Abstract
Recent data highlight the role of the proximal tubule (PT) in reabsorbing, processing, and transcytosing urinary albumin from the glomerular filtrate. Innovative techniques and approaches have provided exciting insights into these processes, and numerous investigators have shown that selective PT cell defects lead to significant albuminuria, even reaching nephrotic range in animal models. Thus, the mechanisms of albumin reabsorption and transcytosis are undergoing intense study. Working in concert with megalin and cubilin, a nonselective multireceptor complex that predominantly directs proteins for lysosomal degradation, the neonatal Fc receptor (FcRn) located at the brush border of the apical membrane has been implicated as the “receptor” mediating albumin transcytosis. The FcRn pathway facilitates reabsorption and mediates transcytosis by its pH-dependent binding affinity in endosomal compartments. This also allows for selective albumin sorting within the PT cell. This reclamation pathway minimizes urinary losses and catabolism of albumin, thus prolonging its serum half-life. It may also serve as a molecular sorter to preserve and reclaim normal albumin while allowing “altered” albumin to be catabolized via lysosomal pathways. Here, we critically review the data supporting this novel mechanism.
Keywords: endocytosis, transcytosis, nephrotic syndrome, FcRn, megalin, cubulin
Although the importance of urinary albumin in disease progression is known, the key mechanisms mediating the presence and toxic effects of albuminuria remain to be determined. Recently, the quantitative role of the glomerular filtration barrier (GFB) and the proximal tubule (PT) cell (PTC) in the development of albuminuria has been reexamined. Different lines of evidence, from multiple investigative teams, now suggest that the filtration of albumin, under physiologic conditions, is greater than previously determined. These data suggest an increased clinical role for the PT in minimizing albuminuria through the reabsorption of albumin. Emerging data also suggest that both glomerular permeability and PTCs play fundamental, physiologic, synergistic, interactive, and dynamic roles in the renal handling of albumin. Furthermore, it appears that PTCs, especially in the S1 segment, have specific mechanisms for efficiently and effectively reabsorbing and transcytosing albumin (reclamation). Therefore, the purpose of this review is to describe the emerging data regarding PTC albumin handling and provide a framework for considering future exciting, insightful, and novel studies with direct clinical relevance.
This review is not intended to debate the important role of the GFB but to emphasize that the PT should be considered important both under physiologic and pathologic conditions. We believe that glomerular or PTC defects can and do result in proteinuria. Specifically, we outline current data supporting PT uptake of albumin and mechanisms of reabsorption and transcytosis, and we propose a mechanism for intracellular sorting between degradation and transcytotic pathways based on pH-dependent binding.
Dysfunctional PTCs Lead to Albuminuria
For nearly 30 years albumin has been known to be reabsorbed by PTCs.1 Albumin is a 66-kD, 585–amino acid, negatively charged globular protein found in plasma of mammals. It is produced and excreted by the liver and is the most abundant protein in plasma. Its half-life is approximately 35–39 hours in rodents and an impressive 19 days in humans.2 Mammals have developed important cellular mechanisms for minimizing albumin turnover, thus enabling a long plasma half-life. Serum albumin is multifunctional as it buffers pH; provides oncotic pressure; and is a carrier protein for a wide range of molecules, including amino acids, fatty acids, inorganic ions, medications, and metabolites.3,4 Preventing or reducing urinary albumin excretion thus makes the kidney a key player in “protecting” the organism from excessive loss of albumin and its ligands. Albumin loss in urine has long been used as a marker of kidney injury, whether it originates from glomerular dysfunction, defective PT reabsorption, or a combination.
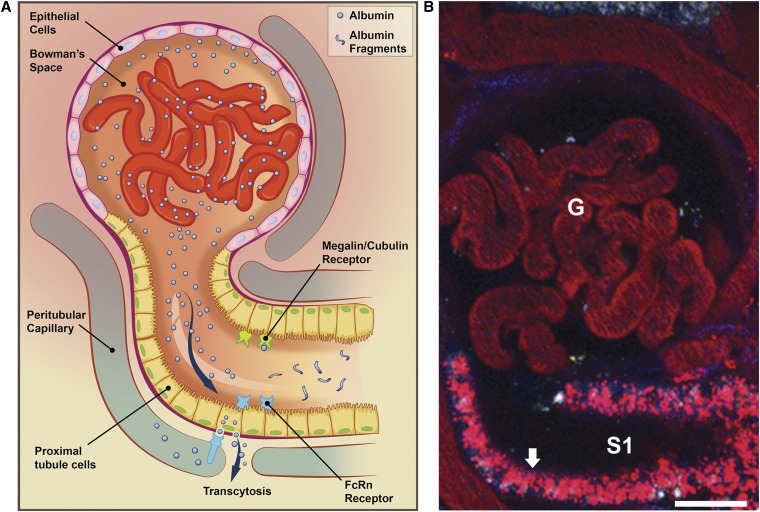
Multiple investigative teams, using various preclinical model systems, have shown the PTCs, especially the S1 segment, have effective and efficient mechanisms of reabsorbing, transcytosing, and processing filtered albumin. Mechanisms for PTC uptake and metabolism of filtered albumin (Figure 1) include receptor-mediated clathrin-dependent endocytosis and fluid-phase endocytosis. Both processes can result in lysosomal targeting for degradation (degradation pathway),5,6 and new data indicate that transcytosis moves albumin across the cell from apical to basolateral membranes into the extracellular fluid (reclamation pathway).7–9 The overall quantitative importance of these processes is indicated by resulting albuminuria when selective defects occur in the involved tubular transport processes (Table 1). Individual disruption of numerous specific PTC processes, have been documented to cause proteinuria and albuminuria. In most cases the increase is mild to moderate, including loss of megalin and cubulin. Defects in the well studied multiligand endocytic receptor complex, megalin and cubilin, yield increased levels of albuminuria and proteinuria, suggesting a role in albumin reabsorption and metabolism.5,6 Bardoxolone methyl, an anti-inflammatory mediator, is known to cause significant albuminuria by decreasing renal expression of megalin but not cubilin.10 Rats receiving total-body irradiation lose the ability of albumin and megalin to bind to cubilin, resulting in albuminuria.11 Knockout mice lacking the apical Na+-H+ exchanger isoform 3 (NHE-3)12 develop albuminuria. Mutations in ClC-5 in apical endosomes in three different mouse models, as seen in Dent disease, have demonstrated defective receptor-mediated endocytosis and fluid-phase endocytosis, deficient endosomal acidification, decreased internalization of the sodium-phosphate cotransporter 2 and NHE-3, and proteinuria.13–15 Defective endocytosis in ClC-5 knockout mice is now known to be due to trafficking defects related to selective loss of brush-border cubilin and megalin, causing albuminuria.16 Rab 38 dysfunction, or lack of function, is suggested to play a significant role in albuminuria in rats by decreasing endocytosis of colloidal gold-coupled albumin without modifying glomerular permeability.17 The degree of proteinuria correlated best with the Rab 38 mutation, rather than the mutation in Fawn Hooded Hypertensive congenic rat strains associated with increased albumin permeability.18,19 Statins have become of interest recently in albumin reabsorption. Studies have shown that statins may inhibit guanosine triphosphatase prenylation, which reduces PT endocytosis and enhances albuminuria and proteinuria.20–22 Finally, in rats with selective PTC injury induced by using d-serine23 or by expressing and activating the diphtheria toxin receptor on PTCs,24–26 heavy albuminuria occurs without associated glomerular morphologic injury, neither histologic nor electron microscopic.25,26
Figure 1.
Albumin filtration across the glomerulus is greater than previously thought and reclaimed by the PTC, especially S1 cells. (A) Albumin filtered at the level of the glomerular capillaries into the Bowman space is taken up after binding by the megalin-cubilin receptor complex or perhaps by the FcRn lining the brush border of proximal tubular cells. Albumin is internalized to PTCs by receptor-mediated endocytosis via clathrin-coated vesicles and fluid-phase endocytosis. From there it can be catabolized via lysosomal degradation or can be transcytosed. Albumin fragments in the urinary lumen result from lysosomal exocytosis or peptide hydrolysis by apical membrane proteases. (B) In vivo image of 25-micron three-dimensional volume showing amounts of Texas red–labeled albumin uptake into PTCs (arrow), especially the S-1 segment (S1). G, glomerular capillaries. Bar=20 µm.
Table 1.
Data implicating a role for the PT in albumin processing and/or albuminuria
| Process Implicated or Defective | Reference |
|---|---|
| d-Serine–induced PTC injury | Carone and Ganote, 197523 |
| Megalin-cubilin complex | Birn, et al., 20005; Christensen and Birn, 20016; Wang et al., 200515 |
| ClC-5 knockout | Piwon et al., 200013; Christensen et al., 200316 |
| Total-body irradiation | Yammani et al., 200211 |
| NHE-3 knockout | Gekle et al., 200412 |
| Statins | Sidaway et al., 200420; Verhulst et al., 200421; Atthobari et al., 200622 |
| Rab 38 | Rangel-Filho et al., 200518; Williams et al., 201119; Rangel-Filho et al., 201317 |
| Increased GSCs | Russo et al., 20077 |
| Transcytosis | Russo et al., 2007,7 Sandoval et al., 20128 |
| FcRn | Sarav et al., 2009100 |
| Carbon nanotubes | Ruggiero et al., 2010126 |
| Bardoxolone | Reisman et al., 201210 |
| Diphtheria toxin–induced PTC injury | Grgic et al., 201225; Sekine et al., 201224; Zhang et al., 201226 |
Multiple PTC defects have been shown to lead to significant albuminuria. Notably, selective PTC injury using dophtheria toxin induction, essentially eliminating any PT uptake of albumin, resulted in severe, but reversible, albuminuria without histologic or electron microscopic changes.
These recent studies with diphtheria toxin, by three independent groups, have emphasized the magnitude of filtered albumin by selectively injuring the PTC, thus causing global PTC dysfunction and allowing all filtered albumin to end up in the urine. Thus, activating the receptor with diphtheria toxin24–26 caused marked and sustained dose-dependent selective PTC injury that resulted in nephrotic range proteinuria.
Importance of PTC Albumin Reabsorption
There remains considerable controversy around glomerular albumin permeability. Numerous techniques and experimental approaches have been used to determine the quantitative role of glomerular albumin permeability in albuminuria. Values for the glomerular sieving coefficient of albumin have ranged from <0.001 to 0.07 under various physiologic and pathologic conditions using different techniques.27 Of particular importance has been the use of Munich–Wistar (MW) rats that have surface glomeruli, allowing for direct dynamic visualization, instrumentation, and manipulation. MW Fromter (MWF) rats have many surface glomeruli, have been used in micropuncture studies, and spontaneously develop hypertension and progressive albuminuria beginning by week 8 and increasing to urinary albumin excretion>300 mg/24 hours by week 32. By week 40, 50% of glomeruli are sclerotic.28–30 The Heymann nephritis rat model has also been used to study urinary protein loss and was significant in identifying megalin, found largely at the apical membrane of the PTC, as an autoantigen in membranous glomerulopathy.31 MW Simonsen rats have fewer surface glomeruli and do not develop age-related spontaneous albuminuria under physiologic conditions. Mice unfortunately lack surface glomeruli, and therefore direct visualization methods cannot be performed, unless pathologic processes, such as ureteral obstruction for several days, are used.32 This results in formation of atubular glomeruli and extensive alterations and remodeling of PT and glomerular cells.
Micropuncture studies in MWF rats with surface glomeruli measured low glomerular filtration of albumin in fasting states, with a glomerular sieving coefficient (GSC) of 0.00057–0.00062, consistent with low amounts of measured albumin observed in excreted urine (<30 mg/d).33–38 This has been attributed to the charge barrier and size selectivity at the glomerular filtration barrier. Previous in vivo rat filtration studies and noninvasive studies using isolated perfused rat kidneys showed a much higher GSC of albumin using [3H]albumin. Measuring total radioactivity in urine and inhibiting protein uptake in the PTC showed that the GSC of albumin may actually be approximately 0.074- to >120-fold greater than previously thought.39 High GSCs for albumin were also observed by another group using glomerular volumetric analysis in rat glomeruli (0.02±0.01).40 This finding was strengthened by other groups showing that high-molecular-weight proteins and dextrans, which have similar radius and molecular mass as albumin (3.6 nm and 66 kDa) and are not reabsorbed through receptor-mediated endocytosis, had similarly high GSCs in normal kidneys (pancreatic isoamylase: 3.4 nm, 45 kD, GSC of 0.0341; horseradish peroxidase: 3.0 nm, 40 kD, GSC of 0.06; Bence-Jones protein: 2.8 nm, 44 kD, GSC of 0.0942). Recent data using enhanced scanning electron microscopy have also shown that podocyte slit-diaphragm pore size is much larger than previously thought and is sufficiently large enough to allow for albumin filtration.43
Intravital in vivo two-photon microscopy studies, which allow four-dimensional analysis (volume and time) of physiologic processes, permit direct visualization and quantification of glomerular filtration and quantitation of PTC uptake.44–46 This has allowed direct visualization and determination of GSCs for albumin (GSCA), subcellular trafficking, transcytosis, catabolism, and reclamation of proteins from glomerular filtrate by PTCs.45,46 Through use of this technique, MW Simonsen rats, which do not develop spontaneous albuminuria, have a GSCA of 0.034 under physiologic fed states, while simultaneously measuring a GSC of 1.0 for inulin and approximately a 500-fold lower GSC for high-molecular-weight dextrans.7,47 The GSCA for MW Simonsen rats in fasting states is considerably lower at 0.016.8 MWF rats, which develop albuminuria spontaneously with aging, have a lower fed GSCA of 0.010 and also display a GSCA reduction in fasting states to 0.007. Because micropuncture studies were always performed on fasting MWF rats, these studies indicate a much closer agreement between micropuncture studies and two-photon studies than previously reported when comparing two different rat strains.8,48,49 They also indicate that feeding has a substantial effect on urinary albumin filtration.8
Controversy remains regarding the extent of glomerular filtration of albumin and similar-sized dextrans, used to model albumin filtration, as observed by two-photon microscopy. Detection of albumin in the glomerular filtrate requires maximizing the signal through use of the correct fluorescent probe, depth of study, site selection, detector sensitivity, and particular detail to background subtraction of existing autofluorescence.8,50 Work by Peti-Peterdi and Tanner using two-photon microscopy reported values significantly lower than we have published,51–53 which are closer to those from micropuncture studies. Peti-Peterdi and Tanner collectively pointed to multiple aspects of our studies as being causes for our elevated values. Addressing their points with data,8 we refuted these assertions. Of particular importance, our most recent publication points to photo-multiplier tube detector offset settings as the factor probably explaining the observed differences.50 These settings determine detector sensitivity and can cause variation in albumin permeability values spanning several orders of magnitude within the same glomerulus. Nakano et al.51 state in their Methods section that the detector offset was adjusted for each individual glomeruli to reduce nonspecific fluorescence (autofluorescence) before and after dye injections. This is a departure from textbook approaches to correctly adjusting detector settings.54 This approach was probably used to constrain tissue autofluorescence intensity to within the same low values derived from electronic detector noise; in fact, autofluorescence is a tangible phenomenon that when minimized by adjusting detector offset results in a progressively marked decrease in detector sensitivity. This lack of sensitivity is supported by a recent publication by Schießl and Castrop,55 which also reports very low permeability values for albumin. In their work, the offset values used in their background image (Figure 1B, blue warning marker indicating values at zero) are an identical match to offset values in our study50 showing decreased detector sensitivity to low-intensity fluorescence. A detailed method of our approach to determining GSC values can be found elsewhere.56 Therefore, it appears the filtration of albumin across the GFB is dynamic with regard to feeding, varies between rat species, and is probably greater than previously determined by micropuncture studies.
Endocytosis by the PT
Classically, cellular uptake of proteins and other molecules by endocytotic pathways has been attributed to receptor-mediated endocytosis by apical membrane-bound receptors, such as megalin and cubilin, clustering into clathrin-coated pits. Coated pits make up between 0.4% and 3.8% of the cell’s surface, depending on the cell type.57 These pathways have been studied extensively, and numerous reviews exist.58,59 In addition, other mechanisms of protein internalization have also been described, including caveolin-dependent internalization and fluid-phase endocytosis. Molecules endocytosed in this manner are similarly routed to the sorting endosomal compartment and either are degraded through lysosomal pathways or undergo transcytosis back into circulation.60 Albumin uptake by nonselective fluid-phase endocytosis is probably a quantitatively important process in PTCs, as shown by the rapid cellular uptake of molecules not having receptors on the apical membrane, such as neutral fluorescent dextrans (markers of fluid-phase endocytosis).61,62
The endocytic apparatus is found throughout the PT, although clathrin-coated pits and vesicles are notably fewer in the S3 segment.63 Expectedly, protein reabsorption and degradation are greatest in the S1 segment of the PTCs and least in the S3 segment.64–66 Kinetic studies of the rat PT have shown that internalization of cargo at the brush border is highly active. The membrane and trapped fluid (luminal fluid) contained in the apical membrane invaginations are internalized within 78 seconds.67 This rapid rate of uptake means a great deal of luminal fluid is internalized via endocytic vesicles and probably indicates an important role for fluid phase endocytosis. However, quantifying the overall importance of fluid-phase endocytosis has been difficult because all endocytic vesicles contain fluid and thus luminal contents.
Albumin degradation can occur in multiple sites. Degraded lysosomal albumin fragments were initially thought to be completely recycled back into circulation.1 But newer studies using isolated perfused rat kidneys,68 in vitro studies with HK-2 cells,69 and in vivo models with Sprague-Dawley rats have shown that albumin can be rapidly degraded into small peptides and released back into the tubular fluid.66 Use of the CD2AP knockout mouse showed that lack of lysosomal PTC albumin degradation resulted in high levels of intact albumin in urine.68,70 Recent technologies using opossum kidney epithelial cells suggest that albumin uptake and degradation are significantly augmented by flow and fluid shear stress, not static conditions.71 Urine proteases at the apical brush border can likewise hydrolyze and degrade tubular albumin into urinary fragments.72 However, a note of caution is necessary. Modeling in vivo endocytosis to that occurring in cultured cells can be very revealing but also misleading. For example, in vivo PTCs have a rate of endocytosis that is far greater in magnitude than that of cultured PTCs.67,73,74 In addition, the rate of apical endocytosis is many times that of basolateral endocytosis in vivo, but the two are equivalent in cell culture.67,74,75 Therefore, one must not overinterpret cell culture data. Finally, intravenously injected albumin remains intact within the serum and is partially catabolized within PTCs.76
The Megalin-Cubilin Complex
The megalin-cubilin receptor complex is well studied, and myriad reviews have described its function and role in protein absorption and metabolism.6,59,77,78 The dissociation constant (Kd) of albumin to cubilin is estimated at 0.63 µM at a pH of 7.0,5 resulting in a high-affinity, low-capacity pathway of endocytosis that primarily targets product to the lysosome for degradation. Subsequent lysosomal processing and trafficking of resulting amino acids back to the basolateral membrane for transport into plasma occur. Megalin and cubilin work in concert to reabsorb >40 filtered molecules.6,59,79–81 Without this mechanism of retrieval and preservation, protein loss, malnutrition, vitamin deficiencies, and other consequences would ensue. Although both megalin82 and cubilin5 can bind albumin, megalin’s principal role seems to be in catalyzing the retrieval and internalization of apical cubilin-albumin complexes from glomerular filtrate.
This multireceptor retrieval system is thought to have the capacity to process approximately 30–50 μg of albumin daily in mice.83 Disruption of this mechanism results in proteinuria and albuminuria. In megalin knockout models, the internalization of endogenous ligands bound to apical cubilin, especially cubilin-albumin complexes, is markedly reduced. Urinary albumin excretion is increased 6-fold in cubilin knockout mouse models83 and in humans,84 although neither reaches nephrotic range, suggesting that an additional mechanism for albumin reabsorption exist. In fact, a missense mutation in one of the CUBN domains that binds megalin was found in microalbuminuric patients in the general population and in patients with diabetes using a genome-wide association study.85 In two siblings with intermittent proteinuria reaching 2 g daily, exome sequencing was used to identify a homozygous frameshift mutation in cubulin, resulting in decreased albumin uptake in the PT.86 Interestingly, mice deficient in megalin in addition to cubilin did not exhibit any more albuminuria than mice with cubilin deficiency alone,83 suggesting that megalin’s principal role is to facilitate cubilin-albumin internalization. In Dab2 knockout mice (Dab2 is a protein involved in coated pit formation), mild proteinuria was seen.87 Patients with type 1 diabetes and albuminuria had significantly elevated urinary levels of megalin and cubilin, suggesting possible PT shedding of these proteins as a contributing factor to albuminuria in patients with diabetes.88 Moreover, in early streptozotocin-induced diabetes in rats, the GSCA was unchanged but PTC uptake of albumin was markedly reduced.47
Neonatal Fc Receptor
The neonatal Fc receptor (FcRn), discovered by Jones and Waldmann in 1972,89 is a heterodimer with class I MHC-like properties that contains a membrane-bound heavy chain and a β2-microglobulin light chain. Its name originates the fact that it was purified and sequenced by Simister and Rees in 198590 from the intestine of an 11-day-old rat. Wild-type FcRn has two distinct and separate binding sites for albumin and IgG91; binding is low affinity and high capacity at a physiologic pH, with increasing affinity occurring at a lower pH. In humans, FcRn is derived from the FCGRT gene encoded on chromosome 19 located outside of the MHC class I locus on chromosome 6. Rat and mice FcRns are 91% identical, and both are encoded on chromosome 7. Human FcRn has one N-glycan moiety, and its molecular mass is approximately 42–44 kD, while rat FcRn’s molecular mass is 48–52 kD (attributable to three additional N-glycan moieties).92 It is known to reside on vascular endothelium; on epithelial cells of the proximal small intestine, liver, spleen, and lung; on placental syncytiotrophoblasts; on polymorphonuclear neutrophils, monocytes, and phagocytes; on dendritic cells; and in the kidney.90,93–98 Within the kidney, FcRn is found in the vascular endothelia, podocytes, cortical collecting duct, and PT epithelial cells.99 It has been labeled by immunofluorescence in human kidney sections at the brush border of PTCs and in endosomes.99
FcRn is known to transport albumin across membranes, preserving albumin’s function and lifespan as a carrier protein, colloid, and buffer, and one that maintains oncotic hemostasis.100 Its role in fetal immunity by transporting maternal IgG across neonatal placenta and by transcytosis across neonatal intestinal cells is well known.90,101–103 In fact, neonatal overexpression of FcRn in transgenic mice and rabbits increases serum albumin concentrations and further augments humoral immunity, resulting in a 3- to 10-fold increase in IgM and IgG concentrations in serum.104,105 It is thought that elevated immunoglobulin levels would not interfere with albumin processing because of the distinct binding sites for albumin and immunoglobulins, but this concept has not been tested. FcRn mediates transcellular IgG transport in maternal milk during lactation to the newborn.106,107 FcRn is also thought to preserve IgG and confers humoral immunity by podocyte clearance of IgG from the GBM.108 Finally, FcRn is felt to mediate transcytosis and recycling of IgG by PTCs back into circulation.109 The mechanism of FcRn-mediated transcytosis has been well studied in the small intestine, and its role in IgG endocytosis via clathrin-coated pits at low luminal pH is known.110,111 In addition, data exist for other tissues for IgG transport, such as pneumocytes,112 endothelia of small arterioles and capillaries of skeletal muscle and skin,93,113 and vascular endothelia of the central nervous system and the choroid plexus.114
Role of FCRN in PTC Albumin Processing
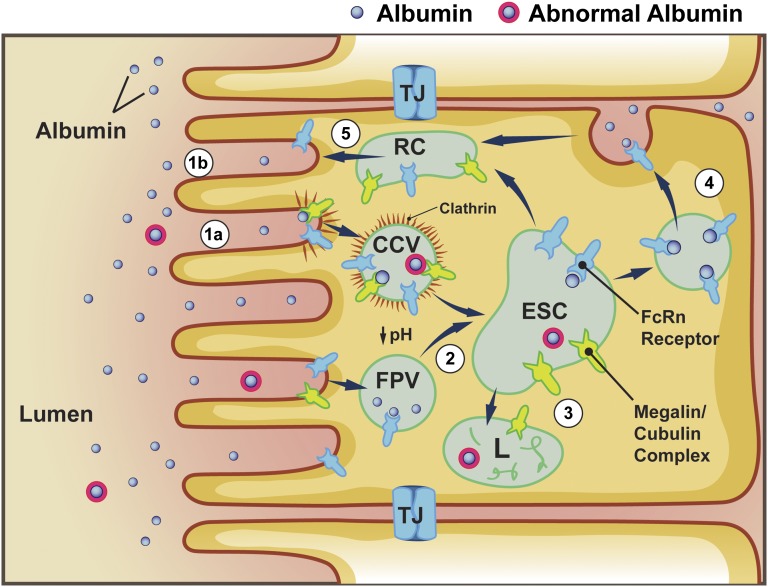
Although not yet fully understood, the role of FcRn appears to be that of intracellular selection, sorting, and preservation of reabsorbed albumin and IgG. FcRn is concentrated into the apical area in the PT. Whether it participates in luminal albumin binding is not known, but this is not favored by the luminal pH. However, the megalin-cubilin–bound albumin within the clathrin-coated pits, and fluid-phase endocytosis vesicles undergo pH reduction to approximately 5.0. At the low pH found in endosomes, albumin dissociates from megalin-cubilin, while FcRn’s affinity to bind both IgG and albumin increases dramatically.90,91,100,115 Thus, albumin is capable of moving from a low-capacity lysosomal degradation pathway39,116,117 to a high-capacity pathway of transcytosis and recycling mediated by FcRn based on inherent binding properties of the receptors.39,118–120 Binding studies have shown that FcRn has a single binding site for albumin that is distinct from the IgG site and that both these interactions are pH dependent. The equilibrium dissociation constant, Kd, is much weaker at a pH of 7.0 (34–408 µM) versus a pH of 5.0 (0.2–0.7 µM).91 Consequently, if albumin is internalized while bound to the megalin-cubulin complex and is trafficked to the late endosomes, it encounters acidic pH and a “handoff” of albumin to the FcRn receptor can occur, thus directing it down the transcytotic pathway. When the transcytotic vesicle fuses with the plasma membrane and encounter neutral physiologic pH, a rapid dissociation of albumin from FcRn will occur, thereby releasing it to the interstitium and ultimately back into the circulation via the FcRn-mediated pathway in the endothelium.110,115,121 The FcRn receptor is recycled back to the apical membrane or apical compartment, ready for another cycle of albumin transcytosis. Of critical importance for albumin dynamics may be how modified albumins (i.e., glycated, carbamylated, and various drugs bound to albumin) affect the albumin–FcRn pH-dependent binding interaction. For instance, increased binding at a neutral pH or decreased binding at an acidic pH may both result in more targeting to lysosomes (Figure 2).
Figure 2.
FcRn mediates pH-dependent transcytosis and intracellular sorting of reabsorbed albumin. Albumin is reabsorbed via both receptor-mediated clathrin-coated pits into vesicles (CCV) (1a) and by fluid-phase (clathrin-negative) endocytosis (1b). Following endocytosis, endosomal acidification occurs (2), causing dissociation of albumin from receptors, such as megalin-cubilin complexes. However, acidification enhances albumin binding to FcRn throughout endocytic compartments; thus, there is exchange of albumin from the megalin-cubulin complex to FcRn. Within the endosomal-sorting compartment (ESC), albumin is directed toward lysosomal degradation or the transcytotic pathway (3). Transcytosis occurs by both vascular and tubular structures mediating albumin delivery to the basolateral membrane (4). Upon fusion with the basolateral membrane, the increase in pH of the extracellular environment causes dissociation of albumin from FcRn; FcRn is then recycled back to the apical membrane via the recycling compartment. It is possible that albumin’s binding to FcRn is reduced by alterations, such as glycosylation and carbamylation; thus, transcytosis of albumin would not occur and albumin would enter the lysosomal pathway. This would provide an intracellular molecular sorting mechanism to preserve physiologic albumin and facilitate catabolism of chemically altered albumin. FPV, fluid-phase vesicle; L, lysosome; RC, recycling compartment; TJ, tight junction.
The first direct evidence for transcytosis of albumin came from PT microperfusion studies.1 Subsequent studies using transmission electron microscopy immunogold studies revealed albumin uptake across the apical membrane and release across the basolateral membrane of PTCs.7 Subsequent two-photon studies showed actual intracellular vesicles and tubules uniting with the basolateral membrane and releasing fluorescently labeled albumin into the interstitium.8 Finally, Tenten et al.9 showed that both negatively charged and neutral albumin released from transgenic podocytes was transcytosed from the filtrate into the blood. Furthermore, genetic deletion of the FcRn receptor in these mice abolished transcytosis of both types of albumin. These data prove FcRn is responsible for mediating albumin transcytosis in the PTC. However, the magnitude of this process remains to be determined.
Functional Significance of PTC Reclamation via FCRN
FcRn knockout mice lacking the neonatal Fc receptor and its ability to recycle filtered protein have been shown to result in plasma albumin with shorter half-lives (reduced to 75% of wild type) and plasma concentrations that are reduced by about 50%.100,122,123 This was shown to result from greater catabolism and clearance of albumin.122 Unfortunately, this study did not quantify urinary albumin excretion. In another study, FcRn knockout mice exhibited more albumin at the brush border and a modest increase in fractional excretion of albumin.100 Mice that have β2-microglobulin or FcRn mutations have reduced half-lives of both IgG and albumin,123,124 and β2-microglobulin knockout mice (which are therefore FcRn deficient) have increased urinary IgG excretion and albuminuria.125
PT-specific FcRn was further implicated when FcRn knockout kidneys were placed in wild-type mice and serum albumin declined to 40%–50% of baseline over 3 weeks. Conversely, serum albumin increased as wild-type kidneys were placed in FcRn knockout mice.100 These data indicate the absence of FcRn results in increased urinary loss of albumin and enhanced catabolism. In PTCs, the lack of transcytotic pathway would shuttle all reabsorbed albumin into the degradation pathway. Further PT-specific studies are needed to more fully examine the exact role of FcRn within the kidney and its function in mediating transcytosis and preventing lysosomal degradation of albumin.
Conclusions
Currently the role of the PTC in albumin reabsorption and reclamation is being rewritten. Numerous single-site alterations cause proteinuria, and complete PTC dysfunction results in a high level of albuminuria, without histologic or electron microscopy structural alterations in the GFB, implying a GSCA greater than previously believed. This has also been shown using intravital two-photon imaging. Reabsorption of filtered albumin involves a high-affinity, low-capacity megalin-cubulin receptor–mediated process and a low-affinity, high-capacity process that we believe is fluid-phase endocytosis. Data on the neonatal Fc receptor within the PT cell suggest that its principal function may be in pH-mediated binding, sorting and intracellular trafficking between transcytosis and degradation pathways. This latter function may be decided based on alterations in albumin binding at low pH to FcRn. Such a mechanism of selective processing and sorting would be evolutionarily critical in reclaiming normal albumin via transcytosis and in the lysosomal catabolism of chemically altered and potentially harmful albumin. Given the quantity of albumin reabsorbed daily, and the prolonged half-life of serum albumin, this is an absolutely essential process. Two-photon intravital studies to delineate the roles of altered GFB permeability, PTC endocytosis, and the intracellular trafficking of albumin are needed in the different animal models available to offer further insight into the renal handling of albumin. Additional reagents and approaches must be developed to allow assessment in human diseases.
Disclosures
None.
Acknowledgments
The authors acknowledge grant support to B.A.M. from the National Institutes of Health (DK 091623 and 079312) and support from the Veterans Administration through a Merit Review award.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Park CH, Maack T: Albumin absorption and catabolism by isolated perfused proximal convoluted tubules of the rabbit. J Clin Invest 73: 767–777, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He XM, Carter DC: Atomic structure and chemistry of human serum albumin. Nature 358: 209–215, 1992 [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharya AA, Grüne T, Curry S: Crystallographic analysis reveals common modes of binding of medium and long-chain fatty acids to human serum albumin. J Mol Biol 303: 721–732, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Kragh-Hansen U: Molecular aspects of ligand binding to serum albumin. Pharmacol Rev 33: 17–53, 1981 [PubMed] [Google Scholar]
- 5.Birn H, Fyfe JC, Jacobsen C, Mounier F, Verroust PJ, Orskov H, Willnow TE, Moestrup SK, Christensen EI: Cubilin is an albumin binding protein important for renal tubular albumin reabsorption. J Clin Invest 105: 1353–1361, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christensen EI, Birn H: Megalin and cubilin: synergistic endocytic receptors in renal proximal tubule. Am J Physiol Renal Physiol 280: F562–F573, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Russo LM, Sandoval RM, McKee M, Osicka TM, Collins AB, Brown D, Molitoris BA, Comper WD: The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: Retrieval is disrupted in nephrotic states. Kidney Int 71: 504–513, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Sandoval RM, Wagner MC, Patel M, Campos-Bilderback SB, Rhodes GJ, Wang E, Wean SE, Clendenon SS, Molitoris BA: Multiple factors influence glomerular albumin permeability in rats. J Am Soc Nephrol 23: 447–457, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tenten V, Menzel S, Kunter U, Sicking EM, van Roeyen CR, Sanden SK, Kaldenbach M, Boor P, Fuss A, Uhlig S, Lanzmich R, Willemsen B, Dijkman H, Grepl M, Wild K, Kriz W, Smeets B, Floege J, Moeller MJ: Albumin is recycled from the primary urine by tubular transcytosis. J Am Soc Nephrol 24: 1966–1980, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reisman SA, Chertow GM, Hebbar S, Vaziri ND, Ward KW, Meyer CJ: Bardoxolone methyl decreases megalin and activates nrf2 in the kidney. J Am Soc Nephrol 23: 1663–1673, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yammani RR, Sharma M, Seetharam S, Moulder JE, Dahms NM, Seetharam B: Loss of albumin and megalin binding to renal cubilin in rats results in albuminuria after total body irradiation. Am J Physiol Regul Integr Comp Physiol 283: R339–R346, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Gekle M, Völker K, Mildenberger S, Freudinger R, Shull GE, Wiemann M: NHE3 Na+/H+ exchanger supports proximal tubular protein reabsorption in vivo. Am J Physiol Renal Physiol 287: F469–F473, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Piwon N, Günther W, Schwake M, Bösl MR, Jentsch TJ: ClC-5 Cl- -channel disruption impairs endocytosis in a mouse model for Dent’s disease. Nature 408: 369–373, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Luyckx VA, Leclercq B, Dowland LK, Yu AS: Diet-dependent hypercalciuria in transgenic mice with reduced CLC5 chloride channel expression. Proc Natl Acad Sci U S A 96: 12174–12179, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang SS, Devuyst O, Courtoy PJ, Wang XT, Wang H, Wang Y, Thakker RV, Guggino S, Guggino WB: Mice lacking renal chloride channel, CLC-5, are a model for Dent’s disease,a nephrolithiasis disorder associated with defective receptor-mediated endocytosis. Hum Mol Genet 9: 2937–2945, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Christensen EI, Devuyst O, Dom G, Nielsen R, Van der Smissen P, Verroust P, Leruth M, Guggino WB, Courtoy PJ: Loss of chloride channel ClC-5 impairs endocytosis by defective trafficking of megalin and cubilin in kidney proximal tubules. Proc Natl Acad Sci U S A 100: 8472–8477, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rangel-Filho A, Lazar J, Moreno C, Geurts A, Jacob HJ: Rab38 modulates proteinuria in model of hypertension-associated renal disease. J Am Soc Nephrol 24: 283–292, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rangel-Filho A, Sharma M, Datta YH, Moreno C, Roman RJ, Iwamoto Y, Provoost AP, Lazar J, Jacob HJ: RF-2 gene modulates proteinuria and albuminuria independently of changes in glomerular permeability in the fawn-hooded hypertensive rat. J Am Soc Nephrol 16: 852–856, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Williams JM, Burke M, Lazar J, Jacob HJ, Roman RJ: Temporal characterization of the development of renal injury in FHH rats and FHH.1BN congenic strains. Am J Physiol Renal Physiol 300: F330–F338, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sidaway JE, Davidson RG, McTaggart F, Orton TC, Scott RC, Smith GJ, Brunskill NJ: Inhibitors of 3-hydroxy-3-methylglutaryl-CoA reductase reduce receptor-mediated endocytosis in opossum kidney cells. J Am Soc Nephrol 15: 2258–2265, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Verhulst A, D’Haese PC, De Broe ME: Inhibitors of HMG-CoA reductase reduce receptor-mediated endocytosis in human kidney proximal tubular cells. J Am Soc Nephrol 15: 2249–2257, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Atthobari J, Brantsma AH, Gansevoort RT, Visser ST, Asselbergs FW, van Gilst WH, de Jong PE, de Jong-van den Berg LT, PREVEND study group : The effect of statins on urinary albumin excretion and glomerular filtration rate: results from both a randomized clinical trial and an observational cohort study. Nephrol Dial Transplant 21: 3106–3114, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Carone FA, Ganote CE: D-serine nephrotoxicity. The nature of proteinuria, glucosuria, and aminoaciduria in acute tubular necrosis. Arch Pathol 99: 658–662, 1975 [PubMed] [Google Scholar]
- 24.Sekine M, Monkawa T, Morizane R, Matsuoka K, Taya C, Akita Y, Joh K, Itoh H, Hayashi M, Kikkawa Y, Kohno K, Suzuki A, Yonekawa H: Selective depletion of mouse kidney proximal straight tubule cells causes acute kidney injury. Transgenic Res 21: 51–62, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grgic I, Campanholle G, Bijol V, Wang C, Sabbisetti VS, Ichimura T, Humphreys BD, Bonventre JV: Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int 82: 172–183, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang MZ, Yao B, Yang S, Jiang L, Wang S, Fan X, Yin H, Wong K, Miyazawa T, Chen J, Chang I, Singh A, Harris RC: CSF-1 signaling mediates recovery from acute kidney injury. J Clin Invest 122: 4519–4532, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Comper WD, Osicka TM, Russo LM: Renal filtration, transport, and metabolism of albumin and albuminuria. In: Seldin and Giebisch's The Kidney, edited by Alpern RJ, Hebert SC, London, Elsevier, Inc., 2007, pp. 2081–2112
- 28.Fassi A, Sangalli F, Maffi R, Colombi F, Mohamed EI, Brenner BM, Remuzzi G, Remuzzi A: Progressive glomerular injury in the MWF rat is predicted by inborn nephron deficit. J Am Soc Nephrol 9: 1399–1406, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Macconi D, Bonomelli M, Benigni A, Plati T, Sangalli F, Longaretti L, Conti S, Kawachi H, Hill P, Remuzzi G, Remuzzi A: Pathophysiologic implications of reduced podocyte number in a rat model of progressive glomerular injury. Am J Pathol 168: 42–54, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schulz A, Hänsch J, Kuhn K, Schlesener M, Kossmehl P, Nyengaard JR, Wendt N, Huber M, Kreutz R: Nephron deficit is not required for progressive proteinuria development in the Munich Wistar Frömter rat. Physiol Genomics 35: 30–35, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Kerjaschki D, Farquhar MG: Immunocytochemical localization of the Heymann nephritis antigen (GP330) in glomerular epithelial cells of normal Lewis rats. J Exp Med 157: 667–686, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forbes MS, Thornhill BA, Chevalier RL: Proximal tubular injury and rapid formation of atubular glomeruli in mice with unilateral ureteral obstruction: A new look at an old model. Am J Physiol Renal Physiol 301: F110–F117, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tojo A, Endou H: Intrarenal handling of proteins in rats using fractional micropuncture technique. Am J Physiol 263: F601–F606, 1992 [DOI] [PubMed] [Google Scholar]
- 34.Lund U, Rippe A, Venturoli D, Tenstad O, Grubb A, Rippe B: Glomerular filtration rate dependence of sieving of albumin and some neutral proteins in rat kidneys. Am J Physiol Renal Physiol 284: F1226–F1234, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Ohlson M, Sörensson J, Haraldsson B: A gel-membrane model of glomerular charge and size selectivity in series. Am J Physiol Renal Physiol 280: F396–F405, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Remuzzi A, Sangalli F, Fassi A, Remuzzi G: Albumin concentration in the Bowman’s capsule: multiphoton microscopy vs micropuncture technique. Kidney Int 72: 1410–1411, author reply 1411, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Rovira-Halbach G, Alt JM, Brunkhorst R, Frei U, Kühn K, Stolte H: Single nephron hyperfiltration and proteinuria in a newly selected rat strain with superficial glomeruli. Ren Physiol 9: 317–325, 1986 [DOI] [PubMed] [Google Scholar]
- 38.Eisenbach GM, Liew JB, Boylan JW, Manz N, Muir P: Effect of angiotensin on the filtration of protein in the rat kidney: A micropuncture study. Kidney Int 8: 80–87, 1975 [DOI] [PubMed] [Google Scholar]
- 39.Osicka TM, Strong KJ, Nikolic-Paterson DJ, Atkins RC, Jerums G, Comper WD: Renal processing of serum proteins in an albumin-deficient environment: An in vivo study of glomerulonephritis in the Nagase analbuminaemic rat. Nephrol Dial Transplant 19: 320–328, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Dileepan KN, Sharma R, Stechschulte DJ, Savin VJ: Effect of superoxide exposure on albumin permeability of isolated rat glomeruli. J Lab Clin Med 121: 797–804, 1993 [PubMed] [Google Scholar]
- 41.Fox JG, Quin JD, O’Reilly DS, Boulton-Jones JM: Assessment of glomerular charge selectivity in man by differential clearance of isoamylases. Clin Sci (Lond) 84: 449–454, 1993 [DOI] [PubMed] [Google Scholar]
- 42.Gregoire F, Lambert PP: The biosynthesis of a labelled Bence-Jones-Protein and its glomerular permeability in the normal dog. Clin Sci 25: 243–248, 1963 [PubMed] [Google Scholar]
- 43.Gagliardini E, Conti S, Benigni A, Remuzzi G, Remuzzi A: Imaging of the porous ultrastructure of the glomerular epithelial filtration slit. J Am Soc Nephrol 21: 2081–2089, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dunn KW, Sandoval RM, Kelly KJ, Dagher PC, Tanner GA, Atkinson SJ, Bacallao RL, Molitoris BA: Functional studies of the kidney of living animals using multicolor two-photon microscopy. Am J Physiol Cell Physiol 283: C905–C916, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Molitoris BA, Sandoval RM: Intravital multiphoton microscopy of dynamic renal processes. Am J Physiol Renal Physiol 288: F1084–F1089, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Sandoval RM, Kennedy MD, Low PS, Molitoris BA: Uptake and trafficking of fluorescent conjugates of folic acid in intact kidney determined using intravital two-photon microscopy. Am J Physiol Cell Physiol 287: C517–C526, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Russo LM, Sandoval RM, Campos SB, Molitoris BA, Comper WD, Brown D: Impaired tubular uptake explains albuminuria in early diabetic nephropathy. J Am Soc Nephrol 20: 489–494, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gekle M: Renal albumin handling: A look at the dark side of the filter. Kidney Int 71: 479–481, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Weyer K, Nielsen R, Christensen EI, Birn H: Generation of urinary albumin fragments does not require proximal tubular uptake. J Am Soc Nephrol 23: 591–596, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sandoval RM, Wang E, Molitoris BA: Finding the bottom and using it. Intravital 2: 1–9, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakano D, Kobori H, Burford JL, Gevorgyan H, Seidel S, Hitomi H, Nishiyama A, Peti-Peterdi J: Multiphoton imaging of the glomerular permeability of angiotensinogen. J Am Soc Nephrol 23: 1847–1856, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanner GA: Glomerular sieving coefficient of serum albumin in the rat: A two-photon microscopy study. Am J Physiol Renal Physiol 296: F1258–F1265, 2009 [DOI] [PubMed] [Google Scholar]
- 53.Peti-Peterdi J: Independent two-photon measurements of albumin GSC give low values. Am J Physiol Renal Physiol 296: F1255–F1257, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pawley J: Handbook of Biological Confocal Microscopy, New York, NY, Springer Science & Business Media, LLC, 2006 [Google Scholar]
- 55.Schießl IM, Castrop H: Angiotensin II AT2 receptor activation attenuates AT1 receptor-induced increases in the glomerular filtration of albumin: A multiphoton microscopy study. Am J Physiol Renal Physiol 305: F1189–F1200, 2013 [DOI] [PubMed] [Google Scholar]
- 56.Sandoval RM, Molitoris BA: Quantifying glomerular permeability of fluorescent macromolecules using 2-photon microscopy in Munich Wistar rats. J Vis Exp 74: e50052, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goldberg RI, Smith RM, Jarett L: Insulin and alpha 2-macroglobulin-methylamine undergo endocytosis by different mechanisms in rat adipocytes: I. Comparison of cell surface events. J Cell Physiol 133: 203–212, 1987 [DOI] [PubMed] [Google Scholar]
- 58.Grant BD, Donaldson JG: Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol 10: 597–608, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Christensen EI, Birn H: Megalin and cubilin: Multifunctional endocytic receptors. Nat Rev Mol Cell Biol 3: 256–266, 2002 [DOI] [PubMed] [Google Scholar]
- 60.Racoosin EL, Swanson JA: Macropinosome maturation and fusion with tubular lysosomes in macrophages. J Cell Biol 121: 1011–1020, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Månsson LE, Melican K, Molitoris BA, Richter-Dahlfors A: Progression of bacterial infections studied in real time—novel perspectives provided by multiphoton microscopy. Cell Microbiol 9: 2334–2343, 2007 [DOI] [PubMed] [Google Scholar]
- 62.Melican K, Boekel J, Månsson LE, Sandoval RM, Tanner GA, Källskog O, Palm F, Molitoris BA, Richter-Dahlfors A: Bacterial infection-mediated mucosal signalling induces local renal ischaemia as a defence against sepsis. Cell Microbiol 10: 1987–1998, 2008 [DOI] [PubMed] [Google Scholar]
- 63.Christensen EI, Nielsen S: Structural and functional features of protein handling in the kidney proximal tubule. Semin Nephrol 11: 414–439, 1991 [PubMed] [Google Scholar]
- 64.Wall DA, Maack T: Endocytic uptake, transport, and catabolism of proteins by epithelial cells. Am J Physiol 248: C12–C20, 1985 [DOI] [PubMed] [Google Scholar]
- 65.Maack T, Camargo MJ, Kleinert HD, Laragh JH, Atlas SA: Atrial natriuretic factor: structure and functional properties. Kidney Int 27: 607–615, 1985 [DOI] [PubMed] [Google Scholar]
- 66.Clapp WL, Park CH, Madsen KM, Tisher CC: Axial heterogeneity in the handling of albumin by the rabbit proximal tubule. Lab Invest 58: 549–558, 1988 [PubMed] [Google Scholar]
- 67.Birn H, Christensen EI, Nielsen S: Kinetics of endocytosis in renal proximal tubule studied with ruthenium red as membrane marker. Am J Physiol 264: F239–F250, 1993 [DOI] [PubMed] [Google Scholar]
- 68.Osicka TM, Comper WD: Protein degradation during renal passage in normal kidneys is inhibited in experimental albuminuria. Clin Sci (Lond) 93: 65–72, 1997 [DOI] [PubMed] [Google Scholar]
- 69.Gudehithlu KP, Pegoraro AA, Dunea G, Arruda JA, Singh AK: Degradation of albumin by the renal proximal tubule cells and the subsequent fate of its fragments. Kidney Int 65: 2113–2122, 2004 [DOI] [PubMed] [Google Scholar]
- 70.Russo LM, Srivatsan S, Seaman M, Suleiman H, Shaw AS, Comper WD: Albuminuria associated with CD2AP knockout mice is primarily due to dysfunction of the renal degradation pathway processing of filtered albumin. FEBS Lett 587: 3738–3741, 2013 [DOI] [PubMed] [Google Scholar]
- 71.Ferrell N, Ricci KB, Groszek J, Marmerstein JT, Fissell WH: Albumin handling by renal tubular epithelial cells in a microfluidic bioreactor. Biotechnol Bioeng 109: 797–803, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kania K, Byrnes EA, Beilby JP, Webb SA, Strong KJ: Urinary proteases degrade albumin: implications for measurement of albuminuria in stored samples. Ann Clin Biochem 47: 151–157, 2010 [DOI] [PubMed] [Google Scholar]
- 73.Bomsel M, Parton R, Kuznetsov SA, Schroer TA, Gruenberg J: Microtubule- and motor-dependent fusion in vitro between apical and basolateral endocytic vesicles from MDCK cells. Cell 62: 719–731, 1990 [DOI] [PubMed] [Google Scholar]
- 74.von Bonsdorff CH, Fuller SD, Simons K: Apical and basolateral endocytosis in Madin-Darby canine kidney (MDCK) cells grown on nitrocellulose filters. EMBO J 4: 2781–2792, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bourdeau JE, Carone FA: Contraluminal serum albumin uptake in isolated perfused renal tubules. Am J Physiol 224: 399–404, 1973 [DOI] [PubMed] [Google Scholar]
- 76.Slattery C, Lee A, Zhang Y, Kelly DJ, Thorn P, Nikolic-Paterson DJ, Tesch GH, Poronnik P: In vivo visualization of albumin degradation in the proximal tubule. Kidney Int 74: 1480–1486, 2008 [DOI] [PubMed] [Google Scholar]
- 77.Russo LM, Bakris GL, Comper WD: Renal handling of albumin: A critical review of basic concepts and perspective. Am J Kidney Dis 39: 899–919, 2002 [DOI] [PubMed] [Google Scholar]
- 78.Moestrup SK, Verroust PJ: Megalin- and cubilin-mediated endocytosis of protein-bound vitamins, lipids, and hormones in polarized epithelia. Annu Rev Nutr 21: 407–428, 2001 [DOI] [PubMed] [Google Scholar]
- 79.Gburek J, Verroust PJ, Willnow TE, Fyfe JC, Nowacki W, Jacobsen C, Moestrup SK, Christensen EI: Megalin and cubilin are endocytic receptors involved in renal clearance of hemoglobin. J Am Soc Nephrol 13: 423–430, 2002 [DOI] [PubMed] [Google Scholar]
- 80.Kozyraki R, Fyfe J, Verroust PJ, Jacobsen C, Dautry-Varsat A, Gburek J, Willnow TE, Christensen EI, Moestrup SK: Megalin-dependent cubilin-mediated endocytosis is a major pathway for the apical uptake of transferrin in polarized epithelia. Proc Natl Acad Sci U S A 98: 12491–12496, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, Melsen F, Christensen EI, Willnow TE: An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell 96: 507–515, 1999 [DOI] [PubMed] [Google Scholar]
- 82.Cui S, Verroust PJ, Moestrup SK, Christensen EI: Megalin/gp330 mediates uptake of albumin in renal proximal tubule. Am J Physiol 271: F900–F907, 1996 [DOI] [PubMed] [Google Scholar]
- 83.Amsellem S, Gburek J, Hamard G, Nielsen R, Willnow TE, Devuyst O, Nexo E, Verroust PJ, Christensen EI, Kozyraki R: Cubilin is essential for albumin reabsorption in the renal proximal tubule. J Am Soc Nephrol 21: 1859–1867, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Storm T, Emma F, Verroust PJ, Hertz JM, Nielsen R, Christensen EI: A patient with cubilin deficiency. N Engl J Med 364: 89–91, 2011 [DOI] [PubMed] [Google Scholar]
- 85.Böger CA, Chen MH, Tin A, Olden M, Köttgen A, de Boer IH, Fuchsberger C, O’Seaghdha CM, Pattaro C, Teumer A, Liu CT, Glazer NL, Li M, O’Connell JR, Tanaka T, Peralta CA, Kutalik Z, Luan J, Zhao JH, Hwang SJ, Akylbekova E, Kramer H, van der Harst P, Smith AV, Lohman K, de Andrade M, Hayward C, Kollerits B, Tönjes A, Aspelund T, Ingelsson E, Eiriksdottir G, Launer LJ, Harris TB, Shuldiner AR, Mitchell BD, Arking DE, Franceschini N, Boerwinkle E, Egan J, Hernandez D, Reilly M, Townsend RR, Lumley T, Siscovick DS, Psaty BM, Kestenbaum B, Haritunians T, Bergmann S, Vollenweider P, Waeber G, Mooser V, Waterworth D, Johnson AD, Florez JC, Meigs JB, Lu X, Turner ST, Atkinson EJ, Leak TS, Aasarød K, Skorpen F, Syvänen AC, Illig T, Baumert J, Koenig W, Krämer BK, Devuyst O, Mychaleckyj JC, Minelli C, Bakker SJ, Kedenko L, Paulweber B, Coassin S, Endlich K, Kroemer HK, Biffar R, Stracke S, Völzke H, Stumvoll M, Mägi R, Campbell H, Vitart V, Hastie ND, Gudnason V, Kardia SL, Liu Y, Polasek O, Curhan G, Kronenberg F, Prokopenko I, Rudan I, Arnlöv J, Hallan S, Navis G, Parsa A, Ferrucci L, Coresh J, Shlipak MG, Bull SB, Paterson NJ, Wichmann HE, Wareham NJ, Loos RJ, Rotter JI, Pramstaller PP, Cupples LA, Beckmann JS, Yang Q, Heid IM, Rettig R, Dreisbach AW, Bochud M, Fox CS, Kao WH, CKDGen Consortium : CUBN is a gene locus for albuminuria. J Am Soc Nephrol 22: 555–570, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ovunc B, Otto EA, Vega-Warner V, Saisawat P, Ashraf S, Ramaswami G, Fathy HM, Schoeb D, Chernin G, Lyons RH, Yilmaz E, Hildebrandt F: Exome sequencing reveals cubilin mutation as a single-gene cause of proteinuria. J Am Soc Nephrol 22: 1815–1820, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Morris SM, Tallquist MD, Rock CO, Cooper JA: Dual roles for the Dab2 adaptor protein in embryonic development and kidney transport. EMBO J 21: 1555–1564, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thrailkill KM, Nimmo T, Bunn RC, Cockrell GE, Moreau CS, Mackintosh S, Edmondson RD, Fowlkes JL: Microalbuminuria in type 1 diabetes is associated with enhanced excretion of the endocytic multiligand receptors megalin and cubilin. Diabetes Care 32: 1266–1268, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jones EA, Waldmann TA: The mechanism of intestinal uptake and transcellular transport of IgG in the neonatal rat. J Clin Invest 51: 2916–2927, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Simister NE, Rees AR: Isolation and characterization of an Fc receptor from neonatal rat small intestine. Eur J Immunol 15: 733–738, 1985 [DOI] [PubMed] [Google Scholar]
- 91.Chaudhury C, Brooks CL, Carter DC, Robinson JM, Anderson CL: Albumin binding to FcRn: distinct from the FcRn-IgG interaction. Biochemistry 45: 4983–4990, 2006 [DOI] [PubMed] [Google Scholar]
- 92.Kuo TT, Baker K, Yoshida M, Qiao SW, Aveson VG, Lencer WI, Blumberg RS: Neonatal Fc receptor: From immunity to therapeutics. J Clin Immunol 30: 777–789, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Borvak J, Richardson J, Medesan C, Antohe F, Radu C, Simionescu M, Ghetie V, Ward ES: Functional expression of the MHC class I-related receptor, FcRn, in endothelial cells of mice. Int Immunol 10: 1289–1298, 1998 [DOI] [PubMed] [Google Scholar]
- 94.Blumberg RS, Koss T, Story CM, Barisani D, Polischuk J, Lipin A, Pablo L, Green R, Simister NE: A major histocompatibility complex class I-related Fc receptor for IgG on rat hepatocytes. J Clin Invest 95: 2397–2402, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vidarsson G, Stemerding AM, Stapleton NM, Spliethoff SE, Janssen H, Rebers FE, de Haas M, van de Winkel JG: FcRn: An IgG receptor on phagocytes with a novel role in phagocytosis. Blood 108: 3573–3579, 2006 [DOI] [PubMed] [Google Scholar]
- 96.Pricop L, Redecha P, Teillaud JL, Frey J, Fridman WH, Sautès-Fridman C, Salmon JE: Differential modulation of stimulatory and inhibitory Fc gamma receptors on human monocytes by Th1 and Th2 cytokines. J Immunol 166: 531–537, 2001 [DOI] [PubMed] [Google Scholar]
- 97.Simister NE, Mostov KE: An Fc receptor structurally related to MHC class I antigens. Nature 337: 184–187, 1989 [DOI] [PubMed] [Google Scholar]
- 98.Yoshida M, Claypool SM, Wagner JS, Mizoguchi E, Mizoguchi A, Roopenian DC, Lencer WI, Blumberg RS: Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity 20: 769–783, 2004 [DOI] [PubMed] [Google Scholar]
- 99.Haymann JP, Levraud JP, Bouet S, Kappes V, Hagège J, Nguyen G, Xu Y, Rondeau E, Sraer JD: Characterization and localization of the neonatal Fc receptor in adult human kidney. J Am Soc Nephrol 11: 632–639, 2000 [DOI] [PubMed] [Google Scholar]
- 100.Sarav M, Wang Y, Hack BK, Chang A, Jensen M, Bao L, Quigg RJ: Renal FcRn reclaims albumin but facilitates elimination of IgG. J Am Soc Nephrol 20: 1941–1952, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Story CM, Mikulska JE, Simister NE: A major histocompatibility complex class I-like Fc receptor cloned from human placenta: Possible role in transfer of immunoglobulin G from mother to fetus. J Exp Med 180: 2377–2381, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Roberts DM, Guenthert M, Rodewald R: Isolation and characterization of the Fc receptor from the fetal yolk sac of the rat. J Cell Biol 111: 1867–1876, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Simister NE, Story CM, Chen HL, Hunt JS: An IgG-transporting Fc receptor expressed in the syncytiotrophoblast of human placenta. Eur J Immunol 26: 1527–1531, 1996 [DOI] [PubMed] [Google Scholar]
- 104.Cervenak J, Bender B, Schneider Z, Magna M, Carstea BV, Liliom K, Erdei A, Bosze Z, Kacskovics I: Neonatal FcR overexpression boosts humoral immune response in transgenic mice. J Immunol 186: 959–968, 2011 [DOI] [PubMed] [Google Scholar]
- 105.Catunda Lemos AP, Cervenak J, Bender B, Hoffmann OI, Baranyi M, Kerekes A, Farkas A, Bosze Z, Hiripi L, Kacskovics I: Characterization of the rabbit neonatal Fc receptor (FcRn) and analyzing the immunophenotype of the transgenic rabbits that overexpresses FcRn. PLoS ONE 7: e28869, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rodewald R: Intestinal transport of antibodies in the newborn rat. J Cell Biol 58: 189–211, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jakoi ER, Cambier J, Saslow S: Transepithelial transport of maternal antibody: Purification of IgG receptor from newborn rat intestine. J Immunol 135: 3360–3364, 1985 [PubMed] [Google Scholar]
- 108.Akilesh S, Huber TB, Wu H, Wang G, Hartleben B, Kopp JB, Miner JH, Roopenian DC, Unanue ER, Shaw AS: Podocytes use FcRn to clear IgG from the glomerular basement membrane. Proc Natl Acad Sci U S A 105: 967–972, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kobayashi N, Suzuki Y, Tsuge T, Okumura K, Ra C, Tomino Y: FcRn-mediated transcytosis of immunoglobulin G in human renal proximal tubular epithelial cells. Am J Physiol Renal Physiol 282: F358–F365, 2002 [DOI] [PubMed] [Google Scholar]
- 110.He W, Ladinsky MS, Huey-Tubman KE, Jensen GJ, McIntosh JR, Björkman PJ: FcRn-mediated antibody transport across epithelial cells revealed by electron tomography. Nature 455: 542–546, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ladinsky MS, Huey-Tubman KE, Bjorkman PJ: Electron tomography of late stages of FcRn-mediated antibody transcytosis in neonatal rat small intestine. Mol Biol Cell 23: 2537–2545, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kim KJ, Fandy TE, Lee VH, Ann DK, Borok Z, Crandall ED: Net absorption of IgG via FcRn-mediated transcytosis across rat alveolar epithelial cell monolayers. Am J Physiol Lung Cell Mol Physiol 287: L616–L622, 2004 [DOI] [PubMed] [Google Scholar]
- 113.Ward ES, Zhou J, Ghetie V, Ober RJ: Evidence to support the cellular mechanism involved in serum IgG homeostasis in humans. Int Immunol 15: 187–195, 2003 [DOI] [PubMed] [Google Scholar]
- 114.Schlachetzki F, Zhu C, Pardridge WM: Expression of the neonatal Fc receptor (FcRn) at the blood-brain barrier. J Neurochem 81: 203–206, 2002 [DOI] [PubMed] [Google Scholar]
- 115.Andersen JT, Daba MB, Berntzen G, Michaelsen TE, Sandlie I: Cross-species binding analyses of mouse and human neonatal Fc receptor show dramatic differences in immunoglobulin G and albumin binding. J Biol Chem 285: 4826–4836, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Greive KA, Nikolic-Paterson DJ, Guimarães MA, Nikolovski J, Pratt LM, Mu W, Atkins RC, Comper WD: Glomerular permselectivity factors are not responsible for the increase in fractional clearance of albumin in rat glomerulonephritis. Am J Pathol 159: 1159–1170, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hilliard LM, Osicka TM, Clavant SP, Robinson PJ, Nikolic-Paterson DJ, Comper WD: Characterization of the urinary albumin degradation pathway in the isolated perfused rat kidney. J Lab Clin Med 147: 36–44, 2006 [DOI] [PubMed] [Google Scholar]
- 118.Koltun M, Nikolovski J, Strong K, Nikolic-Paterson D, Comper WD: Mechanism of hypoalbuminemia in rodents. Am J Physiol Heart Circ Physiol 288: H1604–H1610, 2005 [DOI] [PubMed] [Google Scholar]
- 119.Eppel GA, Osicka TM, Pratt LM, Jablonski P, Howden BO, Glasgow EF, Comper WD: The return of glomerular-filtered albumin to the rat renal vein. Kidney Int 55: 1861–1870, 1999 [DOI] [PubMed] [Google Scholar]
- 120.Koltun M, Comper WD: Retention of albumin in the circulation is governed by saturable renal cell-mediated processes. Microcirculation 11: 351–360, 2004 [DOI] [PubMed] [Google Scholar]
- 121.Prabhat P, Gan Z, Chao J, Ram S, Vaccaro C, Gibbons S, Ober RJ, Ward ES: Elucidation of intracellular recycling pathways leading to exocytosis of the Fc receptor, FcRn, by using multifocal plane microscopy. Proc Natl Acad Sci U S A 104: 5889–5894, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim J, Bronson CL, Hayton WL, Radmacher MD, Roopenian DC, Robinson JM, Anderson CL: Albumin turnover: FcRn-mediated recycling saves as much albumin from degradation as the liver produces. Am J Physiol Gastrointest Liver Physiol 290: G352–G360, 2006 [DOI] [PubMed] [Google Scholar]
- 123.Chaudhury C, Mehnaz S, Robinson JM, Hayton WL, Pearl DK, Roopenian DC, Anderson CL: The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J Exp Med 197: 315–322, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Roopenian DC, Christianson GJ, Sproule TJ, Brown AC, Akilesh S, Jung N, Petkova S, Avanessian L, Choi EY, Shaffer DJ, Eden PA, Anderson CL: The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgG-Fc-coupled drugs. J Immunol 170: 3528–3533, 2003 [DOI] [PubMed] [Google Scholar]
- 125.Junghans RP, Anderson CL: The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proc Natl Acad Sci U S A 93: 5512–5516, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ruggiero A, Villa CH, Bander E, Rey DA, Bergkvist M, Batt CA, Manova-Todorova K, Deen WM, Scheinberg DA, McDevitt MR: Paradoxical glomerular filtration of carbon nanotubes. Proc Natl Acad Sci U S A 107: 12369–12374, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]