran H., Iwamoto Y., Korc M., Williams J. A., Goldfine I. D. Insulin action in pancreatic acini from streptozotocin-treated rats. II. Binding of 125I-insulin to receptors. Am J Physiol. 1981 Jan;240(1):G63–G68. doi: 10.1152/ajpgi.1981.240.1.G63. [DOI] [PubMed] [Google Scholar]
- Schumm D. E., Webb T. E. Effect of adenosine 3':5'-monophosphate and guanosine 3':5'-monophosphate on RNA release from isolated nuclei. J Biol Chem. 1978 Dec 10;253(23):8513–8517. [PubMed] [Google Scholar]
- Schumm D. E., Webb T. E. Insulin-modulated transport of RNA from isolated live nuclei. Arch Biochem Biophys. 1981 Aug;210(1):275–279. doi: 10.1016/0003-9861(81)90190-9. [DOI] [PubMed] [Google Scholar]
- Seals J. R., Jarett L. Activation of pyruvate dehydrogenase by direct addition of insulin to an isolated plasma membrane/mitochondria mixture: evidence for generated of insulin's second messenger in a subcellular system. Proc Natl Acad Sci U S A. 1980 Jan;77(1):77–81. doi: 10.1073/pnas.77.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steer R. C., Wilson M. J., Ahmed K. Phosphoprotein phosphatase activity of rat liver nuclear membrane. Biochem Biophys Res Commun. 1979 Aug 28;89(4):1082–1087. doi: 10.1016/0006-291x(79)92118-1. [DOI] [PubMed] [Google Scholar]
- Steer R. C., Wilson M. J., Ahmed K. Protein phosphokinase activity of rat liver nuclear membrane. Exp Cell Res. 1979 Mar 15;119(2):403–406. doi: 10.1016/0014-4827(79)90372-0. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., King J. Insulin-stimulated ribonucleic acid synthesis and RNA polymerase activity in alloxan-diabetic rat liver. Biochim Biophys Acta. 1966 Jun 22;119(3):510–516. doi: 10.1016/0005-2787(66)90127-4. [DOI] [PubMed] [Google Scholar]
- Vigneri R., Goldfine I. D., Wong K. Y., Smith G. J., Pezzino V. The nuclear envelope. The major site of insulin binding in rat liver nuclei. J Biol Chem. 1978 Apr 10;253(7):2098–2103. [PubMed] [Google Scholar]
- Vorbrodt A., Maul G. G. Cytochemical studies on the relation of nucleoside triphosphatase activity to ribonucleoproteins in isolated rat liver nuclei. J Histochem Cytochem. 1980 Jan;28(1):27–35. doi: 10.1177/28.1.6153190. [DOI] [PubMed] [Google Scholar]
- Walaas O., Walaas E., Lystad E., Alertsen A. R., Horn R. S., Fossum S. A stimulatory effect of insulin on phosphorylation of a peptide in sarcolemma-enriched membrane preparation from rat skeletal muscle. FEBS Lett. 1977 Aug 15;80(2):417–422. doi: 10.1016/0014-5793(77)80489-4. [DOI] [PubMed] [Google Scholar]
- Walsh D. A. Role of the cAMP-dependent protein kinase as the transducer of cAMP action. Biochem Pharmacol. 1978;27(14):1801–1804. doi: 10.1016/0006-2952(78)90022-9. [DOI] [PubMed] [Google Scholar]
- Younkin B., Martin H. Insulin regulation of RNA synthesis. J Theor Biol. 1978 Oct 21;74(4):491–500. doi: 10.1016/0022-5193(78)90236-9. [DOI] [PubMed] [Google Scholar]