Abstract
Age-related hearing loss – presbycusis – is the number one communication disorder and most prevalent neurodegenerative condition of our aged population. Although speech understanding in background noise is quite difficult for those with presbycusis, there are currently no biomedical treatments to prevent, delay or reverse this condition. A better understanding of the cochlear mechanisms underlying presbycusis will help lead to future treatments. Objectives of the present study were to investigate gamma-amino butyric acid A (GABAA) receptor subunit α1, nicotinic acetylcholine (nACh) receptor subunit β2, and N-methyl-D-aspartate (NMDA) receptor subunit NR1 mRNA and protein expression changes in spiral ganglion neurons of the CBA/CaJ mouse cochlea, that occur in age-related hearing loss, utilizing quantitative immunohistochemistry and semi-quantitative RT-PCR techniques. We found that auditory brainstem response (ABR) thresholds shifted over 40 dB from 3–48 kHz in old mice compared to young adults. DPOAE thresholds also shifted over 40 dB from 6–49 kHz in old mice, and their amplitudes were significantly decreased or absent in the same frequency range. Spiral ganglion neuron (SGN) density decreased with age in basal, middle and apical turns, and SGN density of the basal turn declined the most. A positive correlation was observed between SGN density and ABR wave 1 amplitude. mRNA and protein expression of GABAAR α1 and AChR β2 decreased with age in SGNs in the old mouse cochlea. mRNA and protein expression of NMDAR NR1 increased with age in SGNs of the old mice. These findings demonstrate that there are functionally-relevant age-related changes of GABAAR, nAChR, NMDAR expression in CBA mouse SGNs reflecting their degeneration, which may be related to functional changes in cochlear synaptic transmission with age, suggesting biological mechanisms for peripheral age-related hearing loss.
Keywords: Aging, Hearing loss, Cochlea, Spiral Ganglion Neurons, Gene expression, Protein expression
Introduction
Age-related hearing loss is a disorder caused by mixed pathology including both genetic and environmental factors. Schuknecht classified presbycusis into four subtypes: (1) sensory (loss of hair cells), neural (loss of spiral ganglion neurons, [SGNs]), metabolic (atrophy of the stria vascularis and decline of the endolymphatic potential) and mechanical (cochlear anatomical changes, including alterations of the basilar membrane) (Schuknecht. 1964; Schuknecht et al. 1993). “Sensorineural” presbycusis refers to the high-frequency-hearing impairment resulting from loss of hair cells and degeneration of SGNs. It is a major contributor to age-related hearing loss. In addition, SGN degeneration during aging contributes to functional declines in the output of the peripheral auditory system that can induce plasticity changes in the central auditory system. Functionally, SGNs connect hair cells with central auditory system neurons in the cochlear nucleus of the brainstem; the first neurons in the central auditory pathway. Thus, to study the etiologies of SGNs degeneration during aging paves a way to prevent or slow down the progression of age-related hearing loss as well as providing insights for other types of hearing impairment.
Gamma-aminobutyric acid (GABA) is an important inhibitory neurotransmitter in the brain and auditory system. The GABAA receptor (GABAAR) is a key inhibitory receptor; playing a critical role in regulating neuronal excitability and information processing in the nervous system. GABAAR is a pentameric structure formed by its multi-gene family, and there are at least 19 different members of the GABAAR family, including α1-6, β1-3, γ1-3, ρ1-3, δ1, ε1, θ1, and π1. The majority of GABAARs consist of 2α, 2β, and 1γ subunit. In the adult brain, the α1β2γ2 subtype is the major subtype accounting for 43% of all GABAARs (McKernan et al. 1996). The majority of GABAARs are widely expressed in the auditory system (Campos et al. 2001; Yomamoto et al. 2002) and as one of its functions, it plays an important cochlear protective role via the auditory efferent system (Arnold et al. 1998; Murashita et al. 2007). Our previous studies (Tang et al. 2010) showed that the majority of the GABAAR subunits were expressed in the cochlea, and there were age-related changes of GABARs in the mouse cochlea (D’Souza et al. 2008), and central auditory system (Raza et al. 1994). However, age-related changes of GABAAR in the inner ear have not been investigated and it may be that age-related hearing loss is partially due to GABAAR changes with age in both peripheral and central auditory systems.
The N-methyl-D-aspartate receptor (NMDAR) is a glutamate-gated ion channel ubiquitously distributed throughout the brain; and it is fundamental for excitatory neurotransmission. NMDARs are composed of heteromeric complexes of NR1 and NR2 subunits. A functional NMDAR appears to consist of two classes of subunits NR1 and NR2 (AD). NR1 is the fundamental subunit necessary for the NMDAR complex (Watanabe et al. 1992; Wada et al. 2004). NMDA receptors are expressed at hair cell/afferent nerve synapses, playing a crucial role in neurotransmission and synaptic plasticity and excitotoxicity. Age-related changes of NMDARs have been reported in the central auditory system (Osumi et al. 2012). Our previous study showed that all the subunits were expressed in the cochlea (Tang et al. 2010). Investigation of NMDARs expression changes with age will help our understanding of mechanisms of presbycusis.
Acetylcholine (ACh) is a main transmitter released by the medial olivocochlear efferent fibers (Fuchs et al. 1992). nAChRs are mainly located at the synapse between efferent fibers and outer hair cells (OHC). It is now believed that the hair cell cholinergic receptor that mediates synaptic transmission between efferent olivocochlear fibers and hair cells, is formed by both α9 and α10 subunits. The activation of the hair cell nAChR leads to an increase in intracellular Ca+-activated K+ (SK2) channels thus leading to hyperpolarization of hair cells and reduction of electromotility (Dulon et al. 1998; Oliver et al. 2000). Located on the peripheral projections of SGNs are α2, α4-7, and β2-3 nAChR subunits (Housley et al. 1991), and it has been shown that the nAChR β2 subunit is required for the maintenance of SGNs during aging (Bao et al. 2005). Also in the central nervous system, loss of β2 results in region specific alterations in cortex, including neocortical hypotrophy, loss of hippocampal pyramidal neurons, and astro- and micro-gliosis. Spatial learning is significantly impaired as well (Zoli et al. 1999). nAChR β2 is critical in the maintenance of normal neuronal function. However, the exact functions of the nAChRs in SGN synapses remain elusive.
The auditory system of the CBA/CaJ strain has important similarities to the human system, particularly at peripheral and brainstem levels; therefore it has proven to be a very useful model for studying the biological mechanisms of presbycusis. In this present study we report on the detection and age changes of GABAAR α1, nACh β1 and NMDAR NR1 subunits; using protein and mRNA expression level methodologies for young adult (2–3 month) and old age (24–32 month) CBA/CaJ mice to increase our structure-function understanding of pharmacological and electrophysiological characteristics of these receptors.
Materials and methods
Animals
CBA/CaJ mice were bred in-house under 12h:12h light-dark cycle. Young adult (2–3 months, n=12) and old (24–32 months, n=12) were used for the auditory brainstem response (ABR) physiology experiments. Subsets of these physiologically-characterized young adult (Y) and old (O) mice were utilized in the other multidisciplinary experiments as follows. Distortion product otoacoustic emissions (DPOAE): Y, n=6; O, n=6. For the immunocytochemistry procedures: ACh, Y, n=6; O, n=5. NMDA, Y, n=8; O, n=7. GABA, Y, n=4; O, n=4. The opposite-side cochleae were used for the PCR experiments: Y, n=6, and O, n=6. All the mice were examined under the microscope for their external ear canal and tympanic membrane; mice with any symptoms of ear infection were excluded from the study. All the animal procedures were approved by the University of South Florida Committee on Animal Resources and are consistent with US Federal and NIH guidelines. Auditory brainstem response (ABR) and distortion-product emissions recordings were tested prior to sacrificing, and the procedures were the same as our previous reports (Zettel et al. 2007; D’Souza et al. 2008; Frisina et al. 2011).
Auditory brainstem responses (ABR) recordings
Mice were anesthetized with a mixture of ketamine/xylazine (120 and 10mg/kg body weight, respectively, intraperitoneal injection). ABRs were measured in response to tone pips of 3, 6, 12, 16, 20, 24, 32, 48 kHz and wide band noise presented at a rate of 11 bursts/s. Briefly, ABR recordings were obtained with a Tucker-Davis Technologies (TDT) System III workstation running BioSigRP in a sound booth (IAC). ABRs were measured with subcutaneous platinum needle electrodes placed at the vertex (non-inverting input), right mastoid prominence (inverted input), and back (indifferent site). Tone pips of 5 msec duration and 0.5 msec rise-fall time (phase alternating 90°) were utilized. Signals were calibrated in a cavity whose volume approximated the volume of the mouse ear canal using a 1/8″ B&K 4138 microphone. Threshold was defined as the lowest intensity which elicited a clearly replicable response. Normal body temperature was maintained at 37°C with a servo heating pad.
Distortion-product otoacoustic emissions (DPOAEs)
Distortion-product otoacoustic emissions were obtained following ABR audiometry, under anesthesia (ketamine/xylazine, 120 and 10 mg/kg, respectively) a TDT combination microphone/speaker system was placed in the test ear near the tympanic membrane with a small, custom acoustic coupler. Acoustic stimuli were delivered and DPOAE waveforms were recorded using BioSig-RP software and TDT System III hardware. The digitally synthesized stimulus (200 kHz sample rate) consisted of two primary pure-tone frequencies (f1 and f2) differing by a factor of 1.25, at 65 and 50dB SPL, respectively. A fast Fourier transform was performed on the resultant waveform recorded from the insert microphone, and the spectral magnitude of the two primaries, 2f1-f2 distortion product, and the noise floor were determined. The procedure was repeated for f1 frequencies ranging from 5.6 to 44.8 kHz (eight frequencies per octave) to adequately assess the neuroethologically relevant functional range of mouse hearing.
Immunocytochemistry
After anesthetization with ketamine (120 mg/kg) and xylazine (10 mg/kg) for 30 minutes, the mice were sacrificed, and the cochleae were dissected and fixed in 4% paraformaldehyde in PBS (PH=7.4) overnight at 4°C, decalcification in 10% EDTA in PBS for a week at 4°C, then cryoprotected overnight at 4°C in cryoprotectant solution (30% sucrose & 10% polyvinyl-pyrrolidone in 0.1M PB and ethylene glycol). The cochleae were then embedded in degassed OCT overnight at 4°C, oriented into a cryomold with OCT degassed for 1 hour, then frozen at −80°C. Cryosectioning was performed at 5 μm per section (Thermo Scientific Inc., Waltham, MA). The slides were dried, washed with PBS for 3×5 min, then blocked in 0.1% Triton X-100,10% goat serum in PBS for 1 hour at room temperature (20–25°C), then incubated with the primary antibody: GABAARα1 (1:100, rabbit polyclonal, ab33299 Abcam, Cambridge, MA), NMDARNR1 (1:100, rabbit polyclonal, ab17345, Abcam) or nAChRβ2 (1:100, rabbit polyclonal, sc-11372, Santa Cruz Biotechnology, Inc., Dallas, TX) overnight at 4°C. Anti-rabbit secondary antibody (HRP, rabbit, Cell Signaling, Danvers, MA) was used to visualize the primary antibodies; then the slides were stained with DAB (Vector Labs, Burlingame, CA) and mounted with cover glasses using Toluene Solution (Fisher Scientific, Waltham, MA). Images were captured using a digital camera (EOS Rebel T3i, Canon) interfaced with the light microscope (Leica DMR). The quantitative expression of staining for each receptor was calculated by obtaining the mean values of densitometry from 80–100 positively labeled cells from basal, middle and apical turns of the cochlea from young adult and old mice (N=6 per group).
RNA extraction and RT-PCR detection
One cochlea from each subject was used for immunocytochemistry (above) and the other for gene expression: RT-PCR. The temporal bones were dissected under a microscope and under sterile conditions; the bony cover of the cochlea and the spiral ligament was removed, total RNA was extracted from the modiolus using the SV Total RNA Isolation System (Promega, Madison, WI). 10 ng of total RNA was reverse transcribed to complementary DNA and PCR was performed according to instructions from the Enhanced Avian HS RT-PCR 100 Kit (HSR-T20, Sigma, St. Louis, MO). All mRNA sequences were from Genbank. The primers were designed using Primer Premier 5.0 software (Premier, Palo Alto, CA) Forward and reverse primer sequences, from the 5′ to 3′ direction were as follows: β-actin: sense 5′-GCTCTGGCTCCTAGCACCAT-3′, anti-sense 5′-GCCGGACTCATCGTACTCCT-3′, GABAARα1: sense 5′-AATGGGCGGATTGGTGTC-3′, antisense 5′-TCATCTTGGGAGGGCTGT-3′; nAChRβ2 sense 5′-CTGTGGACGGTGTACGCTT-3′, antisense 5′-TCAGAGGGGAGGGAGAATG-3′; NMDARNR1 sense 5′-CAAGTGGGCATCTACAAT-3′, anti-sense 5′-TGACATACACGAAGGGTT-3′. PCR was initiated with an initial denaturation step at 95 °C for 5 minutes, followed by 30 cycles of denaturation at 94 °C for 30 seconds, annealing at 60°C for 30 seconds, extension at 72 °C for 30 seconds, and a final extension at 72 °C for 8 minutes. Each set of reactions always included a no-sample negative control. We usually performed a negative control containing RNA instead of cDNA to rule out genomic DNA contamination. The PCR amplified fragments were analyzed on a 1.5% agarose gel. All the samples were electrophoresed in the same agarose gel and expression levels were quantified by densitometry using Image J (National Institutes of Health, USA); Relative quantities of GABAAR, nAChR and NMDAR mRNA were determined by AGABAAR/nAChR/NMDAR/Aβ-actin ratios.
H&E staining and SGN counts
Sections were prepared for immunocytochemistry as mentioned above and stained with H&E (BBC Biochemical, Histo-Perfect, Mount Vernon, WA). The images of Rosenthal’s canal were acquired at 1.25 × 10× magnification with the Leica bright-field microscope. Rosenthal’s canal outline was traced to determine its total area, and all the SGNs (The cell bodies of the SGNs are found in the cochlear modiolus) from apical, middle and basal turns of young adult and old age mice (N=6 per subject group) were counted with the MetaMorph imaging system (Molecular Devices, Sunnyvale, CA). SGN density (number of SGNs/1000 μm2) was calculated by dividing the number of SGNs by the area measured.
Statistical analysis
Statistical analysis was performed with Prism 6.0 software (GraphPad Software, La Jolla, CA). Repeated measures ANOVA and Student’s t-test were used for group comparisons. Bonferroni post-hoc tests, corrected for multiple comparisons were used for ABR and DPOAE data. Linear regression was used for correlation analyses. A p value of less than 0.5 was regarded as statistically significant.
Results
ABR recordings
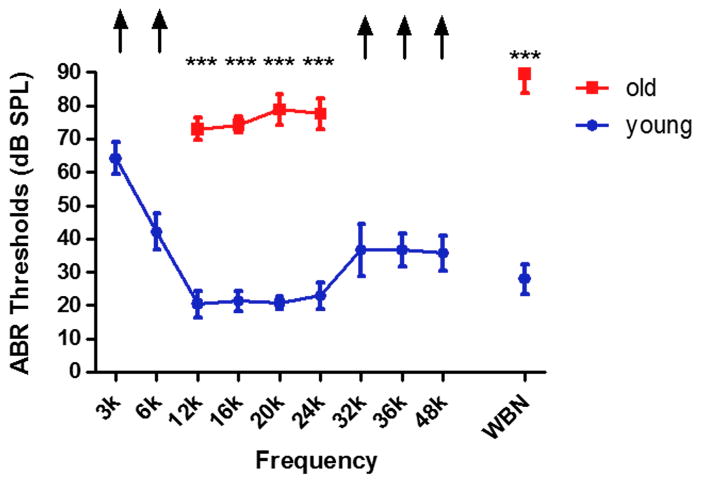
Overall, the ABR thresholds were significantly elevated in old age mice, as shown in Fig. 1 There were statistically significant differences at all frequencies (F1,10=1218, p<0.001) between the young (2–3 months) and old (24–32 months) mice. The age-related threshold shift is over 40 dB from 3–48 kHz.
FIG. 1.
ABR thresholds of young adult and old age mice. ABR thresholds were significantly elevated for the old mice (*** p<0.001). Arrows indicate the thresholds exceeded the upper limits of the TDT ABR system. k = kHz. WNB- wideband noise stimulus.
DPOAEs recordings
DPOAE amplitudes at 12 and 16 kHz were significantly lower in old mice as compared to young, and responses at the other frequencies were absent or in the system noise floor for the old mice (F1,10=672.8, p<0.001; Fig. 2). DPOAEs thresholds were poorer, up to 40 dB for F2 frequencies of 6.7, 13.4, 17.9, 26.8, 33.5 and 49.2 kHz in old mice compared to young adults (F1,10=1598, p<0.001; Fig. 3).
FIG. 2.
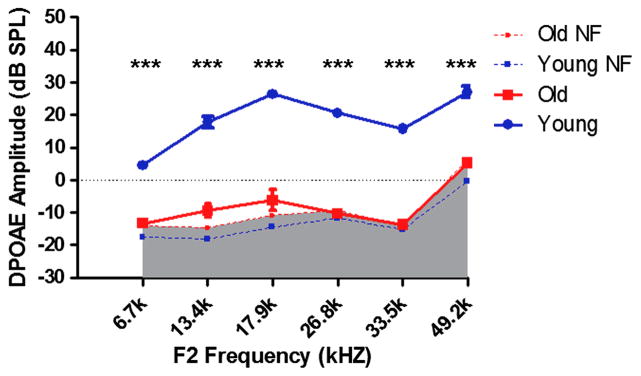
DPOAE amplitudes of young adult exceed those of and old mice, indicating significant loss of outer hair cell function with age. DPOAE (F1=65 dB SPL, F2=50 dB SPL f2/f1=1.25). NF: noise floor, *** p<0.001.
FIG. 3.
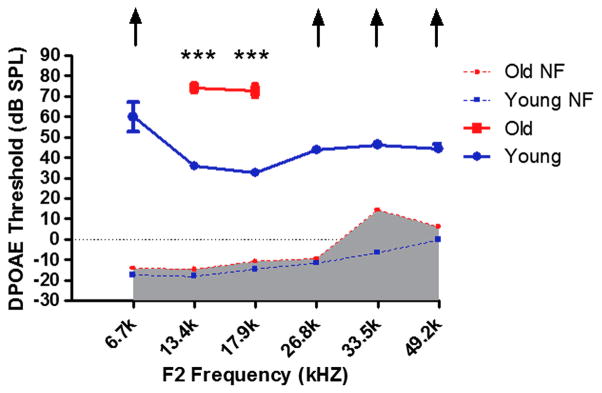
DPOAE thresholds of young adult mice are much lower than for old mice. DPOAE (F1=65 dB SPL, F2=50 dB SPL f2/f1=1.25). NF: noise floor,*** p<0.001. Arrows indicate the thresholds exceeded the upper limits of the TDT DPOAE system.
Gene expression changes with age
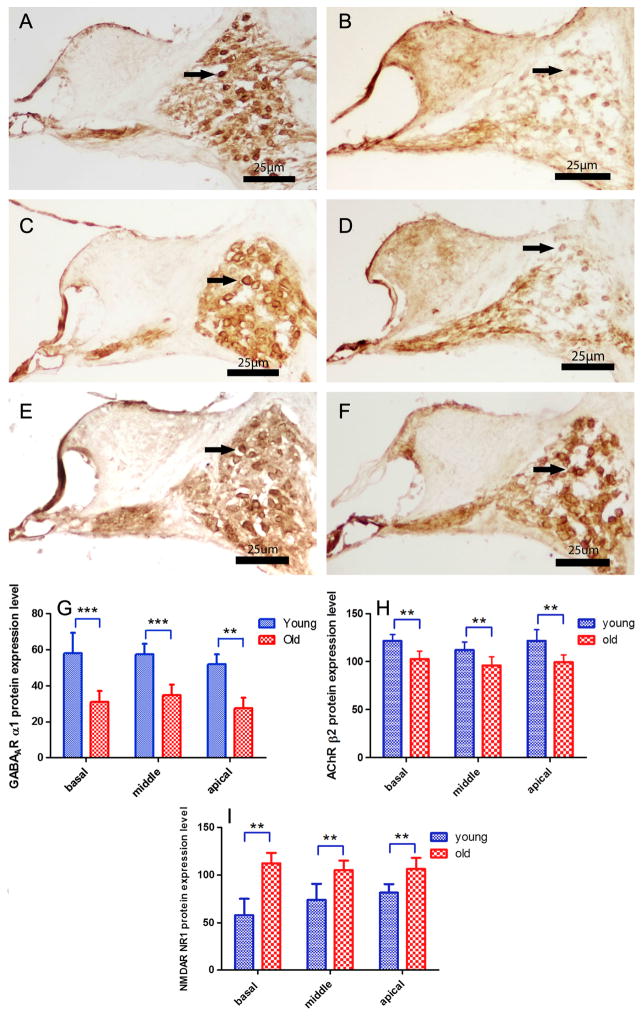
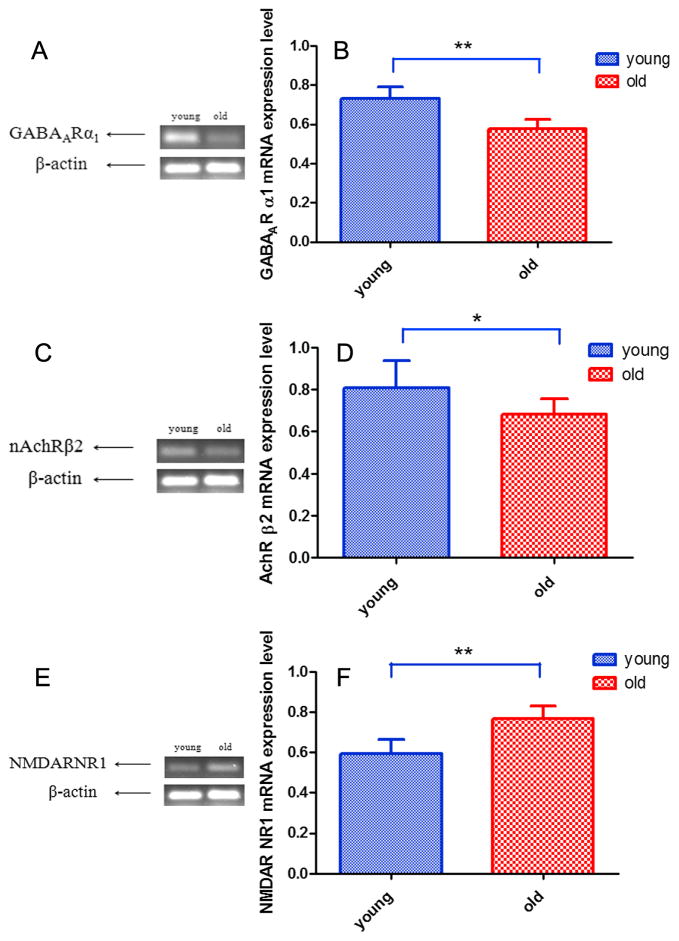
GABAAR α1, nAChR β2 and NMDAR NR1 were detected in SGNs as presented in Figs. 4 and 5. mRNA and protein expression of GABAAR α1 decreased with age in SGNs (F1,10=89.432, p<0.001; Figs. 4A, 4B, 4G, 5A, 5B). mRNA and protein expression of nAChR β2 also decreased in all three turns in the cochlea with age (F1,10=37.073, p<0.001; Figs. 4C, 4D, 4H, 5C, 5D). mRNA and protein expression of NMDAR NR1 increased in all three turns with age in SGNs (F1,10=28.850, p<0.001; Figs. 4E, 4F, 4I, 5E, 5F).
FIG. 4.
Age-related changes of protein expression levels in SGNs. DAB staining (A, B, C, D, E,F) of serial sections from the cochlear middle turn. (A, B) GABAARα1 protein expression decreased with age. (C, D) nAChRβ2 protein expression declined with age. (E, F) NMDARNR1 protein expression increased with age. (G, H, I,) GABAARα1, nAChRβ2 and NMDARNR1 protein expression in basal, middle and apical turns in young adult and old age mice. Bar indicate means ± s.d. for young adult (2–3 month) and old mice (24–32 month). *p<0.5, **p<0.01. Arrow: labeled SGNs. Scale Bar: 25 μm.
FIG. 5.
Age-related changes of mRNA expression in SGNs. (A, B) GABAAR α1 mRNA expression decreased with age. (C, D) nAChR β2 mRNA expression declined with age. (E, F) NMDAR NR1 mRNA expression increased with age. Bar indicate means ± s.d. Young adult (2–3 month), old mice (24–32 month). **p<0.01, ***p<0.001.
Spiral ganglion neuron loss with age
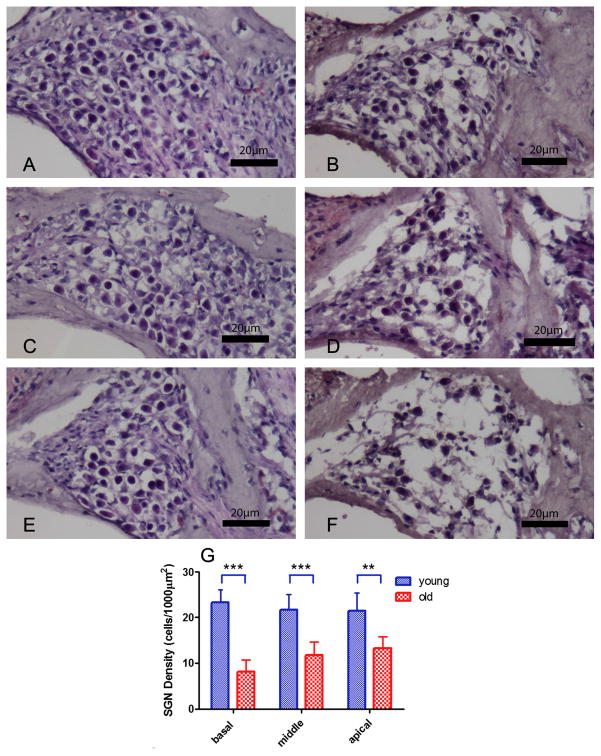
SGN density, as delineated by H&E staining, for basal, middle and apical turns all significantly decreased in the cochlea of old mice (Figs. 6B, 6D, 6F) when compared to young adult mice (F1,10=34.264, p<0.001; Figs. 6A, 6C, 6E, 6G). Overall SGN density of the basal turn decreased the most (F1,10=8.365, p=0.016; Figs. 6E and 6F).
FIG. 6.
H&E staining (A, B [basal turn], C, D [middle turn], E, F [apical turn]) demonstrated spiral ganglion neuron (SGN) loss with age. SGN density declined significantly with age in Rosenthal’s Canal (G). Overall SGN density of the basal turn decreased the most. Error bars indicate means ± s.d. Young adult (2–3 month) and old mice (24–32 month). *p<0.05, **p<0.01,*** p<0.001. Scale Bar: 25 μm.
Correlations between SGN density and ABR amplitude wave I
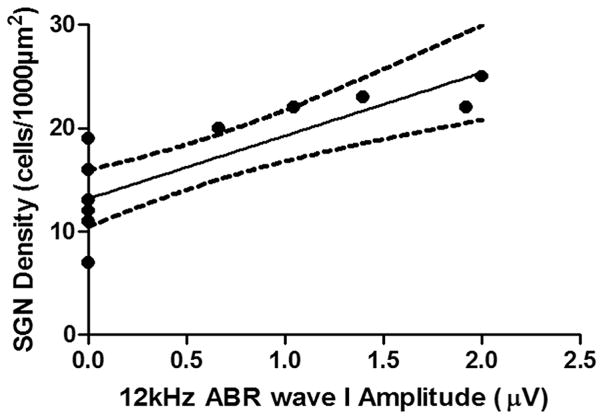
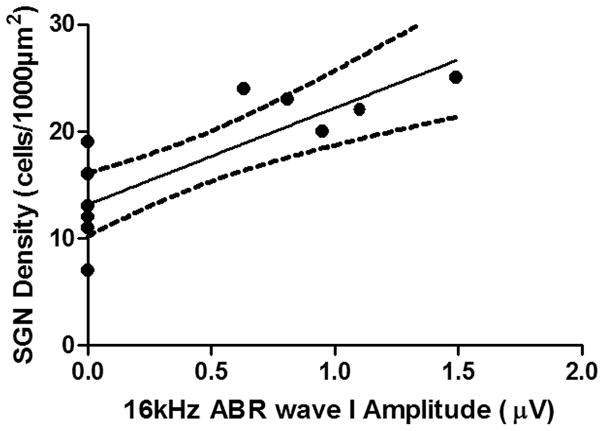
In old mice, ABR thresholds were found to be higher than 90 dB SPL, except for 12 and 16 kHz, which are generated in the middle turn of the cochlea (Muniak et al. 2013). We analyzed the correlations between SGN density and ABR wave I responses, and the results revealed a positive correlation between SGN density and ABR wave I amplitude (Fig. 7 and Fig. 8).
FIG. 7.
Positive correlation between SGN density and 12 kHz ABR wave I amplitude (n=12): r2=0.7410 , F1,10=28.60, p=0.0003.
FIG. 8.
Positive correlation between SGN density and 16 kHz ABR wave I amplitude (n=12): r2=0.6742 , F1,10=20.70, p=0.0011.
Discussion
Functional roles and age-linked changes of NMDAR, GABAAR and nAChR expression in the primary auditory pathway
NMDAR NR1 subunit expression does not change greatly during the development of the cochlear nuclei in chicken (Tang et al. 2004). NR1 expression increases in early development, then falls to and/or remains at an intermediate level into adulthood in rat (Akazawa et al. 1994) and mouse auditory brainstem (Tadros et al. 2007). However, NR1 expression declines in the inferior colliculus (IC) of aging animals, which indicates that NR1 may be involved in the pathogenesis of presbycusis (Osumi et al. 2012). NR1 expression in SGNs of the rat cochlea is up-regulated following kanamycin induced deafness (Hasegawa et al. 2000). Our study showed that NMDAR NR1 increased with age in mouse SGNs, which might be compensatory for decreased neuronal excitability in response to glutamate neurotransmitter system changes in the aging cochlea. Specifically, previous investigations have shown that the content of glutamate in the brain, including the central auditory system (Profant et al., 2013) and in the inner ear (Peng et al., 2013) primarily decrease with age. In contrast, the NMDAR NR1 increases seen in the present study, despite declining numbers of SGNs with age, may be geared towards maintaining neuronal excitability in cochlear afferent signal transduction during aging. Taken together with the previous studies of NMDAR in the auditory pathway, these results suggest that patterns of NMDAR expression changes in conjunction with age-related hearing loss may be different for the peripheral and central auditory systems.
Significant alterations of GABAA R subunits mRNA levels have been reported for the aging Fischer 344 rat IC (Milbrandt et al 1997). The γ1 subunit mRNA significantly increased in IC while the α1, β2 and γ2 subunits mRNA was observed to change very little with age. The alterations of the GABAA R subunits represent compensatory mechanisms in response to GABAergic system changes in the auditory brainstem reported by Caspary and colleagues (Raza et al. 1994; Milbrandt et al. 1997). It was also reported that GABAA R subunit expression showed age-related changes in rat auditory cortex (Cui et al. 1997; Xu et al. 2009; Caspary et al. 2012). In cochlea, GABA reduces NMDA-induced activity of afferent fibers (Bodarky et al. 2009; Moeller et al. 2010) . In addition, GABAAR activation in the cochlea reduces probability of acoustic injury (Murashita et al. 2007). So GABAARs serve a protective role in the peripheral auditory system. Also, GABAergic components of the olivocochlear system contribute to the long-term maintenance of hair cell and neuron survival in the inner ear (Maison et al. 2006). The present study discovered GABAAR α1 subunit expression down-regulation with age, consistent with the idea that the normal protective actions of GABAARs in the cochlea for hair cell and SGN survival are less effective with aging, thus contributing to the deleterious effects of age-related hearing loss in the inner ear.
ACh is a primary efferent neurotransmitter in the cochlea. Efferent protection is mediated via the α9 nAChRs in OHCs, which are responsible for cochlear efferent inhibition and provide some protection of the cochlea from noise over-exposure injury (Maison et al. 2000). SGNs express α2, α4-7, and β2-3 nAChR subunits. Mice lacking the nAChR subunit (β2−/−) have dramatic hearing loss and significant reduction in the number of SGNs, therefore, the β2 nAChR is required for maintenance of SGNs during aging (Bao et al. 2005). Consistent with this, the current study showed that nAChR β2 expression decreased in the presbycusis mouse model, likely contributing to functional hearing changes during aging.
There are synaptic interactions involving ACh, glutamate and GABA in the auditory system (Metherate et al. 1995). For instance, in auditory cortex, activation of NMDA receptors may reduce ACh release, and cholinergic agonists depress EPSPs mediated by glutamate; and ACh can also reduce monosynaptic GABAergic IPSPs. Spontaneous ACh release tonically depresses synaptic potentials mediated by glutamate and GABA. In sum, both the inhibitory and excitatory neurotransmitters and their receptors exist and interact in the auditory pathway for processing acoustic signals, and in the young adult there is an optimal balance between inhibitory and excitatory neurotransmitter systems that becomes disrupted with age. Changes in this delicate balance of inhibitory and excitatory neurotransmitters and their receptors during aging likely results in altered, degraded evoked responses to acoustical stimuli due to synaptic strength and inhibitory processing mis-matches in the auditory system (e.g., Kotak et al. 1997; Mossop et al. 2000).
Spiral ganglion neuron loss with age
The correlation between SGN density and ABR wave I amplitude underscores the possible interactions between SGN survival and ABR deficits and impaired cochlear function in the aging auditory system. Our study revealed that SGNs decrease with age in apical, middle and basal regions, with the basal area most affected. This is consistent with the tendency towards high frequency hearing loss in presbycusis and relations to hair cell pathologies in CBA mice (Spongr et al. 1997). Mechanistically, it is likely that SGNs rely on hair cell synaptic activity for trophic support. But alternatively, SGN loss can occur without damage or death of hair cells (Ryals et al. 1988; White et al. 2000; Linthicum et al. 2009). Indeed, in humans IHC loss does not always lead to SGN loss during aging (Jin et al. 2011). Additionally, mitochondrial damage caused by reactive oxygen species (ROS) can induce age-related SGN death via necrosis or apoptotic, programmed cell death (Yamasoba et al. 2007). Previous studies (Kujawa et al. 2006; Kujawa et al. 2009) have shown that early noise exposure can cause a reversible temporary hearing threshold increase with irreversible SGN loss, therefore early noise exposure is a critical environmental factor for SGN survival during age-related hearing loss. Genetic mutations can also predispose one to more rapid age-related cochlear dysfunction. For example, the glutamate receptor metabotropic 7 (GRM7) expressed in the hair cell/SGN synapse has been identified as one of the first presbycusis genes in humans (Newman et al. 2012). In sum, the present report underscores the importance of hair cell/SGN/efferent synapse neurotransmitter receptor changes with age, but additional research is required to better describe the molecular mechanisms of age-linked synaptic degradation and SGN death, and dysfunction during aging.
Conclusion
The present study demonstrated that there are age-related changes of GABAA, nACh and NMDA receptor expression in CBA mouse primary auditory neurons that might contribute to neural degeneration and cochlear synaptic transmission as a function of age. Preventing or optimally modulating the age-related down-regulation of GABAAR α1, nAChR β2 and up-regulation of NMDAR NR1 expression levels before or during age-related hearing loss could contribute to preventing or minimizing presbycusis. In conclusion, the present report leads to more insights into the functions of GABAAR α1, nAChR β2 and NMDAR NR1 receptor protein regulation in the aging mammalian auditory system, paving the way for novel biomedical interventions to prevent or slow down the progression of age-related hearing loss as well as other types of hearing impairment.
Highlights.
Auditory brainstem response (ABR) thresholds shift over 40 dB from 3–48 kHz in old mice compared to young adults.
DPOAE thresholds shifted over 40 dB in old mice, and their amplitudes were decreased or absent in the same frequency range.
Spiral ganglion neuron (SGN) density decreased with age in the whole cochlea with the largest decline in the basal turn.
A positive correlation between SGN density and ABR wave I amplitude was discovered.
mRNA and protein expression of GABAAR α1 and nAChR β2 decreased while NMDAR NR1 increased with age in SGNs of the old mice.
Acknowledgments
This work supported by NIH Grant: National Institute on Aging P01 AG009524. We thank Shannon Salvog for project support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akazawa C, Shigemoto R, Bessho Y, Nakanishi S, Mizuno N. Differential expression of five N-methyl-D-aspartate receptor subunit mRNAs in the cerebellum of developing and adult rats. J Comp Neurol. 1994;347(1):150–160. doi: 10.1002/cne.903470112. [DOI] [PubMed] [Google Scholar]
- Arnold T, Oestreicher E, Ehrenberger K, Felix D. GABA(A) receptor modulates the activity of inner hair cell afferents in guinea pig cochlea. Hear Res. 1998;125(1–2):147–153. doi: 10.1016/s0378-5955(98)00144-0. [DOI] [PubMed] [Google Scholar]
- Bao J, Lei D, Du Y, Ohlemiller KK, Beaudet AL, Role LW. Requirement of nicotinic acetylcholine receptor subunit beta2 in the maintenance of spiral ganglion neurons during aging. J Neurosci. 2005;25(12):3041–3045. doi: 10.1523/JNEUROSCI.5277-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodarky CL, Halene TB, Ehrlichman RS, Banerjee A, Ray R, Hahn CG, Jonak G, Siegel SJ. Novel environment and GABA agonists alter event-related potentials in N-methyl-D-aspartate NR1 hypomorphic and wild-type mice. J Pharmacol Exp Ther. 2009;331(1):308–318. doi: 10.1124/jpet.109.150938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos ML, de Cabo C, Wisden W, Juiz JM, Merlo D. Expression of GABA(A) receptor subunits in rat brainstem auditory pathways: cochlear nuclei, superior olivary complex and nucleus of the lateral lemniscus. Neuroscience. 2001;102(3):625–638. doi: 10.1016/s0306-4522(00)00525-x. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Hughes LF, Ling LL. 2013;Age-related GABAA receptor changes in rat auditory cortex. Neurobiol Aging. 2013;34(5):1486–1496. doi: 10.1016/j.neurobiolaging.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Lu J, Sun X. Age-related changes in GABAA receptor subunits mRNA expression in rat auditory cortex. J Comp Neurol. 1997;379(3):455–465. [Google Scholar]
- D’Souza M, Zhu X, Frisina RD. Novel approach to select genes from RMA normalized microarray data using functional hearing tests in aging mice. J Neurosci Methods. 2008;171(2):279–287. doi: 10.1016/j.jneumeth.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulon D, Luo L, Zhang C, Ryan AF. Expression of small-conductance calcium activated potassium channels (SK) in outer hair cells of the rat cochlea. Eur J Neurosci. 1998;10:907–915. doi: 10.1046/j.1460-9568.1998.00098.x. [DOI] [PubMed] [Google Scholar]
- Frisina RD, Singh A, Bak M, Bozorg S, Seth R, Zhu X. F1 (CBA-C57) mice show superior hearing in old age relative to their parental strains: hybrid vigor or a new animal model for “golden ears”? Neurobiol Aging. 2011;32(9):1716–24. doi: 10.1016/j.neurobiolaging.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs PA, Murrow BW. Cholinergic inhibition of short (outer) hair cells of the chick’s cochlea. J Neurosci. 1992;12:800–809. doi: 10.1523/JNEUROSCI.12-03-00800.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa T, Doi K, Fuse Y, Fujii K, Uno Y, Nishimura H, Kubo T. Deafness induced up-regulation of GluR2/3 and NR1 in the spiral ganglion cells of the rat cochlea. Neuroreport. 2000;11(11):2515–2519. doi: 10.1097/00001756-200008030-00034. [DOI] [PubMed] [Google Scholar]
- Housley GD, Ashmore JF. Direct measurement of the action of acetylcholine on isolated outer hair cells of the guinea pig cochlea. Proc Biol Sci. 1991;244(1310):161–167. doi: 10.1098/rspb.1991.0065. [DOI] [PubMed] [Google Scholar]
- Jin D, Ohlemiller KK, Lei D, Dong E, Role L, Ryugo DK, Bao J. Age-related neuronal loss in the cochlea is not delayed by synaptic modulation. Neurobiol Aging. 2011;32(12):2321. e13–23. doi: 10.1016/j.neurobiolaging.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Acceleration of age-related hearing loss by early noise exposure: evidence of a misspent youth. J Neurosci. 2006;26(7):2115–2123. doi: 10.1523/JNEUROSCI.4985-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29(45):14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak VC, Sanes DH. Deafferentation weakens excitatory synapses in the developing central auditory system. Eur J Neurosci. 1997;9(11):2340–2347. doi: 10.1111/j.1460-9568.1997.tb01651.x. [DOI] [PubMed] [Google Scholar]
- Linthicum FH, Jr, Fayad JN. Spiral ganglion cell loss is unrelated to segmental cochlear sensory system degeneration in humans. Otol Neurotol. 2009;30(3):418–422. doi: 10.1097/mao.0b013e31819a8827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison SF, Liberman MC. Predicting vulnerability to acoustic injury with a noninvasive assay of olivocochlear reflex strength. J Neurosci. 2000;20(12):4701–4707. doi: 10.1523/JNEUROSCI.20-12-04701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison SF, Rosahl TW, Homanics GE, Liberman MC. Functional role of GABAergic innervation of the cochlea: phenotypic analysis of mice lacking GABA(A) receptor subunits alpha 1, alpha 2, alpha 5, alpha 6, beta 2, beta 3, or delta. J Neurosci. 2006;26(40):10315–10326. doi: 10.1523/JNEUROSCI.2395-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci. 1996;19(4):139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- Metherate R, Ashe JH. Synaptic interactions involving acetylcholine, glutamate, and GABA in rat auditory cortex. Exp Brain Res. 1995;107(1):59–72. doi: 10.1007/BF00228017. [DOI] [PubMed] [Google Scholar]
- Milbrandt JC, Hunter C, Caspary DM. Alterations of GABAA receptor subunit mRNA levels in the aging Fischer 344 rat inferior colliculus. J Comp Neurol. 1997;379(3):455–465. doi: 10.1002/(sici)1096-9861(19970317)379:3<455::aid-cne10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Moeller CK, Kurt S, Happel MF, Schulze H. Long-range effects of GABAergic inhibition in gerbil primary auditory cortex. Eur J Neurosci. 2010;31(1):49–59. doi: 10.1111/j.1460-9568.2009.07039.x. [DOI] [PubMed] [Google Scholar]
- Mossop JE, Wilson MJ, Caspary DM, Moore DR. Down-regulation of inhibition following unilateral deafening. Hear Res. 2000;147(1–2):183–187. doi: 10.1016/s0378-5955(00)00054-x. [DOI] [PubMed] [Google Scholar]
- Muniak MA, Rivas A, Montey KL, May BJ, Francis HW, Ryugo DK. 3D model of frequency representation in the cochlear nucleus of the CBA/J mouse. J Comp Neurol. 2013;521(7):1510–1532. doi: 10.1002/cne.23238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashita H, Tabuchi K, Sakai S, Uemaetomari I, Tsuji S, Hara A. The effect of a GABAA agonist muscimol on acoustic injury of the mouse cochlea. Neurosci Lett. 2007;418(1):18–21. doi: 10.1016/j.neulet.2007.02.060. [DOI] [PubMed] [Google Scholar]
- Newman DL, Fisher LM, Ohmen J, Parody R, Fong CT, Frisina ST, Mapes F, Eddins DA, Robert Frisina D, Frisina RD, Friedman RA. RM7 variants associated with age-related hearing loss based on auditory perception. Hear Res. 2012;294(1–2):125–132. doi: 10.1016/j.heares.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D, Klocker N, Schuck J, Baukrowitz T, Ruppersberg JP, Fakler B. Gating of Ca+-activated K+ channels controls fast inhibitory synaptic transmission at auditory outer hair cells. Neuron. 2000;26:595–601. doi: 10.1016/s0896-6273(00)81197-6. [DOI] [PubMed] [Google Scholar]
- Osumi Y, Shibata SB, Kanda S, Yagi M, Ooka H, Shimano T, Asako M, Kawamoto K, Kuriyama H, Inoue T, Nishiyama T, Yamashita T, Tomoda K. Downregulation of N-methyl-D-aspartate receptor ζ1 subunit (GluN1) gene in inferior colliculus with aging. Brain Res. 2012;1454:23–32. doi: 10.1016/j.brainres.2012.03.018. [DOI] [PubMed] [Google Scholar]
- Peng Z, Wang GP, Zeng R, Guo JY, Chen CF, Gong SS. Temporospatial expression and cellular localization of VGLUT3 in the rat cochlea. Brain Res. 2013;1537:100–110. doi: 10.1016/j.brainres.2013.09.019. [DOI] [PubMed] [Google Scholar]
- Profant O, Balogová Z, Dezortová M, Wagnerová D, Hájek M, Syka J. Metabolic changes in the auditory cortex in presbycusis demonstrated by MR spectroscopy. Exp Gerontol. 2013;48(8):795–800. doi: 10.1016/j.exger.2013.04.012. [DOI] [PubMed] [Google Scholar]
- Raza A, Milbrandt JC, Arneric SP, Caspary DM. Age-related changes in brainstem auditory neurotransmitters : measures of GABA and acetylcholine function. Hear Res. 1994;77(1–2):221–230. doi: 10.1016/0378-5955(94)90270-4. [DOI] [PubMed] [Google Scholar]
- Ryals BM, Westbrook EW. Ganglion cell and hair cell loss in Coturnix quail associated with aging. Hear Res. 1988;36(1):1–8. doi: 10.1016/0378-5955(88)90133-5. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF. Further observations on the pathology of presbycusis. Arch Otolaryngol. 1964;80:369–382. doi: 10.1001/archotol.1964.00750040381003. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF, Gacek MR. Cochlear pathology in presbycusis. Ann Otol Rhinol Laryngol. 1993;102:1–16. doi: 10.1177/00034894931020S101. [DOI] [PubMed] [Google Scholar]
- Spongr VP, Flood DG, Frisina RD, Salvi RJ. Quantitative measures of hair cell loss in CBA and C57B1/6 mice throughout their life spans. J Acoust Soc Am. 1997;101:3546–3553. doi: 10.1121/1.418315. [DOI] [PubMed] [Google Scholar]
- Tadros SF, D’Souza M, Zettel ML, Zhu X, Waxmonsky NC, Frisina RD. Glutamate-related gene expression in CBA mouse inferior colliculus changes with age and hearing loss. Brain Res. 2007;1127:1–9. doi: 10.1016/j.brainres.2006.09.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XL, Gao M, Feng S, Su JP. Gamma-aminobutyric acid A receptor and N-methyl-D-aspartate receptor subunit expression in rat spiral ganglion neurons. Neural Regeneration Res. 2010;5(13):1020–1024. [Google Scholar]
- Tang YZ, Carr CE. Development of NMDA R1 expression in chicken auditory brainstem. Hear Res. 2004;191(1–2):79–89. doi: 10.1016/j.heares.2004.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada K, Sakaguchi H, Jarvis ED, Hagiwara M. Differential expression of glutamate receptors in avian neural pathways for learned vocalization. J Comp Neurol. 2004;476(1):44–64. doi: 10.1002/cne.20201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Inoue Y, Sakimura K, Mishina M. Developmental changes in distribution of NMDA receptor channel subunit mRNAs. Neuroreport. 1992;3(12):1138–1140. doi: 10.1097/00001756-199212000-00027. [DOI] [PubMed] [Google Scholar]
- White JA, Burgess BJ, Hall RD, Nadol JB. Pattern of degeneration of the spiral ganglion cell and its processes in the C57BL/6J mouse. Hear Res. 2000;141(1–2):12–18. doi: 10.1016/s0378-5955(99)00204-x. [DOI] [PubMed] [Google Scholar]
- Xu J, Yu L, Cai R, Zhang J, Sun X. Early continuous white noise exposure alters auditory spatial sensitivity and expression of GAD65 and GABAA receptor subunits in rat auditory cortex. Cereb Cortex. 2010;20(4):804–812. doi: 10.1093/cercor/bhp143. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Matsubara A, Ishii K, Makinae K, Sasaki A, Shinkawa H. Localization of g-Aminobutyric Acid A Receptor Subunits in the Rat Spiral Ganglion and Organ of Corti. Acta Otolaryngol. 2002;122(7):709–714. [PubMed] [Google Scholar]
- Yamasoba T, Someya S, Yamada C, Weindruch R, Prolla TA, Tanokura M. Role of mitochondrial dysfunction and mitochondrial DNA mutations in age-related hearing loss. Hear Res. 2007;226(1–2):185–193. doi: 10.1016/j.heares.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Zettel ML, Zhu X, O’Neill WE, Frisina RD. Age-related decline in Kv3.1b expression in the mouse auditory brainstem correlates with functional deficits in the medial olivocochlear efferent system. J Assoc Res Otolaryngol. 2007;8(2):280–293. doi: 10.1007/s10162-007-0075-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoli M, Picciotto MR, Ferrari R, Ferrari R, Cocchi D, Changeux JP. Increased neurodegeneration during ageing in mice lacking high-affinity nicotine receptors. EMBO J. 1999;18(5):1235–1244. doi: 10.1093/emboj/18.5.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]