Abstract
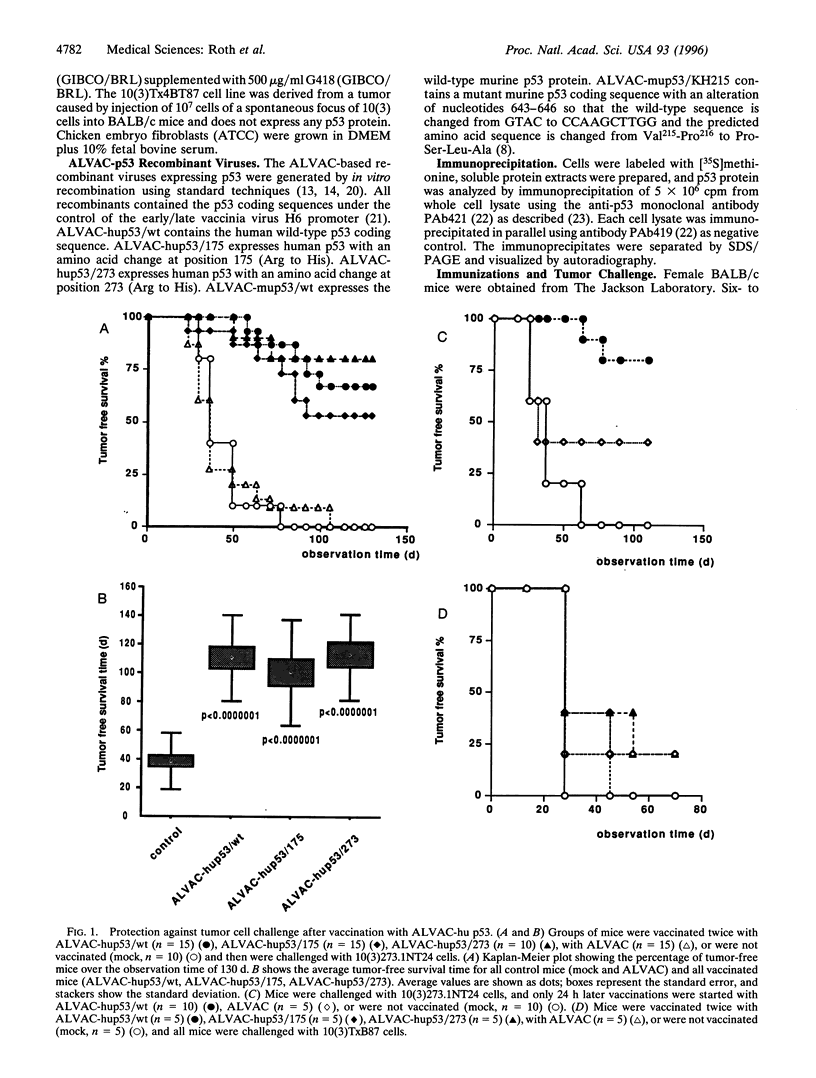
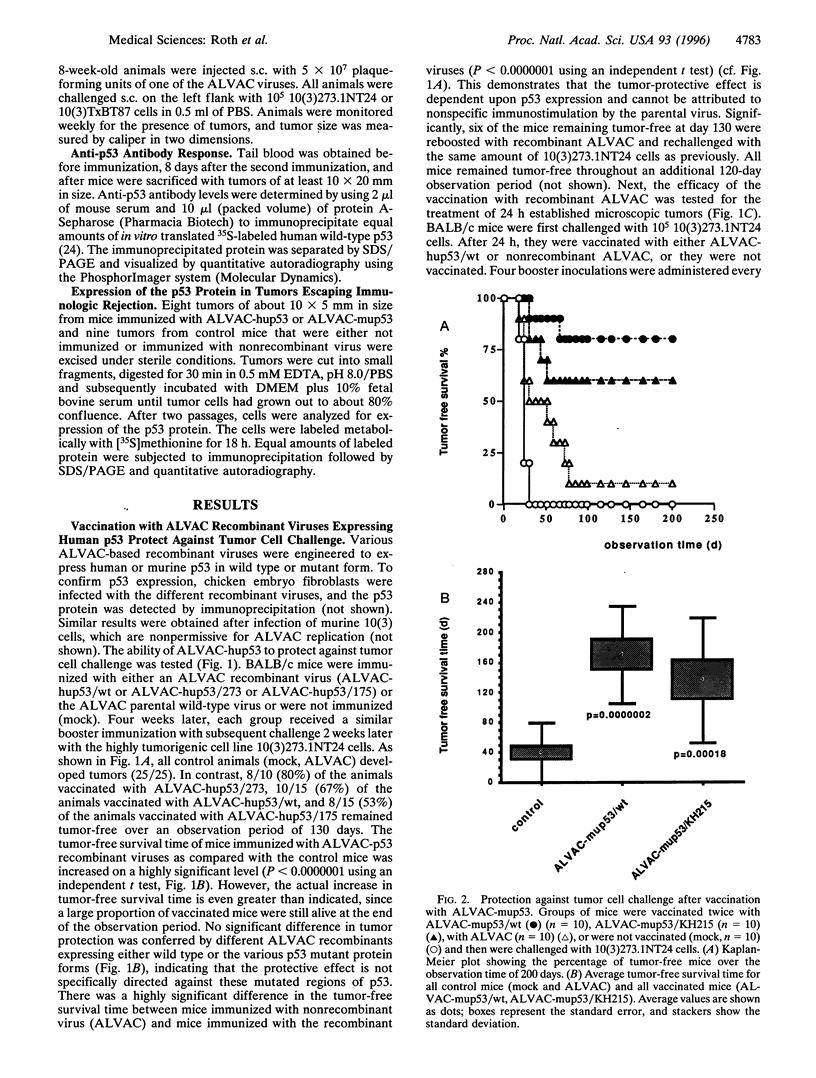
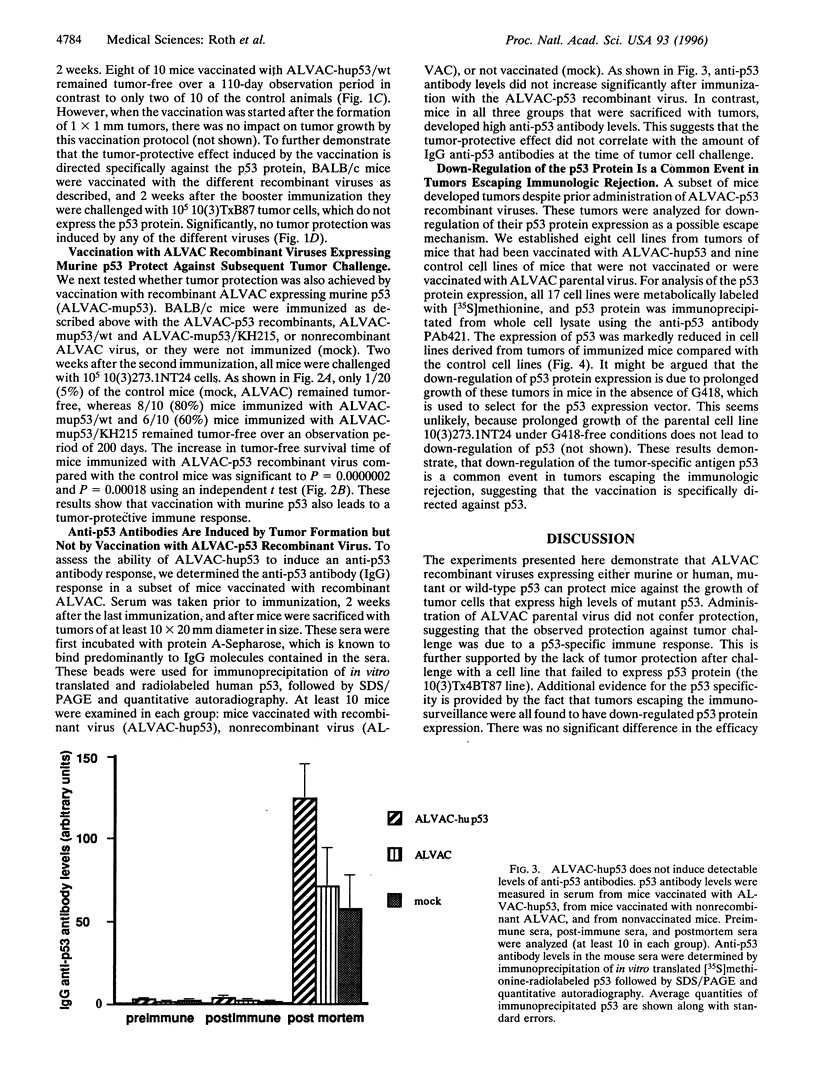
The p53 protein is an attractive target for immunotherapy, because mutations in the p53 gene are the most common genetic alterations found in human tumors. These mutations result in high levels of p53 protein in the tumor cell, whereas the expression level of wild-type p53 in nonmalignant tissue is usually much lower. Several canarypox virus recombinants expressing human or murine p53 in wild-type or mutant form were constructed. Immunization with these viruses protected BALB/c mice from a challenge with an isogenic and highly tumorigenic mouse fibroblast tumor cell line expressing high levels of mutant p53. The tumor protection was equally effective regardless of whether wild-type or mutant p53 was used for the immunization, indicating that the immunologic response was not dependent on any particular p53 mutation and that immunization with this live virus vaccine works effectively against mutant p53 protein expressed in a tumor cell. In tumors escaping immunologic rejection, the expression of the p53 protein was commonly down-regulated.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abimiku A. G., Franchini G., Tartaglia J., Aldrich K., Myagkikh M., Markham P. D., Chong P., Klein M., Kieny M. P., Paoletti E. HIV-1 recombinant poxvirus vaccine induces cross-protection against HIV-2 challenge in rhesus macaques. Nat Med. 1995 Apr;1(4):321–329. doi: 10.1038/nm0495-321. [DOI] [PubMed] [Google Scholar]
- Cadoz M., Strady A., Meignier B., Taylor J., Tartaglia J., Paoletti E., Plotkin S. Immunisation with canarypox virus expressing rabies glycoprotein. Lancet. 1992 Jun 13;339(8807):1429–1432. doi: 10.1016/0140-6736(92)92027-d. [DOI] [PubMed] [Google Scholar]
- Corr M., Boyd L. F., Padlan E. A., Margulies D. H. H-2Dd exploits a four residue peptide binding motif. J Exp Med. 1993 Dec 1;178(6):1877–1892. doi: 10.1084/jem.178.6.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford L. V., Pim D. C., Bulbrook R. D. Detection of antibodies against the cellular protein p53 in sera from patients with breast cancer. Int J Cancer. 1982 Oct 15;30(4):403–408. doi: 10.1002/ijc.2910300404. [DOI] [PubMed] [Google Scholar]
- Dittmer D., Pati S., Zambetti G., Chu S., Teresky A. K., Moore M., Finlay C., Levine A. J. Gain of function mutations in p53. Nat Genet. 1993 May;4(1):42–46. doi: 10.1038/ng0593-42. [DOI] [PubMed] [Google Scholar]
- Dranoff G., Mulligan R. C. Gene transfer as cancer therapy. Adv Immunol. 1995;58:417–454. doi: 10.1016/s0065-2776(08)60624-0. [DOI] [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Stevanović S., Jung G., Rammensee H. G. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991 May 23;351(6324):290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- Feltkamp M. C., Smits H. L., Vierboom M. P., Minnaar R. P., de Jongh B. M., Drijfhout J. W., ter Schegget J., Melief C. J., Kast W. M. Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. Eur J Immunol. 1993 Sep;23(9):2242–2249. doi: 10.1002/eji.1830230929. [DOI] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Levine A. J. The p53 proto-oncogene can act as a suppressor of transformation. Cell. 1989 Jun 30;57(7):1083–1093. doi: 10.1016/0092-8674(89)90045-7. [DOI] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Tan T. H., Eliyahu D., Oren M., Levine A. J. Activating mutations for transformation by p53 produce a gene product that forms an hsc70-p53 complex with an altered half-life. Mol Cell Biol. 1988 Feb;8(2):531–539. doi: 10.1128/mcb.8.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Pim D. C., Crawford L. V. Complex of simian virus 40 large-T antigen and host 53,000-molecular-weight protein in monkey cells. J Virol. 1981 Feb;37(2):564–573. doi: 10.1128/jvi.37.2.564-573.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey D. M., Levine A. J. p53 alteration is a common event in the spontaneous immortalization of primary BALB/c murine embryo fibroblasts. Genes Dev. 1991 Dec;5(12B):2375–2385. doi: 10.1101/gad.5.12b.2375. [DOI] [PubMed] [Google Scholar]
- Hinds P. W., Finlay C. A., Quartin R. S., Baker S. J., Fearon E. R., Vogelstein B., Levine A. J. Mutant p53 DNA clones from human colon carcinomas cooperate with ras in transforming primary rat cells: a comparison of the "hot spot" mutant phenotypes. Cell Growth Differ. 1990 Dec;1(12):571–580. [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Houbiers J. G., Nijman H. W., van der Burg S. H., Drijfhout J. W., Kenemans P., van de Velde C. J., Brand A., Momburg F., Kast W. M., Melief C. J. In vitro induction of human cytotoxic T lymphocyte responses against peptides of mutant and wild-type p53. Eur J Immunol. 1993 Sep;23(9):2072–2077. doi: 10.1002/eji.1830230905. [DOI] [PubMed] [Google Scholar]
- Iggo R., Gatter K., Bartek J., Lane D., Harris A. L. Increased expression of mutant forms of p53 oncogene in primary lung cancer. Lancet. 1990 Mar 24;335(8691):675–679. doi: 10.1016/0140-6736(90)90801-b. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Momand J., Finlay C. A. The p53 tumour suppressor gene. Nature. 1991 Jun 6;351(6326):453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- Lin J., Chen J., Elenbaas B., Levine A. J. Several hydrophobic amino acids in the p53 amino-terminal domain are required for transcriptional activation, binding to mdm-2 and the adenovirus 5 E1B 55-kD protein. Genes Dev. 1994 May 15;8(10):1235–1246. doi: 10.1101/gad.8.10.1235. [DOI] [PubMed] [Google Scholar]
- Lubin R., Schlichtholz B., Bengoufa D., Zalcman G., Trédaniel J., Hirsch A., Caron de Fromentel C., Preudhomme C., Fenaux P., Fournier G. Analysis of p53 antibodies in patients with various cancers define B-cell epitopes of human p53: distribution on primary structure and exposure on protein surface. Cancer Res. 1993 Dec 15;53(24):5872–5876. [PubMed] [Google Scholar]
- Nanda N. K., Sercarz E. E. Induction of anti-self-immunity to cure cancer. Cell. 1995 Jul 14;82(1):13–17. doi: 10.1016/0092-8674(95)90047-0. [DOI] [PubMed] [Google Scholar]
- Noguchi Y., Chen Y. T., Old L. J. A mouse mutant p53 product recognized by CD4+ and CD8+ T cells. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3171–3175. doi: 10.1073/pnas.91.8.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti E., Tartaglia J., Cox W. I. Immunotherapeutic strategies for cancer using poxvirus vectors. Ann N Y Acad Sci. 1993 Aug 12;690:292–300. doi: 10.1111/j.1749-6632.1993.tb44017.x. [DOI] [PubMed] [Google Scholar]
- Pardoll D. New strategies for active immunotherapy with genetically engineered tumor cells. Curr Opin Immunol. 1992 Oct;4(5):619–623. doi: 10.1016/0952-7915(92)90037-f. [DOI] [PubMed] [Google Scholar]
- Peoples G. E., Goedegebuure P. S., Smith R., Linehan D. C., Yoshino I., Eberlein T. J. Breast and ovarian cancer-specific cytotoxic T lymphocytes recognize the same HER2/neu-derived peptide. Proc Natl Acad Sci U S A. 1995 Jan 17;92(2):432–436. doi: 10.1073/pnas.92.2.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkus M. E., Limbach K., Paoletti E. Cloning and expression of foreign genes in vaccinia virus, using a host range selection system. J Virol. 1989 Sep;63(9):3829–3836. doi: 10.1128/jvi.63.9.3829-3836.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber G., Leder G. H., Storkus W. T., Lotze M. T., Modrow S., Székely L., Wolf H., Klein E., Kärre K., Klein G. Identification of wild-type and mutant p53 peptides binding to HLA-A2 assessed by a peptide loading-deficient cell line assay and a novel major histocompatibility complex class I peptide binding assay. Eur J Immunol. 1994 Mar;24(3):765–768. doi: 10.1002/eji.1830240341. [DOI] [PubMed] [Google Scholar]
- Tartaglia J., Cox W. I., Taylor J., Perkus M., Riviere M., Meignier B., Paoletti E. Highly attenuated poxvirus vectors. AIDS Res Hum Retroviruses. 1992 Aug;8(8):1445–1447. doi: 10.1089/aid.1992.8.1445. [DOI] [PubMed] [Google Scholar]
- Tartaglia J., Perkus M. E., Taylor J., Norton E. K., Audonnet J. C., Cox W. I., Davis S. W., van der Hoeven J., Meignier B., Riviere M. NYVAC: a highly attenuated strain of vaccinia virus. Virology. 1992 May;188(1):217–232. doi: 10.1016/0042-6822(92)90752-b. [DOI] [PubMed] [Google Scholar]
- Theobald M., Biggs J., Dittmer D., Levine A. J., Sherman L. A. Targeting p53 as a general tumor antigen. Proc Natl Acad Sci U S A. 1995 Dec 19;92(26):11993–11997. doi: 10.1073/pnas.92.26.11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilkin A. F., Lubin R., Soussi T., Lazar V., Janin N., Mathieu M. C., Lefrère I., Carlu C., Roy M., Kayibanda M. Primary proliferative T cell response to wild-type p53 protein in patients with breast cancer. Eur J Immunol. 1995 Jun;25(6):1765–1769. doi: 10.1002/eji.1830250642. [DOI] [PubMed] [Google Scholar]
- Yanuck M., Carbone D. P., Pendleton C. D., Tsukui T., Winter S. F., Minna J. D., Berzofsky J. A. A mutant p53 tumor suppressor protein is a target for peptide-induced CD8+ cytotoxic T-cells. Cancer Res. 1993 Jul 15;53(14):3257–3261. [PubMed] [Google Scholar]
- van der Bruggen P., Traversari C., Chomez P., Lurquin C., De Plaen E., Van den Eynde B., Knuth A., Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991 Dec 13;254(5038):1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]




