Abstract
Increased markers of oxidative stress and acute-phase inflammation are prevalent in patients undergoing maintenance hemodialysis therapy (MHD), and are associated with increased mortality and hospitalization rates and decreased erythropoietin responsiveness. No adequately powered studies have examined the efficacy of antioxidant therapies on markers of inflammation and oxidative stress. We tested the hypothesis that oral antioxidant therapy over 6 months would decrease selected biomarkers of acute-phase inflammation and oxidative stress and improve erythropoietic response in prevalent MHD patients. In total, 353 patients were enrolled in a prospective, placebo-controlled, double-blind clinical trial and randomly assigned to receive a combination of mixed tocopherols (666 IU/d) plus α-lipoic acid (ALA; 600 mg/d) or matching placebos for 6 months (NCT00237718); 238 patients completed the study. High-sensitivity C-reactive protein (hsCRP) and IL-6 concentration were measured as biomarkers of systemic inflammation, and F2 isoprostanes and isofurans were measured as biomarkers of oxidative stress. The groups did not significantly differ at baseline. At 3 and 6 months, the treatment had no significant effect on plasma hsCRP, IL-6, F2 isoprostane, or isofuran concentrations and did not improve the erythropoietic response. No major adverse events were related to the study drug, and both groups had similar mortality and hospitalization rates during the study. In conclusion, the administration of mixed tocopherols and ALA was generally safe and well tolerated, but did not influence biomarkers of inflammation and oxidative stress or the erythropoietic response.
Elevated biomarkers of increased oxidative stress are highly prevalent in patients undergoing maintenance hemodialysis therapy (MHD), and several lines of evidence point to their association with adverse outcomes.1–11 Elevated biomarkers of the inflammatory response—serum high-sensitivity C-reactive protein (hsCRP) and serum IL-6 concentrations, which are also highly prevalent in this population—are robust predictors of cardiovascular events and mortality and have been linked to increased oxidative stress.12–18 Further, the systemic inflammatory response and increased oxidative stress are associated with poor response to erythropoietin-stimulating agent (ESA) therapy.19 Initiation of MHD does not improve biomarkers of oxidative stress or systemic inflammation, suggesting that maintenance dialysis alone is inadequate to control the proatherogenic metabolic milieu that accompanies uremia.20
Emerging evidence associating oxidative stress biomarkers with inflammation and cardiovascular risk in patients undergoing MHD has led to several pilot trials of antioxidant regimens, with most studies focusing on use of tocopherols or thiol-containing antioxidants. The tocopherols have long been recognized as potent chain-breaking antioxidants, have potent effects on intracellular signaling pathways, and have been reported to have beneficial metabolic effects in patients undergoing dialysis therapy.21–25 More recently, it has been recognized that γ-tocopherol and its principal metabolite are particularly potent in inhibiting inflammatory cell activation and proinflammatory cytokine production.26,27 The SPACE (Secondary Prevention with Antioxidants of Cardiovascular Disease in End-Stage Renal Disease) study demonstrated a clinically and statistically significant reduction in myocardial infarction and other cardiovascular events in patients treated with α-tocopherol compared with placebo.28
Another potential therapeutic approach for patients with ESRD is to administer reduced thiol-containing antioxidants. Studies have demonstrated that low-molecular-weight thiols and plasma protein thiols are markedly oxidized in patients undergoing MHD.29,30 In a prospective randomized clinical trial, administration of the thiol-containing antioxidant N-acetylcysteine markedly decreased the cardiovascular event rate in a cohort of MHD patients, suggesting potential utility for this approach.31 α-Lipoic acid (ALA) is a thiol-containing antioxidant that can replete reduced thiol groups, restore intracellular glutathione levels, and participate in the redox recycling of ascorbate and tocopherols.32
In this study we hypothesized that administration of combined antioxidant therapy (i.e., mixed tocopherols, 666 IU/d, plus ALA, 600 mg/d, over 6 months) will decrease biomarkers of acute-phase inflammation and oxidative stress in MHD patients. We also examined the effects of the intervention on erythropoietic response as a secondary outcome measure. Hospitalization and mortality were examined as safety measures.
Results
Baseline Characteristics
Table 1 shows the baseline demographic data for all study participants, combined and by assigned treatment groups. The average age was 58±12 years; 44% of patients were female, and 58% were African American. Sixty-five percent of the patients were dialyzed with an arteriovenous fistula, 40% had diabetes, and 39% had hypertension. The average time on hemodialysis (vintage) was 51±56 months. There were no significant differences between groups for any of these parameters.
Table 1.
Descriptive statistics of baseline characteristics stratified by treatment
| Characteristic | Placebo Group (n=165) | α-Tocopherol +ALA Group (n=160) | Total (n=325) | P Valuea |
|---|---|---|---|---|
| Age (yr)b | 59 (50, 68) (58±13) | 59 (50, 65) (58±12) | 59 (50, 67) (58±12) | 0.74 |
| Maintenance hemodialysis vintage duration (mo)b | 31 (19, 61) (53±61) | 34 (19, 61) (50±51) | 33 (19, 61) (51±56) | 0.78 |
| Women | 42 (69) | 46 (74) | 44 (143) | 0.42 |
| Men | 58 (96) | 54 (86) | 56 (182) | |
| Race | ||||
| White | 42 (69) | 37 (59) | 39 (128) | 0.085 |
| African American | 55 (90) | 61 (98) | 58 (188) | |
| Asian | 0 (0) | 1 (2) | 1 (2) | |
| Other | 4 (6) | 1 (1) | 2 (7) | |
| Ethnicity | ||||
| Hispanic | ||||
| No | 93 (154) | 94 (151) | 94 (305) | 0.7 |
| Yes | 7 (11) | 6 (9) | 6 (20) | |
| Type of vascular access | ||||
| Graft | 27 (45) | 32 (51) | 30 (96) | 0.46 |
| Fistula | 67 (110) | 64 (102) | 65 (212) | |
| Catheter with graft | 0 (0) | 0 (0) | 0 (0) | |
| Catheter with fistula | 0 (0) | 1 (1) | 0 (1) | |
| Catheter only | 6 (10) | 4 (6) | 5 (16) | |
| Cause of ESRD | ||||
| Diabetes | 38 (63) | 42 (67) | 40 (130) | 0.65 |
| Hypertension | 40 (66) | 39 (62) | 39 (128) | |
| GN | 5 (8) | 5 (8) | 5 (16) | |
| Polycystic kidney disease | 5 (8) | 3 (5) | 4 (13) | |
| Interstitial nephritis | 0 (0) | 1 (2) | 1 (2) | |
| Hereditary nephritis | 0 (0) | 0 (0) | 0 (0) | |
| Other | 12 (20) | 10 (16) | 11 (36) | |
| Diabetes at baseline | ||||
| No | 45 (75) | 45 (72) | 45 (147) | 0.93 |
| Yes | 55 (90) | 55 (88) | 55 (178) | |
| History of atherosclerotic cardiovascular disease | ||||
| No | 53 (88) | 46 (73) | 50 (161) | 0.16 |
| Yes | 47 (77) | 54 (87) | 50 (164) |
Unless otherwise noted, values are the number (percentage) of patients.
Values are expressed as median (interquartile range) and (mean±SD).
P values were obtained from nonparametric Mann–Whitney U tests for continuous variables and chi-squared test for categorical variables.
At baseline enrollment (n=325), 155 (48%) patients were taking angiotensin-converting enzyme inhibitors, 145 (45%) were taking statins, 18 (6%) were taking xanthine oxidase inhibitors, and 28 (9%) were taking thiazolidinediones. In total, 244 (75%) patients were taking at least one of those medications at the time of study enrollment. Of those, 121 (73%) in placebo group and 123 (77%) in the treatment group had at least one such medication.
Among 325 randomly assigned patients, 87 dropped out of the study. Forty-six (53%) of those who dropped out were from the antioxidative treatment group. Treatment assignment group did not differ between patients who dropped out and those who did not. Except for cause of ESRD, baseline characteristics also did not differ between those who dropped out and those who did not. There were also no differences in baseline hsCRP, IL-6, F2 isoprostane, or F2 isofuran concentrations between the patients who dropped out and those who remained in the study.
Concentration of Inflammatory Markers
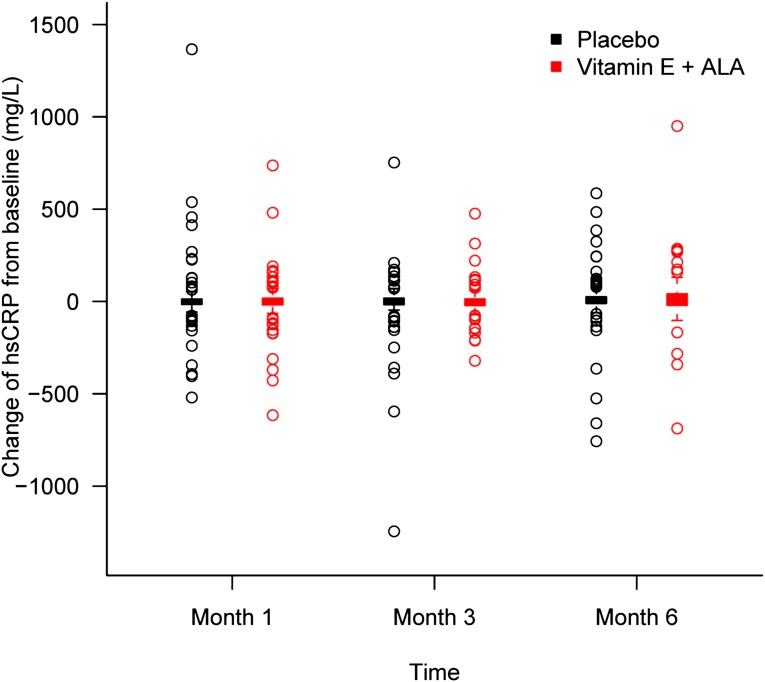
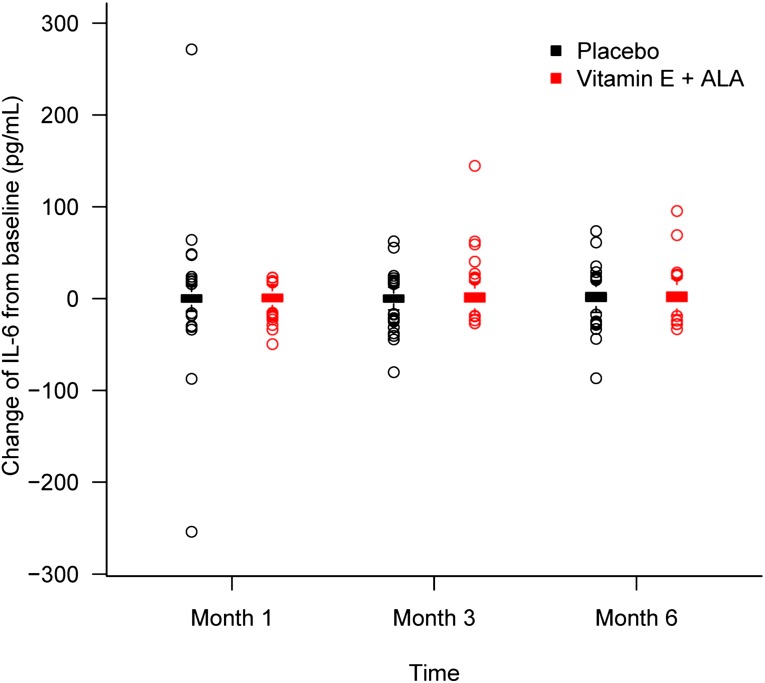
Table 2 shows descriptive data for hsCRP and IL-6 at baseline and monthly thereafter. Figures 1 and 2 depict changes in hsCRP and IL-6 from baseline at months 3 and 6. As shown, both hsCRP and IL-6 levels were elevated at baseline for both groups compared with normative ranges and stayed relatively stable throughout the study for both groups. There were no significant differences between groups at any time point. When we analyzed the data by differences in variables between each time point for each patient, we found no significant differences between groups. Finally, we performed regression analyses for both hsCRP and IL-6 after adjustment for baseline values, diabetes, history of cardiovascular disease, race, and age. Again, the groups did not differ for any of those variables at 3 or 6 months.
Table 2.
hsCRP and IL-6 concentrations at baseline and monthly thereafter stratified by treatment
| Variable | Patients (n) | Placebo Group (n=165) | α-Tocopherol+ALA Group (n=160) | P Valuea | ||
|---|---|---|---|---|---|---|
| Median (IQR) | Mean±SD | Median (IQR) | Mean±SD | |||
| hsCRP (mg/L) | 320 | 31 (16, 84) | 91±235 | 37 (14, 78) | 73±111 | 0.94 |
| Baseline | ||||||
| 1 mo | 282 | 38 (18, 88) | 91±202 | 39 (19, 88) | 75±106 | 0.63 |
| 3 mo | 255 | 36 (15, 85) | 71±103 | 51 (15, 102) | 76±111 | 0.53 |
| 6 mo | 238 | 46 (15, 108) | 92±133 | 45 (20, 141) | 96±132 | 0.48 |
| IL-6 (pg/ml) | 319 | 13.0 (8.4, 20.2) | 19.9±28.2 | 12.8 (7.4, 20.2) | 16.8±19.0 | 0.42 |
| Baseline | ||||||
| 1 mo | 283 | 12.5 (8.0, 20.8) | 19.4±27.5 | 14.1 (9.1, 20.4) | 17.2±17.3 | 0.52 |
| 3 mo | 255 | 12.6 (7.7, 20.3) | 18.5±29.9 | 12.9 (8.4, 22.0) | 19.0±21.8 | 0.5 |
| 6 mo | 238 | 13.9 (8.2, 22.1) | 20.2±27.8 | 14.8 (10.4, 23.7) | 19.3±15.0 | 0.13 |
IQR, interquartile range.
P values were obtained from nonparametric Mann–Whitney U tests.
Figure 1.
Changes in hsCRP from baseline at months 3 and 6. Levels stayed relatively stable throughout the study for both groups. Box and whisker plot (box represents the interquartile range; whiskers extend to the most extreme data point, which is no more than 1.5 times the interquartile range from the box; circles beyond the whiskers are extreme values; and the line within the box represents the median) of change in hsCRP from baseline at 1, 3, and 6 months of α-tocopherol plus ALA treatment (red) and placebo (black) groups.
Figure 2.
Changes in IL-6 from baseline at months 3 and 6. Levels stayed relatively stable throughout the study for both groups. Box and whisker plot (box represents the interquartile range; whiskers extend to the most extreme data point, which is no more than 1.5 times the interquartile range from the box; circles beyond the whiskers are extreme values; and the line within the box represents the median) of change in IL-6 from baseline at 1, 3, and 6 months of α-tocopherol plus ALA treatment (red) and placebo (black) groups.
Plasma F2 Isoprostane and F2 Isofuran Concentrations
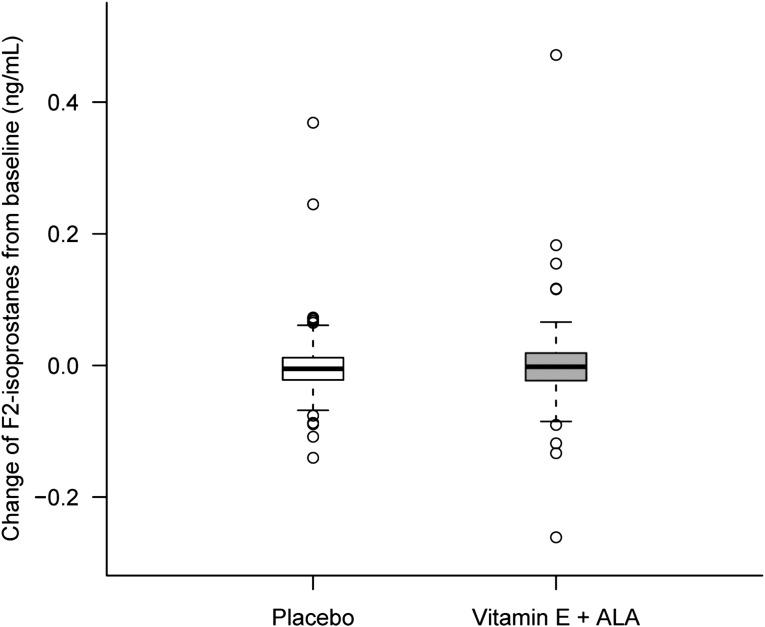
Table 3 shows descriptive data for F2 isoprostanes and F2 isofurans at baseline and at 6 months. Figure 3 depicts changes in F2 isoprostanes from baseline to month 6. As was seen with inflammatory biomarkers, both F2 isoprostanes and F2 isofurans levels were elevated at baseline for both groups compared with normative ranges and stayed high at the end of the study for both groups. There were no significant differences between groups at baseline and the end of the study. When we analyzed differences in variables between time points for each patient, we found no significant difference between groups. Finally, we performed regression analyses for F2 isoprostanes and F2 isofurans after adjustment for baseline values, diabetes, history of cardiovascular disease, race, age, and medication use. There were no significant differences between treatment groups for any of those variables at 6 months.
Table 3.
F2 isoprostane and F2 isofuran concentrations at baseline and 6 months stratified by treatment
| Variable | Patients (n) | Placebo Group (n=128) | α-Tocopherol +ALA Group (n=125) | P Valuea | ||
|---|---|---|---|---|---|---|
| Median (IQR) | Mean±SD | Median (IQR) | Mean±SD | |||
| Baseline | ||||||
| F2 isoprostane (ng/ml) | 247 | 0.060 (0.048, 0.088) | 0.072±0.036 | 0.063 (0.045, 0.088) | 0.074±0.044 | 0.98 |
| F2 isofuran (ng/ml) | 252 | 0.44 (0.25, 0.84) | 0.89±1.91 | 0.39 (0.24, 0.67) | 0.69±1.31 | 0.23 |
| 6 mo | ||||||
| F2 isoprostane (ng/ml) | 249 | 0.058 (0.045, 0.081) | 0.072±0.054 | 0.058 (0.042, 0.086) | 0.074±0.059 | 0.93 |
| F2 isofuran (ng/ml) | 252 | 0.45 (0.26, 0.77) | 0.79±1.36 | 0.38 (0.20, 0.70) | 0.65±0.90 | 0.33 |
IQR, interquartile range.
P values were obtained from nonparametric Mann–Whitney U tests.
Figure 3.
Changes in F2 isoprostanes from baseline at month 6. Levels stayed relatively stable throughout the study for both groups. Box and whisker plot (box represents the interquartile range; whiskers extend to the most extreme data point, which is no more than 1.5 times the interquartile range from the box; circles beyond the whiskers are extreme values; and the line within the box represents the median) of change in F2 isoprostanes from baseline at 6 months of α-tocopherol plus ALA treatment (gray) and placebo (white) groups.
To further confirm the results, we categorized patients into two groups based on their baseline F2 isoprostane levels. Patients were considered as having high oxidative stress if their baseline F2 isoprostane levels were higher than or equal to the population median F2 isoprostane level; they were considered to have low oxidative stress if the levels were below the median. The distribution of such groupings did not differ between treatment groups (P=0.85). There was also no difference in 6-month F2 isoprostane levels between treatment groups among patients with high oxidative stress and among those with low oxidative stress.
Finally, we conducted a subanalysis that classified patients as having significant reduction in oxidative markers (F2 isoprostane at 6 months was ≤60% of the baseline F2 isoprostane level) or having no significant reduction (all patients not meeting the preceding criteria). Forty patients (20 from the treatment group and 20 from the placebo group) were classified as having significant reduction in oxidative markers during the study. Compared with patients who did not have a significant reduction, those who did were more likely to have a higher baseline F2 isoprostane and F2 isofurans levels (P<0.001 and P=0.03, respectively). No other differences in patient characteristics and medication use were seen between the two groups (data not shown).
Erythropoietic Index
Table 4 shows the data for baseline and monthly total ESA dose and monthly ESA dose adjusted by the last hemoglobin concentration of the month. There were no significant differences between groups at baseline (enrollment and 1 month after enrollment) and each month of the study. Area under the curve of the monthly ESA dose and monthly ESA dose adjusted by the last hemoglobin concentration did not differ between the two treatment groups.
Table 4.
ESA use, unadjusted and adjusted for hemoglobin concentration, at each time point between two treatments
| Time Point | Placebo Group | α-Tocopherol+ALA Group | P Valuea | ||
|---|---|---|---|---|---|
| Patients (n) | ESA Use | Patients (n) | ESA Use | ||
| Unadjusted | |||||
| Enrollment | 165 | 36,000 | 160 | 33,600 | 0.54 |
| Baseline | 164 | 36,000 | 159 | 32,400 | 0.37 |
| 1 mo | 154 | 37,200 | 137 | 32,400 | 0.62 |
| 2 mo | 147 | 36,000 | 126 | 31,200 | 0.58 |
| 3 mo | 137 | 36,000 | 118 | 36,000 | 0.48 |
| 4 mo | 130 | 36,000 | 116 | 36,000 | 0.83 |
| 5 mo | 125 | 36,000 | 114 | 40,800 | 0.82 |
| 6 mo | 124 | 40,800 | 114 | 42,000 | 0.62 |
| Adjusted for hemoglobin | |||||
| Enrollment | 165 | 3214 | 159 | 2857 | 0.48 |
| Baseline | 163 | 2070 | 159 | 2656 | 0.34 |
| 1 mo | 154 | 3239 | 137 | 2824 | 0.72 |
| 2 mo | 147 | 3186 | 124 | 2880 | 0.75 |
| 3 mo | 136 | 3144 | 117 | 3429 | 0.50 |
| 4 mo | 129 | 3158 | 114 | 3081 | 0.86 |
| 5 mo | 124 | 3332 | 113 | 3579 | 0.74 |
| 6 mo | 123 | 3495 | 114 | 3578 | 0.71 |
P value was obtained from nonparametric Mann–Whitney U tests. ESA dose in units.
Safety
Four patients died (all in the placebo group) and 111 patients were hospitalized (60 in the placebo group and 51 in the intervention group) during the study period. An additional 10 patients died (4 in the placebo group and 6 in the intervention group) during the 6-month study follow-up period after the intervention was stopped. The overall adverse event rates were 5.16 and 6.11 per patient-year for placebo and study drug, respectively (P=0.02). The study intervention was associated with significantly higher rates of gastrointestinal upset, vascular access–related events, and electrolyte disorders, and a significantly lower rate of fluid overload (Supplemental Table 1).
Discussion
In this randomized, double-blind, placebo-controlled study of patients receiving MHD, the provision of mixed tocopherols and ALA as antioxidant therapy was generally safe and well tolerated. However, the effect of this combination antioxidant therapy on biomarkers of inflammation as a primary endpoint was not statistically significantly different from that of placebo. Additionally, antioxidant therapy had no significant effect on selected oxidative stress biomarkers, dosing requirements of erythropoietic-stimulating agents, or hospitalization or mortality rates.
Cardiovascular disease is the leading cause of death in patients undergoing dialysis, and to date the use of interventions targeting cardiovascular risk factors unique to this patient population have demonstrated only limited effectiveness. Previous observational studies have demonstrated that oxidative stress is increased in dialysis patients, that biomarkers of inflammation and oxidative stress are often closely linked,33–38 and that these biomarkers are also associated with cardiovascular risk in this population. This has led to the suggestion that oxidative stress pathways are a “nontraditional” cardiovascular risk factor for patients receiving dialysis therapy and that antioxidant therapies may be more effective in this population than in studies of primary and secondary risk prevention in the absence of advanced kidney disease.39
We chose to study a combination of mixed tocopherols and ALA as antioxidant therapy for several reasons. First, in two separate pilot studies, the separate provision of α-tocopherol and the thiol-containing antioxidant N-acetyl cysteine had some benefit in reducing cardiovascular events rates in patients receiving MHD.28,31 Second, in previous pharmacokinetic studies, we and others showed that the metabolism of tocopherols and thiol-containing antioxidants is dramatically altered in dialysis patients, leading to prolonged circulating half-lives of the parent antioxidant or biologically active metabolites.26,40–42 In tocopherol metabolism, cytochrome P450 enzymes catalyze the formation of biologically active carboxyethyl hydroxy chroman metabolites.43,44 Carboxyethyl hydroxy chroman metabolites are water-soluble, low-molecular-weight compounds that accumulate in the absence of kidney function and are readily removed by dialysis, thus providing a favorable pharmacokinetic profile for use in a dialysis population. Likewise, low-molecular-weight thiol-containing antioxidants have dramatically increased area under the plasma-concentration curve, probably because of reduced cellular uptake.41 Third, previous data from our group and others have suggested that γ-tocopherol, the major plant-derived dietary form of tocopherols, may possess more anti-inflammatory properties than α-tocopherol. Fourth, previous data from our group and others have suggested that oxidation of thiol groups, particularly in plasma proteins, is especially prominent in persons with kidney disease.29,30 Finally, we hypothesized that combining predominantly lipophilic antioxidants (tocopherols) with more water-soluble antioxidants (ALA) would provide synergistic antioxidant effect.
We chose biomarkers of inflammation as the primary endpoint for this study for several reasons. Inflammatory biomarkers are well studied in patients on dialysis and are among the most potent predictors for cardiovascular morbidity and overall mortality risk. Further, considerable experimental data suggest that increased oxidative stress may be an upstream cause of activation of inflammatory cascades. Our previous data had demonstrated that biomarkers of oxidative stress and inflammation are closely linked in patients receiving dialysis therapy,45 and in earlier stages of kidney disease oxidative stress biomarkers are more frequently elevated than are inflammatory biomarkers.46 Finally, previous pilot studies by our group and others had suggested that similar antioxidant therapies may have anti-inflammatory effects in patients receiving dialysis.26,47
Despite the aforementioned considerations, the results indicated no significant effect of the treatment on inflammatory and oxidative stress markers. These findings could be interpreted to suggest that the proposed interventions have no biologic influence on these pathways in MHD patients. Although discouraging, this is consistent with many other interventions that have failed to show a beneficial effect in this rather complicated patient population. On the other hand, it is also possible that higher doses of antioxidants, or different types of antioxidants, might have had a more beneficial effect on uremia-associated oxidative stress and inflammation than the chosen antioxidants. For example, recent studies have suggested that the antioxidants coenzyme Q10 or phenolic antioxidants derived from red wine or pomegranate juice may have benefit in this patient population and should be further studied.47–49 In addition, it is possible that a longer course of tocopherols plus ALA treatment might have been required to demonstrate a beneficial clinical effect, even in the absence of measurable changes in inflammatory and oxidative stress biomarkers.
Two published studies using a randomized, placebo-controlled design have suggested that antioxidant therapy may provide clinical benefit. In the SPACE study, 196 patients with known cardiovascular disease were randomly assigned to receive α-tocopherol, 800 IU/d, or matching placebo and were followed for an average of 519 days.28 The tocopherol group had a significantly lower occurrence of the composite primary endpoint of myocardial infarction, ischemic stroke, peripheral vascular disease, and unstable angina. Mortality and the secondary endpoints (other than myocardial infarction) did not significantly differ between groups. Comparison of the SPACE study results with our study findings reveals many important differences in the study design and demographic characteristics of the enrolled population that may affect what appear to be divergent results. Participants in the SPACE study were older, less likely to be female, and universally had known cardiovascular disease. They were also substantially less likely to be receiving concomitant therapy with a lipid-lowering agent (13% versus 45%) or an angiotensin-converting enzyme inhibitor (18% versus 48%), which may have independent antioxidant effects.50 Perhaps as a consequence, the mortality rate in the SPACE study (about 22% per year) was much higher than in our study (about 6% per year, including during the 6-month follow-up after discontinuation of the study agent). The reported myocardial infarction rate in the SPACE placebo group (12.3% per year) was exceptionally high. Additionally, in the SPACE study, remarkably few adverse events were reported (approximately 4% of participants), dropouts were not reported, and methods for adjudication of clinical events were not reported. Although the SPACE study did not systematically measure levels of oxidative stress biomarkers in response to therapy, two subsequent reports have not found a significant effect of administering α-tocopherol, 800 IU/d, on plasma oxidized LDL or oxidative protein product concentration.51,52
Similar to the SPACE study, a randomized trial comparing the antioxidant N-acetyl cysteine with placebo in dialysis patients also observed a decrease in the incidence of a composite cardiovascular endpoint, although most observed events were not cardiac but rather stroke and peripheral vascular disease related.31 This study also did not systematically measure biomarkers of oxidative stress in response to therapy, did not report adverse events or dropouts, and did not indicate how cardiovascular events were adjudicated. Commentary on the SPACE study and many other antioxidant trials have emphasized the need for rigorous biomarker assessment of antioxidant therapy and suggested that combinations of antioxidants may have greater utility than individual antioxidants.53,54
The present study has many strengths. It is the largest antioxidant trial conducted to date in patients undergoing hemodialysis. We used a double-blind randomized controlled study design with sufficient power to definitely address the primary endpoint. We used combination antioxidant therapy, with doses chosen on the basis of prior pilot studies that suggested possible benefit. We used harmonized procedures for obtaining and processing biosamples at all sites for endpoint analysis. This is essential for assessment of most oxidative stress biomarkers, which are highly subject to in vitro artifactual oxidation. Lipid peroxidation biomarkers (F2 isoprostanes and F2 isofurans), considered gold standard measures because of their precision, were analyzed at a reference quality laboratory using gas chromatography/mass spectrometry.55,56 The results of this randomized trial supported the null hypothesis, providing relatively strong evidence of a lack of benefit of this antioxidant combination on measured endpoints.
The study also has limitations. In particular, even though the plasma F2 isoprostane and F2 isofuran concentrations were elevated at baseline compared with normative ranges, these oxidative stress biomarkers did not improve with active therapy over the course of the clinical trial. This might be a consequence of inadequate doses or a course of antioxidants shorter than what is necessary to demonstrate a beneficial clinical effect. It is also possible that more clinically relevant outcomes might still be influenced, even in the absence of measurable changes in inflammatory and oxidative stress biomarkers.
In conclusion, the administration of a combination of mixed tocopherols plus ALA did not improve inflammatory or oxidative stress biomarkers in patients with ESRD undergoing MHD. These data highlight the uncertainties surrounding the use of antioxidants in patients receiving dialysis. Although >50 studies investigating antioxidants in patients undergoing hemodialysis have been published, there is no consensus on procedures for optimal study design, and no previous studies have measured both appropriate biomarkers and clinical outcomes in sufficiently large populations to draw meaningful conclusions (reviewed in Coombes and Fassett39). Further studies, probably using different antioxidants and perhaps assessing different endpoints, are warranted to fully test whether antioxidants may confer benefit to this patient population.
Concise Methods
Study Design
This was a prospective, randomized, placebo-controlled, double-blind clinical trial (NCT00237718). After informed consent was obtained, baseline enrollment data and blood work were obtained 1 month before initiation of the study drug. Patients were then assigned to one of two study groups by a permuted block randomization strategy in a 1:1 ratio. Patients were stratified according to the presence or absence of diabetes mellitus and according to having high (≥10 mg/dl) or low (<10 mg/dl) CRP. The study compared combination antioxidant therapy with mixed tocopherols (α, β, γ, and δ), 666 IU/d, plus ALA, 600 mg/d, with matching placebo. Mixed tocopherols were provided as one capsule of 666 IU (Yasoo Health, Inc., Johnson City, TN) and ALA was provided as two capsules of 300 mg each (Jarrow Industries, Inc., Santa Fe Springs, CA). Matching capsules were provided to the placebo group, prepared by the Vanderbilt University Medical Center Investigational Drug Services. Patients took a total of three capsules per day for a total of 6 months.
Patients
Patients were recruited from outpatient dialysis clinics. Recruitment began in May 2006 and continued through July 2009. Criteria for study participation included patients with ESRD receiving thrice-weekly hemodialysis for at least 120 days, age>18 years, life expectancy >1 year, and the ability to understand and provide informed consent for participation in the study. Exclusion criteria included AIDS (HIV seropositivity was not an exclusion criterion); active malignancy, excluding basal cell carcinoma of the skin; gastrointestinal dysfunction requiring parenteral nutrition; history of functional kidney transplant <6 months before study entry; anticipated live donor kidney transplant over the study duration; history of poor adherence to hemodialysis or medical regimen; prisoners, patients with significant mental illness, pregnant women, and other vulnerable populations; patients taking vitamin E supplements≥60 IU/d or vitamin C≥500 mg/d during the past 30 days; patients taking anti-inflammatory medication, except aspirin≤325 mg/d, over the past 30 days; patients using a temporary catheter for dialysis access at baseline or patients receiving a graft/fistula within the 6-month study period; and more than two hospitalizations within the last 90 days or one hospitalization within the 30 days preceding enrollment. Patients underwent study visits at baseline (prior to initiation of study drugs) and then monthly for 6 months. Demographic characteristics, medical history, and blood for routine chemistries and nutritional biomarkers were collected at the baseline visit. Additional blood was collected for biomarkers of inflammation and oxidative stress at baseline and at the monthly visits.
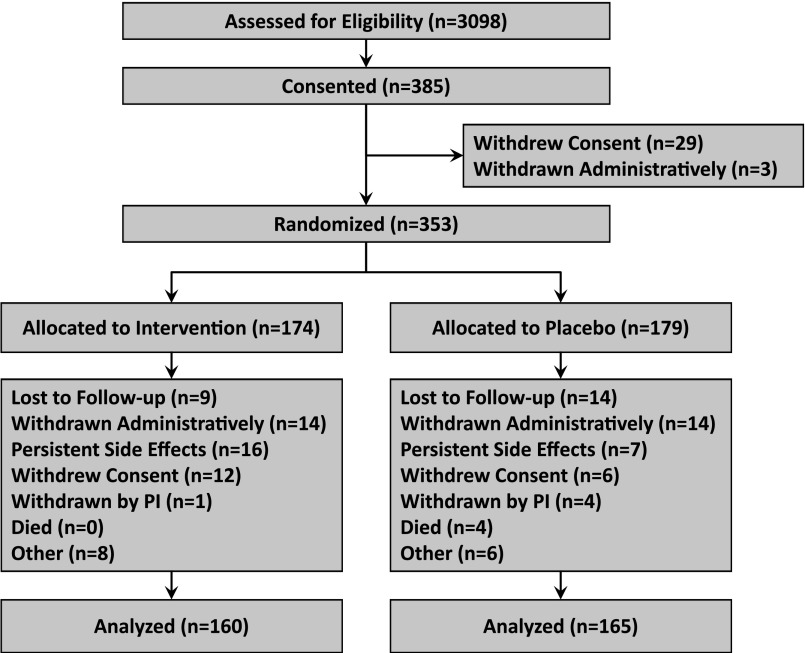
A total of 3098 patients were assessed for eligibility (using the inclusion and exclusion criteria described above) and 385 gave consent (Figure 4). Twenty-nine patients gave consent initially, but voluntarily elected not to participate in the study before randomization, and three patients were administratively withdrawn before randomization because of ineligibility; thus, 174 patients were allocated to the intervention and 179 patients were allocated to placebo. After randomization, 28 patients from a single site (14 in each group) were administratively withdrawn from the study because of numerous protocol violations at that site, which resulted in termination of the site participation. At the end of the study, 160 patients in the intervention group and 165 patients in the placebo group were included in the intention-to-treat analysis. Of these patients, 238 (73%) completed the study (treatment group, 114; placebo group, 124). A data safety monitoring board oversaw the safety profile of the study, and no interim efficacy analyses were planned or conducted. The Western and Vanderbilt University Medical Center institutional review boards approved the study, and all patients provided written informed consent before study enrollment.
Figure 4.
Flow chart of study populations, including the number of patients who were screened, gave consent, underwent randomization, and completed the study treatment or presented the primary variable. “Other” reasons included the following: for the intervention group (n=8 patients): nursing home placement (n=1); patient no longer wishes to take study drug (n=2); patient states he is unable to swallow the pills (n=1); stopped taking study drug (n=4). For the placebo group (6 patients): patient diagnosed with cancer during the study (n=2); patient no longer wishes to take study drug (n=2); patient says she has no more medicine to continue (n=1); nursing home placement (n=1). PI, principal investigator.
Compliance and adverse events were assessed monthly during months 1–6. Drug compliance forms were completed at each of the monthly study visits. Patients were instructed to bring in their drug bottles, and the number of remaining pills were counted and recorded. For both vitamin E and ALA, the overall rate of noncompliance (pills not taken) was 9%. Protocol compliance at the sites was monitored as follows: (1) In-person monitoring visits were conducted at the participating sites. The monitor randomly selected approximately 10% of the enrolled patients and thoroughly reviewed the consent, inclusion/exclusion criteria, and completed case report forms. The monitor reviewed and discussed enrollment, protocol adherence, and adverse events with the investigator and study coordinators as needed. All queries were resolved, and the data clarification information was sent to the data coordinating center (Vanderbilt University)—the only exception being the site that was closed. (2) On a monthly basis, participating sites submitted case report forms to the data coordinating center. Data on these forms were entered into a database, which was double-checked in terms of data entry accuracy. Sites were queried for missing or questionable case report form entries. All queries were resolved, with the exception of the site that was closed. (3) On a monthly basis, participating sites submitted samples to the sample storage facility (Maine Medical Center). The samples were double-checked against the sample worksheets and entered into a sample log, and the samples stored at −80°C. Sites were queried for missing samples or questionable labeling. All queries were resolved, with the exception of the site that was closed.
Outcomes
The primary outcome was a change in plasma hsCRP and IL-6 concentrations. The secondary outcomes were changes in plasma F2-isoprostane and F2-isofuran concentrations, erythropoietic index, and serum albumin concentrations. The erythropoietic index was calculated as monthly ESA dose adjusted by hemoglobin concentration of the previous month.
Analytical Procedures
All blood sampling were performed at the participating dialysis clinics. Blood sample measurements included hsCRP, IL-6 and plasma F2 isoprostanes and isofurans. Routine chemistries were obtained from the patient charts. Blood was drawn into Vacutainer (Becton-Dickinson, Franklin Lakes, NJ) tubes containing EDTA for plasma separation. Samples were transported on ice and immediately centrifuged at 20°C at 3000 rpm for 15 minutes. Supernatants were stored in aliquots locally at −20°C, then shipped on dry ice to the central storage facility where they were stored at −70°C until further use. Plasma hsCRP levels were measured by ELISA using high-sensitivity kits from Diagnostic Systems Laboratories (Webster, TX) and expressed in milligrams per liter. Plasma IL-6 cytokine concentrations were determined by ELISA with kits from BioSource International (Camarillo, CA). Oxidative stress was quantified by simultaneous measurement of plasma F2-isoprostane and isofuran concentrations. Internal standard [2H4]-15-F2T-isoprostane was added to plasma, and the sample was purified by sequential C-18 and silica solid-phase extraction and then derivatized to penta-fluorobenzyl ester, trimethylsilyl ether for gas chromatography/negative ion chemical ionization/mass spectrometry analysis.56
Statistical Analyses
Sample size was computed on the basis of changes in natural log of hsCRP from baseline to 6 months using a t test. According to our preliminary data, including 50 patients with ESRD without any specific therapy who initiated MHD, mean hsCRP concentrations was reduced by 2.2 mg/L in hsCRP values after 6 months of MHD (initial values were from 20.1 mg/L). The intervention was anticipated to reduce hsCRP values by 20%, a change from 20.1 to 16.08 mg/L (d=4.02 mg/L), whereas the control group was expected to have a decrease of only 10% from baseline, a change from 20.1 to 18.09 mg/L. Because hsCRP concentration is known to be skewed, natural log transformed hsCRP was used for estimating mean±SD (SD of log hsCRP, 0.4). A sample size of 175 in each group (total, 350) was estimated to achieve 80% power with a two-sided 5% significance level.
Descriptive statistics are presented with proportions or means ± SDs for categorical variables or medians and interquartile ranges for continuous variables. Patient baseline characteristics were compared using the chi-squared test for categorical variables and the Mann-Whitney U test for continuous variables. Concentration of outcomes of interest at baseline was defined as the mean of the measurement at enrollment and 1 month after enrollment (month 0).
Concentrations of hsCRP, IL-6, and serum albumin between the combination antioxidant therapy and placebo groups were separately compared at baseline and 1, 3, and 6 months using Mann–Whitney U tests. The effect of the combination antioxidant therapy was further assessed by comparing the change in hsCRP, IL-6, and serum albumin at 1 month, 3 months, or 6 months from baseline between the treatment groups using general linear models with bootstrap covariance accounting for correlation among repeated measures within a patient. The difference in change from baseline was assessed in the bootstrap general linear model by including an interaction term between treatment and time. The baseline values of outcome variables were adjusted as a model covariate, as well as other baseline covariates including age, body mass index, race (African American versus non–African American), presence or absence of diabetes mellitus, and history of cardiovascular disease at baseline. The effect of treatment at each of the three time points was assessed only when the global test was rejected.
Concentration of F2 isoprostanes and F2 isofurans were measured at baseline and 6 months of therapy. The effect of the combination antioxidant therapy was compared at each time point using Mann–Whitney U test. An analysis of covariance model was used to compare the change of F2 isoprostanes and F2 isofurans at 6 month of study from baseline between the treatment arms. Baseline concentration of F2 isoprostanes and F2 isofurans was adjusted as a covariate in the model as well as age, body mass index, race (African American versus non–African American), presence or absence of diabetes mellitus, and history of cardiovascular disease at baseline. Residuals of all models were assessed graphically for normality, and dependent variable was transformed to correct non-normal residuals if needed.
ESA dose and monthly ESA dose adjusted by the last hemoglobin concentration of the month were compared every month between the two treatment groups using a Mann–Whitney U test. For each patient, we further calculated the area under the curve of the monthly ESA dose and monthly ESA dose adjusted by the last hemoglobin concentration. A Mann–Whitney U test was used to compare the area under the curve of the monthly ESA dose and monthly ESA dose adjusted by the last hemoglobin concentration between the two treatment groups.
Dropout and missing values were considered in our statistical analyses. Univariate comparison between two treatment groups at each particular time point were limited to patients who contributed data to that time point; however, in our multivariable regression analyses, all 325 patients who ever participated in the study were taken into account and were analyzed until the time at which they dropped out of the study or at the end of the study. By doing this, we aimed to minimize the effect of dropout and missing comparisons to the complete case analyses (n=238) and maximize our power in detecting an effect between treatment groups. All analyses were performed using R-software, version 2.7.2 (www.r-project.org), using two-sided 5% significance level for statistical inference.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors would like to thank the following individuals for participation in the study: Lourdes Terrado, Susan Gerkin, Thomas Woolridge, James Cotton, Hany Rezk, Jeffrey Ryu, Gary Singer, Geoffrey Walker, Michael Akom, Donovan Polack, Vijaykumar Rao, Paul Dykes, and Ronald Hamburger. We would also like to thank Veronica Legg, Phuong Le, Ellen McMonagle, and Elizabeth McMenamin for support for recruitment, laboratory sample processing and measurement, and execution of the study.
This study is sponsored by a nonrestricted grant from Renal Care Group and Fresenius Medical Services-North America, a Clinical Translational Science Award 1UL-1RR024975 from the National Center for Research Resources, K24 DK 62849 from the National Institute of Diabetes and Digestive and Kidney Diseases, R01 HL070938 from the National Heart, Lung, and Blood Institute and Center in Molecular Toxicology, and National Institutes of Health P30 ES000267 from National Institute of Environmental Health Sciences. The sponsors had no influence on the design, execution, analysis and interpretation of the results.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “If Oxidative Stress Is an Appropriate and Specific Target, What Reagent Should We Choose?,” on pages 427–429.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013050545/-/DCSupplemental.
References
- 1.Bayés B, Pastor MC, Bonal J, Juncà J, Hernandez JM, Riutort N, Foraster A, Romero R: Homocysteine, C-reactive protein, lipid peroxidation and mortality in haemodialysis patients. Nephrol Dial Transplant 18: 106–112, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Deicher R, Ziai F, Bieglmayer C, Schillinger M, Hörl WH: Low total vitamin C plasma level is a risk factor for cardiovascular morbidity and mortality in hemodialysis patients. J Am Soc Nephrol 16: 1811–1818, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Himmelfarb J, McMenamin ME, Loseto G, Heinecke JW: Myeloperoxidase-catalyzed 3-chlorotyrosine formation in dialysis patients. Free Radic Biol Med 31: 1163–1169, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM: The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int 62: 1524–1538, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Ikizler TA, Morrow JD, Roberts LJ, Evanson JA, Becker B, Hakim RM, Shyr Y, Himmelfarb J: Plasma F2-isoprostane levels are elevated in chronic hemodialysis patients. Clin Nephrol 58: 190–197, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Maggi E, Bellazzi R, Falaschi F, Frattoni A, Perani G, Finardi G, Gazo A, Nai M, Romanini D, Bellomo G: Enhanced LDL oxidation in uremic patients: An additional mechanism for accelerated atherosclerosis? Kidney Int 45: 876–883, 1994 [DOI] [PubMed] [Google Scholar]
- 7.Stenvinkel P, Diczfalusy U, Lindholm B, Heimbürger O: Phospholipid plasmalogen, a surrogate marker of oxidative stress, is associated with increased cardiovascular mortality in patients on renal replacement therapy. Nephrol Dial Transplant 19: 972–976, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Espe KM, Raila J, Henze A, Blouin K, Schneider A, Schmiedeke D, Krane V, Pilz S, Schweigert FJ, Hocher B, Wanner C, Drechsler C, German Diabetes and Dialysis Study Investigators : Low plasma α-tocopherol concentrations and adverse clinical outcomes in diabetic hemodialysis patients. Clin J Am Soc Nephrol 8: 452–458, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roselaar SE, Nazhat NB, Winyard PG, Jones P, Cunningham J, Blake DR: Detection of oxidants in uremic plasma by electron spin resonance spectroscopy. Kidney Int 48: 199–206, 1995 [DOI] [PubMed] [Google Scholar]
- 10.Jung HH, Choi DH, Lee SH: Serum malondialdehyde and coronary artery disease in hemodialysis patients. Am J Nephrol 24: 537–542, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Handelman GJ, Walter MF, Adhikarla R, Gross J, Dallal GE, Levin NW, Blumberg JB: Elevated plasma F2-isoprostanes in patients on long-term hemodialysis. Kidney Int 59: 1960–1966, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Kaysen GA: The microinflammatory state in uremia: Causes and potential consequences. J Am Soc Nephrol 12: 1549–1557, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Stenvinkel P, Heimbürger O, Paultre F, Diczfalusy U, Wang T, Berglund L, Jogestrand T: Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int 55: 1899–1911, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Yeun JY, Levine RA, Mantadilok V, Kaysen GA: C-Reactive protein predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis 35: 469–476, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Kimmel PL, Phillips TM, Simmens SJ, Peterson RA, Weihs KL, Alleyne S, Cruz I, Yanovski JA, Veis JH: Immunologic function and survival in hemodialysis patients. Kidney Int 54: 236–244, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bologa RM, Levine DM, Parker TS, Cheigh JS, Serur D, Stenzel KH, Rubin AL: Interleukin-6 predicts hypoalbuminemia, hypocholesterolemia, and mortality in hemodialysis patients. Am J Kidney Dis 32: 107–114, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Zimmermann J, Herrlinger S, Pruy A, Metzger T, Wanner C: Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int 55: 648–658, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Pecoits-Filho R, Bárány P, Lindholm B, Heimbürger O, Stenvinkel P: Interleukin-6 is an independent predictor of mortality in patients starting dialysis treatment. Nephrol Dial Transplant 17: 1684–1688, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Gaweda AE, Goldsmith LJ, Brier ME, Aronoff GR: Iron, inflammation, dialysis adequacy, nutritional status, and hyperparathyroidism modify erythropoietic response. Clin J Am Soc Nephrol 5: 576–581, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pupim LB, Himmelfarb J, McMonagle E, Shyr Y, Ikizler TA: Influence of initiation of maintenance hemodialysis on biomarkers of inflammation and oxidative stress. Kidney Int 65: 2371–2379, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Brigelius-Flohé R, Traber MG: Vitamin E: Function and metabolism. FASEB J 13: 1145–1155, 1999 [PubMed] [Google Scholar]
- 22.Islam KN, O’Byrne D, Devaraj S, Palmer B, Grundy SM, Jialal I: Alpha-tocopherol supplementation decreases the oxidative susceptibility of LDL in renal failure patients on dialysis therapy. Atherosclerosis 150: 217–224, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Panzetta O, Cominacini L, Garbin U, Fratta Pasini A, Gammaro L, Bianco F, Davoli A, Campagnola M, De Santis A, Pastorino AM, et al. : Increased susceptibility of LDL to in vitro oxidation in patients on maintenance hemodialysis: Effects of fish oil and vitamin E administration. Clin Nephrol 44: 303–309, 1995 [PubMed] [Google Scholar]
- 24.Yukawa S, Hibino A, Maeda T, Mimura K, Yukawa A, Maeda A, Kishino M, Sonobe M, Mune M, Yamada Y, Nisideet al. : Effect of alpha-tocopherol on in vitro and in vivo metabolism of low-density lipoproteins in haemodialysis patients. Nephrol Dial Transplant 10[Suppl 3]: 1–3, 1995 [DOI] [PubMed] [Google Scholar]
- 25.Saran R, Novak JE, Desai A, Abdulhayoglu E, Warren JS, Bustami R, Handelman GJ, Barbato D, Weitzel W, D’Alecy LG, Rajagopalan S: Impact of vitamin E on plasma asymmetric dimethylarginine (ADMA) in chronic kidney disease (CKD): A pilot study. Nephrol Dial Transplant 18: 2415–2420, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Himmelfarb J, Kane J, McMonagle E, Zaltas E, Bobzin S, Boddupalli S, Phinney S, Miller G: Alpha and gamma tocopherol metabolism in healthy subjects and patients with end-stage renal disease. Kidney Int 64: 978–991, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Jiang Q, Christen S, Shigenaga MK, Ames BN: Gamma-tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am J Clin Nutr 74: 714–722, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Boaz M, Smetana S, Weinstein T, Matas Z, Gafter U, Iaina A, Knecht A, Weissgarten Y, Brunner D, Fainaru M, Green MS: Secondary prevention with antioxidants of cardiovascular disease in endstage renal disease (SPACE): Randomised placebo-controlled trial. Lancet 356: 1213–1218, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Himmelfarb J, McMenamin E, McMonagle E: Plasma aminothiol oxidation in chronic hemodialysis patients. Kidney Int 61: 705–716, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Himmelfarb J, McMonagle E, McMenamin E: Plasma protein thiol oxidation and carbonyl formation in chronic renal failure. Kidney Int 58: 2571–2578, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Tepel M, van der Giet M, Statz M, Jankowski J, Zidek W: The antioxidant acetylcysteine reduces cardiovascular events in patients with end-stage renal failure: A randomized, controlled trial. Circulation 107: 992–995, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Jones W, Li X, Qu ZC, Perriott L, Whitesell RR, May JM: Uptake, recycling, and antioxidant actions of alpha-lipoic acid in endothelial cells. Free Radic Biol Med 33: 83–93, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Himmelfarb J, McMonagle E: Albumin is the major plasma protein target of oxidant stress in uremia. Kidney Int 60: 358–363, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Witko-Sarsat V, Friedlander M, Nguyen Khoa T, Capeillère-Blandin C, Nguyen AT, Canteloup S, Dayer JM, Jungers P, Drüeke T, Descamps-Latscha B: Advanced oxidation protein products as novel mediators of inflammation and monocyte activation in chronic renal failure. J Immunol 161: 2524–2532, 1998 [PubMed] [Google Scholar]
- 35.Nguyen-Khoa T, Massy ZA, De Bandt JP, Kebede M, Salama L, Lambrey G, Witko-Sarsat V, Drüeke TB, Lacour B, Thévenin M: Oxidative stress and haemodialysis: Role of inflammation and duration of dialysis treatment. Nephrol Dial Transplant 16: 335–340, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Bolton CH, Downs LG, Victory JG, Dwight JF, Tomson CR, Mackness MI, Pinkney JH: Endothelial dysfunction in chronic renal failure: Roles of lipoprotein oxidation and pro-inflammatory cytokines. Nephrol Dial Transplant 16: 1189–1197, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Mezzano D, Pais EO, Aranda E, Panes O, Downey P, Ortiz M, Tagle R, González F, Quiroga T, Caceres MS, Leighton F, Pereira J: Inflammation, not hyperhomocysteinemia, is related to oxidative stress and hemostatic and endothelial dysfunction in uremia. Kidney Int 60: 1844–1850, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Kalantar-Zadeh K, Brennan ML, Hazen SL: Serum myeloperoxidase and mortality in maintenance hemodialysis patients. Am J Kidney Dis 48: 59–68, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Coombes JS, Fassett RG: Antioxidant therapy in hemodialysis patients: A systematic review. Kidney Int 81: 233–246, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Smith KS, Lee CL, Ridlington JW, Leonard SW, Devaraj S, Traber MG: Vitamin E supplementation increases circulating vitamin E metabolites tenfold in end-stage renal disease patients. Lipids 38: 813–819, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Nolin TD, Ouseph R, Himmelfarb J, McMenamin ME, Ward RA: Multiple-dose pharmacokinetics and pharmacodynamics of N-acetylcysteine in patients with end-stage renal disease. Clin J Am Soc Nephrol 5: 1588–1594, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galli F, Floridi AG, Floridi A, Buoncristiani U: Accumulation of vitamin E metabolites in the blood of renal failure patients. Clin Nutr 23: 205–212, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Schultz M, Leist M, Elsner A, Brigelius-Flohé R: alpha-Carboxyethyl-6-hydroxychroman as urinary metabolite of vitamin E. Methods Enzymol 282: 297–310, 1997 [DOI] [PubMed] [Google Scholar]
- 44.Schultz M, Leist M, Petrzika M, Gassmann B, Brigelius-Flohé R: Novel urinary metabolite of alpha-tocopherol, 2,5,7,8-tetramethyl-2(2′-carboxyethyl)-6-hydroxychroman, as an indicator of an adequate vitamin E supply? Am J Clin Nutr 62[Suppl]: 1527S–1534S, 1995 [DOI] [PubMed] [Google Scholar]
- 45.Danielski M, Ikizler TA, McMonagle E, Kane JC, Pupim L, Morrow J, Himmelfarb J: Linkage of hypoalbuminemia, inflammation, and oxidative stress in patients receiving maintenance hemodialysis therapy. Am J Kidney Dis 42: 286–294, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Oberg BP, McMenamin E, Lucas FL, McMonagle E, Morrow J, Ikizler TA, Himmelfarb J: Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int 65: 1009–1016, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Castilla P, Echarri R, Dávalos A, Cerrato F, Ortega H, Teruel JL, Lucas MF, Gómez-Coronado D, Ortuño J, Lasunción MA: Concentrated red grape juice exerts antioxidant, hypolipidemic, and antiinflammatory effects in both hemodialysis patients and healthy subjects. Am J Clin Nutr 84: 252–262, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Shema-Didi L, Sela S, Ore L, Shapiro G, Geron R, Moshe G, Kristal B: One year of pomegranate juice intake decreases oxidative stress, inflammation, and incidence of infections in hemodialysis patients: a randomized placebo-controlled trial. Free Radic Biol Med 53: 297–304, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Sakata T, Furuya R, Shimazu T, Odamaki M, Ohkawa S, Kumagai H: Coenzyme Q10 administration suppresses both oxidative and antioxidative markers in hemodialysis patients. Blood Purif 26: 371–378, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Li H, Horke S, Förstermann U: Oxidative stress in vascular disease and its pharmacological prevention. Trends Pharmacol Sci 34: 313–319, 2013 [DOI] [PubMed] [Google Scholar]
- 51.Diepeveen SH, Verhoeven GW, Van Der Palen J, Dikkeschei LD, Van Tits LJ, Kolsters G, Offerman JJ, Bilo HJ, Stalenhoef AF: Effects of atorvastatin and vitamin E on lipoproteins and oxidative stress in dialysis patients: A randomised-controlled trial. J Intern Med 257: 438–445, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Lu L, Erhard P, Salomon RG, Weiss MF: Serum vitamin E and oxidative protein modification in hemodialysis: A randomized clinical trial. Am J Kidney Dis 50: 305–313, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Tsimikas S: In vivo markers of oxidative stress and therapeutic interventions. Am J Cardiol 101[10A]: 34D–42D, 2008 [DOI] [PubMed] [Google Scholar]
- 54.Steinberg D, Witztum JL: Is the oxidative modification hypothesis relevant to human atherosclerosis? Do the antioxidant trials conducted to date refute the hypothesis? Circulation 105: 2107–2111, 2002 [DOI] [PubMed] [Google Scholar]
- 55.Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ, 2nd: A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci U S A 87: 9383–9387, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fessel JP, Porter NA, Moore KP, Sheller JR, Roberts LJ, 2nd: Discovery of lipid peroxidation products formed in vivo with a substituted tetrahydrofuran ring (isofurans) that are favored by increased oxygen tension. Proc Natl Acad Sci U S A 99: 16713–16718, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Milne GL, Yin H, Brooks JD, Sanchez S, Jackson Roberts L, 2nd, Morrow JD: Quantification of F2-isoprostanes in biological fluids and tissues as a measure of oxidant stress. Methods Enzymol 433: 113–126, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.