Abstract
Vasopressin-regulated expression and insertion of aquaporin-2 channels in the luminal membrane of renal principal cells is essential for urine concentration. Lithium affects urine concentrating ability, and approximately 20% of patients treated with lithium develop nephrogenic diabetes insipidus (NDI), a disorder characterized by polyuria and polydipsia. Lithium-induced NDI is caused by aquaporin-2 downregulation and a reduced ratio of principal/intercalated cells, yet lithium induces principal cell proliferation. Here, we studied how lithium-induced principal cell proliferation can lead to a reduced ratio of principal/intercalated cells using two-dimensional and three-dimensional polarized cultures of mouse renal collecting duct cells and mice treated with clinically relevant lithium concentrations. DNA image cytometry and immunoblotting revealed that lithium initiated proliferation of mouse renal collecting duct cells but also increased the G2/S ratio, indicating G2/M phase arrest. In mice, treatment with lithium for 4, 7, 10, or 13 days led to features of NDI and an increase in the number of principal cells expressing PCNA in the papilla. Remarkably, 30%–40% of the PCNA-positive principal cells also expressed pHistone-H3, a late G2/M phase marker detected in approximately 20% of cells during undisturbed proliferation. Our data reveal that lithium treatment initiates proliferation of renal principal cells but that a significant percentage of these cells are arrested in the late G2 phase, which explains the reduced principal/intercalated cell ratio and may identify the molecular pathway underlying the development of lithium-induced renal fibrosis.
Lithium is widely used as a treatment for bipolar disorder, a common chronic psychiatric illness typically requiring treatment for the rest of the patient’s life. An important side effect of lithium treatment, however, is nephrogenic diabetes insipidus (NDI), a disorder in which urine concentration is impaired, resulting in polyuria and polydipsia.1 Although lithium treatment for a period of weeks already reduces urine concentrating ability in humans,2 approximately 20% of patients receiving long-term lithium therapy will develop clinically extreme concentration defects resulting in NDI.3 Nevertheless, cessation of lithium therapy is usually not an option because bipolar disorder has a larger effect on the patient’s quality of life than NDI. Moreover, due to its efficacy, toxicity profile, and low cost, lithium remains the preferred therapy for bipolar disorders.4
Urine concentration is regulated by arginine vasopressin (AVP), which is released from the pituitary in response to hypovolemia or hypernatremia. In the kidney, AVP binds its type-2 receptor at the basolateral membrane of principal cells of the collecting duct, leading to the redistribution of aquaporin (AQP)-2 water channels from intracellular vesicles to the apical membrane. Driven by the transcellular osmotic gradient, water then enters the cell via AQP2 and exits through AQP3 and AQP4 in the basolateral membrane, resulting in correction of the water deficit and in concentrated urine.5
On the basis of studies in rodents, the development of lithium-induced NDI is thought to occur in two phases. In the first short-term phase, lithium causes a decrease in AQP2 expression.6 Lithium mainly enters principal cells through the epithelial sodium channel at the apical surface6,7 and, consequently, accumulates in principal cells due to the low affinity of the basolateral Na+ efflux pump Na+/K+-ATPase for lithium.6,8 How lithium downregulates AQP2 remains unclear but likely involves glycogen synthase kinase type 3β, which is of importance in AVP-regulated antidiuresis and is inhibited by lithium.9–11 Lithium also affects AQP2-mediated water reabsorption by the elevated tubular release of prostaglandin E2.11–13
In a second phase, lithium reduces the percentage of principal cells in the collecting duct, which are “exchanged” for intercalated cells, involved in acid-base homeostasis.14 Paradoxically, but in line with increased Wnt/β-catenin induced activity,15,16 lithium is known to induce proliferation of principal cells.17,18 Apoptosis or principal-to-intercalated cell conversion could be explanations, but Christensen et al. concluded that the number of detected apoptotic events or cells costaining for principal and intercalating cell marker proteins in lithium-induced NDI rats was too low to support these explanations.17 In this study, we provide an explanation for this paradox.
Results
Lithium Initiates Proliferation of Mouse Renal Collecting Duct Cells
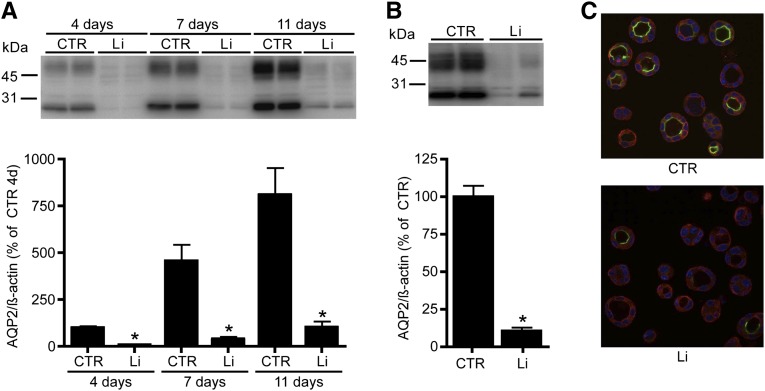
To study lithium-induced NDI in vitro, mouse renal collecting duct (mpkCCD) cells were cultured on two-dimensional (2D) transwell filters as described6,19 and treated with 1 and 10 mM lithium at the basolateral and apical side, respectively, for 4, 7, and 11 days. Immunoblotting showed significant downregulation of the 1-deamino-8-D-arginine vasopressin (dDAVP)–induced expression of endogenous AQP2 at all time points (Figure 1A). The effect of lithium on mpkCCD cells was also studied when grown as spheroids, because this condition mimics the physiologic three-dimensional (3D) structure of the collecting duct.20 Immunoblotting and immunocytochemistry revealed that treatment of spheroid-grown mpkCCD cells with 10 mM lithium for 3 days significantly reduced endogenous AQP2 abundance (Figure 1, B and C).
Figure 1.
Lithium-induced AQP2 downregulation in two mpkCCD cell models. (A) mpkCCD cells are cultured on transwell filters to study the long-term effect of lithium (Li) treatment. After growth to confluence for 96 hours, cells are treated at the basolateral side with 1 mM lithium chloride and at the apical side with 10 mM lithium chloride. After 4, 7, and 11 days of lithium exposure, cells are collected, lysed, and immunoblotted for AQP2 (upper). Quantification is depicted in the lower panel (n=4 for each condition and time point). (B and C) mpkCCD cells are cultured in matrigel and treated with (Li) or without (CTR) 10 mM lithium chloride. After 3 days, cells are lysed and immunoblotted for AQP2 and signals are quantified, corrected for β-actin (n=4 for each condition). (C) Immunocytochemistry of 3D-grown mpkCCD cells. AQP2 expression is visualized in green, whereas α-tubulin and nuclear 4′,6-diamidino-2-phenylindole staining are depicted in red and blue, respectively. *P<0.05, significant difference from control (CTR).
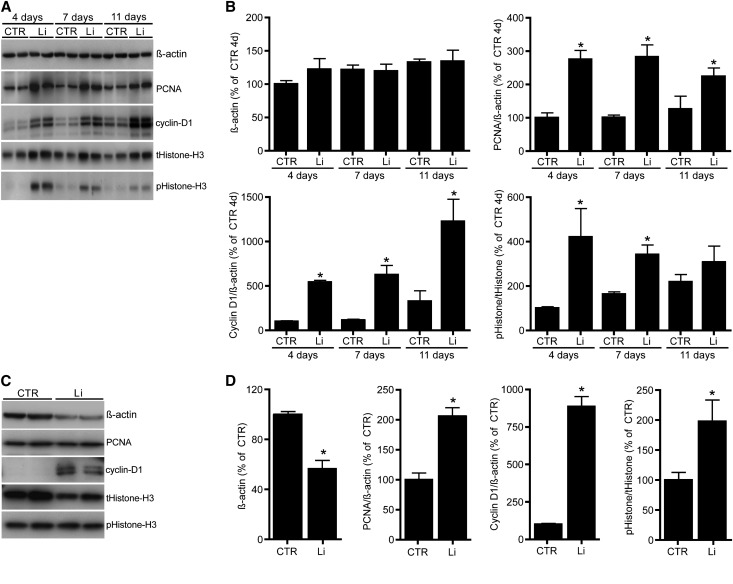
To assess the effect of lithium on proliferation, mpkCCD cell cycle profiles were obtained using DNA image cytometry (Figure 2A) and immunoblotting. In line with lithium-induced proliferation, the 2D model demonstrated a significant decrease in cells in the G0/1 phase, which was accompanied by a significant increase in cells in the S and G2 phases at all days tested (Figure 2B). Similar data were obtained with 3D-grown cells (Figure 2C). Immunoblotting revealed that in our transwell model, lithium significantly increased the abundance of the S/G2 and G2/M phase marker proteins PCNA and phospho-Histone-H3 (pHistone-H3), respectively, but the effect gradually decreased over time (Figure 3, A and B). The abundance of cyclin-D1, a protein essential for the transition from the G0/1 phase to the S phase and whose proteolysis is mediated by glycogen synthase kinase type 3β activity,21 was strongly elevated upon lithium treatment, whereas β-actin levels remained constant. With 3D spheroids, the effects of lithium on PCNA, pHistone-H3, and cyclin-D1 levels were similar to 2D-grown mpkCCD cells, but β-actin abundance was significantly reduced (Figure 3, C and D). The reduction in β-actin levels in lithium-treated spheroids is due to a reduced cell number because the total Histone-H3 abundance and area-equivalents of proteins (not shown) were also reduced.
Figure 2.
Lithium treatment induces proliferation. (A) Representative cell cycle profiles of matrigel-cultured mpkCCD cells treated without (control, left) or with 10 mM lithium chloride for 3 days (right). The x-axis represents the DNA content of the nuclei population, whereas the y-axis identifies the number of nuclei. The DNA content per cell cycle phase is indicated. (B) Distribution of the cell cycle phases of mpkCCD cells cultured on transwell filters for 4, 7, or 11 days treated with (Li) or without (CTR) lithium chloride (n=6 for each condition and time point). (C) Averaged data of the distribution of the cell cycle phases of matrigel-cultured mpkCCD cells treated without (CTR) or with (Li) lithium chloride (n=4 for each condition). *P<0.05, significant difference from control (CTR).
Figure 3.
The effect of lithium on different cell cycle markers. (A and C) mpkCCD cells are cultured and treated with lithium on Transwell filters (A) or in matrigel (C) as described in the legend for Figure 1. After three days, cells are lysed and immunoblotted for PCNA, cyclin D1, Histone H3, pHistone-H3, and β-actin. (B and D) Quantification of protein abundances of filter (A) or matrigel (C) grown cells, corrected for β-actin and related to the abundances of control cells of day 4 (n=4 for each condition and time point). *P<0.05, significant difference from control (CTR).
Lithium Induces a Checkpoint Kinase 1–Dependent Accumulation in the G2 Phase
To ensure that cell division occurs flawlessly, the transition from one phase to the next phase of the cell cycle is controlled at the G1/S and G2/M checkpoints.22 Because lithium increased the number of cells present in the S and G2 phases, we investigated whether lithium affects the G2/M transition step. We therefore assessed the percentage of cells in the G2 phase relative to all cells in the S and G2 phases. Interestingly, in both cell models, we observed a significant accumulation of cells in the G2 phase after lithium treatment compared with the control situation (Figure 4, A and D). Importantly, this percentage remained significantly elevated at all time points tested (Figure 4A), demonstrating that the G2 cell cycle arrest was sustained for these cells. Subsequent assessment of several proteins essential for G2/M transition revealed that cyclin-B1 and phospho-cyclin–dependent kinase 1 (CDK1) protein levels were significantly increased in both models (Figure 4, B, C, E, and F). Comparison between the two model systems demonstrated that the abundance of cyclin-B1 and phospho-CDK1 gradually declined over time in the 2D model and that the abundance of CDK1 was only elevated in the spheroid culture system.
Figure 4.
Lithium treatment of mpkCCD cells causes accumulation in the G2 phase. mpkCCD cells are cultured and treated with lithium on Transwell filters (A–C) or in matrigel (D–F) as described in the legend for Figure 1 and the percentage of cells in the G2 phase is determined (Transwell: n=6 for each condition and time point; matrigel: n=4 for each condition). Cell lysates of Transwell filter-cultured (B) or matrigel-cultured (D) cells are lysed and immunoblotted for cyclin B1, CDK1, pCDK1, and β-actin. (C and F) Quantification of protein abundances of filter (B) or matrigel (E) grown cells, corrected for β-actin and related to the abundances of control cells of day 4 (n=4 for each condition and time point). *P<0.05, significant difference from control (CTR).
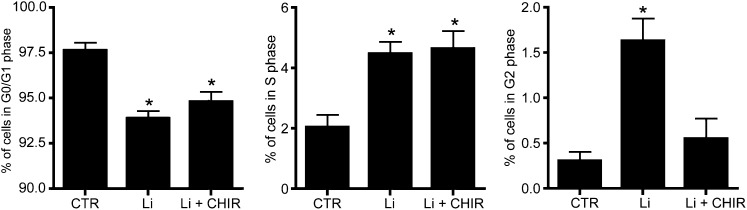
Because a G2 cell cycle arrest is often coupled with activation of checkpoint kinase 1 (Chk1),23 the involvement of Chk1 was studied by use of the selective Chk1 inhibitor CHIR-124. Treatment of mpkCCD cells with 500 nM CHIR-124 for the last 4 days did not affect the lithium-induced reduction of cells in the G0/G1 phase or the increase of cells present in the S phase. However, the lithium-induced increase in cells present in the G2 phase was abolished in cells treated with CHIR-124, demonstrating an essential role for Chk1 in lithium-induced G2 accumulation (Figure 5).
Figure 5.
Chk1 kinase blockage prevents lithium-induced cell cycle arrest. mpkCCD cells are grown and treated with or without lithium (Li) on Transwell filters for 4 days as described in the legend for Figure 1. Cells treated with lithium are cultured in the presence or absence of 500 nM of the Chk blocker CHIR-124. Cells are then trypsinized, stained, and analyzed by DNA image cytometry (n=5). *P<0.05, significant difference from control (CTR).
In Vivo Lithium Treatment Induces a G2 Cell Cycle Arrest of Principal Cells
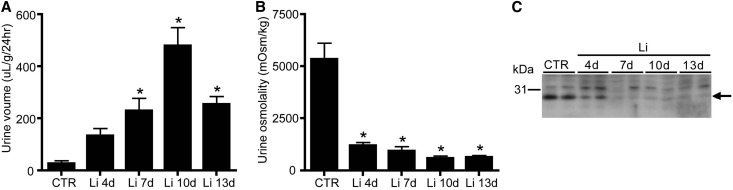
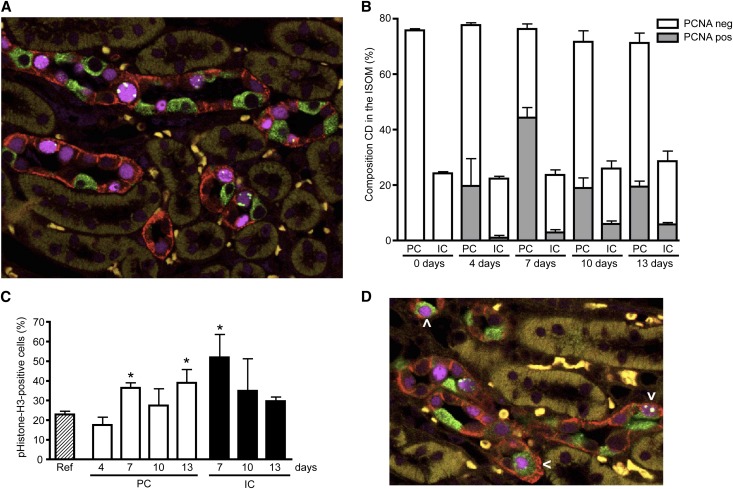
Our in vitro data revealed, besides proliferation, that lithium induced a G2/M phase cell cycle arrest. To investigate whether lithium also caused a G2 cell cycle arrest in vivo, mice were treated with or without 40 mmol lithium/kg food for 4–13 days. As anticipated, lithium treatment caused a time-dependent increase in urine volume (Figure 6A) and a decrease in urine osmolality (Figure 6B). To note, for unexplained reasons, the urine volume decreased at 13 days of lithium treatment compared with 10 days but was still significantly increased compared with controls. Immunoblotting confirmed a parallel reduction in AQP2 abundance (Figure 6C). Subsequently, kidney sections were costained with antibodies against AQP4, H+-ATPase, PCNA, and pHistone-H3 to mark principal cells, intercalated cells, proliferation, and the late G2 phase of the cell cycle, respectively. As previously reported,17 lithium strongly increased the number of cells positive for PCNA, and was most pronounced at the inner stripe of the outer medulla (ISOM). Figure 7A shows a representative staining of the ISOM of mice treated for 7 days with lithium. Counting collecting duct cells (approximately 500 cells/mouse) in the ISOM demonstrated that the principal/intercalated cell ratio had a tendency to decrease with longer lithium exposure (3.1±0.1 at day 0 to 2.6±0.5 at day 13), but did not reach significance (Figure 7B). The percentage of PCNA-positive principal cells changed from 0% at day 0 to 25%, 58%, 26%, and 27% at days 4, 7, 10, and 13, respectively (Figure 7B). This was higher than that of intercalated cells, because the percentage of PCNA-positive intercalated cells changed from 0 at day 0 to 4%, 12%, 23%, and 20% on days 4–13.
Figure 6.
Lithium-treated mice develop NDI. Mice are exposed to food without (CTR) or with 40 mM lithium (Li) chloride/kg food for 4, 7, 10, and 13 days. Mice are placed in metabolic cages for 48 hours and urine output (A) and urine osmolality (B) are determined during the last 24 hours (n=6 per time points for CTR and Li 4–10 days; n=5 for Li 13 days). (C) AQP2 immunoblotting data of whole kidneys of control (CTR) and Li-treated mice. The arrow indicates AQP2. *P<0.05, significant difference from control (CTR).
Figure 7.
Lithium causes a G2 cell cycle arrest of principal cells in vivo. Kidneys of mice treated or not with lithium chloride as described in the legend for Figure 6 are isolated, fixed, paraffin embedded, sectioned, and subjected to immunohistochemistry. Simultaneous staining is done for AQP4 (principal cell, red), H+-ATPase (intercalated cell, green), PCNA (nucleus, purple), and pHistone-H3 (G2 cell cycle phase, green). (A) Representative immunohistochemical staining of ISOM area of a mouse treated for 7 days with lithium. (B) Principal-intercalated cell composition (as a percentage of the total number of cells) of the collecting duct of the ISOM of mice treated for 0–13 days (indicated) with lithium and the percentage of PCNA-positive cells in principal and intercalated cells. For each time point, approximately 500 collecting duct cells per mouse for a total of three mice are counted. (C) Percentage of positive pHistone-H3 cells from the PCNA-positive population at days 4–13. (D) Immunohistochemical staining of a kidney after 10 days of lithium treatment. The arrowhead indicates cells that stain for markers of both intercalated and principal cells. *P<0.05, significant difference from the reference value, obtained from a renal cell population of nontreated mice. CD, collecting duct; IC, intercalated cell; neg, negative; PC, principal cell; pos, positive; ref, reference value.
To assess whether the PCNA-positive principal and/or intercalated cells were accumulating in the late G2 phase, cells with pHistone-H3–positive nuclear foci (Figure 7A) were counted. The percentage of PCNA-positive principal cells in the late G2 phase changed from 17% at day 4 to 36%, 27%, and 38% at days 7, 10, and 13, respectively (Figure 7C). For the intercalated cells, these numbers were 51%, 34%, and 30% at days 7, 10, and 13 (Figure 7C). The percentages for principal cells and intercalated cells at day 7 and for principal cells at day 13 were significantly elevated compared with the percentage of pHistone-H3–positive PCNA-positive cells found in a renal cell population of nontreated mice (22%; reference value obtained from Yang et al.24). Note that percentages for pHiston-H3-PCNA–positive principal cells and intercalated cells for day 0 and for day 4 for intercalated cells could not be given because of the too low number of PCNA-positive cells. To note, a small subset of cells (2.5%) expressed marker proteins of intercalated and principal cells at 10 days (Figure 7D).
Discussion
Lithium Enhances G1/S Cell Cycle Progression
In this study, the 3D spheroid cell model was used for the first time to study the effect of lithium on AQP2 regulation. It is stated that cells grown in three dimensions (i.e., spheroids) are more like renal tubules and can thereby reach a higher level of epithelial polarity compared with 2D cell culture.25 However, in our study, the percentage of 2D cells in the S-G2 phase (2%) was more similar to in vivo compared with spheroids (approximately 12%). Therefore, we see the spheroid-grown cells as an alternative model for 2D-grown cells instead of a better model. Lithium treatment of mpkCCD cells grown as a polarized monolayer or as spheroids increased the number of cells in the S and G2 phases. This was accompanied by an enhanced expression of the proliferation markers PCNA and cyclin-D1, which was also found at the later time points of 7 or 11 days. The sustained effect of lithium on cell cycle progression is in line with the progressive decline of collecting duct function and morphology in rodents treated with lithium.26 In addition to our mpkCCD model, we observed a stimulatory effect of lithium on the initiation of cell division in mice, as demonstrated by the high number of cells positive for PCNA. At days 4 and 7 of lithium treatment a large number of principal cells were positive for PCNA (25% and 58%, respectively), whereas these percentages were much smaller for intercalated cells (4% and 12%) or for both cell types in control mice, in which a negligible number of PCNA-positive cells was found. This is in agreement with earlier findings.9,17 The percentages of PCNA-positive cells are similar between both cell types at 10 and 13 days. The observation that lithium first initiates proliferation of principal cells and only later of intercalated cells indicates that there might be two different mechanisms by which lithium induces proliferation of both cell types.
Lithium Induces a Chk1-Dependent G2 Cell Cycle Arrest
We show here for the first time that lithium causes a G2 phase arrest of polarized collecting duct cells at clinically relevant concentrations (1 mM basolateral and 10 mM apical). This cell cycle arrest is likely the cause of the reduced β-actin abundance in the lithium-treated spheroids because it prevents the continuation of cell division, leading to a reduced number of cells. Protein levels of cyclin-B1 and CDK1 in the lithium-treated mpkCCD cells were elevated, suggesting that CDK1-cyclin complex formation, essential for cell cycle progression through the G2 phase, was not affected by lithium. Next, we observed that lithium caused an accumulation of the Tyr15-phosphorylated form of CDK1. CDK1 phosphorylation should indeed take place after complex formation; however, this complex will remain inactive until the phosphate is removed. This final and essential step of the removal of the inhibitory phosphate is performed by cdc25 phosphatase, which in turn is negatively regulated by Chk1.27 The accumulation of pCDK1 upon lithium treatment suggests that removal of the phosphate did not take place, supporting a role for the cdc25-Chk1 signaling pathway in lithium-induced G2 phase arrest. Indeed, treatment with 500 nM CHIR-124, a selective Chk1 inhibitor,28 prevented the G2 phase arrest induced by lithium, indicating the involvement of the Chk1 kinase in the lithium-induced G2 phase arrest. These findings are in line with data from Wang et al., who showed that lithium enhanced the activity of Chk1 kinase in 7721 cells.29 How lithium increases Chk1 activity is unknown.
Besides mpkCCD cells, our data indicate that lithium also induced a G2 cell cycle arrest in mice. Using the pHistone-H3–positive foci as a marker for late G2 phase, we found that 30%–40% of principal cells were in the late G2 phase during the 7–13 days of lithium treatment, which is markedly higher than found in renal tubular cells (approximately 20%) in nontreated male BALB/c mice aged 8–10 weeks.24 Thus, lithium also induced a G2 phase arrest in vivo. The question regarding whether chronic lithium treatment for months or years would lead to a sustained cell cycle arrest was not answered by our study, because the mice received lithium for a maximum of 13 days. However, we did not see any decrease in the percentage of arrested principal cells during the time course of 7–13 days in mice or in cells treated for 4–11 days with lithium. In this respect, Kling et al. demonstrated an increased presence of nuclear variation (irregular size and shape) of collecting duct cells in rats treated with lithium for 3, 9, and 18 weeks.26 These data are consistent with cells arrested in the G2 phase because an altered nuclear morphology in G2-arrested cells was previously documented.30–32 The consequences of such a prolonged G2 cell cycle arrest are not fully understood, although recent investigations of different AKI models indicate that activation of c-Jun NH2-terminal signaling in G2-arrested cells can lead to renal fibrosis due to the upregulation of profibrotic cytokines, including TGF-β1.24 Because fibrosis and CKD are also observed after long-term lithium treatment,33 and lithium has been shown to activate c-Jun NH2-terminal signaling16 and stimulate TGF-β1 production in the collecting duct,33 the role of G2-arrested cells in lithium-induced fibrosis should be further investigated.
Various studies demonstrated that lithium increased the number of principal cells incorporated with 3H-thymidine or positive for PCNA and concluded that lithium enhances proliferation of principal cells.17,26,34 Our study reveals that PCNA-positive cells are not necessarily proliferating and that additional proof is required to assess whether cells are actually dividing or whether their cell cycle is arrested. We also noted that the small number of PCNA-positive intercalated cells also exhibited a higher percentage of pHistone-H3 positivity than cells with unaffected cell division. Considering the generally accepted view that lithium cannot enter intercalated cells, we do not have an explanation for cell cycle arrest of the intercalated cells. The altered cell polarization/proliferation status of its neighboring principal cells might also possibly affect the intercalated cells. Another explanation may be that these intercalated cells are derived from principal cells, because principal cells can transit to intercalated cells.35 Perhaps the pHistone-H3–positive intercalated cells were originally principal cells, but transformed into intercalated cells upon lithium entry. In line with this hypothesis, a small subset of ISOM collecting duct cells (2.5%) expressed marker proteins of intercalated and principal cells at day 10 of lithium treatment.
The G2 Arrest of Principal Cells May Contribute to Collecting Duct Remodeling in Lithium-Induced NDI
In addition to the loss of AQP2 expression, lithium treatment induces collecting duct remodeling, in which the ratio of principal-to-intercalated cells decreases.14,36 We found a tendency for collecting duct remodeling after 13 days of lithium treatment. The paradoxical finding that lithium induces cell proliferation of principal cells, but nevertheless ends up in a reduced principal/intercalated cell ratio, is not understood. Christensen et al. were aware of this paradox and thus investigated different options but only sparsely observed apoptosis of principal cells or conversion into intercalated cells during lithium treatment; therefore, the authors excluded these as potential causes for the reduced percentage of principal cells.17 Our study provides an explanation for this paradox as the arrest of a large proportion of PCNA-positive principal cells in the late G2 phase, indicating that they do not further divide.
We, however, believe that the G2-phase arrest of principal cells is not the sole factor leading to collecting duct cell remodeling. Previous studies demonstrated that lithium treatment of rats induced an increased number of cells in the collecting duct.26,34,37 The increased number of cells likely constitutes mostly intercalated cells. The question remains as to why intercalated cells proliferate. Interestingly, because lithium treatment is known to cause a metabolic acidosis,38–40 this might be the trigger for intercalated cells to divide to remove excess acid. Accordingly, an acetazolamide-induced metabolic acidosis did indeed stimulate the proliferation of intercalated cells, resulting in similar collecting duct remodeling as observed during lithium-inducted NDI.41 Thus, lithium-induced collecting duct remodeling could be explained by a rather stable population of principal cells, which are partly in a cell cycle arrest, and a proliferating population of intercalated cells.
In conclusion, this study demonstrates that lithium not only induces principal cell division in vitro and in vivo, but also, and for the first time, that lithium induces a cell cycle arrest in the late G2 phase, likely involving inhibited Chk1 activity. The lithium-induced cell cycle arrest is likely to contribute to the reduced percentage of principal cells and collecting duct remodeling in developed lithium-induced NDI.
Concise Methods
Animal Experiments
Mice (C57bl6/j) weighing 15–20 g were obtained from Harlan Laboratories (Horst, The Netherlands) and maintained at the Radboud University Medical Centre animal facility. Mice were divided in five groups of three male mice and three female mice. Mice in group 1 (control) received a normal rodent diet (ssniff R/M-H V1534; ssniff Spezialdiaten GmbH, Soest, Germany) for 7 days. Mice in groups 2–5 received lithium chloride at a concentration of 40 mmol/kg of chow and were euthanized after 4 days (group 2), 7 days (group 3), 10 days (group 4), or 13 days (group 5) of lithium exposure. All mice had free access to water, food, and a sodium chloride block. For the last 48 hours of the experiment, mice were housed in metabolic cages in order to measure water intake and urine output during the last 24 hours. Mice were anesthetized with isoflurane and euthanized by cervical dislocation, after which the kidneys were removed. Urine samples were analyzed for osmolality using a Micro-Osmometer Model 3320 (Advanced Instruments, Inc., Norwood, MA). Complete methods are available in the Supplemental Material.
Cell Culture and Lithium Treatment Assay
In the 2D filter model, mpkCCD cells were cultured as previously described.6,19 After 72 hours, the cells were treated with 1 nM dDAVP at the basolateral side to induce AQP2 expression. Simultaneously, cells were incubated with 1 mM lithium chloride at the basolateral side and 10 mM lithium chloride at the apical side. After 4, 7, or 11 days of lithium exposure, the cells were either trypsinized, collected in medium, and then used for the DNA image cytometry or were pelleted and prepared for immunoblotting.
In the 3D spheroid model, mpkCCD cells were prepared at a concentration of 12×105 cells/ml in medium containing 2 nM dDAVP with or without 20 mM lithium chloride, and mixed 1:1 (vol/vol) with matrigel (Becton Dickinson, Bedford, UK). Then, 0.36 ml aliquots were plated into Costar 48-well plates (Corning, Corning, NY). The cell/matrigel mix was incubated at 37°C for 2 hours to allow gelling. Afterward, the wells were filled with dDAVP-containing medium with or without lithium. After 3 days, the cells were collected in Recovery medium (Becton Dickinson) and used for DNA image cytometry or pelleted and prepared for immunoblotting.
DNA Image Cytometry
DNA image cytometry was performed as previously described.42 In short, after fixation of cells in Böhm fixative and staining with Schiff reagent, DNA image cytometry was performed with Q-path DNA software to determine DNA ploidy of the nuclei (Leica Imaging Systems Ltd, Cambridge, UK).
Immunoblotting
Cells were lysed in Laemmli buffer, incubated at 37°C for 30 minutes, sonicated with a Branson Sonifier (Branson Ultrasonics Corporation, Danbury, CT), and analyzed by SDS-PAGE and immunoblotting as previously described.43
Immunocytochemistry
Culture medium was removed from matrigel-cultured mpkCCD cells and cells were subsequently washed three times (ice-cold PBS, 0.5 mM CaCl2, and 1.0 mM MgCl2) and fixed for 30 minutes at room temperature with 4% w/v paraformaldehyde in PBS. After washing twice with PBS, cells were treated with permeabilization buffer (0.5% v/v Triton X-100 and in PBS with 0.7% w/v gelatin) for 30 minutes. Cells were then incubated at 4°C overnight with primary antibodies (1:50 Rb5 AQP2 and 1:500 α-tubulin; Invitrogen, Camarillo, CA) in permeabilization buffer and after washing three times with secondary antibodies conjugated to Alexa dyes (1:500; Invitrogen, Paisley, UK) for 2–3 hours at 37°C. Finally, cells were mounted in Vectashield containing 4′,6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA) and imaged by confocal microscopy (FV1000; Olympus, Center Valley, PA).
Immunohistochemistry
Immunohistochemistry was performed as previously described.44 In short, kidney sections were incubated with blocking reagent (NEN Life Science Products, Zaventem, Belgium) and incubated overnight (O/N) with 1:1000 rabbit anti–pHistone-H3. Sections were then incubated for 60 minutes with biotin-labeled secondary anti-rabbit antibody (1:1000) and after that for 30 minutes with 1:100 streptavidin-horseradish peroxidase (TSA Fluorescein System; PerkinElmer, Waltham, MA). After incubation O/N with 1:100 mouse PCNA and 1:400 rabbit H+-ATPase (gift from Dr. S. Nielsen, Aarhus University, Denmark) sections were incubated with secondary antibodies conjugated to Alexa dyes (1:1000) for 1 hour and incubated O/N with 1:100 guinea pig AQP4. The next day, sections were washed, incubated with an Alexa-conjugated secondary antibody against guinea pig for 1 hour and incubated for 8 minutes with 1:50 fluorescein tyramide in amplification diluent (TSA Fluorescein System; PerkinElmer). After washing and incubation with 4′,6-diamidino-2-phenylindole (1:10,000) for 30 minutes, sections were embedded in Fluoromount G (Southern Biotech Associates, Birmingham, AL).
Statistical Analyses
The difference between groups was tested by the t test and one-way ANOVA corrected by the Newman–Keuls multiple-comparisons procedure. A P value<0.05 was considered statistically significant
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank Dr. Karolina Andralojc for analysis of the microscopic images.
This project was supported by grants from The Netherlands Organization for Scientific Research (VICI 865.07.002 to P.M.T.D), Radboud University Nijmegen Medical Centre (2004.55), Society of Experimental Laboratory Medicine (to P.M.T.D.), and the European Union FP7/2009 SYSCILIA Consortium (241955 to R.H.G.), as well as a Niels Stensen Fellowship (to T.d.G.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Lithium in Kidney Diseases: Big Roles for the Smallest Metal,” on pages 421–423.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013090988/-/DCSupplemental.
References
- 1.Timmer RT, Sands JM: Lithium intoxication. J Am Soc Nephrol 10: 666–674, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Walker RJ, Weggery S, Bedford JJ, McDonald FJ, Ellis G, Leader JP: Lithium-induced reduction in urinary concentrating ability and urinary aquaporin 2 (AQP2) excretion in healthy volunteers. Kidney Int 67: 291–294, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Bendz H, Aurell M: Drug-induced diabetes insipidus: Incidence, prevention and management. Drug Saf 21: 449–456, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Geddes JR, Miklowitz DJ: Treatment of bipolar disorder. Lancet 381: 1672–1682, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boone M, Deen PM: Physiology and pathophysiology of the vasopressin-regulated renal water reabsorption. Pflugers Arch 456: 1005–1024, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kortenoeven ML, Li Y, Shaw S, Gaeggeler HP, Rossier BC, Wetzels JF, Deen PM: Amiloride blocks lithium entry through the sodium channel thereby attenuating the resultant nephrogenic diabetes insipidus. Kidney Int 76: 44–53, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Christensen BM, Zuber AM, Loffing J, Stehle JC, Deen PM, Rossier BC, Hummler E: alphaENaC-mediated lithium absorption promotes nephrogenic diabetes insipidus. J Am Soc Nephrol 22: 253–261, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunham PB, Senyk O: Lithium efflux through the Na/K pump in human erythrocytes. Proc Natl Acad Sci U S A 74: 3099–3103, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kjaersgaard G, Madsen K, Marcussen N, Christensen S, Walter S, Jensen BL: Tissue injury after lithium treatment in human and rat postnatal kidney involves glycogen synthase kinase-3β-positive epithelium. Am J Physiol Renal Physiol 302: F455–F465, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Rao R, Patel S, Hao C, Woodgett J, Harris R: GSK3beta mediates renal response to vasopressin by modulating adenylate cyclase activity. J Am Soc Nephrol 21: 428–437, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rao R, Zhang MZ, Zhao M, Cai H, Harris RC, Breyer MD, Hao CM: Lithium treatment inhibits renal GSK-3 activity and promotes cyclooxygenase 2-dependent polyuria. Am J Physiol Renal Physiol 288: F642–F649, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Kortenoeven ML, Schweer H, Cox R, Wetzels JF, Deen PM: Lithium reduces aquaporin-2 transcription independent of prostaglandins. Am J Physiol Cell Physiol 302: C131–C140, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Kwon TH: Dysregulation of renal cyclooxygenase-2 in rats with lithium-induced nephrogenic diabetes insipidus. Electrolyte Blood Press 5: 68–74, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christensen BM, Marples D, Kim YH, Wang W, Frøkiaer J, Nielsen S: Changes in cellular composition of kidney collecting duct cells in rats with lithium-induced NDI. Am J Physiol Cell Physiol 286: C952–C964, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Rao AS, Kremenevskaja N, Resch J, Brabant G: Lithium stimulates proliferation in cultured thyrocytes by activating Wnt/beta-catenin signalling. Eur J Endocrinol 153: 929–938, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Nielsen J, Hoffert JD, Knepper MA, Agre P, Nielsen S, Fenton RA: Proteomic analysis of lithium-induced nephrogenic diabetes insipidus: Mechanisms for aquaporin 2 down-regulation and cellular proliferation. Proc Natl Acad Sci U S A 105: 3634–3639, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christensen BM, Kim YH, Kwon TH, Nielsen S: Lithium treatment induces a marked proliferation of primarily principal cells in rat kidney inner medullary collecting duct. Am J Physiol Renal Physiol 291: F39–F48, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Rojek A, Nielsen J, Brooks HL, Gong H, Kim YH, Kwon TH, Frøkiaer J, Nielsen S: Altered expression of selected genes in kidney of rats with lithium-induced NDI. Am J Physiol Renal Physiol 288: F1276–F1289, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Hasler U, Mordasini D, Bens M, Bianchi M, Cluzeaud F, Rousselot M, Vandewalle A, Feraille E, Martin PY: Long term regulation of aquaporin-2 expression in vasopressin-responsive renal collecting duct principal cells. J Biol Chem 277: 10379–10386, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Otto EA, Hurd TW, Airik R, Chaki M, Zhou W, Stoetzel C, Patil SB, Levy S, Ghosh AK, Murga-Zamalloa CA, van Reeuwijk J, Letteboer SJ, Sang L, Giles RH, Liu Q, Coene KL, Estrada-Cuzcano A, Collin RW, McLaughlin HM, Held S, Kasanuki JM, Ramaswami G, Conte J, Lopez I, Washburn J, Macdonald J, Hu J, Yamashita Y, Maher ER, Guay-Woodford LM, Neumann HP, Obermüller N, Koenekoop RK, Bergmann C, Bei X, Lewis RA, Katsanis N, Lopes V, Williams DS, Lyons RH, Dang CV, Brito DA, Dias MB, Zhang X, Cavalcoli JD, Nürnberg G, Nürnberg P, Pierce EA, Jackson PK, Antignac C, Saunier S, Roepman R, Dollfus H, Khanna H, Hildebrandt F: Candidate exome capture identifies mutation of SDCCAG8 as the cause of a retinal-renal ciliopathy. Nat Genet 42: 840–850, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diehl JA, Cheng M, Roussel MF, Sherr CJ: Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev 12: 3499–3511, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Houtgraaf JH, Versmissen J, van der Giessen WJ: A concise review of DNA damage checkpoints and repair in mammalian cells. Cardiovasc Revasc Med 7: 165–172, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Lossaint G, Besnard E, Fisher D, Piette J, Dulić V: Chk1 is dispensable for G2 arrest in response to sustained DNA damage when the ATM/p53/p21 pathway is functional. Oncogene 30: 4261–4274, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV: Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med 16: 535–543, 1p following 143, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Brien LE, Jou TS, Pollack AL, Zhang Q, Hansen SH, Yurchenco P, Mostov KE: Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat Cell Biol 3: 831–838, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Kling MA, Fox JG, Johnston SM, Tolkoff-Rubin NE, Rubin RH, Colvin RB: Effects of long-term lithium administration on renal structure and function in rats. A distinctive tubular lesion. Lab Invest 50: 526–535, 1984 [PubMed] [Google Scholar]
- 27.Lam MH, Rosen JM: Chk1 versus Cdc25: Chking one’s levels of cellular proliferation. Cell Cycle 3: 1355–1357, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Tse AN, Rendahl KG, Sheikh T, Cheema H, Aardalen K, Embry M, Ma S, Moler EJ, Ni ZJ, Lopes de Menezes DE, Hibner B, Gesner TG, Schwartz GK: CHIR-124, a novel potent inhibitor of Chk1, potentiates the cytotoxicity of topoisomerase I poisons in vitro and in vivo. Clin Cancer Res 13: 591–602, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Wang XM, Li J, Feng XC, Wang Q, Guan DY, Shen ZH: Involvement of the role of Chk1 in lithium-induced G2/M phase cell cycle arrest in hepatocellular carcinoma cells. J Cell Biochem 104: 1181–1191, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Yang L, Wu S, Zhang Q, Liu F, Wu P: 23,24-Dihydrocucurbitacin B induces G2/M cell-cycle arrest and mitochondria-dependent apoptosis in human breast cancer cells (Bcap37). Cancer Lett 256: 267–278, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Solhaug A, Holme JA, Haglund K, Dendele B, Sergent O, Pestka J, Lagadic-Gossmann D, Eriksen GS: Alternariol induces abnormal nuclear morphology and cell cycle arrest in murine RAW 264.7 macrophages. Toxicol Lett 219: 8–17, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Deng L, Zhong H, Wang Y, Jiang X, Chen J: Natural plant extract tubeimoside I promotes apoptosis-mediated cell death in cultured human hepatoma (HepG2) cells. Biol Pharm Bull 34: 831–838, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Walker RJ, Leader JP, Bedford JJ, Gobe G, Davis G, Vos FE, deJong S, Schollum JB: Chronic interstitial fibrosis in the rat kidney induced by long-term (6-mo) exposure to lithium. Am J Physiol Renal Physiol 304: F300–F307, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Jacobsen NO, Olesen OV, Thomsen K, Ottosen PD, Olsen S: Early changes in renal distal convoluted tubules and collecting ducts of lithium-treated rats: Light microscopy, enzyme histochemistry, and 3H-thymidine autoradiography. Lab Invest 46: 298–305, 1982 [PubMed] [Google Scholar]
- 35.Wu H, Chen L, Zhou Q, Zhang X, Berger S, Bi J, Lewis DE, Xia Y, Zhang W: Aqp2-expressing cells give rise to renal intercalated cells. J Am Soc Nephrol 24: 243–252, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ecelbarger CA: Lithium treatment and remodeling of the collecting duct. Am J Physiol Renal Physiol 291: F37–F38, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Ottosen PD, Nyengård JR, Jacobsen NO, Christensen S: A morphometric and ultrastructural study of lithium-induced changes in the medullary collecting ducts of the rat kidney. Cell Tissue Res 249: 311–315, 1987 [DOI] [PubMed] [Google Scholar]
- 38.Roscoe JM, Goldstein MB, Halperin ML, Wilson DR, Stinebaugh BJ: Lithium-induced impairment of urine acidification. Kidney Int 9: 344–350, 1976 [DOI] [PubMed] [Google Scholar]
- 39.Perez GO, Oster JR, Vaamonde CA: Incomplete syndrome of renal tubular acidosis induced by lithium carbonate. J Lab Clin Med 86: 386–394, 1975 [PubMed] [Google Scholar]
- 40.Kim YH, Kwon TH, Christensen BM, Nielsen J, Wall SM, Madsen KM, Frøkiaer J, Nielsen S: Altered expression of renal acid-base transporters in rats with lithium-induced NDI. Am J Physiol Renal Physiol 285: F1244–F1257, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Welsh-Bacic D, Nowik M, Kaissling B, Wagner CA: Proliferation of acid-secretory cells in the kidney during adaptive remodelling of the collecting duct. PLoS ONE 6: e25240, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van der Avoort IA, van de Nieuwenhof HP, Otte-Höller I, Nirmala E, Bulten J, Massuger LF, van der Laak JA, Slootweg PJ, de Hullu JA, van Kempen LC: High levels of p53 expression correlate with DNA aneuploidy in (pre)malignancies of the vulva. Hum Pathol 41: 1475–1485, 2010 [DOI] [PubMed] [Google Scholar]
- 43.Kamsteeg EJ, Wormhoudt TA, Rijss JP, van Os CH, Deen PM: An impaired routing of wild-type aquaporin-2 after tetramerization with an aquaporin-2 mutant explains dominant nephrogenic diabetes insipidus. EMBO J 18: 2394–2400, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Balkom BW, Boone M, Hendriks G, Kamsteeg EJ, Robben JH, Stronks HC, van der Voorde A, van Herp F, van der Sluijs P, Deen PM: LIP5 interacts with aquaporin 2 and facilitates its lysosomal degradation. J Am Soc Nephrol 20: 990–1001, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.