Abstract
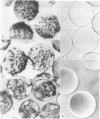
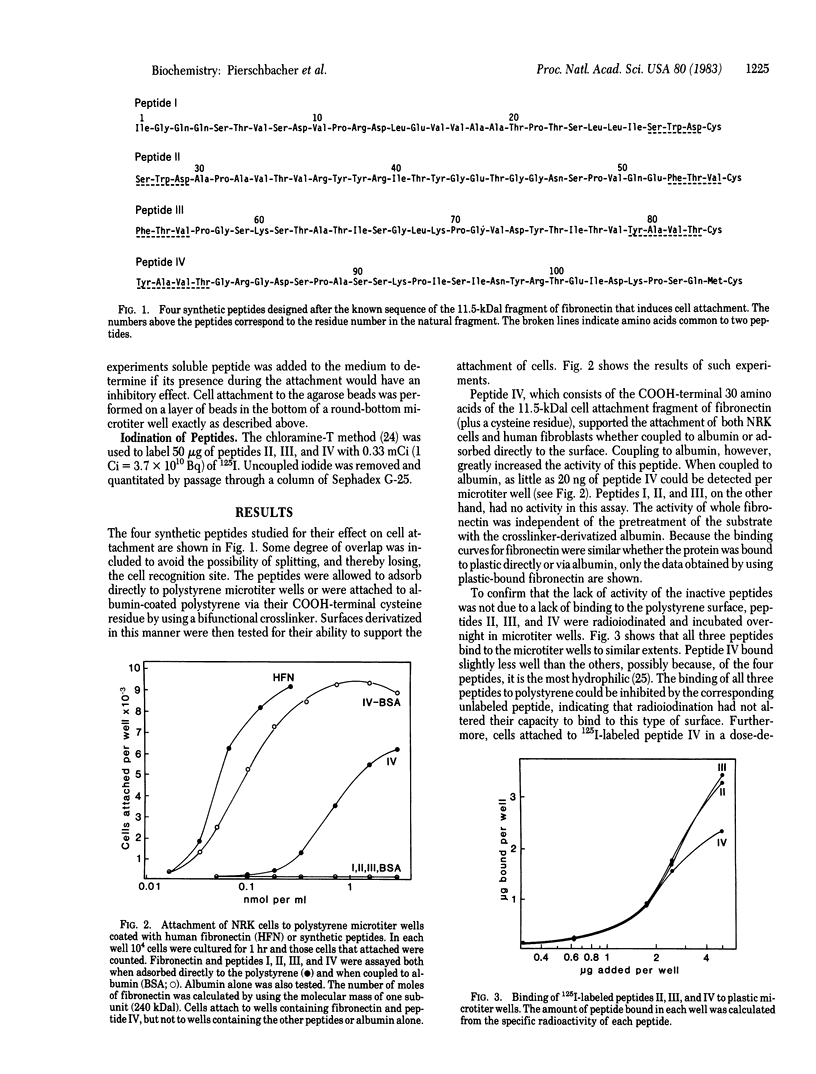
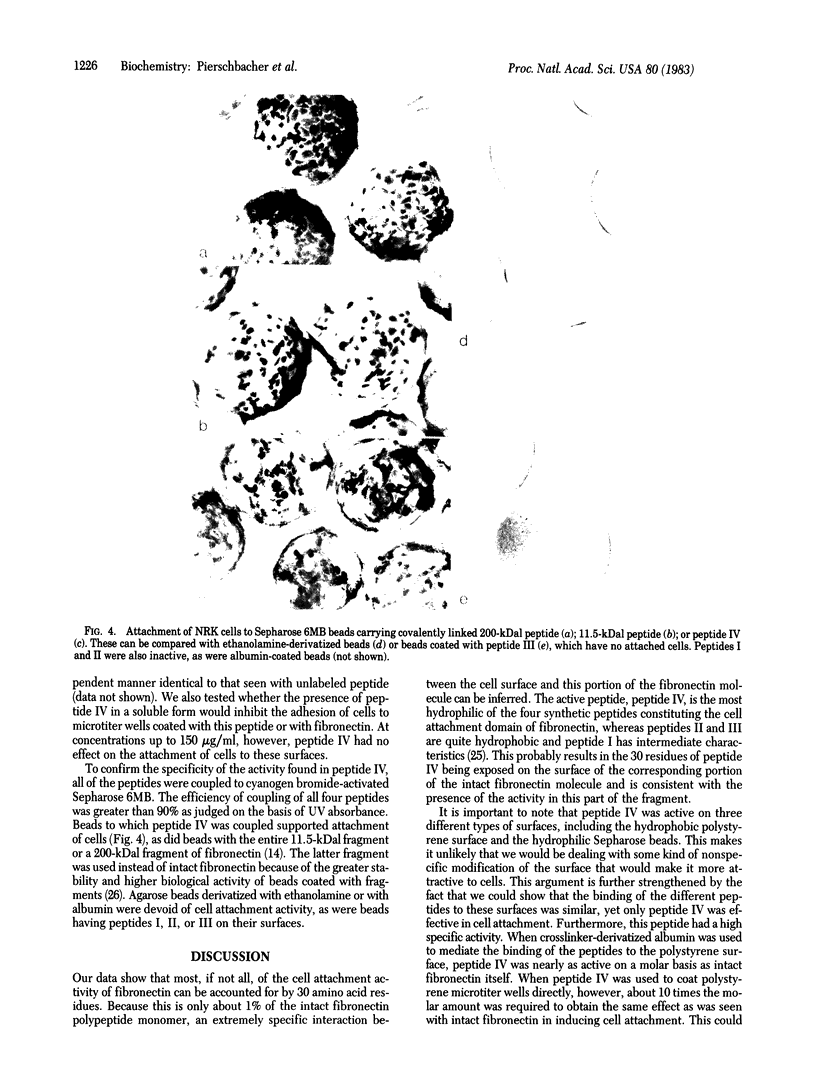
Four synthetic peptides that together constitute the cell attachment domain of fibronectin [Pierschbacher, M.D., Ruoslahti, E., Sundelin, J., Lind, P. & Peterson, P. (1982) J. Biol. Chem. 257, 9593-9597] were constructed and tested for their ability to induce cell attachment and spreading. One of these peptides, consisting of the 30 amino acid residues nearest the COOH terminus of the domain, contained all of the cell attachment activity of the whole domain. Under suitable conditions the peptide was approximately as active as intact fibronectin on a molar basis. The activity could be demonstrated by binding the peptide to polystyrene directly, or via albumin, or by coupling it to agarose beads. This synthetic peptide will be useful in the elucidation of the molecular details of the attachment of cells to fibronectin and could allow manipulation of the adhesive properties of cell culture surfaces and prosthetic materials.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dessau W., Adelmann B. C., Timpl R. Identification of the sites in collagen alpha-chains that bind serum anti-gelatin factor (cold-insoluble globulin). Biochem J. 1978 Jan 1;169(1):55–59. doi: 10.1042/bj1690055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrismann R., Roth D. E., Eppenberger H. M., Turner D. C. Arrangement of attachment-promoting, self-association, and heparin-binding sites in horse serum fibronectin. J Biol Chem. 1982 Jul 10;257(13):7381–7387. [PubMed] [Google Scholar]
- Engvall E., Bell M. L., Carlsson R. N., Miller E. J., Ruoslahti E. Nonhelical, fibronectin-binding basement-membrane collagen from endodermal cell culture. Cell. 1982 Jun;29(2):475–482. doi: 10.1016/0092-8674(82)90164-7. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F. Fibroblast receptor for cell-substratum adhesion: studies on the interaction of baby hamster kidney cells with latex beads coated by cold insoluble globulin (plasma fibronectin). J Cell Biol. 1980 Jul;86(1):104–112. doi: 10.1083/jcb.86.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F. Fibroblast receptor for cell-substratum adhesion: studies on the interaction of baby hamster kidney cells with latex beads coated by cold insoluble globulin (plasma fibronectin). J Cell Biol. 1980 Jul;86(1):104–112. doi: 10.1083/jcb.86.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn L. H., Yamada K. M. Isolation and biological characterization of active fragments of the adhesive glycoprotein fibronectin. Cell. 1979 Dec;18(4):1043–1051. doi: 10.1016/0092-8674(79)90217-4. [DOI] [PubMed] [Google Scholar]
- Jilek F., Hörmann H. Fibronectin (cold-insoluble globulin), VI. Influence of heparin and hyaluronic acid on the binding of native collagen. Hoppe Seylers Z Physiol Chem. 1979 Apr;360(4):597–603. doi: 10.1515/bchm2.1979.360.1.597. [DOI] [PubMed] [Google Scholar]
- Keski-Oja J., Yamada K. M. Isolation of an actin-binding fragment of fibronectin. Biochem J. 1981 Feb 1;193(2):615–620. doi: 10.1042/bj1930615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuusela P. Fibronectin binds to Staphylococcus aureus. Nature. 1978 Dec 14;276(5689):718–720. doi: 10.1038/276718a0. [DOI] [PubMed] [Google Scholar]
- Lahav J., Hynes R. O. Involvement of fibronectin, Von Willebrand factor, and fibrinogen in platelet interaction with solid substrata. J Supramol Struct Cell Biochem. 1981;17(4):299–311. doi: 10.1002/jsscb.380170402. [DOI] [PubMed] [Google Scholar]
- Mosher D. F. Fibronectin. Prog Hemost Thromb. 1980;5:111–151. [PubMed] [Google Scholar]
- Mosher D. F., Proctor R. A. Binding and factor XIIIa-mediated cross-linking of a 27-kilodalton fragment of fibronectin to Staphylococcus aureus. Science. 1980 Aug 22;209(4459):927–929. doi: 10.1126/science.7403857. [DOI] [PubMed] [Google Scholar]
- Pearlstein E. Substrate activation of cell adhesion factor as a prerequisite for cell attachment. Int J Cancer. 1978 Jul 15;22(1):32–35. doi: 10.1002/ijc.2910220108. [DOI] [PubMed] [Google Scholar]
- Perkins M. E., Ji T. H., Hynes R. O. Cross-linking of fibronectin to sulfated proteoglycans at the cell surface. Cell. 1979 Apr;16(4):941–952. doi: 10.1016/0092-8674(79)90109-0. [DOI] [PubMed] [Google Scholar]
- Pierschbacher M. D., Hayman E. G., Ruoslahti E. Location of the cell-attachment site in fibronectin with monoclonal antibodies and proteolytic fragments of the molecule. Cell. 1981 Oct;26(2 Pt 2):259–267. doi: 10.1016/0092-8674(81)90308-1. [DOI] [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E., Sundelin J., Lind P., Peterson P. A. The cell attachment domain of fibronectin. Determination of the primary structure. J Biol Chem. 1982 Aug 25;257(16):9593–9597. [PubMed] [Google Scholar]
- Plow E. F., Ginsberg M. H. Specific and saturable binding of plasma fibronectin to thrombin-stimulated human platelets. J Biol Chem. 1981 Sep 25;256(18):9477–9482. [PubMed] [Google Scholar]
- Rose G. D. Prediction of chain turns in globular proteins on a hydrophobic basis. Nature. 1978 Apr 13;272(5654):586–590. doi: 10.1038/272586a0. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Engvall E. Complexing of fibronectin glycosaminoglycans and collagen. Biochim Biophys Acta. 1980 Aug 13;631(2):350–358. doi: 10.1016/0304-4165(80)90308-6. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Hayman E. G., Engvall E., Cothran W. C., Butler W. T. Alignment of biologically active domains in the fibronectin molecule. J Biol Chem. 1981 Jul 25;256(14):7277–7281. [PubMed] [Google Scholar]
- Ruoslahti E., Hayman E. G., Pierschbacher M., Engvall E. Fibronectin: purification, immunochemical properties, and biological activities. Methods Enzymol. 1982;82(Pt A):803–831. doi: 10.1016/0076-6879(82)82103-4. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Hayman E. G. Two active sites with different characteristics in fibronectin. FEBS Lett. 1979 Jan 15;97(2):221–224. doi: 10.1016/0014-5793(79)80088-5. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Vaheri A. Interaction of soluble fibroblast surface antigen with fribrinogen and fibrin. J Exp Med. 1975 Feb 1;141(2):497–501. doi: 10.1084/jem.141.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonen E. M., Vaheri A. Immobilization of viral and mycoplasma antigens and of immunoglobulins on polystyrene surface for immunoassays. J Immunol Methods. 1979;30(3):209–218. doi: 10.1016/0022-1759(79)90095-4. [DOI] [PubMed] [Google Scholar]
- Sekiguchi K., Fukuda M., Hakomori S. Domain structure of hamster plasma fibronectin. Isolation and characterization of four functionally distinct domains and their unequal distribution between two subunit polypeptides. J Biol Chem. 1981 Jun 25;256(12):6452–6462. [PubMed] [Google Scholar]
- Stathakis N. E., Mosesson M. W., Chen A. B., Galanakis D. K. Cryoprecipitation of fibrin-fibrinogen complexes induced by the cold-insoluble globulin of plasma. Blood. 1978 Jun;51(6):1211–1222. [PubMed] [Google Scholar]
- Stathakis N. E., Mosesson M. W. Interactions among heparin, cold-insoluble globulin, and fibrinogen in formation of the heparin-precipitable fraction of plasma. J Clin Invest. 1977 Oct;60(4):855–865. doi: 10.1172/JCI108840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemberger A., Hörmann H. Affinity chromatography on immobilized fibrinogen and fibrin monomer, II. The behavior of cold-insoluble globulin [1]. Hoppe Seylers Z Physiol Chem. 1976 Jul;357(7):1003–1005. [PubMed] [Google Scholar]