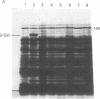
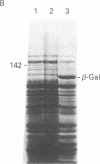
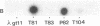Abstract
A sensitive and general technique has been devised for the dual purposes of cloning genes by using antibodies as probes and isolating unknown proteins encoded by cloned DNA. The method uses an expression vector, lambda gt11 (lac5 nin5 cI857 S100), that permits insertion of foreign DNA into the beta-galactosidase structural gene lacZ and promotes synthesis of hybrid proteins. Efficient screening of antigen-producing clones in lambda gt11 recombinant cDNA libraries is achieved through lysogeny of the phage library in hflA (high-frequency lysogeny) mutant cells of Escherichia coli; lysogens produce detectable quantities of antigen on induction, even when plated at high cell densities. The vector is also designed to facilitate the isolation of proteins specified by previously cloned gene sequences. Hybrid proteins encoded by recombinant phage accumulate in strains defective in protein degradation (lon mutants) in amounts amenable to large-scale purification. Antibodies produced against the portion of the hybrid encoded by foreign DNA could in turn be used to isolate the native polypeptide from eukaryotic cells.
Full text
PDF

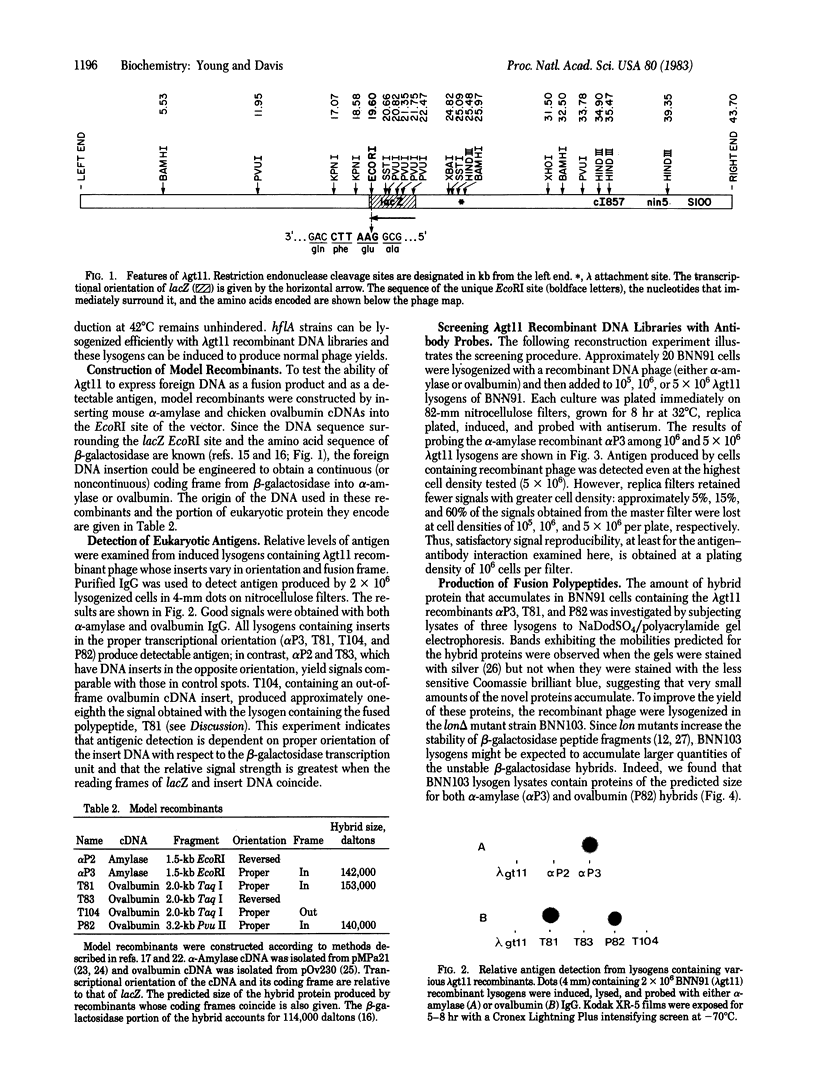


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belfort M., Wulff D. L. An analysis of the processes of infection and induction of E. coli mutant hfl-1 by bacteriophage lambda. Virology. 1973 Sep;55(1):183–192. doi: 10.1016/s0042-6822(73)81020-7. [DOI] [PubMed] [Google Scholar]
- Broome S., Gilbert W. Immunological screening method to detect specific translation products. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2746–2749. doi: 10.1073/pnas.75.6.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhari A. I., Zipser D. Mutants of Escherichia coli with a defect in the degradation of nonsense fragments. Nat New Biol. 1973 Jun 20;243(129):238–241. doi: 10.1038/newbio243238a0. [DOI] [PubMed] [Google Scholar]
- Charnay P., Gervais M., Louise A., Galibert F., Tiollais P. Biosynthesis of hepatitis B virus surface antigen in Escherichia coli. Nature. 1980 Aug 28;286(5776):893–895. doi: 10.1038/286893a0. [DOI] [PubMed] [Google Scholar]
- Edman J. C., Hallewell R. A., Valenzuela P., Goodman H. M., Rutter W. J. Synthesis of hepatitis B surface and core antigens in E. coli. Nature. 1981 Jun 11;291(5815):503–506. doi: 10.1038/291503a0. [DOI] [PubMed] [Google Scholar]
- Erlich H. A., Cohen S. N., McDevitt H. O. A sensitive radioimmunoassay for detecting products translated from cloned DNA fragments. Cell. 1978 Apr;13(4):681–689. doi: 10.1016/0092-8674(78)90218-0. [DOI] [PubMed] [Google Scholar]
- Galas D. J., Calos M. P., Miller J. H. Sequence analysis of Tn9 insertions in the lacZ gene. J Mol Biol. 1980 Nov 25;144(1):19–41. doi: 10.1016/0022-2836(80)90213-2. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Goldberg A. R., Howe M. New mutations in the S cistron of bacteriophage lambda affecting host cell lysis. Virology. 1969 May;38(1):200–202. doi: 10.1016/0042-6822(69)90148-2. [DOI] [PubMed] [Google Scholar]
- Hagenbüchle O., Bovey R., Young R. A. Tissue-specific expression of mouse-alpha-amylase genes: nucleotide sequence of isoenzyme mRNAs from pancreas and salivary gland. Cell. 1980 Aug;21(1):179–187. doi: 10.1016/0092-8674(80)90125-7. [DOI] [PubMed] [Google Scholar]
- Hitzeman R. A., Clarke L., Carbon J. Isolation and characterization of the yeast 3-phosphoglycerokinase gene (PGK) by an immunological screening technique. J Biol Chem. 1980 Dec 25;255(24):12073–12080. [PubMed] [Google Scholar]
- Hoyt M. A., Knight D. M., Das A., Miller H. I., Echols H. Control of phage lambda development by stability and synthesis of cII protein: role of the viral cIII and host hflA, himA and himD genes. Cell. 1982 Dec;31(3 Pt 2):565–573. doi: 10.1016/0092-8674(82)90312-9. [DOI] [PubMed] [Google Scholar]
- Itakura K., Hirose T., Crea R., Riggs A. D., Heyneker H. L., Bolivar F., Boyer H. W. Expression in Escherichia coli of a chemically synthesized gene for the hormone somatostatin. Science. 1977 Dec 9;198(4321):1056–1063. doi: 10.1126/science.412251. [DOI] [PubMed] [Google Scholar]
- Kemp D. J., Cowman A. F. Direct immunoassay for detecting Escherichia coli colonies that contain polypeptides encoded by cloned DNA segments. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4520–4524. doi: 10.1073/pnas.78.7.4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lerner R. A. Tapping the immunological repertoire to produce antibodies of predetermined specificity. Nature. 1982 Oct 14;299(5884):593–596. doi: 10.1038/299592a0. [DOI] [PubMed] [Google Scholar]
- McReynolds L., O'Malley B. W., Nisbet A. D., Fothergill J. E., Givol D., Fields S., Robertson M., Brownlee G. G. Sequence of chicken ovalbumin mRNA. Nature. 1978 Jun 29;273(5665):723–728. doi: 10.1038/273723a0. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Switzer R. C., Van Keuren M. L. Trace polypeptides in cellular extracts and human body fluids detected by two-dimensional electrophoresis and a highly sensitive silver stain. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4335–4339. doi: 10.1073/pnas.76.9.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount D. W. The genetics of protein degradation in bacteria. Annu Rev Genet. 1980;14:279–319. doi: 10.1146/annurev.ge.14.120180.001431. [DOI] [PubMed] [Google Scholar]
- Neubauer Z., Calef E. Immunity phase-shift in defective lysogens: non-mutational hereditary change of early regulation of lambda prophage. J Mol Biol. 1970 Jul 14;51(1):1–13. doi: 10.1016/0022-2836(70)90265-2. [DOI] [PubMed] [Google Scholar]
- Sanzey B., Mercereau O., Ternynck T., Kourilsky P. Methods for identification of recombinants of phage lambda. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3394–3397. doi: 10.1073/pnas.73.10.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schibler U., Tosi M., Pittet A. C., Fabiani L., Wellauer P. K. Tissue-specific expression of mouse alpha-amylase genes. J Mol Biol. 1980 Sep 5;142(1):93–116. doi: 10.1016/0022-2836(80)90208-9. [DOI] [PubMed] [Google Scholar]
- Skalka A., Shapiro L. In situ immunoassays for gene translation products in phage plaques and bacterial colonies. Gene. 1976;1(1):65–79. doi: 10.1016/0378-1119(76)90007-x. [DOI] [PubMed] [Google Scholar]
- Villa-Komaroff L., Efstratiadis A., Broome S., Lomedico P., Tizard R., Naber S. P., Chick W. L., Gilbert W. A bacterial clone synthesizing proinsulin. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3727–3731. doi: 10.1073/pnas.75.8.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]