Abstract
Background
Increasingly, women with a strong family history of breast cancer are seeking genetic testing as a starting point to making significant decisions regarding management of their cancer risks. Individuals who are found to be carriers of a BRCA1 or BRCA2 mutation have a substantially elevated risk for breast cancer and are frequently faced with the decision of whether or not to undergo risk reducing mastectomy.
Objective
In order to provide BRCA1/2 carriers with ongoing decision support for breast cancer risk management, a computer-based interactive decision aid was developed and tested against usual care in a randomized controlled trial.
Design
Following genetic counseling, 214 female (aged 21-75) BRCA1/2 mutation carriers were randomized to Usual Care (UC; N=114) or Usual Care plus Decision Aid (DA; N=100) arms. UC participants received no further intervention; DA participants were sent the CD-ROM based decision aid to view at home.
Main Outcome Measures
The authors measured general distress, cancer specific distress and genetic testing specific distress at 1-, 6- and 12-month follow up time points, post-randomization.
Results
Longitudinal analyses revealed a significant longitudinal impact of the DA on cancer specific distress (B= 5.67, z = 2.81, p = 0.005) which varied over time (DA group by time; B = -2.19, z = -2.47, p = 0.01) and on genetic testing specific distress (B = 5.55, z = 2.46, p = 0.01) which also varied over time (DA group by time; B= -2.46, z = -2.51, p = 0.01). Individuals randomized to UC reported significantly decreased distress in the month following randomization, whereas individuals randomized to the DA maintained their post-disclosure distress over the short-term. By 12-months, the overall decrease in distress between the two groups was similar.
Conclusion
This report provides new insight into the long-term longitudinal effects of DAs.
Introduction
Increasingly, women with a strong family history of breast or ovarian cancer are seeking genetic testing as a starting point for making significant decisions regarding management of their cancer risks. Lifetime breast cancer risk in women found to carry a BRCA1 or BRCA2 (BRCA1/2) mutation is estimated to be as high as 75%, and ovarian cancer risk may be as high as 40% (1,2). For BRCA1/2 carriers, management guidelines recommend breast cancer surveillance with yearly mammograms and breast magnetic resonance imaging beginning between the ages of 25 and 30, regular clinician breast exams and consideration of risk-reducing mastectomy (3,4). For ovarian cancer risk management, risk reducing oophorectomy is recommended upon completion of childbearing or by age 35. Women who have their ovaries removed prior to age 50 also experience a significant reduction in breast cancer risk (5,6).
After a woman learns that she carries a BRCA1/2 mutation, she is confronted with a cascade of decisions regarding her breast cancer risk management; most prominently, decisions between risk-reducing mastectomy and cancer surveillance (7). These complex decisions are preference-driven and involve multiple tradeoffs between competing options (8). Given the complexity and preference-driven nature of these decisions, decision aids have been developed to help BRCA1/2 carriers decide among their risk management options.
Decision Aids (DA) are designed to help individuals reach preference-based medical decisions (9). They provide information about decision options and possible outcomes along with value clarification tools designed to facilitate informed and preference-sensitive decision making. DAs have been employed for a variety of medical decisions such as decisions about hormone replacement therapy (10), treatment for breast and prostate cancers (11-14), and prostate cancer screening (15,16). These DAs have been consistently shown to result in increased knowledge and reduced decisional conflict (17). Similarly, DAs for cancer risk management decisions among women at increased risk for cancer have led to increased knowledge and decreased decisional conflict (18-20). In a previous report, we demonstrated that an interactive DA for BRCA1/2 carriers led to an increased probability of reaching a breast cancer management decision, decreased decisional conflict, and increased decision satisfaction among those who were initially undecided about their breast cancer risk management (21). We also documented that the DA impacted the trajectory of risk management decisions, suggesting more deliberative decision making for the DA group (21).
Despite substantial evidence for the efficacy of DAs at improving short-term decision making outcomes, there is little evidence documenting an impact on more distal outcomes such as long term distress or quality of life. Studies that have measured the impact of decision support on global measures of distress such as anxiety and depression have typically found no effect on either outcome (11,19). The few studies that have employed measures of cancer specific distress have also failed to demonstrate an impact of decision support (18-20). Given the lack of research on the longitudinal impact of decision support, there is an emerging consensus that evaluation of DAs should extend beyond short-term measures of decision making such as decisional conflict and knowledge. Indeed, the assumption that short-term reductions in decisional conflict indicate better decision making has been questioned (22).
In understanding the impact of DAs on distress, it may be important to consider the trajectory of distress. For example, management decision making among BRCA1/2 carriers often takes place over an extended period. By fostering extended deliberation, the potential impact of decision support on distress could be delayed. Thus, the impact of a DA on distress may depend upon the time point at which distress is assessed. Consistent with this theory, a randomized trial of in-person decision counseling for breast cancer risk management found that decision counseling led to decreased cancer specific distress nine months after exposure to the intervention (8). Other studies have reported stronger effects on longer term measures related to satisfaction and health outcomes (12,23). Thus, measuring the longitudinal impact of decision support may be crucial to gaining a full understanding of its impact.
This report focuses on the impact of decision support on the longitudinal trajectory of psychosocial outcomes of BRCA1/2 mutation carriers. As we have previously reported (21), we developed our DA using the Ottawa framework for informed decision making (10,24). The DA included information about breast cancer risk management options and a value-clarification exercise designed to help participants weigh the risks and benefits of each option for breast cancer risk reduction and/or surveillance. As discussed above, the DA led to improved decision making outcomes among carriers who were initially undecided about how to manage their breast cancer risk (21). Here we report on the longitudinal impact of the DA on the trajectory of general, cancer specific, and genetic testing distress. We hypothesized that the longitudinal trajectories of distress between the DA and usual care (UC) groups would differ. Specifically, we predicted that the impact of the DA on distress would be most apparent over the long-term.
Methods
Participants
Participants were 214 women between age 25-75 years who received a positive BRCA1/2 gene test result through the clinical research program at one of the participating sites [Lombardi Comprehensive Cancer Center (Washington, DC), Mount Sinai School of Medicine (New York), and Englewood Hospital and Medical Center (Englewood, NJ)] from 2001 to 2005. Eligible participants had not had prior bilateral mastectomy and did not have metastatic breast or ovarian cancer. Genetic counseling was provided free of charge.
Participants were self- or physician-referred to the genetic counseling programs at each site. As displayed in Figure 1, a total of 1,223 individuals contacted the clinical research programs to inquire about genetic counseling services across all sites, 91% (N=1,109 out of 1223) of whom consented to complete a baseline interview as a part of their participation in an observational study of patients interested in genetic counseling and testing. Subsequently, 89% (N=992 out of 1,109) completed pretest genetic counseling and received BRCA1/2 test results. Overall, there were 333 positive BRCA1/2 test results. Of the 333 BRCA1/2 carriers, 106 were ineligible for randomization for the following reasons: male (N=46), history of prior bilateral mastectomy (N=44), under age 25 (N=2), age 75 or older (N=6), history of metastatic disease (N=7) and non-English speaker (N=1). Of the 227 women who were eligible for randomization, 3 (1.3%) declined randomization, 8 (3.5%) did not complete the post-disclosure baseline interview, and 2 (0.9%) were excluded from the study due to clerical errors. The remaining 214 (94% of 227) women completed the baseline interview and were randomized to either UC (N=114) or DA (N=100). This final sample size was based on power analyses which suggested a minimum N of 100 per group in order to yield greater than 80% power to detect medium size (d=0.40 SDs) group differences on our primary study outcomes.
Figure 1. Study Flow Chart.
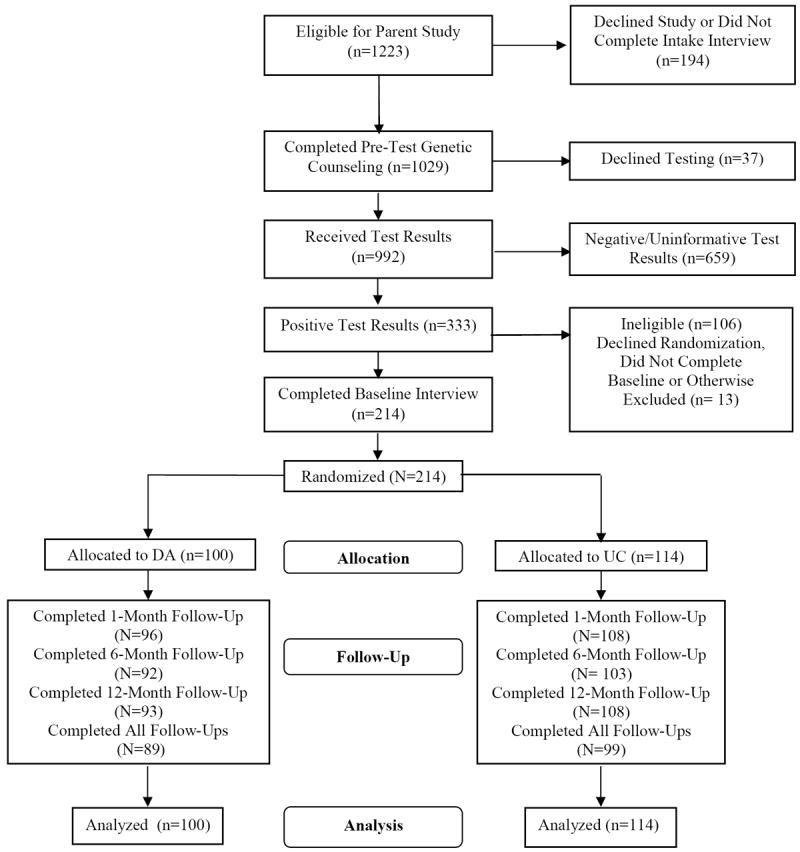
Of the 214 women included in these analyses (Table 1), 93% were White, 76% were college educated, 67% were married, 48% were employed full-time and 49% were Ashkenazi Jewish (and thus at increased risk for carrying a BRCA1/2 mutation because of the high frequency of BRCA1/2 mutations among individuals of Ashkenazi Jewish descent). The mean age of participants was 44 years (Range: 21-74 years). Thirty-seven percent were affected with breast cancer and 10% with ovarian cancer (mean time since diagnosis of either cancer = 7.7 years).
Table 1.
Baseline Group Comparisons
| Characteristic | Usual Care (n=114) | Decision Aid (n=100) |
|---|---|---|
| Mean Age (SD) | 43.5 (10.6) | 44.3 (11.2) |
| Education | ||
| <College (%) | 33 (29) | 19 (19) |
| College + (%) | 81(71) | 81(81) |
| Religion/Ethnicity | ||
| Jewish (%) | 56 (49) | 49(49) |
| Non-Jewish (%) | 58 (51) | 51(51) |
| Employment status | ||
| Full time (%) | 55 (48.3) | 48 (48) |
| < Full Time (%) | 59 (51.7) | 52 (52) |
| Race | ||
| Caucasian (%) | 107 (94) | 92 (92) |
| Non-Caucasian (%) | 7 (6) | 8 (8) |
| Marital Status | ||
| Married /Partner (%) | 79 (69) | 64 (64) |
| Single/Widow/Divorced | 35 (31) | 36 (36) |
| Affected with Breast Cancer (%) | ||
| Yes(%) | 44 (39) | 34 (34) |
| No (%) | 69 (61) | 66 (66) |
| Affected with Ovarian Cancer (%) | ||
| Yes(%) | 7 (6) | 15 (15) |
| No (%) | 107 (94) | 85 (85)* |
| Past or Current Chemotherapy (% of Affected) | ||
| Yes(%) | 38 (71.7) | 41 (78.8) |
| No (%) | 15 (28.3) | 11 (21.1) |
| Prior Oophorectomy (%) | ||
| Yes(%) | 13 (11.4) | 27 (27) |
| No (%) | 101 (88.6) | 73 (73) ** |
| Past Breast Biopsy (%) | ||
| Yes(%) | 58 (51) | 48 (48) |
| No (%) | 56 (49) | 52 (52) |
| First Degree Relatives with Breast or Ovarian Cancer | ||
| <2 (%) | 87(76) | 77 (77) |
| 2+ (%) | 27 (24) | 23 (23) |
| Reached Final Management Decision | ||
| Yes (%) | 56 (49) | 47(47) |
| No (%) | 58 (51) | 53 (53) |
| Mean Baseline Cancer Specific Distress – IES (SD) | 21.2 (17.6) | 19.6 (17.0) |
| Mean Baseline General Distress – BSI (SD) | 17.8 (6.7) | 17.5 (6.3) |
| Mean Baseline Genetic Testing Distress - MICRA (SD) | 33.8 (16.0) | 36.3 (19.1) |
p <0.05,
p <0.01
Procedure
This randomized trial was nested within a larger observational study assessing the outcomes of BRCA1/2 testing. Before receiving genetic counseling, participants consented to participation in the observational study and to participation in the DA trial if they were determined eligible. Following pretest genetic counseling, and once BRCA1/2 test results were available, participants completed a genetic counseling disclosure session during which the counselor discussed test result implications, risk-management options/recommendations and physician referrals. Since this was a high-risk population, data regarding the efficacy of various screening and risk-reducing options, including risk-reducing surgeries, were reviewed and recommendations were consistent with guidelines for high-risk individuals. Following the disclosure session participants were contacted for a routine 2-week follow-up telephone call and were mailed a summary letter outlining all guidelines and recommendations specific to their test result and personal/family history of breast and ovarian cancer.
One month following the disclosure session, participants were contacted by a research assistant for a baseline interview to assess behaviors, management intentions, and psychosocial outcomes (general distress, cancer specific and genetic testing specific distress). After completing this interview, eligible participants provided verbal consent for participation in the randomized control trial and were randomized by the research assistant in a 1:1 ratio to either the UC (N=114) or usual care plus DA (DA; N=100) arm. Randomization sequences were generated for each research site in blocks of four using the SAS software package, and research assistants were kept blind to the randomization sequences until the interventions were assigned. Participants assigned to the UC arm received no further intervention while participants in the DA arm were mailed the CD-ROM-based interactive DA via priority mail. We completed follow-up telephone interviews with all participants at 1-, 6- and 12-months post-randomization.
UC
All participants received standard genetic counseling, as described above; see previous reports for a more detailed description of standard genetic counseling for BRCA1/2 provided through this protocol (25).
DA Intervention
After completing UC, DA participants were sent the CD-ROM-based interactive DA via priority mail. We have previously described the content and development of the DA (26). Following a brief introduction and tutorial, the user completed a series of questions that were used to tailor the content based on age, menopausal status, breast cancer history, tamoxifen use, and oophorectomy history. The informational content of the DA was divided into four sections: 1) The “Breast Cancer Information” section reviewed information on breast cancer causes, prevention and treatment; 2) The “Risk Communication” section provided individually tailored breast and ovarian cancer risk information; 3) The “Risk Management Options” section provided a detailed overview of the pros and cons of each management option with a particular focus on the advantages and disadvantages of choosing one option over another; 4) The “Interactive Decision Task” guided participants through a multi-attribute value model (27) and provided feedback on the management option that the participant appeared to favor based on the results of this task.
Measures
Control Variables
Sociodemographics
In the pre-counseling interview, we assessed age, race, education level, marital status, employment status, health insurance status, and ethnic background.
Medical / Family History
In the pre-counseling interview, we assessed personal and family history of cancer, cancer screening behavior, and surgical history.
Outcome Variables
We assessed outcome variables at the time of randomization and in the 1-, 6-and 12-month post-randomization interviews.
General Distress
We used the12-item Brief Symptom Inventory (BSI) to measure general distress (28). The BSI is a five-item Likert-style scale used to assess anxiety and depressive symptoms. We used a modified four-point scale (not at all-extremely) to determine the discomfort experienced during the previous two weeks (21).
Cancer Specific Distress
We measured cancer specific distress with the Likert-style 15-item Impact of Event Scale (IES) (29). The IES is designed to assess the stress associated with a specific life event, in this case risk for cancer, indicating avoidant and intrusive feelings, thoughts or behaviors.
Genetic Testing Distress
We used the Multidimensional Impact of Cancer Risk Assessment Questionnaire (MICRA) to evaluate post-disclosure genetic testing distress (30). The MICRA is a 25-item scale developed to measure specific responses to the receipt of genetic test results; it uses a four-point scale to assess three main factors (Distress, Uncertainty, Positive Experiences) that are combined as a total score.
Management Decision
At the post-disclosure baseline and each follow-up interview, we asked participants ‘Have you made a final decision about how to manage your risk for breast cancer?’ We also asked participants whether they had obtained a risk-reducing mastectomy since the previous assessment. Participants responded either yes or no to each question.
Statistical Analyses
We conducted preliminary bivariate analyses to identify baseline group differences. We then evaluated the impact of study site and of recruiting multiple members of the same family on our outcomes. As the impact of these variables was negligible, we did not include them in subsequent analyses. We compared mean scores on all outcome measures at follow up time points, adjusting for baseline scores on these measures, using a repeated measure ANOVA. We conducted linear regression using Generalized Estimating Equations (GEE) to evaluate the longitudinal impact of the DA intervention (31). Our primary analyses used an intention-to-treat approach in which all participants were included regardless of whether or not they viewed the intervention. We also conducted follow-up per-protocol analyses in which we removed individuals who had not viewed the intervention. We conducted all analyses controlling for baseline scores on the outcome of interest and for potential baseline confounders, including whether the patient had had their ovaries removed at baseline and whether the patient had a history of breast cancer. We also controlled for whether or not the participant had made a decision regarding breast cancer risk management at baseline, as it was an important moderator of DA impact in our previous report (21). In each model we included a time factor and the group by time interaction term to evaluate the longitudinal effect of group. We also conducted follow-up point-to-point analyses with standard multiple linear regression to examine changes from baseline to 1-month post-randomization, from 1-month to 6-months and from 6-months to 12-months. For point-to-point regression models, only women who completed 1-, 6- and 12-month post-randomization interviews were included (88% of randomized participants, N=188).
Results
Baseline Comparisons
We randomized 214 individuals to the DA (N=100) or UC (N=114) (Table 1). At the time of randomization, DA participants were more likely to have been diagnosed with ovarian cancer (15% vs. 6%, χ2 (df =1, N=214) =4.5, p=0.03) and to have had an oophorectomy (27% vs. 13%, χ2 (df =1, N=214) =8.0, p=0.005). Since these variables were highly confounded, we chose to control for the stronger effect, baseline ovarian status (i.e. whether the participant had their ovaries at the time of baseline), in multivariate analyses. Inclusion of the potential confounders of baseline decision status (i.e., whether the participant reported having made a final breast cancer risk management decision at the time of randomization) and whether or not the patient had a history of breast cancer did not significantly impact either the main effects of our models, not did it impact the interaction terms, and as a result, these variables were excluded from the final multivariate models. All other demographic and medical history variables were comparable between the groups. Differences in distress levels were not observed by research site.
Intent to Treat Analyses
General Distress
Means for general distress by randomization group across the year following testing are displayed in Figure 2A. To evaluate the impact of the DA on general distress, we conducted a linear regression using GEE (controlling for general distress, and baseline ovary status). As displayed in Table 2, neither the main effect of DA group assignment (B= -0.46, z = -0.54, p = 0.59) nor the DA group by time interaction effect (B= -0.17, z = -0.43, p = 0.67) attained statistical significance.
Figure 2. Mean Distress Levels by Randomization Group.
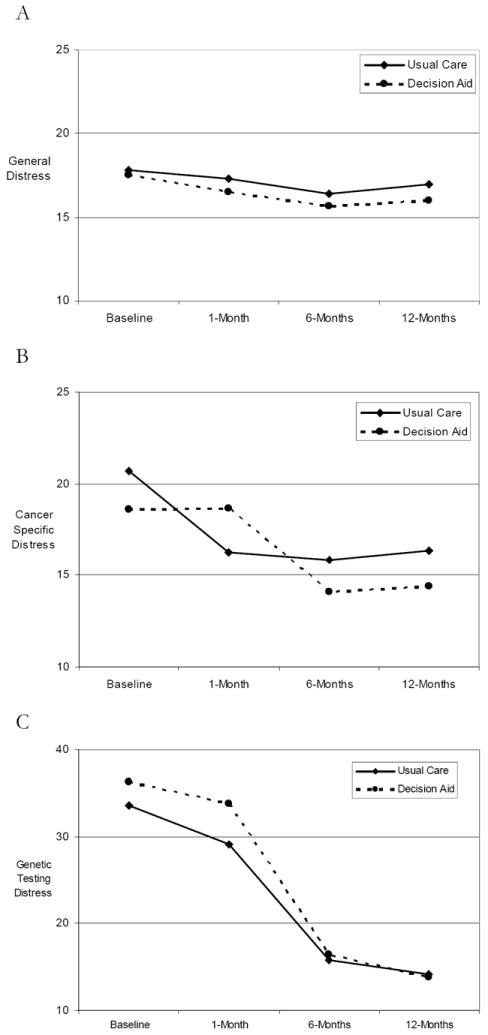
Table 2.
Linear Regression Models of Distress Outcomes
| Outcome Variable | General Distress | Cancer Specific Distress | Genetic Testing Distress | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Predictor | Coefficient (SE) | z | Coefficient (SE) | z | Coefficient (SE) | z |
| Ovary Status at Baseline | -0.08 (0.53) | -0.15 | -1.30 (1.46) | -0.89 | -0.52 (1.02) | -0.51 |
| General Distress at Baseline | 0.59 (0.05)*** | 11.34 | ||||
| Cancer Specific Distress at Baseline | 0.68 (0.04)*** | 15.89 | ||||
| Genetic Testing Distress at Baseline | 0.50 (0.03)*** | 19.86 | ||||
| Time | -0.12 (0.26) | -0.48 | 0.16 (0.58) | 0.27 | -7.53 (0.66)*** | -11.41 |
| Decision Aid Group | -0.46 (0.86) | -0.54 | 5.67 (2.02)** | 2.81 | 5.55 (2.26)* | 2.46 |
| Decision Aid Group × Time | -0.17 (0.39) | -0.43 | -2.19 (0.89)* | -2.47 | -2.46 (0.98)* | -2.51 |
p <0.05,
p<0.01,
p<0.001
Cancer Specific Distress
As displayed in Table 2, multiple linear regression with GEE (controlling for cancer specific distress, ovary status and decision status at the time of randomization) revealed a significant longitudinal impact of the DA (B= 5.67, z = 2.81, p = 0.005) which varied over time (DA by time; B = -2.19, z = -2.47, p = 0.01) (see Figure 2B). To characterize this interaction effect, we conducted point-to-point analyses using multiple linear regression (controlling for ovary status and management decision at randomization). These analyses included only the individuals who completed 1-, 6- and 12-month post-randomization interviews (N= 89 in DA group, 99 in UC group). From baseline (i.e., post disclosure and pre-randomization) to 1-month post-randomization, controlling for pre-randomization distress, the UC group exhibited decreased distress relative to the DA group (B= 3.95, z = 2.61, p = 0.01). In contrast, from 1- to 6-months, controlling for distress at 1-month post-randomization, the DA group exhibited significantly decreased distress relative to the UC group (B = -3.71, z = -2.35, p= 0.02). There was no significant difference between the groups from 6- to 12-months (B = -1.05, z = -0.67, p= 0.51). Importantly, the only time point in which the mean adjusted cancer specific distress scores for the two groups differed significantly was at the 1-month post-randomization point (p = 0.02)
Genetic Testing Specific Distress
As displayed in Table 2, multiple linear regression with GEE (controlling for genetic testing distress, ovary status and decision status at the time of randomization), revealed a significant longitudinal impact of the DA on genetic testing specific distress (B = 5.55, z = 2.46, p = 0.01) which was modified by a significant DA by time interaction (DA by time; B = -2.46, z = -2.51, p = 0.01) (Figure 2C).
To further characterize the time by DA interaction, we again conducted point-to-point regression analyses. The UC group exhibited decreased distress relative to the DA group from baseline to 1-month post-randomization (B= 3.08, z = 2.01, p = 0.04). From 1- to 6-months post-randomization (controlling for distress at 1-month), the groups did not differ on change in genetic testing distress (B= -1.35, z = -1.08, p = 0.28). Similarly, from 6- to 12-months post randomization (controlling for distress at 6-months), the groups did not differ on change in distress (B= -0.32, z = -0.25, p = 0.80). As was the case for cancer specific distress, the only time point at which the mean adjusted genetic testing specific distress scores for the DA and UC groups differed significantly on distress was at 1-month post-randomization (p = 0.04).
Use of DA
Of the 100 DA participants included in these analyses, 36 (36%) reported that they did not use the DA. Thus, we conducted follow-up per-protocol analyses to evaluate the impact of the DA among individuals who reported using it (N= 64). As in the intent-to-treat analyses, we adjusted for group differences in ovarian status at randomization. Table 3 describes the results of the GEE models comparing DA-users to UC. As in the intent-to-treat analyses, DA use was not associated with general distress (B = -0.78, z = -0.82, p = 0.41). For cancer specific and genetic testing specific distress, the trajectories of distress were identical to the intent-to-treat sample; however, eliminating participants who did not use the DA strengthened the effects. Specifically, use of the DA relative to UC was longitudinally associated with cancer specific distress (B = 7.42, z = 3.30, p = 0.001) and this effect varied over time (DA by time; B = -2.95, z = -2.79, p = 0.005). Similarly, for genetic testing distress, there was a longitudinal effect of DA use (B = 8.23, z = 3.17, p = 0.001) that varied across time points (DA by time; B = -3.89, z = -3.41, p =0.001). In these analyses, mean scores of cancer specific and genetic testing specific distress adjusted for baseline levels were also greater among the DA group at 1-month post-randomization (p= 0.009 and 0.04, respectively) and individuals in the DA group who viewed the DA reported significantly lower genetic testing specific distress 12-months post-randomization than did the UC group (p = 0.03).
Table 3.
Linear Regression Models of Distress Outcomes Removing Individuals Who Did Not View the DA (N=178, 114 UC/64 DA)
| Outcome Variable | General Distress | Cancer Specific Distress | Genetic Testing Distress | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Predictor | Coefficient (SE) | z | Coefficient (SE) | z | Coefficient (SE) | z |
| Ovary Status at Baseline | 0.35 (0.51) | 0.68 | -1.14 (1.62) | -0.71 | 0.01 (1.17) | 0.00 |
| General Distress at Baseline | 0.55 (0.05)*** | 10.91 | ||||
| Cancer Specific Distress at Baseline | 0.69 (0.05)*** | 14.12 | ||||
| Genetic Testing Distress at Baseline | 0.49 (0.03)*** | 16.37 | ||||
| Time | -0.13 (0.26) | -0.50 | 0.15 (0.58) | 0.26 | -7.54 (0.66)*** | -11.41 |
| Decision Aid Group | -0.78 (0.95) | -0.82 | 7.42 (2.25)*** | 3.30 | 8.23 (2.60)** | 3.17 |
| Decision Aid Group × Time | -0.12 (0.47) | -0.27 | -2.95 (1.06)** | -2.79 | -3.89 (1.14)*** | -3.41 |
p <0.05,
p<0.01,
p<0.001
Discussion
Deciding how to manage breast cancer risk following a positive BRCA1/2 test result can be a complex and emotion-laden process. In the absence of definitive guidelines, many women are left to make decisions on the basis of their own knowledge and preferences. In this study we examined the impact of a computer-based interactive DA among women who had recently received a positive BRCA1/2 result on distress over the year following randomization to the DA or UC intervention. We identified different distress trajectories in the DA and the UC groups. Individuals randomized to UC reported significantly decreased distress in the month following randomization. In contrast, individuals randomized to the DA maintained levels of distress similar to their post-randomization baseline levels. By 12-months, the overall decrease in distress between the two groups was similar.
Our finding that the psychosocial impact of the DA varied over time contrasts with prior studies. These studies have been inconsistent in their measurement of distress. For example, some studies have not measured distress at all (12,32,33). Others have measured distress at only one time point (18) or with a single measure (11,34). These inconsistencies may have obscured the activity of decision support interventions on measures of distress. This may partly explain the failure of meta-analyses to detect effects of decision support on distress (17).
Although the UC and DA groups did not differ on distress at 12-months post-randomization, our study suggests that decision support impacted the trajectory of distress among BRCA1/2 carriers. One of the goals of decision support is to foster deliberation about decision options (35). Indeed the sustained distress at 1-month following randomization among women in the DA group may reflect ongoing cognitive processing and extended deliberation about their management options. This is consistent with our previous report in which UC participants were more likely to opt for risk-reducing mastectomy in the month following randomization but DA participants were more likely to opt for risk-reducing mastectomy after deliberating for 6-12 months following randomization (21).
If short-term increases in cancer specific and genetic testing distress are a reflection of ongoing deliberation or cognitive processing, this could have clinical implications. For example, if sustained levels of short-term distress indicate better, more deliberative decision making, it would be important to incorporate this clinically as a functional element of the decision making process. Our previous report from this trial provides some evidence for this. In that report, we found that women in the DA group ultimately reported improved satisfaction and reduced decisional conflict over the long term (21). There is a considerable body of research suggesting that intrusive thoughts or stressor-specific distress may be necessary for adequate cognitive processing of traumatic events (36). If this were the case we would expect the DA group to exhibit greater distress in the short-term, as they are more actively processing their risk-reduction and surveillance options, and then decreased distress over longer periods of time. Although our intent-to-treat results suggest that the UC and DA groups did not differ on our distress measures at 12-months, when we consider only those DA participants who viewed the DA, we do see significantly lower genetic testing distress at 12-months relative to UC participants. This finding is consistent with the possibility of sustained psychosocial benefits for individual exposed to the DA. If the DA fostered cognitive processing, we might also expect to see DA participants reporting more positive outcomes such as traumatic growth or meaning finding once they have made their management decisions. Unfortunately our study did not include measures of traumatic growth.
It is also possible that the benefits of deliberative decision making may vary across decisions and individuals. There is experimental evidence that non-deliberative decision making can be more effective in some situations (37). However, these studies have been challenged by further experimental data which, in some cases, suggest that deliberation may be beneficial (38,39). More research is needed to understand this, and to apply it towards delivering the most effective support to the patients who are most likely to benefit from it. Additionally, our knowledge of the longer term outcomes of DAs remains limited. Perhaps decision support provided at the right time for the right decisions could have an impact on the way that patients experience future events related to, or stemming from, their decisions (cancer diagnoses, preventative surgeries, etc).
This study has a number of limitations. There were a relatively small number of women who obtained risk-reducing mastectomy during the study. Thus, we were underpowered to examine actual decision making outcomes. Further, actual usage of the DA was not tracked. As such it is unclear which parts of the DA may be responsible for the effects seen in this study. Further research is needed to identify the components of DAs which most profoundly impact outcomes of interest. Also, the DA focused only on breast cancer risk management. It is possible that a broader focus on breast and ovarian cancer risk management might have led to different outcomes. Finally, the lack of diversity in the sample population limits the generalizability of these results; whether these results could be replicated in a lower SES population remains to be seen.
Despite these limitations, this report provides new insight into the long-term longitudinal effects of DAs, and raises intriguing possibilities for mechanisms of deliberative decision making. Future research should further refine our understanding of the decision making trajectory, the factors which influence that trajectory, and outcomes reflective of each stage along that trajectory.
Acknowledgments
We thank Michael Green, Lisa Moss and Sharon Hecker for their contributions to this research.
IRB approval was granted for this study and all participants provided informed consent. This research was supported by National Cancer Institute Grant R01 CA1846 and by the Jess and Mildred Fisher Center for Familial Cancer Research.
Footnotes
The authors do not have any financial conflicts to disclose.
Contributor Information
Gillian W. Hooker, Georgetown University, Lombardi Comprehensive Cancer Center
Kara-Grace Leventhal, Georgetown University, Lombardi Comprehensive Cancer Center.
Tiffani DeMarco, Georgetown University, Lombardi Comprehensive Cancer Center.
Beth N. Peshkin, Georgetown University, Lombardi Comprehensive Cancer Center
Clinton Finch, Georgetown University, Lombardi Comprehensive Cancer Center.
Erica Wahl, Section of Genetics and Metabolism, Albany Medical Center.
Jessica Rispoli Joines, University of Maryland Greenebaum Cancer Center.
Karen Brown, Department of Genetic and Genomic Sciences, Mount Sinai School of Medicine.
Heiddis Valdimarsdottir, Department of Oncological Sciences, Mount Sinai School of Medicine.
Marc D. Schwartz, Georgetown University, Lombardi Comprehensive Cancer Center
References
- 1.Antoniou AC, Pharoah PD, Easton DF, Evans DG. BRCA1 and BRCA2 cancer risks. J Clin Oncol. 2006;24(20):3312–3. doi: 10.1200/JCO.2006.06.7934. [DOI] [PubMed] [Google Scholar]
- 2.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25(11):1329–33. doi: 10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network. [4 January 2010];NCCN Clinical Practice Guidelines in Oncology. Genetic/Familial High-Risk Assessment: Breast and Ovarian--v.1.2006. http://www.nccn.org/professionals/physician_gls/PDF/genetics_screening.pdf.
- 4.Nusbaum R, Isaacs C. Management updates for women with a BRCA1 or BRCA2 mutation. Mol Diagn Ther. 2007;11(3):133–44. doi: 10.1007/BF03256234. [DOI] [PubMed] [Google Scholar]
- 5.Kauff ND, Satagopan JM, Robson ME, Scheuer L, Hensley M, Hudis CA, et al. Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2002;346(21):1609–15. doi: 10.1056/NEJMoa020119. [DOI] [PubMed] [Google Scholar]
- 6.Rebbeck TR. Prophylactic oophorectomy in BRCA1 and BRCA2 mutation carriers. Eur J Cancer. 2002;38(Suppl 6):S15–S17. doi: 10.1016/s0959-8049(02)00269-1. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz MD, Peshkin BN, Tercyak KP, Taylor KL, Valdimarsdottir H. Decision making and decision support for hereditary breast-ovarian cancer susceptibility. Health Psychol. 2005;24(4 Suppl):S78–S84. doi: 10.1037/0278-6133.24.4.S78. [DOI] [PubMed] [Google Scholar]
- 8.van Roosmalen MS, Stalmeier PF, Verhoef LC, Hoekstra-Weebers JE, Oosterwijk JC, Hoogerbrugge N, et al. Randomized trial of a shared decision-making intervention consisting of trade-offs and individualized treatment information for BRCA1/2 mutation carriers. J Clin Oncol. 2004;22(16):3293–301. doi: 10.1200/JCO.2004.05.066. [DOI] [PubMed] [Google Scholar]
- 9.Elwyn G, O’Connor A, Stacey D, Volk R, Edwards A, Coulter A, et al. Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. BMJ. 2006;333(7565):417. doi: 10.1136/bmj.38926.629329.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Connor AM, Tugwell P, Wells GA, Elmslie T, Jolly E, Hollingworth G, et al. A decision aid for women considering hormone therapy after menopause: decision support framework and evaluation. Patient Educ Couns. 1998;33(3):267–79. doi: 10.1016/s0738-3991(98)00026-3. [DOI] [PubMed] [Google Scholar]
- 11.Goel V, Sawka CA, Thiel EC, Gort EH, O’Connor AM. Randomized trial of a patient decision aid for choice of surgical treatment for breast cancer. Med Decis Making. 2001;21(1):1–6. doi: 10.1177/0272989X0102100101. [DOI] [PubMed] [Google Scholar]
- 12.Molenaar S, Sprangers MA, Rutgers EJ, Luiten EJ, Mulder J, Bossuyt PM, et al. Decision support for patients with early-stage breast cancer: effects of an interactive breast cancer CDROM on treatment decision, satisfaction, and quality of life. J Clin Oncol. 2001;19(6):1676–87. doi: 10.1200/JCO.2001.19.6.1676. [DOI] [PubMed] [Google Scholar]
- 13.Waljee JF, Rogers MA, Alderman AK. Decision aids and breast cancer: do they influence choice for surgery and knowledge of treatment options? J Clin Oncol. 2007;25(9):1067–73. doi: 10.1200/JCO.2006.08.5472. [DOI] [PubMed] [Google Scholar]
- 14.Whelan T, Levine M, Willan A, Gafni A, Sanders K, Mirsky D, et al. Effect of a decision aid on knowledge and treatment decision making for breast cancer surgery: a randomized trial. JAMA. 2004;292(4):435–41. doi: 10.1001/jama.292.4.435. [DOI] [PubMed] [Google Scholar]
- 15.Gattellari M, Ward JE. A community-based randomised controlled trial of three different educational resources for men about prostate cancer screening. Patient Educ Couns. 2005;57(2):168–82. doi: 10.1016/j.pec.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Volk RJ, Spann SJ, Cass AR, Hawley ST. Patient education for informed decision making about prostate cancer screening: a randomized controlled trial with 1-year follow-up. Ann Fam Med. 2003;1(1):22–8. doi: 10.1370/afm.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Brien MA, Whelan TJ, Villasis-Keever M, Gafni A, Charles C, Roberts R, et al. Are cancer-related decision aids effective? A systematic review and meta-analysis. J Clin Oncol. 2009;27(6):974–85. doi: 10.1200/JCO.2007.16.0101. [DOI] [PubMed] [Google Scholar]
- 18.Metcalfe KA, Poll A, O’Connor A, Gershman S, Armel S, Finch A, et al. Development and testing of a decision aid for breast cancer prevention for women with a BRCA1 or BRCA2 mutation. Clin Genet. 2007;72(3):208–17. doi: 10.1111/j.1399-0004.2007.00859.x. [DOI] [PubMed] [Google Scholar]
- 19.Tiller K, Meiser B, Gaff C, Kirk J, Dudding T, Phillips KA, et al. A randomized controlled trial of a decision aid for women at increased risk of ovarian cancer. Med Decis Making. 2006;26(4):360–72. doi: 10.1177/0272989X06290486. [DOI] [PubMed] [Google Scholar]
- 20.van Roosmalen MS, Stalmeier PF, Verhoef LC, Hoekstra-Weebers JE, Oosterwijk JC, Hoogerbrugge N, et al. Randomised trial of a decision aid and its timing for women being tested for a BRCA1/2 mutation. Br J Cancer. 2004;90(2):333–42. doi: 10.1038/sj.bjc.6601525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartz MD, Valdimarsdottir HB, DeMarco TA, Peshkin BN, Lawrence W, Rispoli J, et al. Randomized trial of a decision aid for BRCA1/BRCA2 mutation carriers: impact on measures of decision making and satisfaction. Health Psychol. 2009;28(1):11–9. doi: 10.1037/a0013147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson WL, Han PK, Fagerlin A, Stefanek M, Ubel PA. Rethinking the objectives of decision aids: a call for conceptual clarity. Med Decis Making. 2007;27(5):609–18. doi: 10.1177/0272989X07306780. [DOI] [PubMed] [Google Scholar]
- 23.Barry MJ, Cherkin DC, Chang Y, Fowler FJ, Skates S. A randomized trial of a multimedia shared decision-making program for men facing a treatment decision for benign prostatic hyperplasia. Dis Manag Clin Outcomes. 1997;1(1):5–14. [Google Scholar]
- 24.O’Connor AM, Fiset V, DeGrasse C, Graham ID, Evans W, Stacey D, et al. Decision aids for patients considering options affecting cancer outcomes: evidence of efficacy and policy implications. J Natl Cancer Inst Monogr. 1999;(25):67–80. doi: 10.1093/oxfordjournals.jncimonographs.a024212. [DOI] [PubMed] [Google Scholar]
- 25.Peshkin BN, Schwartz MD, Isaacs C, Hughes C, Main D, Lerman C. Utilization of breast cancer screening in a clinically based sample of women after BRCA1/2 testing. Cancer Epidemiol Biomarkers Prev. 2002;11(10 Pt 1):1115–8. [PubMed] [Google Scholar]
- 26.Kaufman EM, Peshkin BN, Lawrence WF, Shelby R, Isaacs C, Brown K, et al. Development of an interactive decision aid for female BRCA1/BRCA2 carriers. Journal of Genetic Counseling. 2003;12(2):109–29. doi: 10.1023/A:1022698112236. [DOI] [PubMed] [Google Scholar]
- 27.Keeney RL, Raiffa H. Decisions with Multiple Objectives: Preferences and Value Tradeoffs. Cambridge, UK: Cambridge University Press; 1993. [Google Scholar]
- 28.Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med. 1983;13(3):595–605. [PubMed] [Google Scholar]
- 29.Horowitz M, Wilner N, Alvarez W. Impact of Event Scale: a measure of subjective stress. Psychosom Med. 1979;41(3):209–18. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Cella D, Hughes C, Peterman A, Chang CH, Peshkin BN, Schwartz MD, et al. A brief assessment of concerns associated with genetic testing for cancer: the Multidimensional Impact of Cancer Risk Assessment (MICRA) questionnaire. Health Psychol. 2002;21(6):564–72. [PubMed] [Google Scholar]
- 31.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44(4):1049–60. [PubMed] [Google Scholar]
- 32.Dolan JG, Frisina S. Randomized controlled trial of a patient decision aid for colorectal cancer screening. Med Decis Making. 2002;22(2):125–39. doi: 10.1177/0272989X0202200210. [DOI] [PubMed] [Google Scholar]
- 33.Ozanne EM, Annis C, Adduci K, Showstack J, Esserman L. Pilot trial of a computerized decision aid for breast cancer prevention. Breast J. 2007;13(2):147–54. doi: 10.1111/j.1524-4741.2007.00395.x. [DOI] [PubMed] [Google Scholar]
- 34.Green MJ, Peterson SK, Baker MW, Harper GR, Friedman LC, Rubinstein WS, et al. Effect of a computer-based decision aid on knowledge, perceptions, and intentions about genetic testing for breast cancer susceptibility: a randomized controlled trial. JAMA. 2004;292(4):442–52. doi: 10.1001/jama.292.4.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Connor AM, Wennberg JE, Legare F, Llewellyn-Thomas HA, Moulton BW, Sepucha KR, et al. Toward the ‘tipping point’: decision aids and informed patient choice. Health Aff (Millwood) 2007;26(3):716–25. doi: 10.1377/hlthaff.26.3.716. [DOI] [PubMed] [Google Scholar]
- 36.Lepore SJ. A social-cognitive processing model of emotional adjustment to cancer. In: Baum A, Andersen BL, editors. Psychosocial Interventions for Cancer. Washington, DC: American Psychological Association; 2001. pp. 99–116. [Google Scholar]
- 37.Dijksterhuis A, Bos MW, Nordgren LF, van Baaren RB. On making the right choice: the deliberation-without-attention effect. Science. 2006;311(5763):1005–7. doi: 10.1126/science.1121629. [DOI] [PubMed] [Google Scholar]
- 38.Acker F. New findings on unconscious versus conscious thought in decision making: additional empirical data and meta-analysis. Judgm Decis Mak. 2008;3(4):292–303. [Google Scholar]
- 39.Newell BR, Wong KY, Cheung JC, Rakow T. Think, blink or sleep on it? The impact of modes of thought on complex decision making. Q J Exp Psychol (Colchester) 2009;62(4):707–32. doi: 10.1080/17470210802215202. [DOI] [PubMed] [Google Scholar]


